HDL-C Role in Acquired Aortic Valve Stenosis Patients and Its Relationship with Oxidative Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Populations
2.2. Stenosis Assessment
- aortic jet velocity—Vmax (m/s),
- mean pressure gradient—PG mean (mmHg),
- aortic valve area—AVA (cm2),
- indexed aortic valve area—indexed AVA (cm2/m2).
2.3. Laboratory Assay
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics of Study Patients and Control Group Individuals
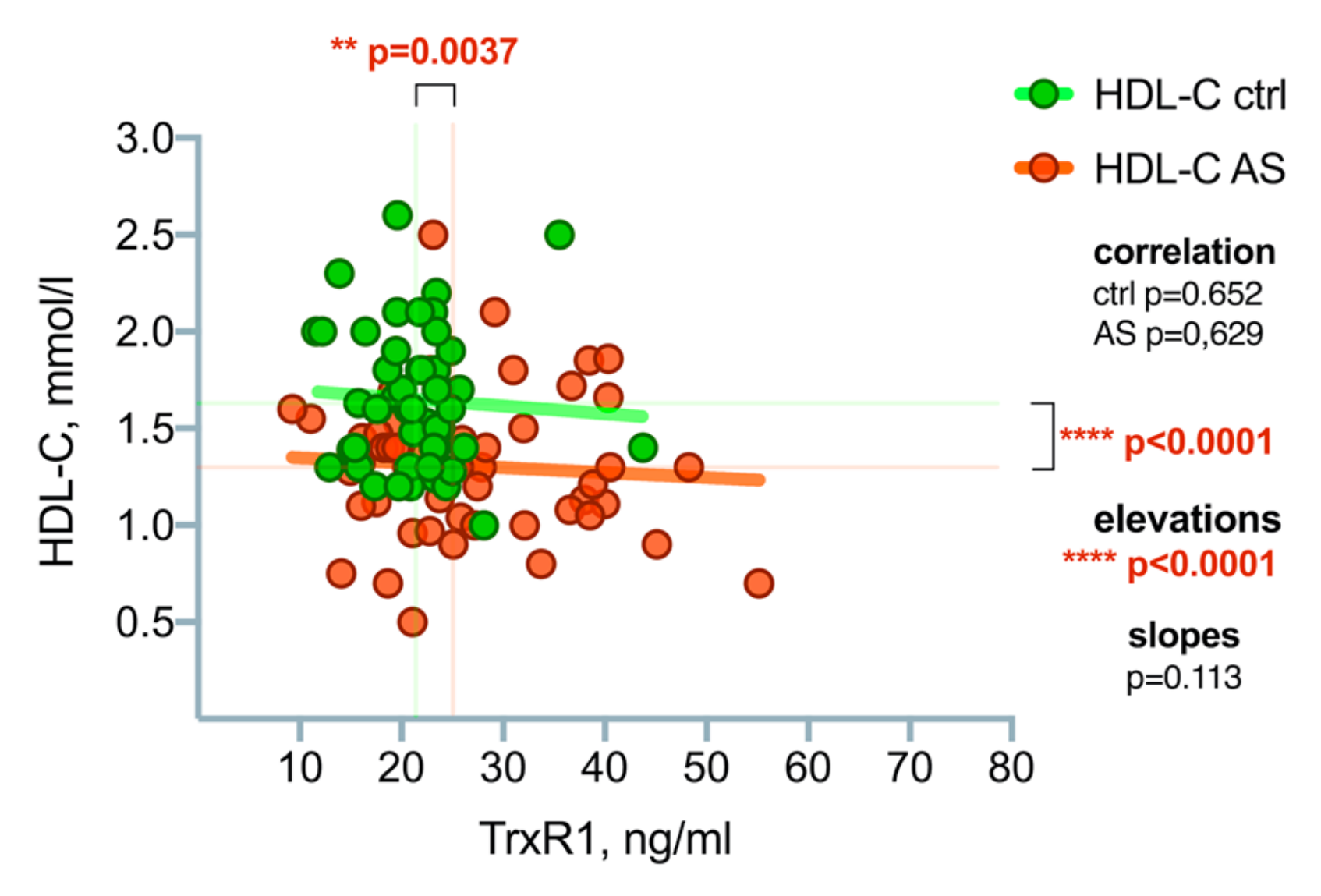
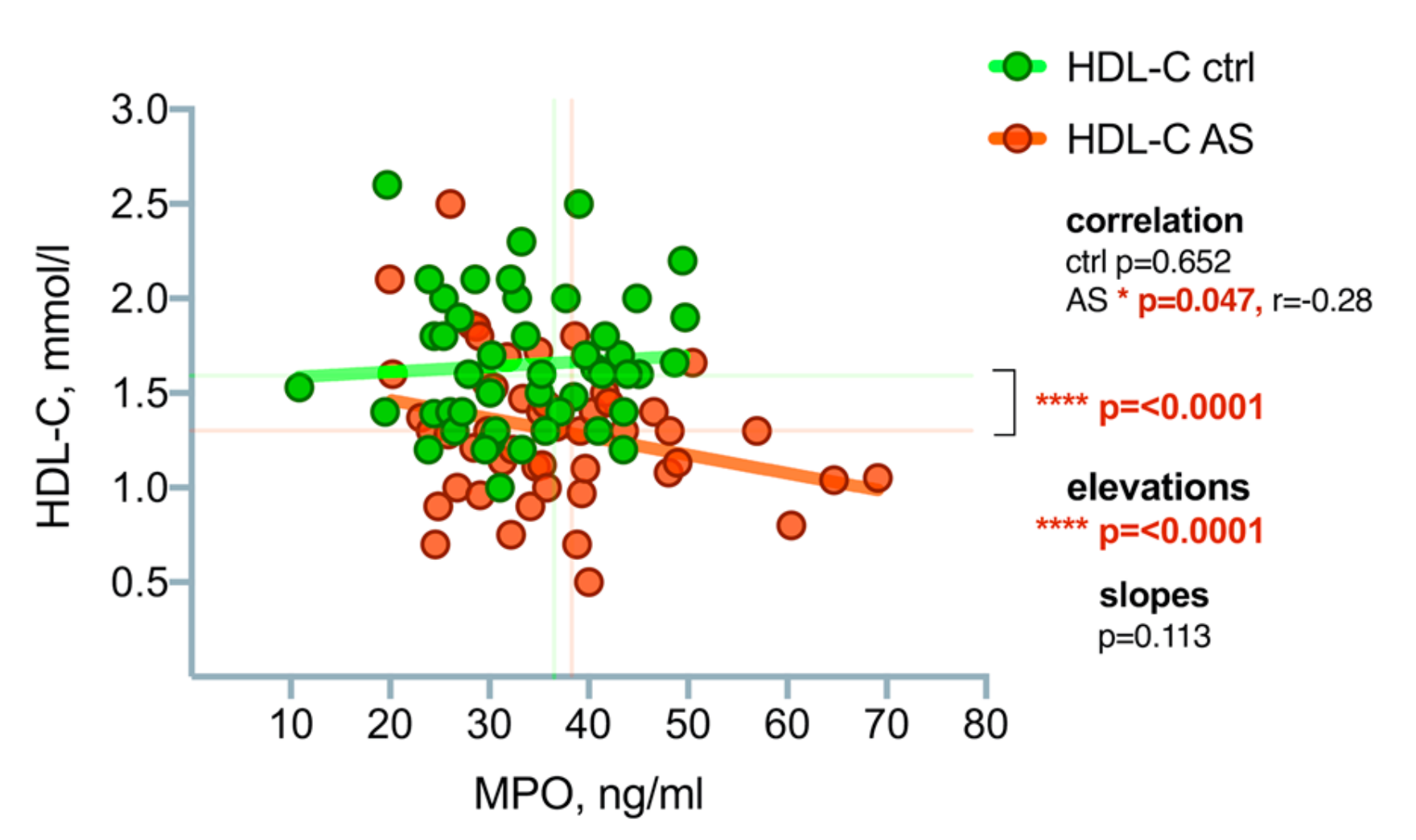
3.2. High-Density Lipoprotein Cholesterol (HDL-C) Level Differences between the Patient Groups
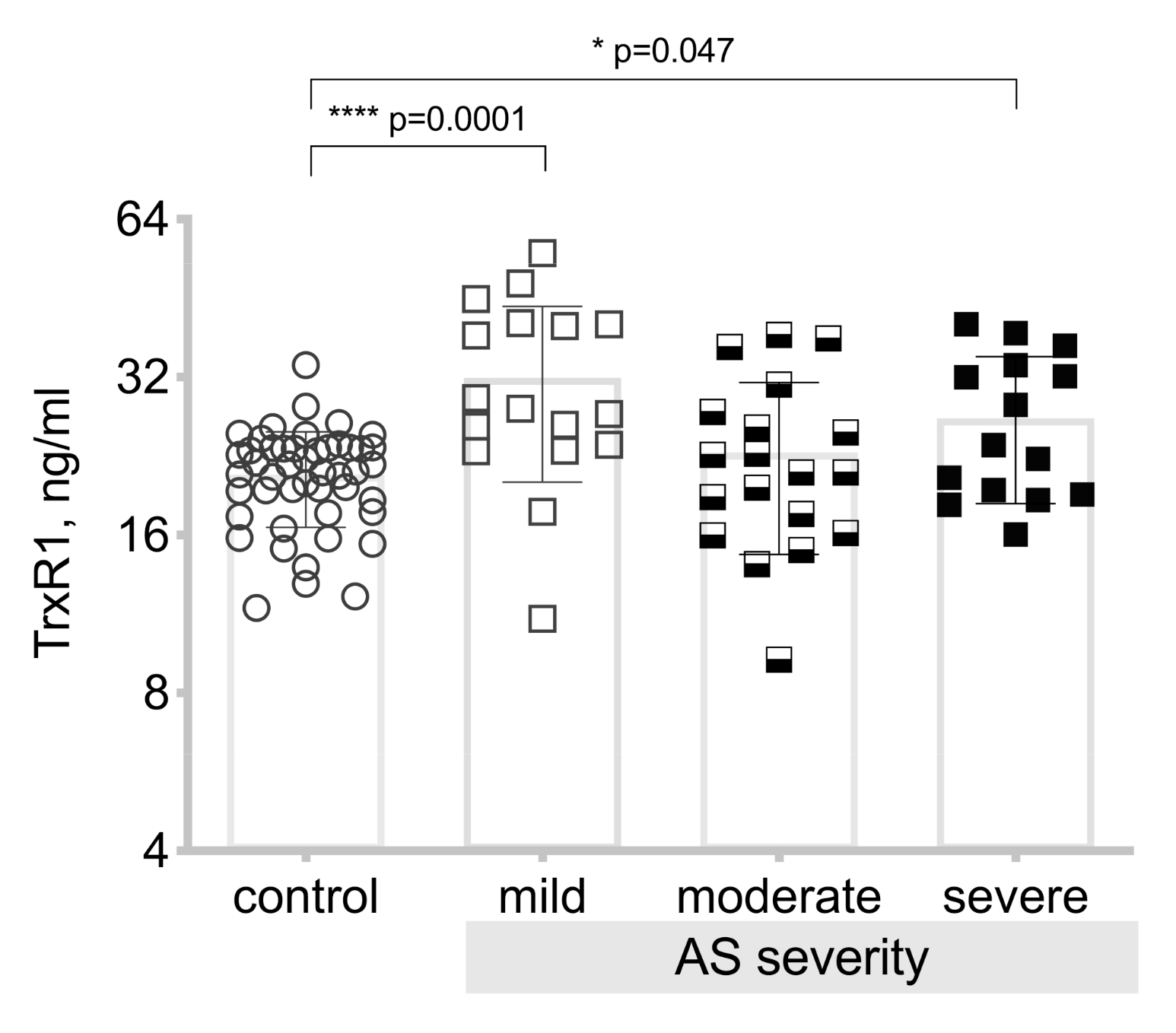
3.3. Thioredoxin Reductase-1(TrxR1) Level Differences between the Patient Groups
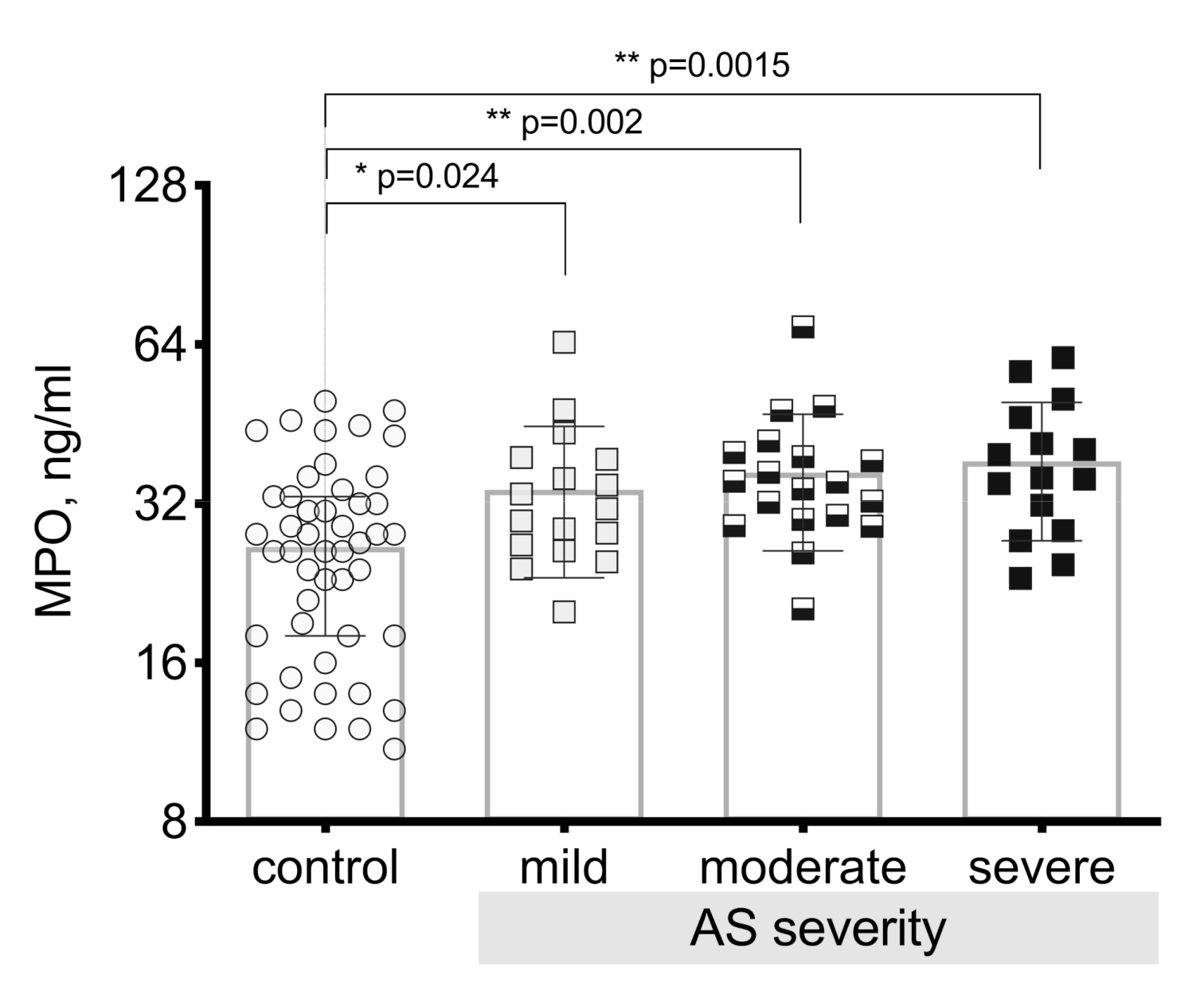
3.4. Myeloperoxidase (MPO) Level Differences between the Patient Groups
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lindman, B.R.; Clavel, M.A.; Mathieu, P.; Lung, B.; Lancellotti, P.; Otto, C.M.; Pibarot, P. Calcific aortic stenosis. Nat. Rev. Dis. Primers 2016, 2, 16006. [Google Scholar] [CrossRef] [PubMed]
- Pawade, T.A.; Newby, D.E.; Dweck, M.R. Calcification in Aortic Stenosis. The Skeleton Key. J. Am. Coll. Cardiol. 2015, 66, 561–577. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Song, R.; Fullerton, D.A.; Ao, L.; Zhai, Y.; Li, S.; Ballak, D.B.; Cleveland, J.C.; Reece, T.B.; McKinsey, T.A.; et al. Interleukin-37 suppresses the osteogenic responses of human aortic valve interstitial cells in vitro and alleviates valve lesions in mice. Proc. Natl. Acad. Sci. USA 2017, 114, 1631–1636. [Google Scholar] [CrossRef] [PubMed]
- Kanwar, A.; Thaden, J.J.; Nkomo, V.T. Management of Patients with Aortic Valve Stenosis. Mayo Clin. Proc. 2018, 93, 488–508. [Google Scholar] [CrossRef] [PubMed]
- Eveborn, G.W.; Schirmer, H.; Heggelund, G.; Lunde, P.; Rasmussen, K. The evolving epidemiology of valvular aortic stenosis. The Tromsø study. Heart 2013, 99, 396–400. [Google Scholar] [CrossRef] [PubMed]
- Perera, S.; Wijesinghe, N.; Ly, E.; Devlin, G.; Pasupati, S. Outcomes of patients with untreated severe aortic stenosis in real-world practice. N. Z. Med. J. 2011, 124, 40–48. [Google Scholar] [PubMed]
- Otto, C.M.; Prendergast, B. Aortic Valve Stenosis-From Patients at Risk to Severe Valve Obstruction. N. Engl. J. Med. 2014, 371, 744–756. [Google Scholar] [CrossRef] [PubMed]
- Heistad, D.D.; Wakisaka, Y.; Miller, J.; Chu, Y.; Pena-Silva, R. Novel Aspects of Oxidative Stress in Cardiovascular Diseases. Circ. J. 2009, 73, 201–207. [Google Scholar] [CrossRef]
- Pena Silva, R.A. Cardiovascular Oxidative Stress: Recent Findings on ACE2 and MAO. Ph.D. Thesis, University of Iowa, Iowa, IA, USA, 2012. [Google Scholar]
- Lommi, J.I.; Kovanen, P.T.; Jauhiainen, M.; Lee-Rueckert, M.; Kupari, M.; Helske, S. High-density lipoproteins (HDL) are present in stenotic aortic valves and may interfere with the mechanisms of valvular calcification. Atherosclerosis 2011, 219, 538–544. [Google Scholar] [CrossRef]
- Barter, P.J.; Nicholls, S.; Rye, K.A.; Anantharamaiah, G.M.; Navab, M.; Fogelman, A.M. Antiinflammatory Properties of HDL. Circ. Res. 2004, 95, 764–772. [Google Scholar] [CrossRef]
- Schindhelm, R.K.; van der Zwan, L.P.; Teerlink, T.; Scheffer, P.G. Myeloperoxidase: A Useful Biomarker for Cardiovascular Disease Risk Stratification? Clin. Chem. 2009, 55, 1462–1470. [Google Scholar] [CrossRef]
- Matsushita, T.; Matsumura, Y.; Nomura, N.; Nakatsuji, K.; Kagawa, S.; Matsuo, M.; Nakagawa, M.; Shimeno, K.; Matsumoto, R.; Abe, Y.; et al. Plasma Myeloperoxidase Levels are Inversely Correlated with Serum High-Density Lipoprotein-Associated Paraoxonase-1 Levels in Patients with Aortic Valve Stenosis. In Proceedings of the 82nd Annual Scientific Meeting of the Japanese Circulation Society, Osaka International Convention Center, Osaka, Japan, 23–25 March 2018; Available online: https://www.micenavi.jp/jcs2018/search/detail_program/id:1629 (accessed on 20 June 2019).
- Strzepa, A.; Pritchard, K.A.; Dittel, B.N. Myeloperoxidase: A new player in autoimmunity. Cell Immunol. 2017, 317, 1–8. [Google Scholar] [CrossRef]
- Wada, S.; Sugioka, K.; Naruko, T.; Kato, Y.; Shibata, T.; Inoue, T.; Inaba, M.; Ohsawa, M.; Yoshiyama, M.; Ueda, M. Myeloperoxidase and progression of aortic valve stenosis in patients undergoing hemodialysis. J. Heart Valve Dis. 2013, 22, 640–647. [Google Scholar]
- Gorreta, F.; Runfola, T.P.; VanMeter, A.J.; Barzaghi, D.; Chandhoke, V.; Del Giacco, L. Identification of thioredoxin reductase 1-regulated genes using small interference RNA and cDNA microarray. Cancer Biol. Ther. 2005, 4, 1079–1088. [Google Scholar] [CrossRef]
- Lee, S.; Kim, S.M.; Lee, R.T. Thioredoxin and Thioredoxin Target Proteins: From Molecular Mechanisms to Functional Significance. Antioxid. Redox Signal. 2013, 18, 1165–1207. [Google Scholar] [CrossRef]
- Vahanian, A.; Alfieri, O.; Andreotti, F.; Antunes, M.J.; Barón-Esquivias, G.; Baumgartner, H.; Borger, M.A.; Carrel, T.P.; De Bonis, M.; Evangelista, A.; et al. Committee for Practice Guidelines (CPG); Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC); European Association for Cardio-Thoracic Surgery (EACTS). Eur J. Cardiothorac. Surg. 2012, 42, 1–44. [Google Scholar] [CrossRef]
- Aboyans, V.; Criqui, M.H.; Abraham, P.; Allison, M.A.; Creager, M.A.; Diehm, C.; Fowkes, F.G.; Hiatt, W.R.; Jönsson, B.; Lacroix, P.; et al. American Heart Association Council on Peripheral Vascular Disease; Council on Epidemiology and Prevention; Council on Clinical Cardiology; Council on Cardiovascular Nursing; Council on Cardiovascular Radiology and Intervention; and Council on Cardiovascular Surgery and Anesthesia. Measurement and interpretation of the ankle-brachial index: A scientific statement from the American Heart Association. Circulation 2012, 126, 2890–2909. [Google Scholar] [CrossRef]
- Lurins, J.; Lurina, D.; Tretjakovs, P.; Mackevics, V.; Lejnieks, A.; Rapisarda, V.; Baylon, V. Increased serum chemerin level to predict early onset of aortic valve stenosis. Biomed. Rep. 2018, 8, 31–36. [Google Scholar] [CrossRef]
- Domouzoglou, E.M.; Naka, K.K.; Vlahos, A.P.; Papafaklis, M.I.; Michalis, L.K.; Tsatsoulis, A.; Maratos-Flier, E. Fibroblast growth factors in cardiovascular disease: The emerging role of FGF21. Am. J. Physiol Heart Circ. Physiol. 2015, 309, 1029–1038. [Google Scholar] [CrossRef]
- Yamamoto, M.; Yang, G.; Hong, C.; Liu, J.; Holle, E.; Yu, X.; Wagner, T.; Vatner, S.F.; Sadoshima, J. Inhibition of endogenous thioredoxin in the heart increases oxidative stress and cardiac hypertrophy. J. Clin. Investig. 2003, 112, 1395–1406. [Google Scholar] [CrossRef]
- Jekell, A.; Hossain, A.; Alehagen, U.; Dahlström, U.; Rosén, A. Elevated circulating levels of thioredoxin and stress in chronic heart failure. Eur. J. Heart Failure 2004, 6, 883–890. [Google Scholar] [CrossRef]
- Cowell, S.J.; Newby, D.E.; Prescott, R.J.; Bloomfield, P.; Reif, J.; Northridge, D.B.; Boon, N.A. Scottich Aortic Stenosis and Lipid Lowering Trial, Inpact on Regression (SALTIRE) Investigators. A randomized trial of intensive lipid-lowering therapy in calcific aortic stenosis. N. Engl. J. Med. 2005, 352, 89–2397. [Google Scholar] [CrossRef]
- Rossebo, A.B.; Pedersen, T.R.; Allen, C.; Boman, K.; Chambers, J.; Egstrup, K.; Gerdts, E.; Gohlke-Barwolf, C.; Holme, I.; Wachtell, K.; et al. Design and baseline characteristics of the simvastatin and ezetemibe in aortic stenosis (SEAS) study. Am. J. Cardiol. 2007, 99, 970–973. [Google Scholar] [CrossRef][Green Version]
- Chan, K.L.; Teo, K.; Dumesnil, J.G.; Ni, A.; Tam, J. ASTRONOMER Investigator. Effect of lipid lowering with rosuvastatin on progression of aortic stenosis: Results of the aortic stenosis progression observation: Measuring effects of rosuvastatin (ASTRONOMER) Trial. Circulation 2010, 121, 306–314. [Google Scholar] [CrossRef]
- Olgun Küçük, H.; Küçük, U.; Demirtaş, C.; Özdemir, M. Role of serum high density lipoprotein levels and functions in calcific aortic valve stenosis progression. J. Clin. Exp Med. 2015, 8, 22543. [Google Scholar]
- Arsenault, B.J.; Dubé, M.P.; Brodeur, M.R.; de Oliveira Moraes, A.B.; Lavoie, V.; Kernaleguen, A.E. Evaluation of Links between High-Density Lipoprotein Genetics, Functionality, and Aortic Valve Stenosis Risk in Humans. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 457–462. [Google Scholar] [CrossRef]
- Gebhard, C.; Maafi, F.; Stähli, B.; Bonnefoy, A.; Gebhard, C.E.; Nachar, W.; de Oliveira Moraes, A.B.; Mecteau, M.; Mihalache-Avram, T.; Lavoie, V.; et al. Beneficial Effects of High-Density Lipoproteins on Acquired von Willebrand Syndrome in Aortic Valve Stenosis. Thromb. Haemost. 2018, 118, 288–297. [Google Scholar] [CrossRef]
- Tardif, J.C. An Important Discovery at the Montreal Heart Institute: A New Approach to Treat the Most Common Heart Valve Disease in Western Countries. 2013. Available online: https://www.icm-mhi.org/en/new-approach-treat-most-common-heart-valve-disease-western-countries (accessed on 20 June 2019).
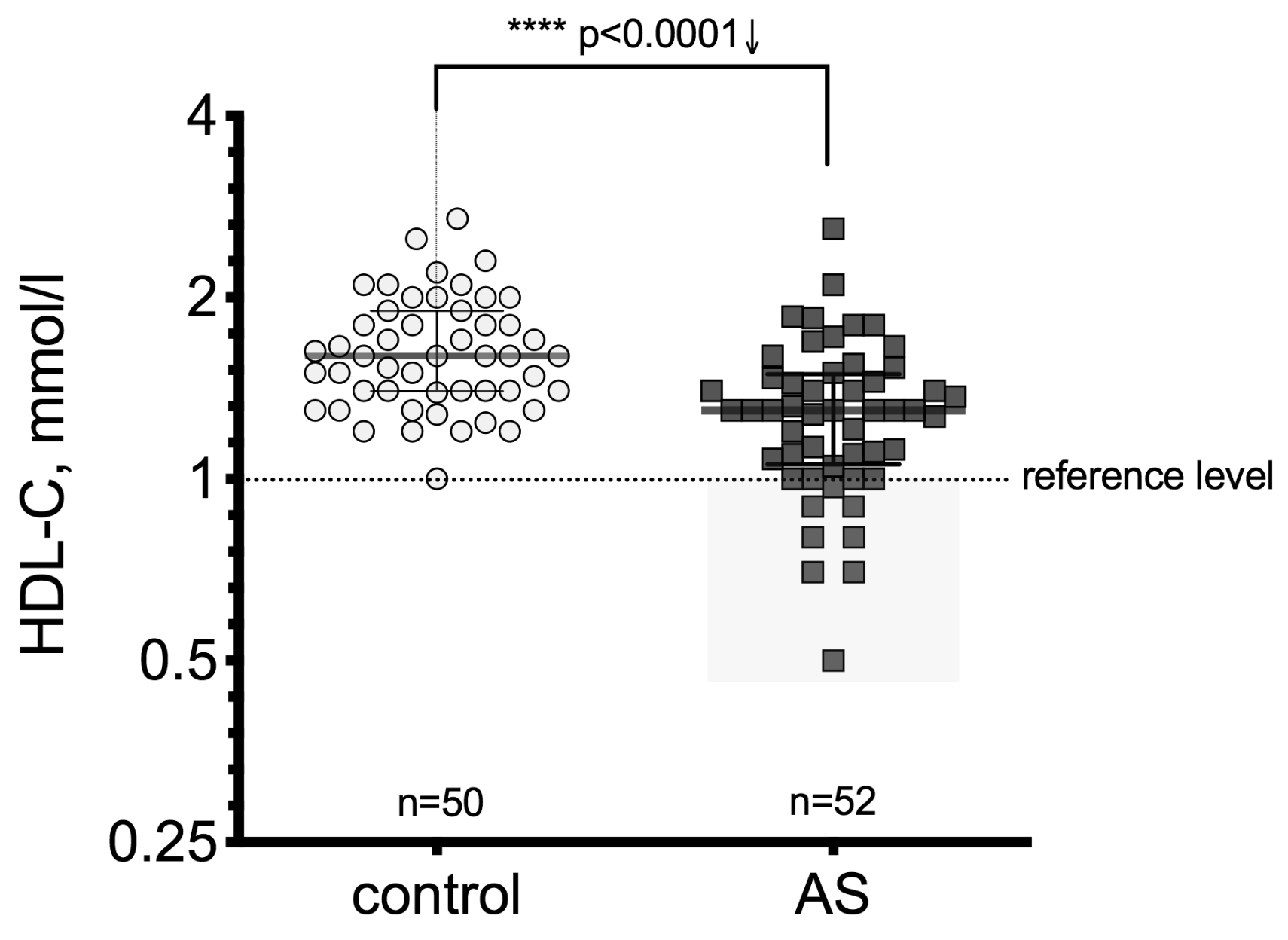
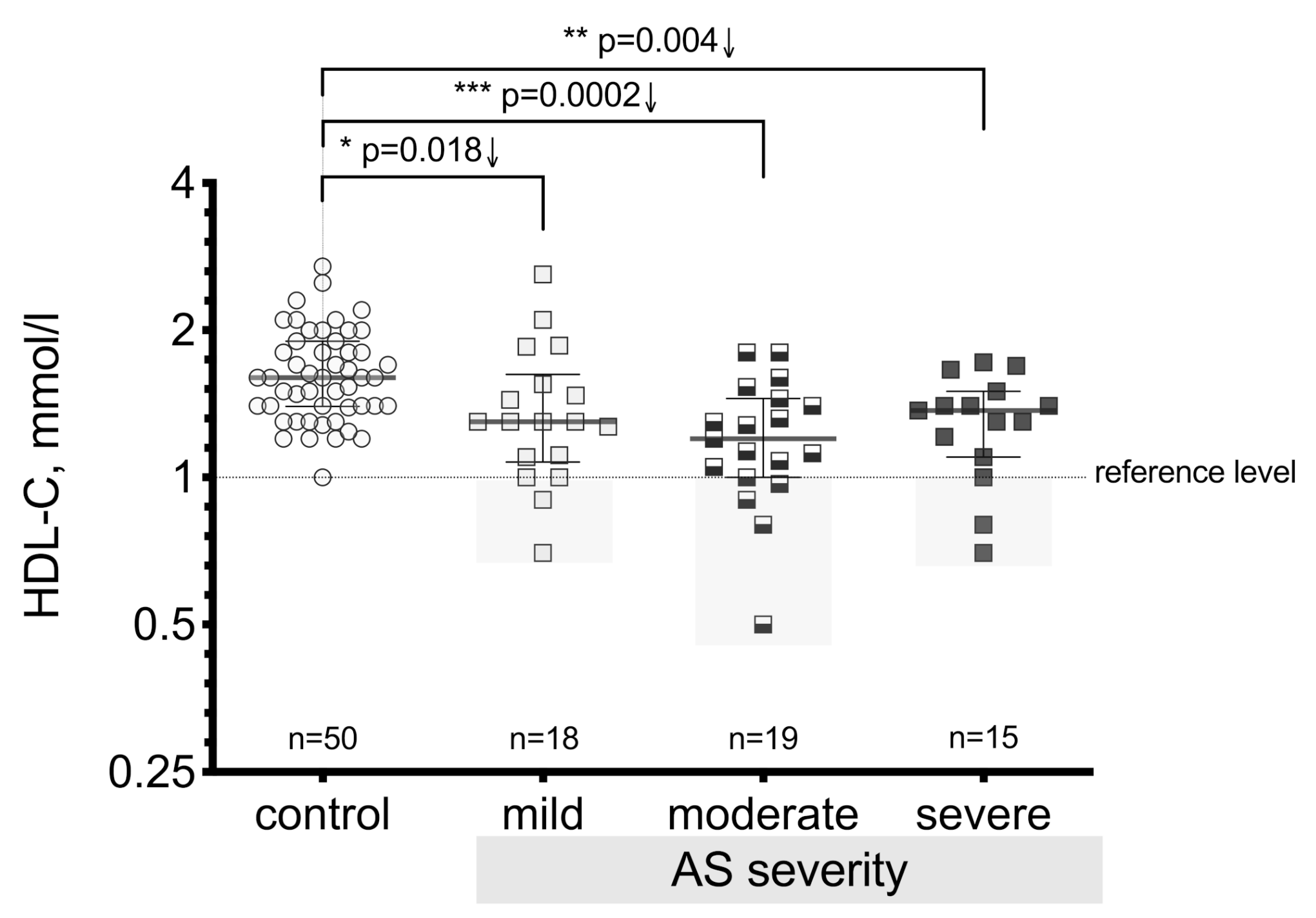
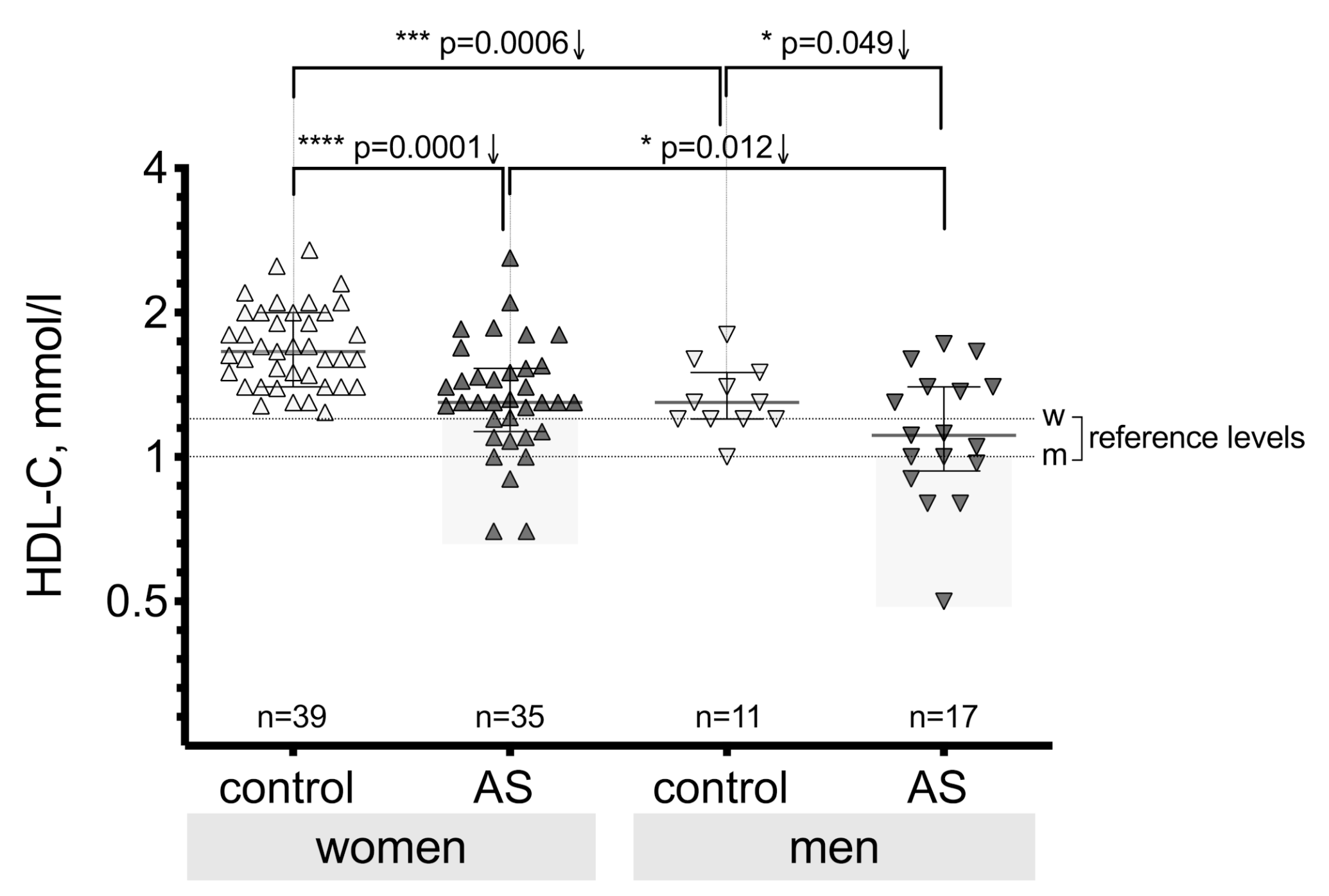
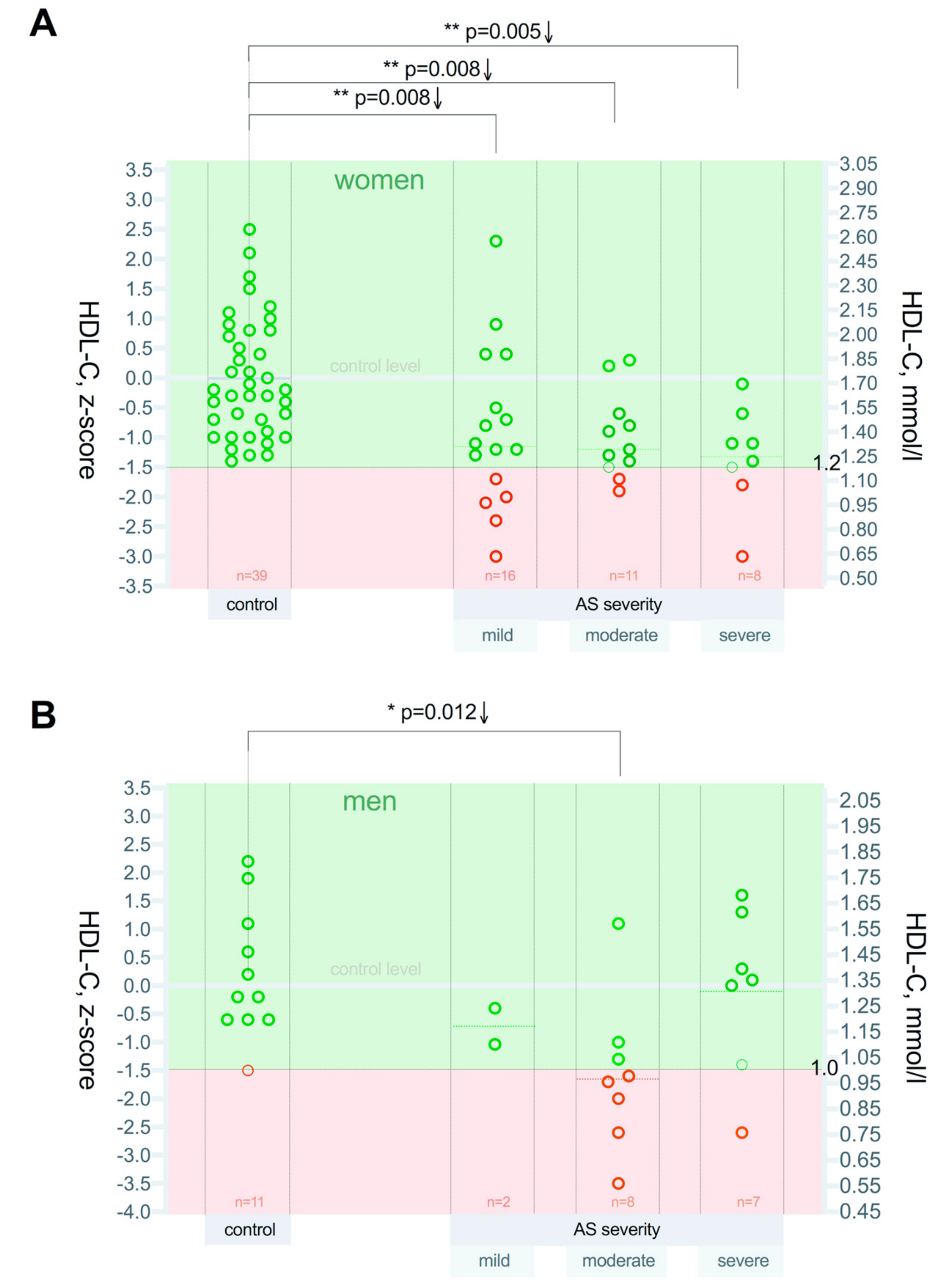
| Control, n = 50 | AV Mild Stenosis, n = 18 | AV Moderate Stenosis, n = 19 | AV Severe Stenosis, n = 15 | ||
|---|---|---|---|---|---|
| Gender, (%) | Male Female | 11 (22.0) 39 (78.0) | 2 (11.1) 16 (88.9) | 8 (42.1) 11 (57.9) | 7 (46.7) 8 (53.3) |
| Age, years | Mdn (IQR) | 64 (57–75) | 71 (65–75) | 74 (65–79) | 65 (60–74) |
| Control, n = 50 | AV Mild Stenosis, n = 18 | AV Moderate Stenosis, n = 19 | AV Severe Stenosis, n = 15 | |||
|---|---|---|---|---|---|---|
| 1 BMI | M (± SD) | 26.04 (4.31) | 27.39 (3.10) p = 0.399 | 25.81 (4.58) p = 0.682 | 27.40 (3.18) p = 0.869 | |
| p-value vs. control | ||||||
| 2 LDL-C, mmol/L | M (± SD) | 3.28 (1.18) | 3.05 (0.97) p > 0.999 | 2.59 (0.92) p = 0.057 | 3.10 (1.12) p > 0.999 | |
| p-value vs. control | ||||||
| 3 TG, mmol/L | M (± SD) | 1.47 (0.71) | 1.64 (0.84) p = 0.406 | 1.11 (0.56) p = 0.178 | 1.27 (0.57) p = 0.406 | |
| p-value vs. control | ||||||
| 4 TC, mmol/L | M (± SD) | 5.49 (1.28) | 5.01 (1.34) p = 0.056 | 4.21 (1.18) ** p = 0.001 | 4.68 (1.08) * p = 0.016 | |
| p-value vs. control | ||||||
| 5 SV, mL | Mdn (IQR) | 96.5 (90.0–106.3) | 100.0 (90.0–110.0) p = 0.716 | 96.0 (88.0–100.0) p = 0.375 | 90.0 (88.0–95.0) p = 0.103 | |
| p-value vs. control | ||||||
| 6 EF % | Mdn (IQR) | 63.5 (57.7–68.0) | 60.0 (57.5–63.5) p = 0.347 | 61.0 (58.0–66.0) p = 0.981 | 60.0 (57.0–64.0) p = 0.347 | |
| p-value vs. control | ||||||
| 7 SVI | Mdn (IQR) | 52.2 (46.3–59.1) | 53.6 (49.6–60.2) p = 0.767 | 49.4 (47.4–52.1) p = 0.288 | 49.7 (42.9–52.7) p = 0.157 | |
| p-value vs. control | ||||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hofmanis, J.; Hofmane, D.; Svirskis, S.; Mackevics, V.; Tretjakovs, P.; Lejnieks, A.; Signorelli, S.S. HDL-C Role in Acquired Aortic Valve Stenosis Patients and Its Relationship with Oxidative Stress. Medicina 2019, 55, 416. https://doi.org/10.3390/medicina55080416
Hofmanis J, Hofmane D, Svirskis S, Mackevics V, Tretjakovs P, Lejnieks A, Signorelli SS. HDL-C Role in Acquired Aortic Valve Stenosis Patients and Its Relationship with Oxidative Stress. Medicina. 2019; 55(8):416. https://doi.org/10.3390/medicina55080416
Chicago/Turabian StyleHofmanis, Juris, Dace Hofmane, Simons Svirskis, Vitolds Mackevics, Peteris Tretjakovs, Aivars Lejnieks, and Salvatore Santo Signorelli. 2019. "HDL-C Role in Acquired Aortic Valve Stenosis Patients and Its Relationship with Oxidative Stress" Medicina 55, no. 8: 416. https://doi.org/10.3390/medicina55080416
APA StyleHofmanis, J., Hofmane, D., Svirskis, S., Mackevics, V., Tretjakovs, P., Lejnieks, A., & Signorelli, S. S. (2019). HDL-C Role in Acquired Aortic Valve Stenosis Patients and Its Relationship with Oxidative Stress. Medicina, 55(8), 416. https://doi.org/10.3390/medicina55080416







