Association of Breastfeeding Duration with Susceptibility to Allergy, Influenza, and Methylation Status of TLR1 Gene
Abstract
1. Introduction
2. Materials and Methods
2.1. Blood Samples Collection and Clinical History of Volunteers
2.2. Criteria for Defining Different Diseases
2.3. Naïve B Cell Isolation
2.4. DNA Extraction and Bisulfite Treatment
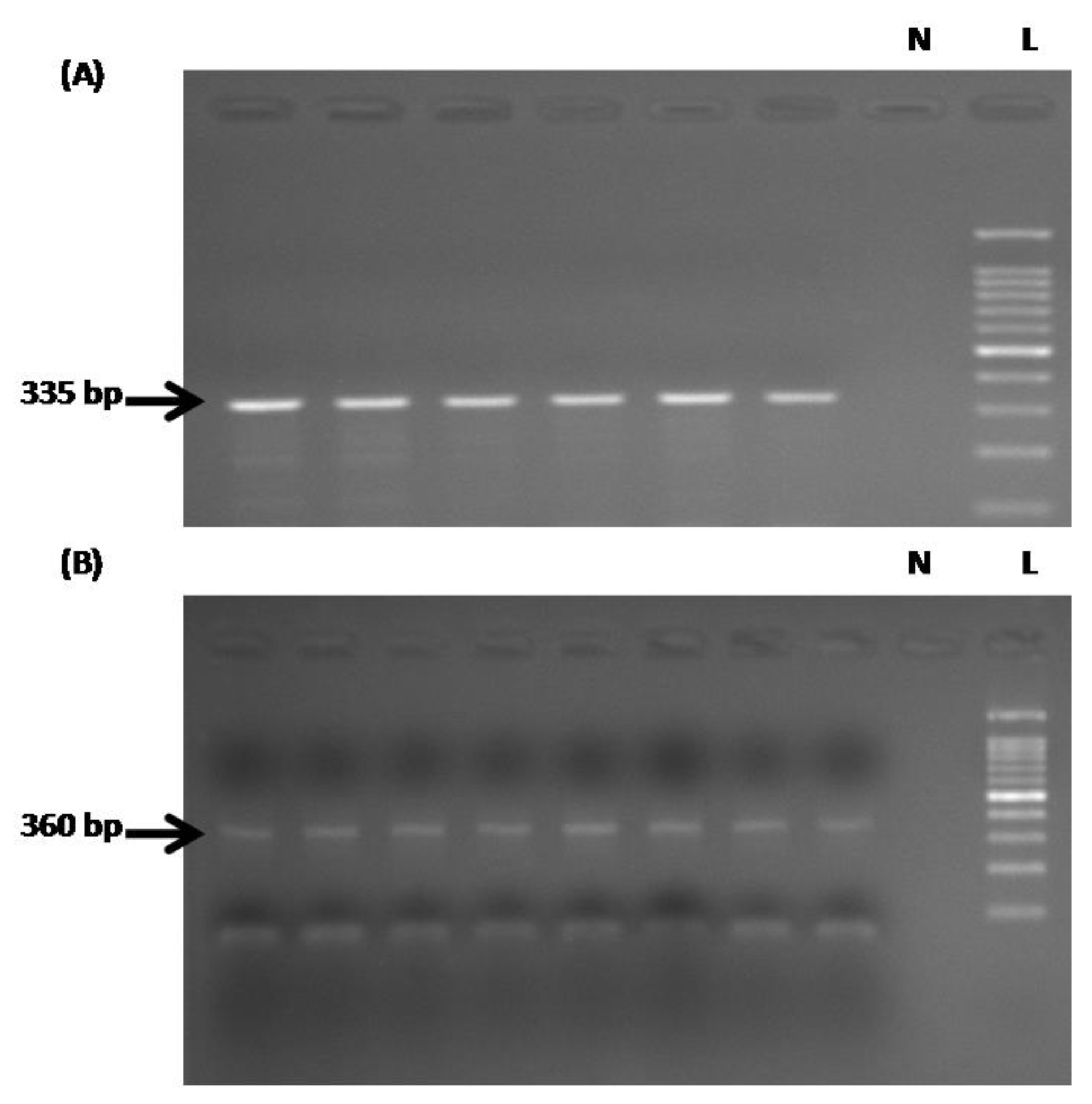
2.5. PCR Amplification of the TLR1 Promoter Before and After Bisulfate Treatment
2.6. DNA Sequencing and Analysis
2.7. Statistical Analysis
3. Results
3.1. Association of Breastfeeding with Susceptibility to Influenza and Allergies
3.2. DNA Methylation at AP-1Binding Sites
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Labayen, I.; Ruiz, J.R.; Ortega, F.B.; Loit, H.M.; Harro, J.; Villa, I.; Veidebaum, T.; Sjostrom, M. Exclusivebreastfeedingdurationandcardiorespiratoryfitnessinchildrenandadolescents. Am. J. Clin. Nutr. 2012, 95, 498–505. [Google Scholar] [CrossRef]
- Jackson, K.M.; Nazar, A.M. Breastfeeding, the immune response, and long-term health. J. Am.Osteopat. Assoc. 2006, 106, 203–207. [Google Scholar]
- Altorki, S. Milk-Kinship in Arab Society. An Unexplored Problem in the Ethnography of Marriage. Ethnology 1980, 19, 233–244. [Google Scholar] [CrossRef]
- Ozkan, H.; Tuzun, F.; Kumral, A.; Duman, N. Milk kinship hypothesis in light of epigenetic knowledge. Clin.Epigenet. 2012, 4, 14. [Google Scholar] [CrossRef] [PubMed]
- Newburg, D.S. Innate immunity and human milk. J. Nutr. 2005, 135, 1308–1312. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Sayed, N.; Hunter, A.; Au, K.F.; Wong, W.H.; Mocarski, E.S.; Pera, R.R.; Yakubov, E.; Cooke, J.P. Activation of innate immunity is required for efficient nuclear reprogramming. Cell 2012, 151, 547–558. [Google Scholar] [CrossRef]
- Civardi, E.; Garofoli, F.; Mazzucchelli, I.; Angelini, M.; Manzoni, P.; Stronati, M. Enteral nutrition, and infections: The role of human milk. Early Hum. Dev. 2014, 90, S57–S59. [Google Scholar] [CrossRef]
- Khan, J.; Vessel, L.; Bahl, R.; Martines, J.C. Timing of breastfeeding initiation and exclusivity of breastfeeding during the first month of life: Effects on neonatal mortality and morbidity—A systematic review and meta-analysis. Matern. Child Health J. 2015, 19, 468–479. [Google Scholar] [CrossRef]
- Mathivanan, S.; Ji, H.; Simpson, R.J. Exosomes: Extracellular organelles important in intercellular communication. J. Proteomics 2010, 73, 1907–1920. [Google Scholar] [CrossRef]
- Cheng, L.C.; Tavazoie, M.; Doetsch, F. Stem cells: From epigenetics to microRNAs. Neuron 2005, 46, 363–367. [Google Scholar] [CrossRef]
- Thery, C.; Ostrowski, M.; Segura, E. Membrane vesicles as conveyors of immune responses. Nat. Rev. Immunol. 2009, 9, 581–593. [Google Scholar] [CrossRef]
- Xiao, C.; Rajewsky, K. MicroRNA control in the immune system: Basic principles. Cell 2009, 136, 26–36. [Google Scholar] [CrossRef]
- Weber, J.A.; Baxter, D.H.; Zhang, S.; Huang, D.Y.; Huang, K.H.; Lee, M.J.; Galas, D.J.; Wang, K. The microRNA spectrum in 12 body fluids. Clin. Chem. 2010, 56, 1733–1741. [Google Scholar] [CrossRef]
- Sarkar, K.; Miller, F.W. Possible roles and determinants of microchimerism in autoimmune and other disorders. Autoimmun. Rev. 2004, 3, 454–463. [Google Scholar] [CrossRef]
- Zhou, Q.; Li, M.; Wang, X.; Li, Q.; Wang, T.; Zhu, Q.; Zhou, X.; Wang, X.; Gao, X.; Li, X. Immune-related microRNAs are abundant in breast milk exosomes. Int. J. Biol. Sci. 2012, 8, 118–123. [Google Scholar] [CrossRef]
- Cedar, H.; Bergman, Y. Linking DNA methylation and histone modification: Patterns and paradigms. Nat. Rev. Genet. 2009, 10, 295–304. [Google Scholar] [CrossRef]
- Hlady, R.A.; Novakova, S.; Opavska, J.; Klinkebiel, D.; Peters, S.L.; Bies, J.; Hannah, J.; Iqbal, J.; Anderson, K.M.; Siebler, H.M.; et al. Loss of Dnmt3b function upregulates the tumor modifier Ment and accelerates mouse lymphomagenesis. J. Clin. Investig. 2012, 122, 163–177. [Google Scholar] [CrossRef]
- Hemmi, H.; Takeuchi, O.; Kawai, T.; Kaisho, T.; Sato, S.; Sanjo, H.; Matsumoto, M.; Hoshino, K.; Wagner, H.; Takeda, K.; et al. A Toll-like receptor recognizes bacterial DNA. Nature 2000, 408, 740–745. [Google Scholar] [CrossRef]
- Rolls, A.; Shechter, R.; London, A.; Ziv, Y.; Ronen, A.; Levy, R.; Schwartz, M. Toll-like receptors modulate adult hippocampal neurogenesis. Nat. Cell Biol. 2007, 9, 1081–1088. [Google Scholar] [CrossRef]
- Dowling, J.K.; Mansell, A. Toll-like receptors: The swiss army knife of immunity and vaccine development. Clin. Transl. Immunol. 2016, 5, e85. [Google Scholar] [CrossRef]
- He, Y.; Lawlor, N.T.; Newburg, D.S. Human Milk Components Modulate Toll-Like Receptor-Mediated Inflammation. Adv. Nutr. 2016, 7, 102–111. [Google Scholar] [CrossRef]
- Buescher, E.S. Anti-inflammatory characteristics of human milk: How; Where; Why. Adv. Exp. Med. Biol. 2001, 501, 207–222. [Google Scholar]
- Chen, C.Z.; Schaffert, S.; Fragoso, R.; Loh, C. Regulation of immune responses and tolerance: The microRNA perspective. Immunol. Rev. 2013, 253, 112–128. [Google Scholar] [CrossRef]
- Shaulian, E.; Karin, M. AP-1 as a regulator of cell life and death. Nat. Cell Biol. 2002, 4, E131–E136. [Google Scholar] [CrossRef]
- Hata, T.; Murakami, K.; Nakatani, H.; Yamamoto, Y.; Matsuda, T.; Aoki, N. Isolation of bovine milk-derived microvesicles carrying mRNAs and microRNAs. Biochem. Biophys. Res. Commun. 2010, 396, 528–533. [Google Scholar] [CrossRef]
- Izadi, H.; Motameni, A.T.; Bates, T.C.; Olivera, E.R.; Villar-Suarez, V.; Joshi, I.; Garg, R.; Osborne, B.A.; Davis, R.J.; Rincón, M.; et al. c-Jun N-terminal kinase1 is required for Toll-like receptor 1 gene expression in macrophages. Infect. Immun. 2007, 75, 5027–5034. [Google Scholar] [CrossRef]
- Tulchinsky, E.M.; Georgiev, G.P.; Lukanidin, E.M. Novel AP-1 binding site created by DNA-methylation. Oncogene 1996, 12, 1737–1745. [Google Scholar]
- Tirado-Magallanes, R.; Rebbani, K.; Lim, R.; Pradhan, S.; Benoukraf, T. Whole genome DNA methylation: Beyond genes silencing. Oncotarget 2017, 8, 5629–5637. [Google Scholar] [CrossRef]
- WHO. Global Strategy for Infant and Young Child Feeding; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Woicka-Kolejwa, K.; Zaczeniuk, M.; Majak, P.; Pawłowska-Iwanicka, K.; Kopka, M.; Stelmach, W.; Jerzyńska, J.; Stelmach, I. Food allergy is associated with recurrent respiratory tract infections during childhood. Postepy Dermatol. Alergol. 2016, 2, 109–113. [Google Scholar] [CrossRef]
- de Oliveira, T.; Klering, E.; da Veiga, A. Is recurrent respiratory infection associated with allergic respiratory disease? J. Asthma 2018, 56, 160–166. [Google Scholar] [CrossRef]
- Schwarze, J.; Gelfand, E. Respiratory viral infections as promoters of allergic sensitization and asthma in animal models. Eur. Respir. J. 2002, 19, 341–349. [Google Scholar] [CrossRef]
- Asthma and Allergy Foundation of America. Available online: https://www.aafa.org/respiratory–infections–flu–cold–asthm (accessed on 5 August 2012).
- Wang, J. The sticky relationship between allergies and infections. Asia Pac. Allergy 2015, 5, 133. [Google Scholar] [CrossRef][Green Version]
- Nicholson, K.G.; Wood, J.M.; Zambon, M. Influenza. Lancet 2003, 362, 1733–1745. [Google Scholar] [CrossRef]
- Kelishadi, R.; Farajian, S. The protective effects of breastfeeding on chronic non-communicable diseases in adulthood: A review of evidence. Adv. Biomed. Res. 2014, 3, 3. [Google Scholar] [CrossRef]
- Ip, S.; Chung, M.; Raman, G.; Chew, P.; Magula, N.; DeVine, D.; Trikalinos, T.; Lau, J. Breastfeeding and Maternal and Infant Health Outcomes in Developed Countries; Agency for Healthcare Research and Qualit; Evidence Report/Technology Assessment (Full Report) 153; Rockville, MD, USA, 2007; pp. 1–186. [Google Scholar]
- Newburg, D.S.; Walker, W.A. Protection of the neonate by the innate immune system of developing gut and of human milk. Pediatr. Res. 2007, 61, 2–8. [Google Scholar] [CrossRef]
- Jensen-Jarolim, E.; Untersmayr, E. Gender-medicine aspects in allergology. Allergy 2008, 63, 610–615. [Google Scholar] [CrossRef]
- Zein, J.; Erzurum, S. Asthma is Different in Women. Curr. Allergy Asthma Rep. 2015, 15, 28. [Google Scholar] [CrossRef]
- Day, A. Asthma in men and women: Two different diseases? Gend. Med. 2006, 3, S36. [Google Scholar] [CrossRef]
- Lauriello, M.; Angelone, A.M.; Di RienzoBusinco, L.; Passali, D.; Bellussi, L.M.; Passali, F.M. Correlation between female sex and allergy was significant in patients presenting with dysphonia. Acta Otorhinolaryngol. Ital. 2011, 31, 161–166. [Google Scholar]
- Thong, B.; Tan, T. Epidemiology and risk factors for drug allergy. Br. J. Clin. Pharmacol. 2011, 71, 684–700. [Google Scholar] [CrossRef]
- Sakaguchi, S.; Wing, K.; Miyara, M. Regulatory T cells—A brief history and perspective. Eur. J. Immunol. 2007, 37, 116–123. [Google Scholar] [CrossRef]
- Greer, F.R.; Sicherer, S.H.; Burks, A.W. American Academy of Pediatrics Committee on N.; American Academy of Pediatrics Section on A.; Immunology. Effects of early nutritional interventions on the development of atopic disease in infants and children: The role of maternal dietary restriction; breastfeeding; timing of introduction of complementary foods; and hydrolyzed formulas. Pediatrics 2008, 121, 183–191. [Google Scholar]
- Togias, A.; Cooper, S.F.; Acebal, M.L.; Assa’ad, A.; Baker, J.R.; Beck, L.A.; Block, J.; Byrd-Bredbenner, C.; Chan, E.S.; Eichenfield, L.F.; et al. Addendum guidelines for the prevention of peanut allergy in the United States: Report of the National Institute of Allergy and Infectious Diseases-sponsored expert panel. J. Pediatr. Nurs. 2017, 32, 91–98. [Google Scholar] [CrossRef]
- Thygarajan, A.; Burks, A.W. American Academy of Pediatrics recommendations on the effects of early nutritional interventions on the development of atopic disease. Curr. Opin. Pediatr. 2008, 20, 698–702. [Google Scholar] [CrossRef]
- Mikeska, T.; Craig, J.M. DNA methylation biomarkers: Cancer and beyond. Genes 2014, 5, 821–864. [Google Scholar] [CrossRef]
- Yang, C.A.; Chiang, B.L. Toll-like receptor 1 N248S polymorphism affects T helper 1 cytokine production and is associated with serum immunoglobulin E levels in Taiwanese allergic patients. J. Microbiol. Immunol. Infect. 2017, 50, 112–117. [Google Scholar] [CrossRef]
| Disease | Category | <6 months | ≥6 months | p-Value | ||
|---|---|---|---|---|---|---|
| Yes | No | Yes | No | |||
| Influenza | Total | 27 (20) | 28 (8) | 6 (6) | 39 (19) | 0.00 (0.00) |
| Males | 13 | 10 | 3 | 13 | 0.04 | |
| Females | 14 | 18 | 3 | 26 | 0.01 | |
| Allergies | Total | 24 (10) | 31 (18) | 9 (4) | 36 (21) | 0.02 (0.12) |
| Males | 9 | 14 | 3 | 13 | 0.31 | |
| Females | 15 | 17 | 5 | 24 | 0.03 | |
| Disease | Methylation at the Two Sites Together | Unmethylation of Least One Site | p-Value | ||
|---|---|---|---|---|---|
| Yes | No | Yes | No | ||
| Influenza | 12 | 5 | 13 | 18 | 0.08 |
| Allergies | 6 | 11 | 7 | 24 | 0.50 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hatmal, M.M.; Issa, N.N.; Alshaer, W.; Al-Ameer, H.J.; Abuyaman, O.; Tayyem, R.; Hijjawi, N.S. Association of Breastfeeding Duration with Susceptibility to Allergy, Influenza, and Methylation Status of TLR1 Gene. Medicina 2019, 55, 535. https://doi.org/10.3390/medicina55090535
Hatmal MM, Issa NN, Alshaer W, Al-Ameer HJ, Abuyaman O, Tayyem R, Hijjawi NS. Association of Breastfeeding Duration with Susceptibility to Allergy, Influenza, and Methylation Status of TLR1 Gene. Medicina. 2019; 55(9):535. https://doi.org/10.3390/medicina55090535
Chicago/Turabian StyleHatmal, Ma’mon M., Nada N. Issa, Walhan Alshaer, Hamzeh J. Al-Ameer, Omar Abuyaman, Reema Tayyem, and Nawal S. Hijjawi. 2019. "Association of Breastfeeding Duration with Susceptibility to Allergy, Influenza, and Methylation Status of TLR1 Gene" Medicina 55, no. 9: 535. https://doi.org/10.3390/medicina55090535
APA StyleHatmal, M. M., Issa, N. N., Alshaer, W., Al-Ameer, H. J., Abuyaman, O., Tayyem, R., & Hijjawi, N. S. (2019). Association of Breastfeeding Duration with Susceptibility to Allergy, Influenza, and Methylation Status of TLR1 Gene. Medicina, 55(9), 535. https://doi.org/10.3390/medicina55090535








