Thromboembolic Events Following Atrial Fibrillation Cardioversion and Ablation: What’s the Culprit?
Abstract
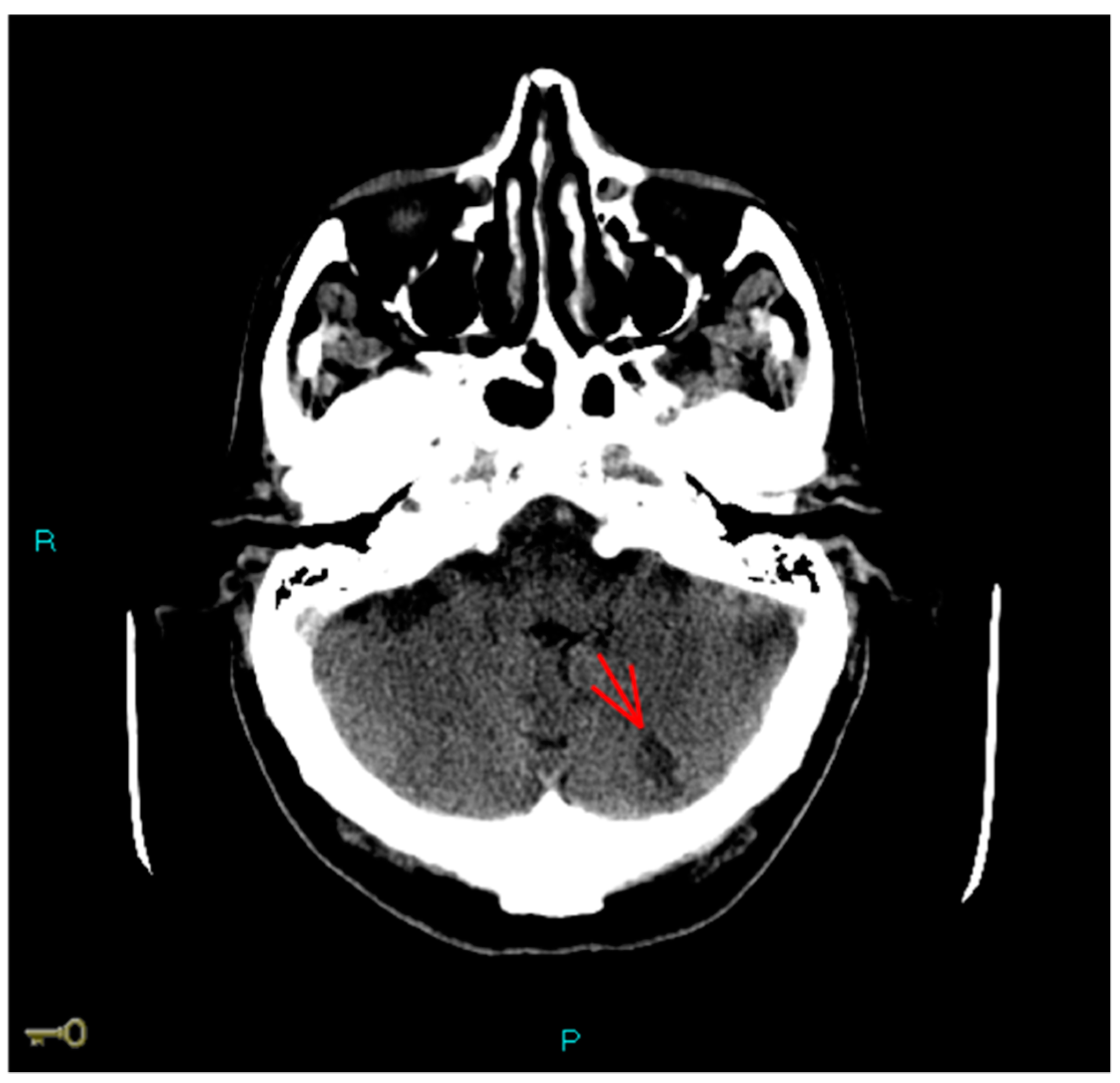
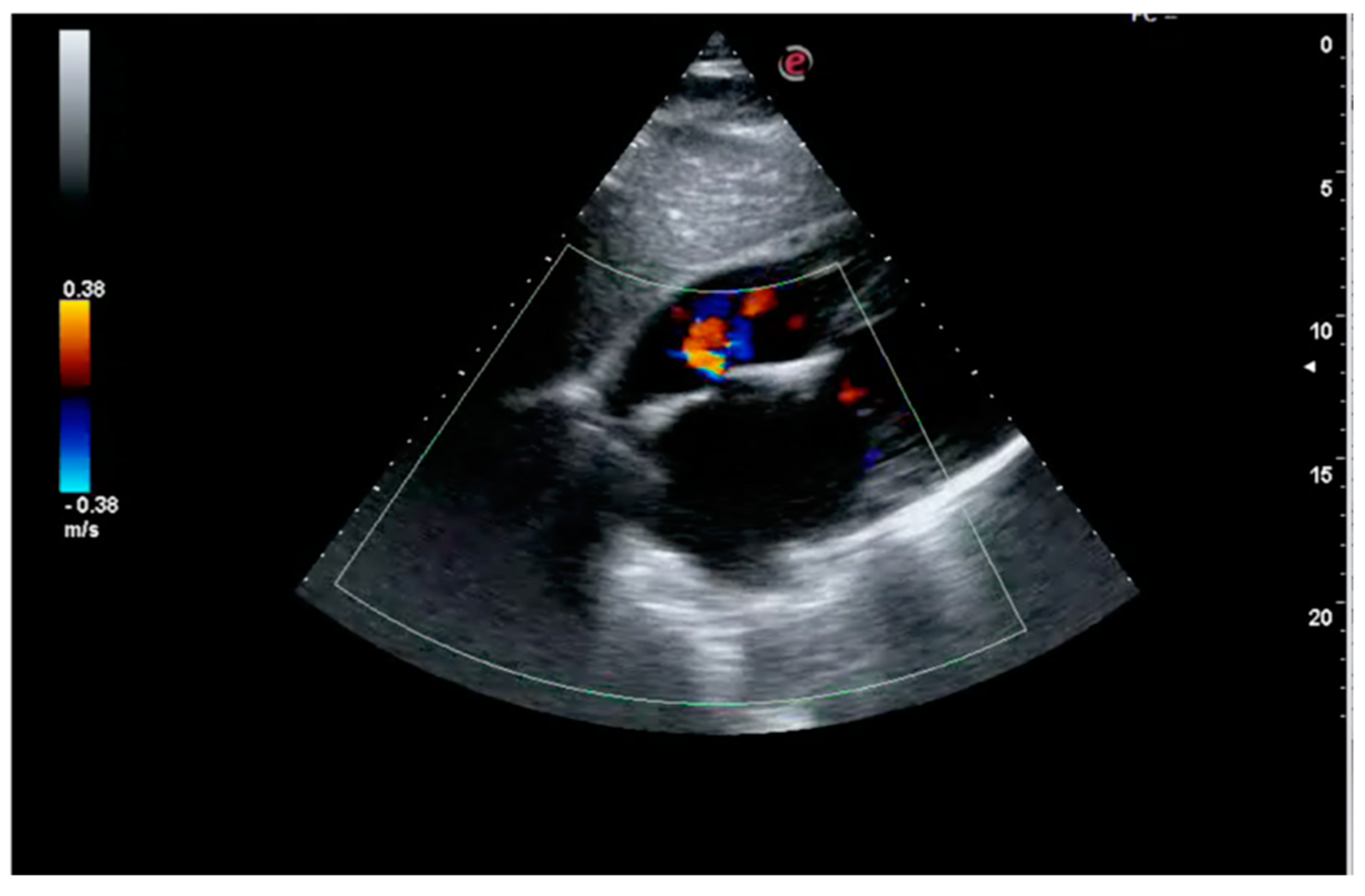
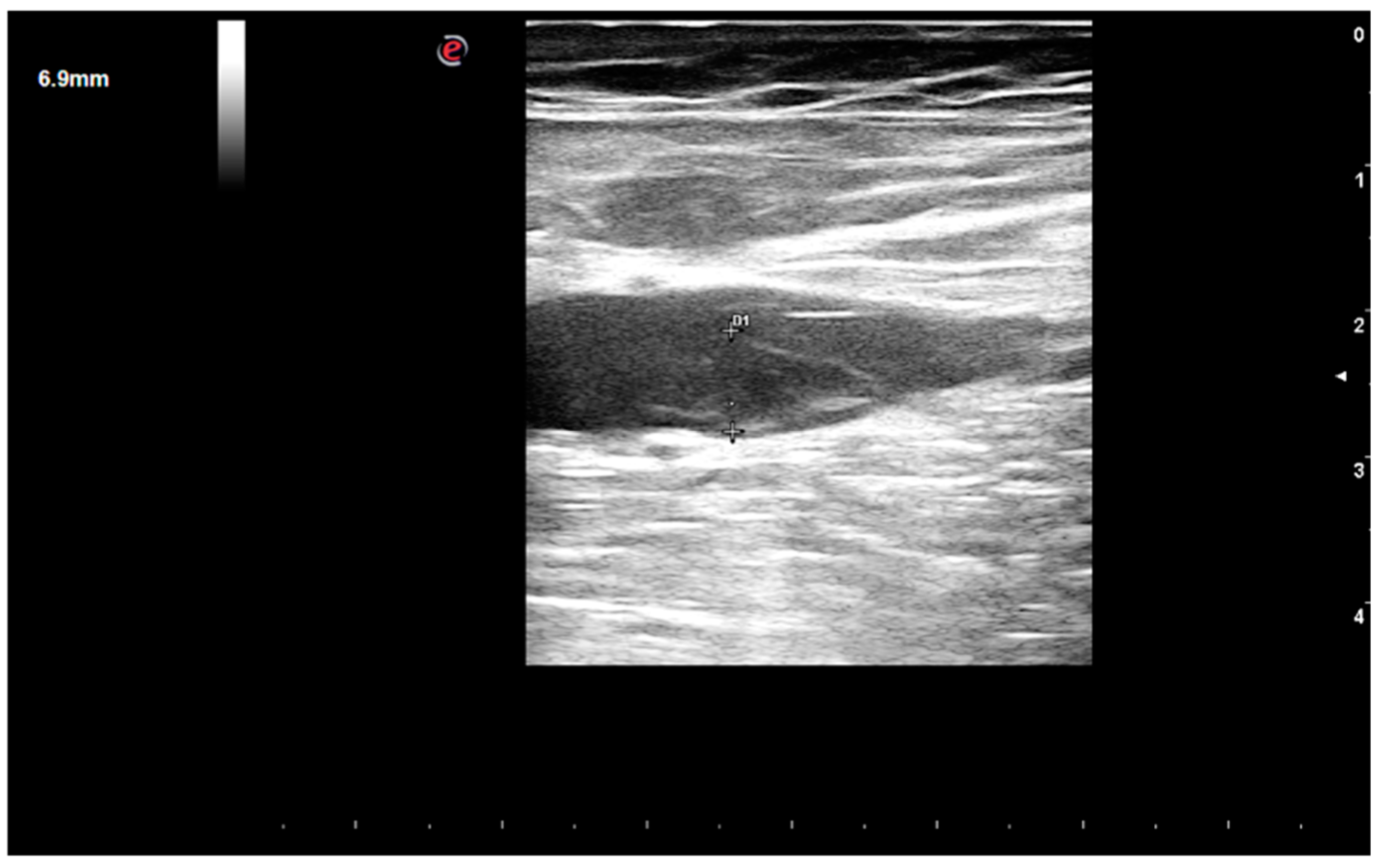
1. Case Report
2. Case Discussion
2.1. Radiofrequency Lesion Set and Ablation “Per Se”
2.2. Management of Anticoagulant Therapy in the Periprocedural Period
2.3. Iatrogenic Interatrial Septal Defect, In Situ Thrombosis and Paradoxical Embolism
3. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Friberg, L.; Tabrizi, F.; Englund, A. Catheter ablation for atrial fibrillation is associated with lower incidence of stroke and death: Data from Swedish health registries. Eur. Heart J. 2016, 37, 2478–2487. [Google Scholar] [CrossRef] [PubMed]
- Saliba, W.; Schliamser, J.E.; Lavi, I.; Barnett-Griness, O.; Gronich, N.; Rennert, G. Catheter ablation of atrial fibrillation is associated with reduced risk of stroke and mortality: A propensity score-matched analysis. Heart Rhythm 2017, 14, 635–642. [Google Scholar] [CrossRef] [PubMed]
- Hunter, R.J.; McCready, J.; Diab, I.; Page, S.P.; Finlay, M.; Richmond, L.; French, A.; Sporton, S.; Lee, G.; Chow, A.; et al. Maintenance of sinus rhythm with an ablation strategy in patients with atrial fibrillation is associated with a lower risk of stroke and death. Heart 2012, 98, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Haeusler, K.G.; Kirchhof, P.; Endres, M. Left atrial catheter ablation and ischemic stroke. Stroke 2012, 43, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Calkins, H.; Hindricks, G.; Cappato, R.; Kim, Y.H.; Saad, E.B.; Aguinaga, L.; Akar, J.G.; Badhwar, V.; Brugada, J.; Camm, J.; et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Europace 2018, 20, e1–e160. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Jiang, C.Y.; Betts, T.R.; Chen, J.; Deisenhofer, I.; Mantovan, R.; Macle, L.; Morillo, C.A.; Haverkamp, W.; Weerasooriya, R.; et al. STAR AF II Investigators. Approaches to catheter ablation for persistent atrial fibrillation. N. Engl. J. Med. 2015, 372, 1812–1822. [Google Scholar] [CrossRef]
- Rillig, A.; Meyerfeldt, U.; Tilz, R.R.; Talazko, J.; Arya, A.; Zvereva, V.; Birkemeyer, R.; Miljak, T.; Hajredini, B.; Wohlmuth, P.; et al. Incidence and long-term follow-up of silent cerebral lesions after pulmonary vein isolation using a remote robotic navigation system as compared with manual ablation. Circ. Arrhythm. Electrophysiol. 2012, 5, 15–21. [Google Scholar] [CrossRef]
- Herm, J.; Fiebach, J.B.; Koch, L.; Kopp, U.A.; Kunze, C.; Wollboldt, C.; Brunecker, P.; Schultheiss, H.P.; Schirdewan, A.; Endres, M.; et al. Neuropsychological effects of MRI- detected brain lesions after left atrial catheter ablation for atrial fibrillation: Long-term results of the MACPAF study. Circ. Arrhythmia Electrophysiol. 2013, 6, 843–850. [Google Scholar] [CrossRef]
- Gaita, F.; Caponi, D.; Pianelli, M.; Scaglione, M.; Toso, E.; Cesarani, F.; Boffano, C.; Gandini, G.; Valentini, M.C.; de Ponti, R.; et al. Radiofrequency catheter ablation of atrial fibrillation: A cause of silent thromboembolism? Magnetic resonance imaging assessment of cerebral thromboembolism in patients undergoing ablation of atrial fibrillation. Circulation 2010, 122, 1667–1673. [Google Scholar] [CrossRef]
- Russo, V.; Rago, A.; Papa, A.A.; D’Onofrio, A.; Golino, P.; Nigro, G. Efficacy and safety of dabigatran in patients with atrial fibrillation scheduled for transoesophageal echocardiogram-guided direct electrical current cardioversion: A prospective propensity score-matched cohort study. J. Thromb. Thrombolysis 2018, 45, 206–212. [Google Scholar] [CrossRef]
- Rago, A.; Papa, A.A.; Cassese, A.; Arena, G.; Magliocca, M.C.G.; D’Onofrio, A.; Golino, P.; Nigro, G.; Russo, V. Clinical Performance of Apixaban vs. Vitamin K Antagonists in Patients with Atrial Fibrillation Undergoing Direct Electrical Current Cardioversion: A Prospective Propensity Score-Matched Cohort Study. Am. J. Cardiovasc. Drugs 2019, 19, 421–427. [Google Scholar] [CrossRef] [PubMed]
- McCready, J.; Chow, A.W.; Lowe, M.D.; Segal, O.R.; Ahsan, S.; de Bono, J.; Dhaliwal, M.; Mfuko, C.; Ng, A.; Rowland, E.R.; et al. Safety and efficacy of multipolar pulmonary vein ablation catheter vs. irrigated radiofrequency ablation for paroxysmal atrial fibrillation: A randomized multicentre trial. Europace 2014, 16, 1145–1153. [Google Scholar] [CrossRef] [PubMed]
- Wieczorek, M.; Lukat, M.; Hoeltgen, R.; Condie, C.; Hilje, T.; Missler, U.; Hirsch, J.; Scharf, C. Investigation into causes of abnormal cerebral MRI findings following PVAC duty-cycled, phased RF ablation of atrial fibrillation. J. Cardiovasc. Electrophysiol. 2013, 24, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, S.; Kajiyama, T.; Yamao, K.; Hada, M.; Yamaguchi, M.; Nakamura, H.; Hachiya, H.; Tada, H.; Hirao, K.; Iesaka, Y. Silent cerebral events/lesions after second-generation cryoballoon ablation: How can we reduce the risk of silent strokes? Heart Rhythm 2019, 16, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Bertaglia, E.; Anselmino, M.; Zorzi, A.; Russo, V.; Toso, E.; Peruzza, F.; Rapacciuolo, A.; Migliore, F.; Gaita, F.; Cucchini, U.; et al. NOACs and atrial fibrillation: Incidence and predictors of left atrial thrombus in the real world. Int. J. Cardiol. 2017, 249, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Hussein, A.A.; Martin, D.O.; Saliba, W.; Patel, D.; Karim, S.; Batal, O.; Banna, M.; Williams-Andrews, M.; Sherman, M.; Kanj, M.; et al. Radiofrequency ablation of atrial fibrillation under therapeutic international normalized ratio: A safe and efficacious periprocedural anticoagulation strategy. Heart Rhythm 2009, 6, 1425–1429. [Google Scholar] [CrossRef]
- De Sensi, F.; Miracapillo, G.; Addonisio, L.; Breschi, M.; Paneni, F. A call for safety during electrophysiological procedures: US in, why not US out? Europace 2017, 19, 2048. [Google Scholar] [CrossRef] [PubMed]
- De Sensi, F.; Miracapillo, G.; Addonisio, L.; Breschi, M.; Scalese, M.; Cresti, A.; Paneni, F.; Limbruno, U. Predictors of Successful Ultrasound Guided Femoral Vein Cannulation in Electrophysiological Procedures. J. Atr. Fibrillation. 2018, 11, 2083. [Google Scholar] [CrossRef]
- Calkins, H.; Willems, S.; Gerstenfeld, E.P.; Verma, A.; Schilling, R.; Hohnloser, S.H.; Okumura, K.; Serota, H.; Nordaby, M.; Guiver, K.; et al. RE-CIRCUIT Investigators. Uninterrupted Dabigatran versus Warfarin for Ablation in Atrial Fibrillation. N. Engl. J. Med. 2017, 376, 1627–1636. [Google Scholar] [CrossRef]
- Cappato, R.; Marchlinski, F.E.; Hohnloser, S.H.; Naccarelli, G.V.; Xiang, J.; Wilber, D.J.; Ma, C.S.; Hess, S.; Wells, D.S.; Juang, G.; et al. VENTURE-AF Investigators. Uninterrupted rivaroxaban vs. uninterrupted vitamin K antagonists for catheter ablation in non-valvular atrial fibrillation. Eur. Heart J. 2015, 36, 1805–1811. [Google Scholar] [CrossRef]
- Reynolds, M.R.; Allison, J.S.; Natale, A.; Weisberg, I.L.; Ellenbogen, K.A.; Richards, M.; Hsieh, W.H.; Sutherland, J.; Cannon, C.P. A Prospective Randomized Trial of Apixaban Dosing During Atrial Fibrillation Ablation: The AEIOU Trial. JACC Clin. Electrophysiol. 2018, 4, 580–588. [Google Scholar] [CrossRef] [PubMed]
- Hohnloser, S.H.; Camm, J.; Cappato, R.; Diener, H.C.; Heidbüchel, H.; Mont, L.; Morillo, C.A.; Abozguia, K.; Grimaldi, M.; Rauer, H.; et al. Uninterrupted edoxaban vs. vitamin K antagonists for ablation of atrial fibrillation: The ELIMINATE-AF trial. Eur. Heart J. 2019, 11. [Google Scholar] [CrossRef] [PubMed]
- Kirchhof, P.; Benussi, S.; Kotecha, D.; Ahlsson, A.; Atar, D.; Casadei, B.; Castella, M.; Diener, H.C.; Heidbuchel, H.; Hendriks, J.; et al. ESC Scientific Document Group. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur. Heart J. 2016, 37, 2893–2962. [Google Scholar] [CrossRef] [PubMed]
- Hammerstingl, C.; Lickfett, L.; Jeong, K.; Troatz, C.; Wedekind, J.A.; Tiemann, K.; Lüderitz, B.; Lewalter, T. ; Lüderitz, B.; Lewalter, T. Persistence of iatrogenic atrial septal defect after pulmonary vein isolation—An under- estimated risk? Am. Heart J. 2006, 152, 362–365. [Google Scholar] [CrossRef] [PubMed]
- Rillig, A.; Meyerfeldt, U.; Birkemeyer, R.; Treusch, F.; Kunze, M.; Jung, W. Persistent iatrogenic atrial septal defect after pulmonary vein isolation: Incidence and clinical implications. J. Interv. Card. Electrophysiol. 2008, 22, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Fitchet, A.; Turkie, W.; Fitzpatrick, A.P. Transeptal approach to ablation of left-sided arrhythmias does not lead to persisting interatrial shunt: A transesophageal echocardiographic study. Pacing Clin. Electrophysiol. 1998, 21, 2070–2072. [Google Scholar] [CrossRef] [PubMed]
- Obel, O.; Mansour, M.; Picard, M.; Ruskin, J.; Keane, D. Persistence of septal defects after transeptal puncture for pulmonary vein isolation procedures. Pacing Clin. Electrophysiol. 2004, 27, 1411–1414. [Google Scholar] [CrossRef]
- Anselmino, M.; Scaglione, M.; Battaglia, A.; Muccioli, S.; Sardi, D.; Azzaro, G.; Garberoglio, L.; Miceli, S.; Gaita, F. Iatrogenic atrial septal defects following atrial fibrillation transcatheter ablation: A relevant entity? Europace 2014, 16, 1562–1568. [Google Scholar] [CrossRef]
- Mugnai, G.; Sieira, J.; Ciconte, G.; Hervas, M.S.; Irfan, G.; Saitoh, Y.; Hünük, B.; Ströker, E.; Velagic, V.; Wauters, K.; et al. One Year Incidence of Atrial Septal Defect after PV Isolation: A Comparison Between Conventional Radiofrequency and Cryoballoon Ablation. Pacing Clin. Electrophysiol. 2015, 38, 1049–1057. [Google Scholar] [CrossRef]
- Sieira, J.; Chierchia, G.B.; di Giovanni, G.; Conte, G.; de Asmundis, C.; Sarkozy, A.; Droogmans, S.; Baltogiannis, G.; Saitoh, Y.; Ciconte, G. One-year incidence of iatrogenic atrial septal defect after cryoballoon ablation for atrial fibrillation. J. Cardiovasc. Electrophysiol. 2014, 25, 11–15. [Google Scholar] [CrossRef]
- Cronin, E.M.; Collier, P.; Wazni, O.M.; Griffin, B.P.; Jaber, W.A.; Saliba, W.I. Persistence of atrial septal defect after cryoballoon ablation of atrial fibrillation. J. Am. Coll. Cardiol. 2013, 62, 1491–1492. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rillig, A.; Meyerfeldt, U.; Kunze, M.; Birkemeyer, R.; Miljak, T.; Jäckle, S.; Hajredini, B.; Treusch, F.; Jung, W. Persistent iatrogenic atrial septal defect after a single-puncture, double-transseptal approach for pulmonary vein isolation using a remote robotic navigation system: Results from a prospective study. Europace 2010, 12, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Davutoglu, V.; Kervancioglu, S.; Dinckal, H.; Soydinc, S.; Turkmen, S.; Akdemir, I.; Aksoy, M. High incidence of occult femoral vein thrombosis related to multiple venous sheaths during electrophysiology study. Heart 2004, 90, 1061–1062. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chen, J.Y.; Chang, K.C.; Lin, Y.C.; Chou, H.T.; Hung, J.S. Safety and outcomes of short-term multiple femoral venous sheath placement in cardiac electrophysiological study and radiofrequency catheter ablation. Jpn. Heart J. 2004, 45, 257–264. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Blanc, J.J.; Almendral, J.; Brignole, M.; Fatemi, M.; Gjesdal, K.; Gonzalez-Torrecilla, E.; Wolpert, C. Consensus document on antithrombotic therapy in the setting of electrophysiological procedures. Europace 2008, 10, 513–527. [Google Scholar] [CrossRef] [PubMed]
- De Sensi, F.; Cresti, A.; Addonisio, L. Migration of femoral vein thrombus to the right ventricle: An undesiderable complication in patients undergoing electrophysiological procedures. Europace 2017, 19, 1131. [Google Scholar] [CrossRef] [PubMed]
- Nasrin, S.; Aaysha Cader, F.; Salahuddin, M.; Nazrin, T.; Iqbal, J.; Jannat, M.; Shafi, P. Pulmonary embolism as a complication of an electrophysiological study: A case report. J. Med. Case Rep. 2016, 10, 89. [Google Scholar] [CrossRef][Green Version]
- Alizadeh, A.; Rad, M.A.; Emkanjoo, Z.; Saravi, M.; Sadeghi, G.; Sadr-Ameli, M.A. Free floating right atrial thrombus in two asymptomatic patients after electrophysiological study: Role of routine echocardiography after ablation. Europace 2010, 12, 587–588. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Sensi, F.; Miracapillo, G.; Addonisio, L.; Breschi, M.; Cresti, A.; Baratta, P.; Paneni, F.; Limbruno, U. Thromboembolic Events Following Atrial Fibrillation Cardioversion and Ablation: What’s the Culprit? Medicina 2019, 55, 505. https://doi.org/10.3390/medicina55080505
De Sensi F, Miracapillo G, Addonisio L, Breschi M, Cresti A, Baratta P, Paneni F, Limbruno U. Thromboembolic Events Following Atrial Fibrillation Cardioversion and Ablation: What’s the Culprit? Medicina. 2019; 55(8):505. https://doi.org/10.3390/medicina55080505
Chicago/Turabian StyleDe Sensi, Francesco, Gennaro Miracapillo, Luigi Addonisio, Marco Breschi, Alberto Cresti, Pasquale Baratta, Francesco Paneni, and Ugo Limbruno. 2019. "Thromboembolic Events Following Atrial Fibrillation Cardioversion and Ablation: What’s the Culprit?" Medicina 55, no. 8: 505. https://doi.org/10.3390/medicina55080505
APA StyleDe Sensi, F., Miracapillo, G., Addonisio, L., Breschi, M., Cresti, A., Baratta, P., Paneni, F., & Limbruno, U. (2019). Thromboembolic Events Following Atrial Fibrillation Cardioversion and Ablation: What’s the Culprit? Medicina, 55(8), 505. https://doi.org/10.3390/medicina55080505





