Abstract
Combined pulmonary fibrosis and emphysema (CPFE) has been increasingly recognized over the past 10–15 years as a clinical entity characterized by rather severe imaging and gas exchange abnormalities, but often only mild impairment in spirometric and lung volume indices. In this review, we explore the gas exchange and mechanical pathophysiologic abnormalities of pulmonary emphysema, pulmonary fibrosis, and combined emphysema and fibrosis with the goal of understanding how individual pathophysiologic observations in emphysema and fibrosis alone may impact clinical observations on pulmonary function testing (PFT) patterns in patients with CPFE. Lung elastance and lung compliance in patients with CPFE are likely intermediate between those of patients with emphysema and fibrosis alone, suggesting a counter-balancing effect of each individual process. The outcome of combined emphysema and fibrosis results in higher lung volumes overall on PFTs compared to patients with pulmonary fibrosis alone, and the forced expiratory volume in one second (FEV1)/forced vital capacity (FVC) ratio in CPFE patients is generally preserved despite the presence of emphysema on chest computed tomography (CT) imaging. Conversely, there appears to be an additive deleterious effect on gas exchange properties of the lungs, reflecting a loss of normally functioning alveolar capillary units and effective surface area available for gas exchange, and manifested by a uniformly observed severe reduction in the diffusing capacity for carbon monoxide (DLCO). Despite normal or only mildly impaired spirometric and lung volume indices, patients with CPFE are often severely functionally impaired with an overall rather poor prognosis. As chest CT imaging continues to be a frequent imaging modality in patients with cardiopulmonary disease, we expect that patients with a combination of pulmonary emphysema and pulmonary fibrosis will continue to be observed. Understanding the pathophysiology of this combined process and the abnormalities that manifest on PFT testing will likely be helpful to clinicians involved with the care of patients with CPFE.
1. Introduction
Pulmonary emphysema and pulmonary fibrosis are common chronic lung diseases which may result in mild pulmonary impairment or end-stage lung disease with chronic respiratory failure. Emphysema is most often caused by long-term exposure to cigarette smoke and is one of the major pathobiologic processes leading to the clinical phenotype of chronic obstructive pulmonary disease (COPD). Pulmonary fibrosis results from numerous diverse environmental, occupational or autoimmune etiologies and is included within the broad category of interstitial lung disease (ILD). Consequently, emphysema and fibrosis are often considered distinct entities with unique pathophysiologic manifestations, but over the past 10–15 years, there has been increasing recognition that these two processes may coexist in individual patients, and this overlapping disorder has often been termed combined emphysema and fibrosis or combined pulmonary fibrosis and emphysema (CPFE). During this time, there have been numerous original research studies, review articles and commentaries discussing the phenotype and physiologic characteristics of patients with CPFE, as well as the prognosis and clinical outcomes in these patients [1,2,3].
A common observation in patients with combined emphysema and fibrosis has been rather severe gas exchange abnormalities, manifested by hypoxemia and an almost universal moderate to severe reduction in diffusing capacity for carbon monoxide (DLCO) [1,2,3,4]. Despite these gas exchange abnormalities, one of the interesting and consistent findings across many studies has been only mild impairment in spirometric and lung volume indices, along with a normal forced expiratory volume in one second (FEV1)/forced vital capacity (FVC) ratio despite the presence of emphysema on chest CT imaging [1,2,3,4]. The presence of only mild impairment in spirometry along with a preserved FEV1/FVC ratio has led to the consideration that the abnormal respiratory mechanics of emphysema are being counterbalanced in some manner by the abnormal respiratory mechanics of pulmonary fibrosis. This observation has been sometimes referred to as pseudo-normalization of spirometry and lung volume parameters in these patients with CPFE [5,6].
The purpose of this review is to discuss pathophysiologic disturbances in pulmonary emphysema, pulmonary fibrosis, and combined emphysema and fibrosis, and to discuss the results of numerous studies which have described pulmonary function (PFT) parameters in these CPFE patients. Hopefully this discussion will help the reader better understand PFT findings and gas exchange abnormalities in patients with combined emphysema and fibrosis.
2. Pulmonary Emphysema
Emphysema is a chronic lung disease characterized pathologically by destruction of extracellular matrix and enlargement of airspaces distal to the terminal bronchiole [7]. Emphysema is most commonly caused by exposure to cigarette smoke, although may be caused by exposure to a wide array of environmental vapors, fumes or dusts, and is most commonly identified by low attenuation areas of the lung parenchyma without clearly definable walls on computed tomography (CT) scan of the chest [8,9,10].
The presence of emphysema has several pathophysiologic consequences which cause both gas exchange and mechanical abnormalities. Within emphysematous spaces, ventilation is impaired, but perfusion is generally impaired to a greater degree or may be completely absent, leading to the widespread development of alveolar capillary units with high ventilation-perfusion ratios [11,12]. Alveolar capillary units with high ventilation-perfusion ratios lead to increased physiologic dead space, inefficient (or wasted) ventilation, and increased minute ventilation requirements which are necessary to preserve alveolar ventilation. Worsening hyperinflation of the lungs during an exacerbation in patients with emphysema may additionally contribute to further ventilation-perfusion imbalance [13]. An inability over time to maintain a normal level of alveolar ventilation will lead to both chronic hypoxemia and hypercapnia [14,15]. High minute ventilation requirements in emphysema result in increased work of breathing, increased overall energy expenditure, and at times cachexia in patients with advanced emphysema [16]. Due to the destruction of extracellular matrix in emphysema, the number of normally functioning alveolar capillary units is reduced, resulting in a loss of effective surface area for gas exchange. Anatomic loss of effective surface area will be manifested by a reduced diffusing capacity of the lung for carbon monoxide (DLCO) and will be associated physiologically with ventilation-perfusion inequality, which in emphysema will be mostly areas of high ventilation-perfusion mismatch. A reduced DLCO in emphysema has been correlated with overall severity of disease and oxygen desaturation with exercise [17,18].
In regard to lung mechanics, destruction of extracellular matrix and enlargement of air spaces in emphysema causes a reduction in elastic recoil forces of the lung [11,19]. Due to the inverse relationship between elastance and compliance, lung compliance (distensibility of the lung) in emphysema is increased. A reduction in elastic recoil forces of the lung in emphysema results in several unwanted outcomes. First, airway collapse on forced expiration is accentuated, resulting in a reduced FEV1 and reduced FEV1/FVC ratio, and thus an obstructive ventilatory defect on spirometry. Along with intrinsic airway resistance, lung elastic recoil forces are a major determinant of the amount of airflow on expiration. Elastic recoil forces provide radial traction support to small airways during the breathing cycle, in addition to their effect on lung compliance. When elastic recoil forces are reduced, radial traction forces on small airways are reduced, and on subsequent forced expiration, airway collapse is accentuated and FEV1, FEV1/FVC, and rates of airflow are all reduced. All normal healthy persons have some degree of airway collapse on forced expiration, manifested on spirometry as a FEV1/FVC ratio of approximately 0.75–0.80 in healthy middle-aged adults, but in patients with emphysema, airway collapse on forced expiration is excessive, and the expiratory time constant, a product of lung compliance and airway resistance, is prolonged [20].
Second, a reduction in elastic recoil forces results in an increased functional residual capacity (FRC) and total lung capacity (TLC), often termed static hyperinflation [11,21]. Resting FRC (or end-expiratory lung volume (EELV)) is defined as the lung volume at which elastic recoil forces of the lung inward are numerically equal but opposite to elastic recoil forces of the chest wall outward [14,21]. Since elastic recoil forces in emphysema are reduced, the resting volume at which inward lung forces equilibrate with outward chest wall forces occurs at a higher lung volume. Similarly, TLC is often increased in emphysema due to reduced inward elastic recoil forces of the lung at full inspiration, as assessed by pressure-volume curves in emphysema patients. [11,21]. Since TLC is usually only mildly increased, which is due to normal chest wall mechanics and compliance in emphysema limiting the extent to which the thorax can ultimately expand, an elevated FRC reduces inspiratory capacity (IC) and limits the increase in tidal volume (VT) which occurs in response to increased ventilatory demands from exercise or a variety of other stressors. Additionally, in emphysema, EELV may increase even further during exercise (termed dynamic hyperinflation), which occurs due to expiratory airflow limitation coupled with shortened expiratory time, and which further reduces IC, alters lung compliance, and limits potential increases in VT [21]. Dynamic hyperinflation can additionally impair venous return to the thorax, further impairing gas exchange and cardiopulmonary hemodynamics.
Third, residual volume (RV) is increased in emphysema, often termed air trapping. Residual volume in the normal lung results from closure of airways at the end of exhalation, and is elevated in emphysema since reduced elastic recoil forces lead to airway closure at a lung volume which is abnormally high [19]. Increased propensity to airway closure in emphysema is also reflected in an abnormally high closing volume (CV) and closing capacity (CC), both of which can be determined from the single breath nitrogen washout test and refer to the lung volume above RV at which the onset of airway closure occurs [22,23]. A substantially elevated RV in the presence of a mildly increased TLC leads to an elevated RV/TLC ratio in emphysema, and results in a significantly reduced vital capacity (VC). Overall, in addition to gas exchange abnormalities which are present, reduced elastic recoil forces in emphysema result in static hyperinflation, dynamic hyperinflation, and excessive airflow limitation on expiration, all of which are likely major contributors to resting dyspnea and reduced exercise capacity in patients with emphysema [21,24].
3. Pulmonary Fibrosis
In contrast to emphysema, pulmonary fibrosis has most often been described as a pathobiologic process in which an excess of extracellular matrix accumulates, and is characterized by a combination of excessive extracellular matrix production, alveolar epithelial cell loss, and permanent alveolar collapse [25,26,27,28]. The effects of pulmonary fibrosis on gas exchange and mechanical properties of the lung are well characterized. Pulmonary fibrosis may be caused by a myriad of environmental exposures, medication toxicities, or autoimmune mechanisms, or may develop without a well-defined etiology and be identified as idiopathic pulmonary fibrosis (IPF) [29]. Pulmonary fibrosis is identified on chest CT imaging by a combination of findings including reticulation, volume loss, traction bronchiectasis, and honeycombing [30].
The development of pulmonary fibrosis alters ventilation-perfusion relationships within the lung [12,15,19]. In the fibrotic lung, there are likely numerous areas in which alveolar capillary units demonstrate low ventilation-perfusion ratios, characterized by greater impairment in ventilation compared to perfusion at the alveolar level. Alveolar capillary units with low ventilation-perfusion ratios and those with shunt (absent ventilation with preserved perfusion) cause hypoxemia both at rest and with exertion, and patients with advanced pulmonary fibrosis almost always require supplemental oxygen. Although ventilation-perfusion mismatch is likely the major cause of hypoxemia in patients with pulmonary fibrosis, a diffusion impairment in oxygen transfer may additionally contribute to hypoxemia both at rest and on exertion in patients with severe disease as a result of increased thickness of the alveolar capillary membrane [14,15,31]. In other areas of the fibrotic lung, there are likely alveolar capillary units with high ventilation-perfusion ratios, and along with areas of true dead space (absent perfusion with preserved ventilation), result in inefficient ventilation and higher minute ventilation requirements in order to preserve alveolar ventilation. Similar to emphysema but albeit by different mechanisms, the number of normally functioning alveolar capillary units in pulmonary fibrosis is reduced, likely resulting from a combination of excess production of extracellular matrix, pulmonary vascular abnormalities, alveolar epithelial cell loss and permanent alveolar collapse. This loss of normally functioning alveolar capillary units results in a loss of effective surface area for gas exchange, ventilation-perfusion inequality, and a reduced DLCO [12,32,33]. A reduced DLCO is associated with severity of disease and poor outcomes in patients with pulmonary fibrosis [34,35].
In contrast to emphysema, the development of pulmonary fibrosis results in increased elastic recoil forces of the lung (thus increased elastance) and therefore reduced lung compliance (reduced distensibility of the lung) [19,31]. The increased elastance and reduced compliance result in overall low lung volumes in most patients, manifested as a reduction in FEV1, VC, FRC, and TLC [19,31,36], and thus full pulmonary function testing (PFTs) will manifest a restrictive ventilatory defect. Resting FRC is reduced since the increased inward recoil force of the lung will equilibrate with the outward recoil force of the chest wall at a lower lung volume, and TLC is mildly to moderately reduced due to markedly increased inward elastic recoil forces of the lung at full inspiration [36]. RV is often relatively preserved in pulmonary fibrosis, especially if cystic change (honeycombing) and/or small airway disease are present as part of the fibrotic process [36]. Since TLC is usually decreased proportionally to a greater degree than RV, the RV/TLC ratio is often elevated and the VC is usually significantly reduced [33,36,37]. Lung volumes including VC and TLC may be relatively preserved in early or mild fibrotic disease due to normal chest wall compliance and normal respiratory muscle strength, even though lung elastance is uniformly increased. The FEV1/FVC ratio in pulmonary fibrosis is preserved or even increased above normal in most instances, even though the absolute values of FEV1 and FVC are reduced [32,33,36]. This occurs as a result of increased elastic recoil forces in pulmonary fibrosis which tether small airways open and therefore reduce the tendency for airway collapse on forced expiration. A significantly elevated FEV1/FVC ratio should alert the clinician to the possibility of ILD and/or pulmonary fibrosis.
Work of breathing is also elevated in patients with pulmonary fibrosis during normal breathing and with exercise as a result of the work required to overcome the reduced compliance and increased elastance [36,38]. The constellation of gas exchange abnormalities, increased lung elastance, reduced ventilatory capacity and increased work of breathing all lead to dyspnea and reduced exercise capacity in patients with pulmonary fibrosis.
4. Combined Emphysema and Fibrosis
There has been increasing recognition over the past 10–15 years that pulmonary emphysema and pulmonary fibrosis may coexist in individual patients and has been most often referred to as CPFE. In the above sections, we have discussed the abnormalities in elastance and compliance that occur in isolated emphysema and isolated pulmonary fibrosis. From a simplistic view, one could postulate that the mechanical abnormalities resulting from a combination of emphysema and fibrosis would be additive in a deleterious way, resulting in a further decline in spirometric and lung volume indices compared to the presence of either process alone. This additive concept appears to be valid when applied to gas exchange properties of the lungs, as patients with CPFE have severe gas exchange abnormalities manifested by a severe reduction in DLCO (Table 1). This DLCO observation seems very plausible since both emphysema and fibrosis reduce the number of normally functioning alveolar capillary units and thus reduce the effective surface area available for gas exchange. In regard to mechanical properties of the lungs however, patients with CPFE have been shown to most often have only mild impairment in spirometric and lung volume indices despite rather severe imaging and gas exchange abnormalities. This observation suggests the abnormal lung mechanics of each individual process are likely being counter-balanced which results in relative preservation of lung volumes and elastic recoil properties [1,2,4,5,6,39].
The precise pathobiology which leads to CPFE in individual patients likely has various mechanisms, although cigarette smoke appears to have a major role. From a genetic standpoint, numerous studies have examined genetic profiling in patients with advanced emphysema, and abnormalities have been observed in mechanisms related to inflammation, oxidative stress, extracellular matrix synthesis, epithelial and endothelial cell function, cell-cell signaling, cell migration and cell senescence [40,41]. Recent genome-wide association studies have also identified numerous loci related to the function of endothelial cells, type I and II alveolar epithelial cells, fibroblasts and smooth muscle cells in COPD [42] and familial forms of emphysema have been caused by mutations related to telomere biology and protease-anti-protease imbalance (alpha-1 antitrypsin) [43]. In pulmonary fibrosis, numerous studies have likewise examined gene expression profiling, and abnormalities in mechanisms related to inflammation, immune system activation, intracellular metabolic processes, alveolar epithelial cells, mesenchymal cells and cell senescence have been observed [28,44,45]. Additionally, mutations have been identified in individual genes in patients with familial forms of pulmonary fibrosis, and have included mutations related to surfactant protein biology, telomere biology, and mucin biology [46,47]. Based on the above, there appear to be numerous overlapping mechanistic abnormalities in gene expression in patients with pulmonary emphysema and patients with pulmonary fibrosis [48,49]. To our knowledge, only a few studies have examined genetics related to CPFE. One recent study demonstrated enrichment of immunoglobulin associated genes in the fibrotic areas of CPFE, whereas enrichment of genes related to cell membrane structures and vascular growth was observed in the emphysematous areas [50]. Based on all current available evidence of genetic profiling in patients with these diseases, it is unknown at the present time why individual patients with a history of smoking develop pulmonary emphysema, pulmonary fibrosis, or CPFE.
Three potential timelines of disease development in CPFE could be postulated. Pulmonary emphysema may develop initially over many years as the initial injury process due to cigarette smoking and followed subsequently at some later time point by development of pulmonary fibrosis. The development of pulmonary fibrosis in this scenario may also be a result of long-term smoking, since cigarette smoke is a well-documented, but likely often overlooked, cause of excess collagen accumulation and fibrosis in the lung [51,52]. Alternatively, the development of fibrosis in this timeline may result from any number of environmental/avocational/occupational exposures or autoimmune mechanisms which are unrelated to both cigarette smoking and the presence of emphysema. Second, emphysema and fibrosis may both develop concurrently as a result of cigarette smoking, and in this instance, the findings of emphysema and fibrosis are likely to be located in a similar geographic distribution within the lung. Third, pulmonary fibrosis may be the initial process to develop which is then followed subsequently by the development of emphysema. This scenario may not seem quite as plausible based on clinical observations, but inflammation, fibrosis, and destruction of small airways as part of cigarette smoke-induced injury has been postulated as a precursor to development of parenchymal emphysema in some instances [53,54]. Additionally, centrilobular emphysema is generally considered the prototype of cigarette smoking-induced emphysema, but many patients with pulmonary fibrosis exhibit significant paraseptal emphysema, suggesting that the fibrotic process in some manner may be contributing to the development of paraseptal emphysema [55,56,57].
Various CT imaging patterns have been observed in patients with combined emphysema and fibrosis (Figure 1). Patients may demonstrate predominantly emphysema in the upper lobes and predominantly pulmonary fibrosis in the lower lobes (Figure 1C), emphysema in the upper lobes with diffuse ground-glass opacities and fibrotic change both in the upper and lower lobes, or enlarged emphysematous spaces surrounded by thickened fibrotic walls often in a predominantly upper lobe distribution (Figure 1D). In this latter pattern, the findings of emphysema and fibrosis exist in a similar geographic distribution within the lung, and this pattern has often been referred to as airspace enlargement with fibrosis [58,59]. Each of these different radiographic patterns can be described under the broad heading of CPFE, which highlights the lack of a universal precise characterization of patients with CPFE. One could speculate on whether there is a correlation between a particular imaging pattern and a particular timeline of disease development as described in the preceding paragraph, but an accurate association in this regard is unknown at present.
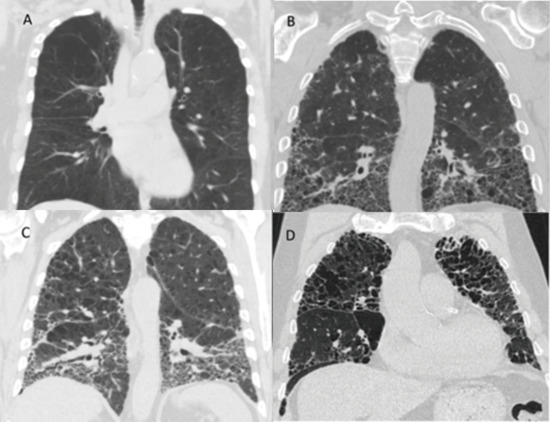
Figure 1.
Representative chest computed tomography (CT) images from selected patients from the authors’ institution with emphysema alone (A), pulmonary fibrosis alone (B), upper lobe emphysema with lower lobe pulmonary fibrosis (C), and upper lobe emphysema with thickened fibrotic walls, also referred to as air space enlargement with fibrosis (D).
Elastic recoil properties of the lungs and lung compliance are difficult to measure routinely in the clinical setting, but the effects of these processes can be assessed with standard spirometry and lung volume testing. In the ensuing sections, we will discuss the results of published studies which have compared standard PFT indices in patients with CPFE to those with pulmonary fibrosis alone. Despite differing imaging patterns of combined emphysema and fibrosis, it seems plausible that the resulting effects on lung mechanics manifested on standard PFT testing would be similar, although patients with basilar fibrosis in CPFE may be expected to have more gas exchange impairment due to greater lower lobe perfusion in general.
Table 1 shows the results of 11 studies over the past 15 years in which PFT parameters in patients with pulmonary fibrosis alone (PF) were compared to patients with CPFE, and each study had at least ten patients in the CPFE group. The primary objectives of each of these studies were not always identical, as some were aimed at outcomes in these two patient groups, some at the value of a composite physiologic index (CPI) for patients with fibrosis and emphysema, and some at longitudinal PFT changes in CPFE patients, but this published data does allow a reasonable comparison of PFT parameters from these two patient groups. Most studies that have examined PFTs in CPFE have compared CPFE to PF, but one recent study did compare patients with CPFE to patients with COPD [57] and extending the comparison of pulmonary function in CPFE to emphysema alone seems reasonable given the numerous published PFT studies in patients with emphysema over many years [60,61].
5. Total Lung Capacity (TLC)
As seen in Table 1, TLC was higher in patients with CPFE compared to patients with PF alone, and TLC was either normal or close to normal in CPFE patients in most of the studies. TLC is elevated in most patients with emphysema [61] and mildly to moderately reduced in most patients with PF alone, as seen in Table 1, and thus reduced elastic recoil forces associated with emphysema are likely resulting in a higher TLC in CPFE patients compared to patients with PF alone. One other study demonstrated that in patients with IPF, a greater number of pack-years of smoking correlated with an increased TLC, although this study did not directly compare patients with PF alone to CPFE [62]. Overall, most published data support the concept that patients with CPFE usually have an intermediate value for TLC which rests between higher values associated with emphysema alone and lower values associated with fibrosis alone.
6. Functional Residual Capacity (FRC)
Only one study in Table 1 published values for FRC, and thus we did not include FRC results in the Table. In this study, FRC percent predicted was 76 ± 22 in PF compared to 78 ± 19 in CPFE, which was not statistically different [63]. In our previous study which is shown in Table 1, we did not originally publish our FRC data, but values recorded for FRC percent predicted were 54 ± 19 in PF compared to 75 ± 32 in CPFE (p = 0.0014) [55]. Similar to TLC, a prior study demonstrated that a greater number of pack-years of smoking in patients with IPF correlated with an increased FRC, although again this study did not directly compare patients with PF alone to CPFE [62]. Overall, based on principles of elastic recoil, it would seem plausible that FRC values in patients with CPFE would be higher than patients with PF alone, but there is not much published data in this regard.
7. Residual Volume (RV)
Six of the studies in Table 1 reported values for RV. In three of the studies, RV was significantly higher in patients with CPFE compared to PF alone, whereas RV was statistically unchanged in the remaining three. Similar to FRC and TLC, a greater number of pack-years of smoking correlated with an increased RV in patients with IPF [62] Since patients with advanced emphysema will uniformly have an elevated RV [60,61], it seems possible that the reduced elastic recoil forces related to the emphysematous component in CPFE would lead to lead to a higher RV in these patients compared to PF alone. However, RV may be relatively preserved even in patients with advanced pulmonary fibrosis [36], which may lead to not much overall difference in RV when comparing CPFE patients to those with PF alone. Overall, there is not a lot of published data on RV in patients with CPFE.
8. Forced Vital Capacity (FVC)
As seen in Table 1, FVC was uniformly higher in patients with CPFE compared to those with PF alone, having reached statistical significance in seven of the 11 studies and trended higher in the remaining four. Since FVC is the difference in volume between TLC and RV, patients with CPFE must in some combination have a greater volume difference between TLC and RV. As seen in Table 1, the majority of studies demonstrate higher TLC values in patients with CPFE compared to PF alone, whereas RV results have been either variable or not reported. More published data on RV values in patients with CPFE would likely help clarify conclusions in regard to relationships between FVC, TLC and RV in these patients.
9. Forced Expiratory Volume in One Second (FEV1)/Forced Vital Capacity (FVC) Ratio
Although the FEV1/FVC ratio was generally preserved in CPFE patients and above 0.70 in most studies, the ratio was rather uniformly reduced in patients with CPFE compared to PF alone, having reached statistical significance in nine of the 11 studies and trended lower in the remaining two, as seen in Table 1. The lower FEV1/FVC ratio in patients with CPFE compared to patients with PF alone suggests differing elastic recoil forces in these two groups of patients, which may potentially be related to the emphysema component in patients with CPFE.
10. Forced Expiratory Volume in One Second (FEV1)
As shown in Table 1, eight studies reported FEV1 and all but one of the studies showed no statistically significant difference in the FEV1 between the CPFE and PF groups. This may be a little counterintuitive if all lung volumes in general are higher in CPFE patients, but is consistent with the data for FVC and FEV1/FVC ratio described above. Even though FVC is higher on average in patients with CPFE compared to PF, the FEV1/FVC ratio is lower on average, and thus having an FEV1 value which is not different between the two groups seems very plausible. Overall, FEV1 values expressed as percent predicted appear normal or mildly reduced in most studies of CPFE.
11. Diffusing Capacity for Carbon Monoxide (DLCO)
The DLCO is moderate to severely low in patients with CPFE, as shown in Table 1, which is a very consistent and characteristic finding in all studies of CPFE patients [2], and is consistent with the effects of each individual process (emphysema and fibrosis) reducing the amount of effective surface area that is available for gas exchange. In five of the studies, a statistically lower DLCO value was observed in the CPFE compared to the PF patients, consistent with an additive effect of emphysema combined with fibrosis, whereas DLCO was not different between the two groups in the remaining studies.
Although not specific to CPFE, one concept which deserves mentioning in regard to assessment of DLCO is the DLCO/VA [31,64,65,66], as the DLCO/VA has been reported in some studies of CPFE patients in addition to reporting DLCO. The DLCO is calculated by multiplying the alveolar volume (VA) by the KCO, which is the rate constant for uptake of CO from alveolar gas. Therefore, KCO can be and is often expressed as DLCO/VA. Evaluating and drawing conclusions from DLCO/VA has been discussed for many years in pulmonary medicine, likely with the goal of attempting to assess whether a reduced DLCO is merely a reflection of reduced lung volume or is in fact reduced as a result of abnormal gas exchange properties of the lung in addition to any lung volume reduction. As many authors have pointed out, interpreting the DLCO/VA can be complex. It has been well-established that DLCO/VA (KCO) is not constant as lung volume changes, should not be interpreted as a correction for lung volume, and actually increases as lung volume decreases from TLC to FRC in the healthy adult [31,64,65]. With standard PFT testing, predicted values for DLCO/VA are provided for a normal TLC volume, thus there are no routinely provided predicted values for DLCO/VA at differing lung volumes in individual patients. Consequently, in our judgment, assessing the measured DLCO as opposed to the DLCO/VA is likely to provide a more accurate assessment of the true gas exchange properties of the lungs in patients with CPFE.

Table 1.
Published pulmonary function test (PFT) parameters comparing patients with pulmonary fibrosis alone (PF) to patients with combined pulmonary fibrosis and emphysema (CPFE).
Table 1.
Published pulmonary function test (PFT) parameters comparing patients with pulmonary fibrosis alone (PF) to patients with combined pulmonary fibrosis and emphysema (CPFE).
| Year | FVC % Predicted ± SD | FEV1 % Predicted ± SD | FEV1/FVC Ratio ± SD | TLC % Predicted ± SD | RV % Predicted ± SD | DLCO % Predicted ± SD | Fibrosis Score | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PF | CPFE | PF | CPFE | PF | CPFE | PF | CPFE | PF | CPFE | PF | CPFE | ||
| 2006 [67] | 70 ± 22 | 77 ± 20 | 77 ± 26 | 76 ± 31 | 0.83 ± 0.07 | 0.74 ± 0.18 | 71 ± 18 | 95 ± 25 * | 73 ± 29 | 111 ± 49 * | 49 ± 18 | 48 ± 26 | similar |
| 2009 [56] | 59 ± 18 | 62 ± 16 | 67 ± 20 | 70 ± 15 | 0.93 ± 0.11 | 0.91 ± 0.09 | nr | nr | nr | nr | nr | nr | * |
| 2009 [63] | 73 ± 19 | 86 ± 24 * | nr | nr | 0.85 ± 0.07 | 0.77 ± 0.09 * | 70 ± 16 | 78 ± 17 | 76 ± 27 | 75 ± 23 | 61 ± 20 | 45 ± 15 * | nr |
| 2010 [68] | 62 ± 16 | 77 ± 14 * | 67 ± 15 | 71 ± 20 | 0.78 ± 0.09 | 0.67 ± 0.12 * | 66 ± 15 | 76 ± 11 * | 73 ± 30 | 75 ± 24 | 50 ± 22 | 29 ± 11 * | nr |
| 2010 [69] | 72 ± 19 | 87 ± 17 * | 87 ± 21 | 88 ± 20 | 0.81 ± 0.08 | 0.70 ± 0.12 * | 77 ± 16 | 94 ± 17 * | nr | nr | 74 ± 20 | 65 ± 21 * | similar |
| 2011 [70] | 65 ± 14 | 76 ± 15 | 77 ± 17 | 84 ± 16 | 0.84 ± 0.06 | 0.78 ± 0.07 * | nr | nr | nr | nr | 46 ± 14 | 42 ± 16 | nr |
| 2011 [55] § | 51 ± 17 | 64 ± 19 * | 59 ± 20 | 66 ± 15 | 0.84 ± 0.06 | 0.78 ± 0.10 * | 50 ± 16 | 66 ± 16 * | 49 ± 19 | 77 ± 35 *,† | 30 ± 15 | 29 ± 14 | similar |
| 2013 [1] | 65 ± 17 | 80 ± 16 * | 71 ± 18 | 80 ± 17 * | 0.83 ± 0.07 | 0.74 ± 0.06 * | 66 ± 13 | 79 ± 14 * | 66 ± 23 | 74 ± 30 | 44 ± 15 | 37 ± 14 * | * |
| 2014 [71] | 74 ± 19 | 94 ± 22 * | nr | nr | 0.86 ± 0.07 | 0.78 ± 0.12 * | 72 ± 15 | 90 ± 16 * | 78 ± 22 | 99 ± 27 * | 54 ± 18 | 50 ± 14 | * |
| 2014 [72] | 68 ± 28 | 83 ± 22 | nr | nr | 0.82 ± 0.04 | 0.77 ± 0.03 * | nr | nr | nr | nr | 57 ± 27 | 37 ± 18 * | nr |
| 2019 [73] | 75 ± 16 | 93 ± 12 * | 88 ± 18 | 96 ± 14 | 0.83 ± 0.08 | 0.72 ± 0.10 * | 74 ± 13 | 90 ± 11 * | nr | nr | 67± 19 | 58 ± 21 | similar |
FVC = Forced vital capacity; FEV1 = Forced expiratory volume in one second; TLC = Total lung capacity; RV = Residual volume; DLCO = Diffusing capacity for carbon monoxide; SD = Standard deviation; PF = Pulmonary fibrosis alone; CPFE = Combined pulmonary fibrosis and emphysema; * indicates significant difference (p < 0.05); nr = not reported; § data originally published as median with quartiles, but recalculated and presented here as mean ± SD; † residual volume data collected as part of initial study, but not reported with prior publication.
12. Overall Observations from Table 1
The published studies in Table 1 provide an overview of PFT parameters in patients with CPFE compared to PF alone, but one potential limitation in drawing conclusions from these studies relates to assessing the degree of parenchymal fibrosis in each group. Fibrosis scoring is generally performed utilizing a scoring system based on chest CT imaging, although scoring fibrosis in the presence of emphysema may be difficult [1]. If the degree of parenchymal fibrosis in PF patients is higher compared to CPFE patients, the higher lung volumes observed in CPFE patients may merely result from less fibrosis as opposed to changes in elastic recoil forces due to the emphysema component. As seen in the far right column in Table 1, three studies did show a statistical difference in fibrosis scores between the two groups of patients, whereas there was no difference or were not reported in the remaining studies. Additionally, some of the conflicting results among the published studies may result from patients having different geographic forms of emphysema and fibrosis, again highlighting that there is no universal precise characterization of patients with CPFE. Despite these limitations, overall observations in aggregate from the studies shown in Table 1 and other studies of patients with CPFE have demonstrated that the abnormal lung mechanics of each individual process, emphysema and fibrosis, likely cause a counter-balancing effect and result in relative preservation of spirometric and lung volume indices.
13. Conclusions
In this Review, we have examined the physiologic characteristics that occur with pulmonary emphysema, pulmonary fibrosis, and CPFE. As we have hopefully demonstrated, the preponderance of literature suggests that patients with CPFE have relative preservation of spirometric and lung volume indices, but have severe gas exchange abnormalities [2,3,4]. From a physiologic standpoint, these observations suggest that elastic recoil properties of the lungs in patients with CPFE are likely intermediate between those of patients with pulmonary fibrosis and those with emphysema, suggesting a counter-balancing effect. As emphasized in many prior publications, patients with CPFE, who may be severely functionally impaired with advanced illness and a poor prognosis, may have normal or only mildly impaired spirometry and lung volume indices, which may provide a false sense of normalcy in a patient with substantially abnormal pulmonary physiology. In all studies of CPFE patients, DLCO has been uniformly profoundly reduced, suggesting that DLCO does likely reflect the severity of illness in these patients and emphasizes the importance of the DLCO measurement when the diagnosis of CPFE is considered. As chest CT imaging continues to be a frequent imaging modality in patients with cardiopulmonary disease, we expect that patients with a combination of pulmonary emphysema and pulmonary fibrosis will continue to be observed, and understanding the abnormalities that manifest on PFT testing can be helpful to understanding the overall pathophysiology of disease in these patients.
Author Contributions
D.E.A. and N.W.T. conceptualized the manuscript, developed the content, and wrote the entire original drafts and final manuscript. S.P.A. assisted with conceptualization, content, editing, and manuscript preparation. N.D., J.D., S.E.H., and J.R.G. assisted with manuscript content, and manuscript review and editing,
Funding
This work was supported by the NIH R01HL126897 and VA Merit Award I01BX002499.
Conflicts of Interest
All authors declare no conflicts of interest related to the work in this manuscript. The NIH and VA funding agencies had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
References
- Ryerson, C.J.; Hartman, T.; Elicker, B.M.; Ley, B.; Lee, J.S.; Abbritti, M.; Jones, K.D.; King, T.E., Jr.; Ryu, J.; Collard, H.R. Clinical features and outcomes in combined pulmonary fibrosis and emphysema in idiopathic pulmonary fibrosis. Chest 2013, 144, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Jankowich, M.D.; Rounds, S.I.S. Combined pulmonary fibrosis and emphysema syndrome: A review. Chest 2012, 141, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Papaioannou, A.I.; Kostikas, K.; Manali, E.D.; Papadaki, G.; Roussou, A.; Kolilekas, L.; Borie, R.; Bouros, D.; Papiris, S.A. Combined pulmonary fibrosis and emphysema: The many aspects of a cohabitation contract. Respir. Med. 2016, 117, 14–26. [Google Scholar] [CrossRef] [PubMed]
- Malli, F.; Papakosta, D.; Antoniou, K.; Dimadi, M.; Polychronopoulos, V.; Malagari, K.; Oikonomou, A.; Bouros, D.E.; Daniil, Z. Combined pulmonary fibrosis and emphysema characteristics in a Greek cohort. ERJ Open Res. 2019, 5. [Google Scholar] [CrossRef] [PubMed]
- Lambert, A.A.; Dransfield, M.T. COPD Overlap Syndromes: Asthma and Beyond. Chronic. Obstr. Pulm. Dis. 2016, 3, 459–465. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cano-Jimenez, E.; Hernandez Gonzalez, F.; Peloche, G.B. Comorbidities and Complications in Idiopathic Pulmonary Fibrosis. Med. Sci. (Basel) 2018, 6, 71. [Google Scholar] [CrossRef] [PubMed]
- Snider, G.L.; Kleinerman, J.; Thurlbeck, W.M.; Bengali, Z.H. The definition of emphysema. Report of a National Heart, Lung, and Blood Institute, Division of Lung Diseases workshop. Am. Rev. Respir. Dis. 1985, 132, 182–185. [Google Scholar] [CrossRef]
- Raherison, C.; Girodet, P.O. Epidemiology of COPD. Eur. Respir. Rev. 2009, 18, 213–221. [Google Scholar] [CrossRef]
- Lynch, D.A.; Moore, C.M.; Wilson, C.; Nevrekar, D.; Jennermann, T.; Humphries, S.M.; Austin, J.H.M.; Grenier, P.A.; Kauczor, H.U.; Han, M.K.; et al. CT-based Visual Classification of Emphysema: Association with Mortality in the COPDGene Study. Radiology 2018, 288, 859–866. [Google Scholar] [CrossRef]
- Petsonk, E.L.; Rose, C.; Cohen, R. Coal mine dust lung disease. New lessons from old exposure. Am. J. Respir. Crit. Care Med. 2013, 187, 1178–1185. [Google Scholar] [CrossRef]
- Robins, A.G. Pathophysiology of emphysema. Clin. Chest Med. 1983, 4, 413–420. [Google Scholar] [PubMed]
- West, J.B. State of the art: Ventilation-perfusion relationships. Am. Rev. Respir. Dis. 1977, 116, 919–943. [Google Scholar] [CrossRef] [PubMed]
- Hajian, B.; De Backer, J.; Vos, W.; van Geffen, W.H.; De Winter, P.; Usmani, O.; Cahn, T.; Kerstjens, H.A.; Pistolesi, M.; De Backer, W. Changes in ventilation-perfusion during and after an COPD exacerbation: An assessment using fluid dynamic modeling. Int. J. Chron. Obstruct. Pulmon. Dis. 2018, 13, 833–842. [Google Scholar] [CrossRef] [PubMed]
- West, J.B. Respiratory Physiology: The Essentials, 9th ed.; Wolters Kluwer Health/Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2012. [Google Scholar]
- Wagner, P.D. The physiological basis of pulmonary gas exchange: Implications for clinical interpretation of arterial blood gases. Eur. Respir. J. 2015, 45, 227–243. [Google Scholar] [CrossRef] [PubMed]
- Remels, A.H.; Gosker, H.R.; Langen, R.C.; Schols, A.M. The mechanisms of cachexia underlying muscle dysfunction in COPD. J. Appl. Physiol. (1985) 2013, 114, 1253–1262. [Google Scholar] [CrossRef] [PubMed]
- Mohsenifar, Z.; Lee, S.M.; Diaz, P.; Criner, G.; Sciurba, F.; Ginsburg, M.; Wise, R.A. Single-breath diffusing capacity of the lung for carbon monoxide: A predictor of PaO2, maximum work rate, and walking distance in patients with emphysema. Chest 2003, 123, 1394–1400. [Google Scholar] [CrossRef] [PubMed]
- Morrison, N.J.; Abboud, R.T.; Ramadan, F.; Miller, R.R.; Gibson, N.N.; Evans, K.G.; Nelems, B.; Muller, N.L. Comparison of single breath carbon monoxide diffusing capacity and pressure-volume curves in detecting emphysema. Am. Rev. Respir. Dis. 1989, 139, 1179–1187. [Google Scholar] [CrossRef]
- West, J.B. Pulmonary Pathophysiology: The Essentials, 8th ed.; Wolters Kluwer/Lippincott Williams & Wilkins Health: Philadelphia, PA, USA, 2012. [Google Scholar]
- Fessler, H.E.; Scharf, S.M.; Ingenito, E.P.; McKenna, R.J., Jr.; Sharafkhaneh, A. Physiologic basis for improved pulmonary function after lung volume reduction. Proc. Am. Thorac. Soc. 2008, 5, 416–420. [Google Scholar] [CrossRef]
- Ferguson, G.T. Why does the lung hyperinflate? Proc. Am. Thorac. Soc. 2006, 3, 176–179. [Google Scholar] [CrossRef]
- Demedts, M.; Cosemans, J.; De Roo, M.; Billiet, L.; van de Woestijne, K.P. Emphysema with minor airway obstruction and abnormal tests of small airway disease. Respiration 1978, 35, 148–157. [Google Scholar] [CrossRef]
- Mottram, C. Ruppel’s Manual of Pulmonary Function Testing, 11th ed.; Elsevier: St. Louis, MO, USA, 2018. [Google Scholar]
- O’Donnell, D.E. Hyperinflation, dyspnea, and exercise intolerance in chronic obstructive pulmonary disease. Proc. Am. Thorac. Soc. 2006, 3, 180–184. [Google Scholar] [CrossRef] [PubMed]
- Wynn, T.A. Integrating mechanisms of pulmonary fibrosis. J. Exp. Med. 2011, 208, 1339–1350. [Google Scholar] [CrossRef] [PubMed]
- Todd, N.W.; Atamas, S.P.; Luzina, I.G.; Galvin, J.R. Permanent alveolar collapse is the predominant mechanism in idiopathic pulmonary fibrosis. Expert Rev. Respir. Med. 2015, 9, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Lutz, D.; Gazdhar, A.; Lopez-Rodriguez, E.; Ruppert, C.; Mahavadi, P.; Gunther, A.; Klepetko, W.; Bates, J.H.; Smith, B.; Geiser, T.; et al. Alveolar derecruitment and collapse induration as crucial mechanisms in lung injury and fibrosis. Am. J. Respir. Cell Mol. Biol. 2015, 52, 232–243. [Google Scholar] [CrossRef] [PubMed]
- Luzina, I.G.; Salcedo, M.V.; Rojas-Pena, M.L.; Wyman, A.E.; Galvin, J.R.; Sachdeva, A.; Clerman, A.; Kim, J.; Franks, T.J.; Britt, E.J.; et al. Transcriptomic evidence of immune activation in macroscopically normal-appearing and scarred lung tissues in idiopathic pulmonary fibrosis. Cell Immunol. 2018, 325, 1–13. [Google Scholar] [CrossRef]
- Kalchiem-Dekel, O.; Galvin, J.R.; Burke, A.P.; Atamas, S.P.; Todd, N.W. Interstitial Lung Disease and Pulmonary Fibrosis: A Practical Approach for General Medicine Physicians with Focus on the Medical History. J. Clin. Med. 2018, 7, 476. [Google Scholar] [CrossRef]
- Raghu, G.; Remy-Jardin, M.; Myers, J.L.; Richeldi, L.; Ryerson, C.J.; Lederer, D.J.; Behr, J.; Cottin, V.; Danoff, S.K.; Morell, F.; et al. Diagnosis of Idiopathic Pulmonary Fibrosis. An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2018, 198, e44–e68. [Google Scholar] [CrossRef]
- Plantier, L.; Cazes, A.; Dinh-Xuan, A.T.; Bancal, C.; Marchand-Adam, S.; Crestani, B. Physiology of the lung in idiopathic pulmonary fibrosis. Eur. Respir. Rev. 2018, 27. [Google Scholar] [CrossRef]
- Richeldi, L.; du Bois, R.M.; Raghu, G.; Azuma, A.; Brown, K.K.; Costabel, U.; Cottin, V.; Flaherty, K.R.; Hansell, D.M.; Inoue, Y.; et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N. Engl. J. Med. 2014, 370, 2071–2082. [Google Scholar] [CrossRef]
- King, T.E., Jr.; Bradford, W.Z.; Castro-Bernardini, S.; Fagan, E.A.; Glaspole, I.; Glassberg, M.K.; Gorina, E.; Hopkins, P.M.; Kardatzke, D.; Lancaster, L.; et al. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N. Engl. J. Med. 2014, 370, 2083–2092. [Google Scholar] [CrossRef]
- Flaherty, K.R.; Andrei, A.C.; Murray, S.; Fraley, C.; Colby, T.V.; Travis, W.D.; Lama, V.; Kazerooni, E.A.; Gross, B.H.; Toews, G.B.; et al. Idiopathic pulmonary fibrosis: Prognostic value of changes in physiology and six-minute-walk test. Am. J. Respir. Crit. Care Med. 2006, 174, 803–809. [Google Scholar] [CrossRef] [PubMed]
- Lynch, D.A.; Godwin, J.D.; Safrin, S.; Starko, K.M.; Hormel, P.; Brown, K.K.; Raghu, G.; King, T.E., Jr.; Bradford, W.Z.; Schwartz, D.A.; et al. High-resolution computed tomography in idiopathic pulmonary fibrosis: Diagnosis and prognosis. Am. J. Respir. Crit. Care Med. 2005, 172, 488–493. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, M.I.; King, T.E. Interstitial Lung Disease, 4th ed.; B.C. Decker: Hamilton, ON, Canada; Lewiston, NY, USA, 2003. [Google Scholar]
- Idiopathic Pulmonary Fibrosis Clinical Research Network; Martinez, F.J.; de Andrade, J.A.; Anstrom, K.J.; King, T.E., Jr.; Raghu, G. Randomized trial of acetylcysteine in idiopathic pulmonary fibrosis. N. Engl. J. Med. 2014, 370, 2093–2101. [Google Scholar] [CrossRef] [PubMed]
- West, J.R.; Alexander, J.K. Studies on respiratory mechanics and the work of breathing in pulmonary fibrosis. Am. J. Med. 1959, 27, 529–544. [Google Scholar] [CrossRef]
- Mori, K.; Shirai, T.; Mikamo, M.; Shishido, Y.; Akita, T.; Morita, S.; Asada, K.; Fujii, M.; Hozumi, H.; Suda, T.; et al. Respiratory mechanics measured by forced oscillation technique in combined pulmonary fibrosis and emphysema. Respir. Physiol. Neurobiol. 2013, 185, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Spira, A.; Beane, J.; Pinto-Plata, V.; Kadar, A.; Liu, G.; Shah, V.; Celli, B.; Brody, J.S. Gene expression profiling of human lung tissue from smokers with severe emphysema. Am. J. Respir. Cell Mol. Biol. 2004, 31, 601–610. [Google Scholar] [CrossRef]
- Castaldi, P.J.; Cho, M.H.; San Jose Estepar, R.; McDonald, M.L.; Laird, N.; Beaty, T.H.; Washko, G.; Crapo, J.D.; Silverman, E.K.; Investigators, C.O. Genome-wide association identifies regulatory Loci associated with distinct local histogram emphysema patterns. Am. J. Respir. Crit. Care Med. 2014, 190, 399–409. [Google Scholar] [CrossRef] [PubMed]
- Sakornsakolpat, P.; Prokopenko, D.; Lamontagne, M.; Reeve, N.F.; Guyatt, A.L.; Jackson, V.E.; Shrine, N.; Qiao, D.; Bartz, T.M.; Kim, D.K.; et al. Genetic landscape of chronic obstructive pulmonary disease identifies heterogeneous cell-type and phenotype associations. Nat. Genet. 2019, 51, 494–505. [Google Scholar] [CrossRef]
- Stanley, S.E.; Merck, S.J.; Armanios, M. Telomerase and the Genetics of Emphysema Susceptibility. Implications for Pathogenesis Paradigms and Patient Care. Ann. Am. Thorac. Soc. 2016, 13 (Suppl 5), S447–S451. [Google Scholar] [CrossRef]
- Kaur, A.; Mathai, S.K.; Schwartz, D.A. Genetics in Idiopathic Pulmonary Fibrosis Pathogenesis, Prognosis, and Treatment. Front. Med. (Lausanne) 2017, 4, 154. [Google Scholar] [CrossRef]
- Reyfman, P.A.; Walter, J.M.; Joshi, N.; Anekalla, K.R.; McQuattie-Pimentel, A.C.; Chiu, S.; Fernandez, R.; Akbarpour, M.; Chen, C.I.; Ren, Z.; et al. Single-Cell Transcriptomic Analysis of Human Lung Provides Insights into the Pathobiology of Pulmonary Fibrosis. Am. J. Respir. Crit. Care Med. 2019, 199, 1517–1536. [Google Scholar] [CrossRef] [PubMed]
- Wolters, P.J.; Collard, H.R.; Jones, K.D. Pathogenesis of idiopathic pulmonary fibrosis. Annu. Rev. Pathol. 2014, 9, 157–179. [Google Scholar] [CrossRef] [PubMed]
- Kropski, J.A.; Young, L.R.; Cogan, J.D.; Mitchell, D.B.; Lancaster, L.H.; Worrell, J.A.; Markin, C.; Liu, N.; Mason, W.R.; Fingerlin, T.E.; et al. Genetic Evaluation and Testing of Patients and Families with Idiopathic Pulmonary Fibrosis. Am. J. Respir. Crit. Care Med. 2017, 195, 1423–1428. [Google Scholar] [CrossRef] [PubMed]
- Chilosi, M.; Poletti, V.; Rossi, A. The pathogenesis of COPD and IPF: Distinct horns of the same devil? Respir. Res. 2012, 13, 3. [Google Scholar] [CrossRef]
- Gauldie, J.; Kolb, M.; Ask, K.; Martin, G.; Bonniaud, P.; Warburton, D. Smad3 signaling involved in pulmonary fibrosis and emphysema. Proc. Am. Thorac. Soc. 2006, 3, 696–702. [Google Scholar] [CrossRef] [PubMed]
- Hanaoka, M.; Ito, M.; Droma, Y.; Ushiki, A.; Kitaguchi, Y.; Yasuo, M.; Kubo, K. Comparison of gene expression profiling between lung fibrotic and emphysematous tissues sampled from patients with combined pulmonary fibrosis and emphysema. Fibrogenesis Tissue Repair 2012, 5, 17. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Auerbach, O.; Garfinkel, L.; Hammond, E.C. Relation of smoking and age to findings in lung parenchyma: A microscopic study. Chest 1974, 65, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Katzenstein, A.L.; Mukhopadhyay, S.; Zanardi, C.; Dexter, E. Clinically occult interstitial fibrosis in smokers: Classification and significance of a surprisingly common finding in lobectomy specimens. Hum. Pathol. 2010, 41, 316–325. [Google Scholar] [CrossRef]
- Salazar, L.M.; Herrera, A.M. Fibrotic response of tissue remodeling in COPD. Lung 2011, 189, 101–109. [Google Scholar] [CrossRef]
- McDonough, J.E.; Yuan, R.; Suzuki, M.; Seyednejad, N.; Elliott, W.M.; Sanchez, P.G.; Wright, A.C.; Gefter, W.B.; Litzky, L.; Coxson, H.O.; et al. Small-airway obstruction and emphysema in chronic obstructive pulmonary disease. N. Engl. J. Med. 2011, 365, 1567–1575. [Google Scholar] [CrossRef]
- Todd, N.W.; Jeudy, J.; Lavania, S.; Franks, T.J.; Galvin, J.R.; Deepak, J.; Britt, E.J.; Atamas, S.P. Centrilobular emphysema combined with pulmonary fibrosis results in improved survival. Fibrogenesis Tissue Repair 2011, 4, 6. [Google Scholar] [CrossRef] [PubMed]
- Mejia, M.; Carrillo, G.; Rojas-Serrano, J.; Estrada, A.; Suarez, T.; Alonso, D.; Barrientos, E.; Gaxiola, M.; Navarro, C.; Selman, M. Idiopathic pulmonary fibrosis and emphysema: Decreased survival associated with severe pulmonary arterial hypertension. Chest 2009, 136, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Kitaguchi, Y.; Fujimoto, K.; Hanaoka, M.; Kawakami, S.; Honda, T.; Kubo, K. Clinical characteristics of combined pulmonary fibrosis and emphysema. Respirology 2010, 15, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Kawabata, Y.; Hoshi, E.; Murai, K.; Ikeya, T.; Takahashi, N.; Saitou, Y.; Kurashima, K.; Ubukata, M.; Takayanagi, N.; Sugita, H.; et al. Smoking-related changes in the background lung of specimens resected for lung cancer: A semiquantitative study with correlation to postoperative course. Histopathology 2008, 53, 707–714. [Google Scholar] [CrossRef]
- Yamada, T.; Nakanishi, Y.; Homma, T.; Uehara, K.; Mizutani, T.; Hoshi, E.; Shimizu, Y.; Kawabata, Y.; Colby, T.V. Airspace enlargement with fibrosis shows characteristic histology and immunohistology different from usual interstitial pneumonia, nonspecific interstitial pneumonia and centrilobular emphysema. Pathol. Int. 2013, 63, 206–213. [Google Scholar] [CrossRef]
- Naunheim, K.S.; Wood, D.E.; Mohsenifar, Z.; Sternberg, A.L.; Criner, G.J.; DeCamp, M.M.; Deschamps, C.C.; Martinez, F.J.; Sciurba, F.C.; Tonascia, J.; et al. Long-term follow-up of patients receiving lung-volume-reduction surgery versus medical therapy for severe emphysema by the National Emphysema Treatment Trial Research Group. Ann. Thorac. Surg. 2006, 82, 431–443. [Google Scholar] [CrossRef]
- Fishman, A.; Martinez, F.; Naunheim, K.; Piantadosi, S.; Wise, R.; Ries, A.; Weinmann, G.; Wood, D.E.; National Emphysema Treatment Trial Research Group. A randomized trial comparing lung-volume-reduction surgery with medical therapy for severe emphysema. N. Engl. J. Med. 2003, 348, 2059–2073. [Google Scholar] [CrossRef]
- Schwartz, D.A.; Merchant, R.K.; Helmers, R.A.; Gilbert, S.R.; Dayton, C.S.; Hunninghake, G.W. The influence of cigarette smoking on lung function in patients with idiopathic pulmonary fibrosis. Am. Rev. Respir. Dis. 1991, 144, 504–506. [Google Scholar] [CrossRef]
- Akagi, T.; Matsumoto, T.; Harada, T.; Tanaka, M.; Kuraki, T.; Fujita, M.; Watanabe, K. Coexistent emphysema delays the decrease of vital capacity in idiopathic pulmonary fibrosis. Respir. Med. 2009, 103, 1209–1215. [Google Scholar] [CrossRef]
- Johnson, D.C. DLCO: Adjust for lung volume, standardised reporting and interpretation. Eur. Respir J. 2017, 50. [Google Scholar] [CrossRef]
- Hughes, J.M.; Pride, N.B. Examination of the carbon monoxide diffusing capacity (DL(CO)) in relation to its KCO and VA components. Am. J. Respir. Crit. Care Med. 2012, 186, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Frans, A.; Nemery, B.; Veriter, C.; Lacquet, L.; Francis, C. Effect of alveolar volume on the interpretation of single breath DLCO. Respir. Med. 1997, 91, 263–273. [Google Scholar] [CrossRef]
- Mura, M.; Zompatori, M.; Pacilli, A.M.; Fasano, L.; Schiavina, M.; Fabbri, M. The presence of emphysema further impairs physiologic function in patients with idiopathic pulmonary fibrosis. Respir. Care 2006, 51, 257–265. [Google Scholar] [PubMed]
- Jankowich, M.D.; Rounds, S. Combined pulmonary fibrosis and emphysema alters physiology but has similar mortality to pulmonary fibrosis without emphysema. Lung 2010, 188, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Kurashima, K.; Takayanagi, N.; Tsuchiya, N.; Kanauchi, T.; Ueda, M.; Hoshi, T.; Miyahara, Y.; Sugita, Y. The effect of emphysema on lung function and survival in patients with idiopathic pulmonary fibrosis. Respirology 2010, 15, 843–848. [Google Scholar] [CrossRef]
- Schmidt, S.L.; Nambiar, A.M.; Tayob, N.; Sundaram, B.; Han, M.K.; Gross, B.H.; Kazerooni, E.A.; Chughtai, A.R.; Lagstein, A.; Myers, J.L.; et al. Pulmonary function measures predict mortality differently in IPF versus combined pulmonary fibrosis and emphysema. Eur. Respir. J. 2011, 38, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Sugino, K.; Ishida, F.; Kikuchi, N.; Hirota, N.; Sano, G.; Sato, K.; Isobe, K.; Sakamoto, S.; Takai, Y.; Homma, S. Comparison of clinical characteristics and prognostic factors of combined pulmonary fibrosis and emphysema versus idiopathic pulmonary fibrosis alone. Respirology 2014, 19, 239–245. [Google Scholar] [CrossRef]
- Inomata, M.; Ikushima, S.; Awano, N.; Kondoh, K.; Satake, K.; Masuo, M.; Kusunoki, Y.; Moriya, A.; Kamiya, H.; Ando, T.; et al. An autopsy study of combined pulmonary fibrosis and emphysema: Correlations among clinical, radiological, and pathological features. BMC Pulm. Med. 2014, 14, 104. [Google Scholar] [CrossRef]
- Yoon, H.Y.; Kim, T.H.; Seo, J.B.; Lee, S.M.; Lim, S.; Lee, H.N.; Kim, N.; Han, M.; Kim, D.S.; Song, J.W. Effects of emphysema on physiological and prognostic characteristics of lung function in idiopathic pulmonary fibrosis. Respirology 2019, 24, 55–62. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).