Multifunctional Platforms Based on Graphene Oxide and Natural Products
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of Graphene Oxide
2.2. Loading of Biological Active Agent (BAA)
2.3. Analysis of Functional Groups of Graphene Oxide
3. Results
4. Discussion
4.1. Fourier Transform Infrared Spectroscopy (FTIR)
4.2. Raman Spectroscopy
4.3. Thermogravimetric Analysis of Graphene Oxide Loaded with Active Compounds
4.4. Release Behavior of Active Compounds
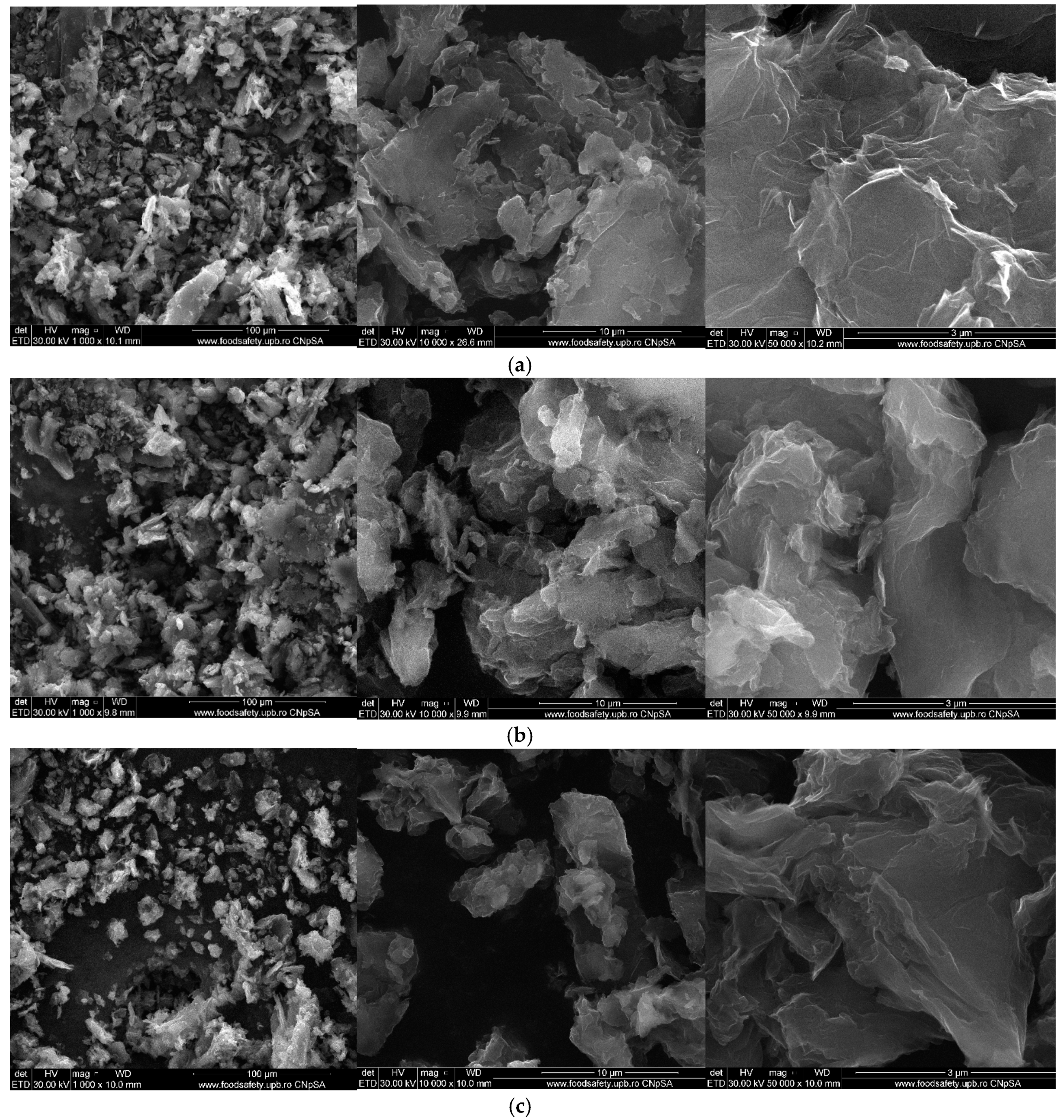
4.5. Scanning Electron Microscopy (SEM)
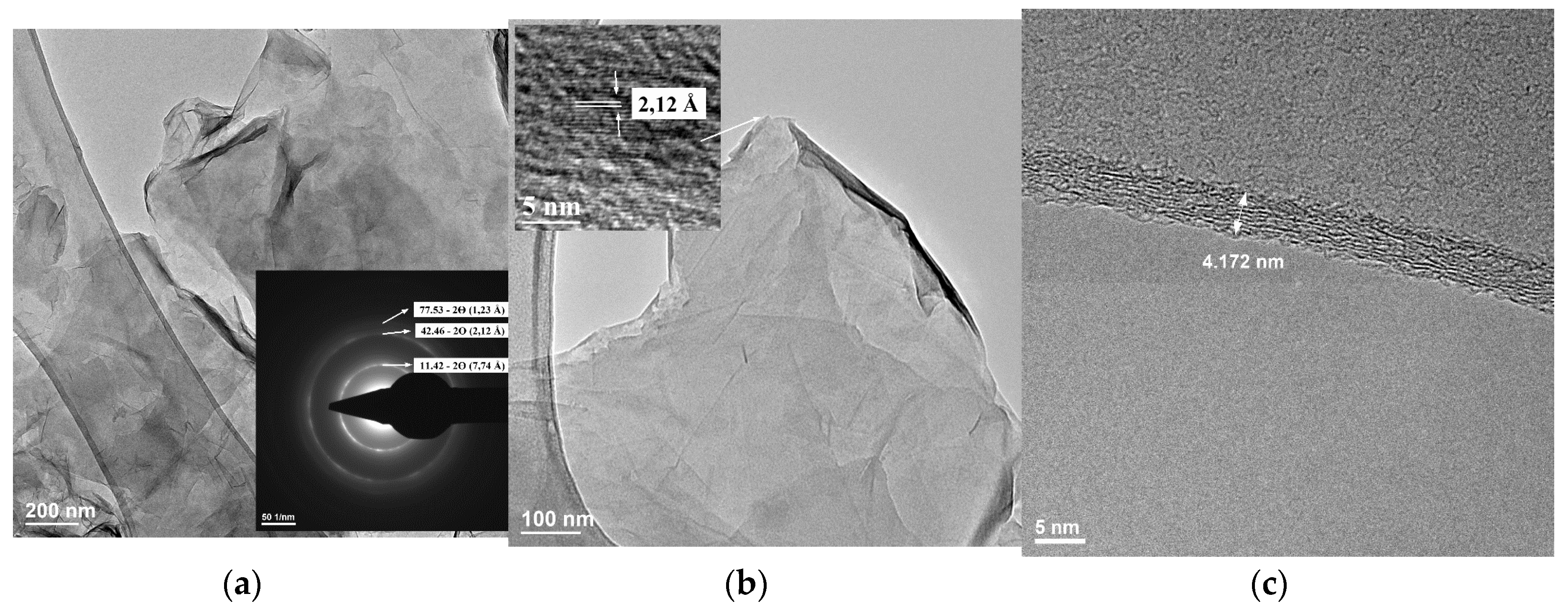
4.6. Transmission Electron Microscopy (TEM)
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Marascu-Klein, V. The applications of carbon materials in advanced technologies. Int. Conf. Econ. Eng. Manuf. Syst. 2007, 8, 313–316. [Google Scholar]
- Yang, L.; Zhang, L.; Webster, T.J. Carbon nanostructures for orthopedic medical applications. Nanomedicine 2011, 6, 1231–1244. [Google Scholar] [CrossRef]
- Biagiotti, G.; Lange, V.; Ligi, C.; Caporali, S.; Muniz-Miranda, M.; Flis, A.; Pietrusiewicz, K.M.; Ghini, G.; Brandi, A.; Cicchi, S. Nanostructured carbon materials decorated with organophosphorus moieties: Synthesis and application. Beilstein J. Nanotechnol. 2017, 8, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.Y.; Zhang, L.N.; Yan, M.; Yu, J.H. Carbon nanostructures in biology and medicine. J. Mater. Chem. B 2017, 5, 6437–6450. [Google Scholar] [CrossRef]
- Ugnivenko, A.; Perepelytsina, O.; Sydorenko, M.V.; Ostapchenko, L.I. Carbon nanotubes in delivery of bioactive substances. J. Bionanosci. 2017, 1475, 1–43. [Google Scholar] [CrossRef]
- Urie, R.; Ghosh, D.; Ridha, I.; Rege, K. Inorganic nanomaterials for soft tissue repair and regeneration. Annu. Rev. Biomed. Eng. 2018, 20, 353–374. [Google Scholar] [CrossRef] [PubMed]
- Alshehri, R.; Ilyas, A.M.; Hasan, A.; Arnaout, A.; Ahmed, F.; Memic, A. Carbon nanotubes in biomedical applications: Factors, mechanisms, and remedies of toxicity. J. Med. Chem. 2016, 59, 8149–8167. [Google Scholar] [CrossRef] [PubMed]
- Kaur, R.; Verma, S.; Joshi, P.; Singh, S.P.; Singh, M. Cytotoxicity of graphene oxide (GO) and graphene oxide conjugated losartan potassium (GO-LP) on neuroblastoma (NB41A3) Cells. J. Nanosci. Nanotechnol. 2018, 18, 6765–6775. [Google Scholar] [CrossRef]
- Bidram, E.; Sulistio, A.; Cho, H.J.; Amini, A.; Harris, T.; Zarrabi, A.; Qiao, G.; Stewart, A.; Dunstan, D.E. Targeted graphene oxide networks: Cytotoxicity and synergy with anticancer agents. Acs Appl. Mater. Interfaces 2018, 10, 43523–43532. [Google Scholar] [CrossRef]
- Mali, N.; Jadhav, S.; Karpe, M.; Kadam, V. Carbon nanotubes as carrier for delivery of bioactive and therapeutic agents: An overview. Int. J. Pharm. Pharm. Sci. 2011, 3, 45–52. [Google Scholar]
- Liu, Z.; Tabakman, S.; Welsher, K.; Dai, H. Carbon nanotubes in biology and medicine: In vitro and in vivo detection, imaging and drug delivery. Nano Res. 2009, 2, 85–120. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Mehra, N.K.; Jain, K.; Jain, N.K. Biomedical applications of carbon nanotubes: A critical review. Curr. Drug Deliv. 2016, 13, 796–817. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Sahoo, N.G.; Li, L. The application of graphene oxide in drug delivery. Expert Opin. Drug Deliv. 2012, 9, 1365–1376. [Google Scholar] [CrossRef] [PubMed]
- Kuila, T.; Bose, S.; Mishra, A.K.; Khanra, P.; Kim, N.H.; Lee, J.H. Chemical functionalization of graphene and its applications. Prog. Mater. Sci. 2012, 57, 1061–1105. [Google Scholar] [CrossRef]
- Sahoo, N.G.; Bao, H.Q.; Pan, Y.Z.; Pal, M.; Kakran, M.; Cheng, H.K.F.; Li, L.; Tan, L.P. Functionalized carbon nanomaterials as nanocarriers for loading and delivery of a poorly water-soluble anticancer drug: A comparative study. Chem. Commun. 2011, 47, 5235–5237. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Wan, J.M.; Zhang, S.A.; Zhang, Y.J.; Lee, S.T.; Liu, Z.A. In vivo pharmacokinetics, long-term biodistribution, and toxicology of PEGylated graphene in mice. ACS Nano 2011, 5, 516–522. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.Q.; Yu, J.H.; Li, Y.Y.; Zhao, J.; Dong, H.Q. Engineering of a novel pluronic F127/graphene nanohybrid for pH responsive drug delivery. J. Biomed. Mater. Res. Part A 2012, 100A, 141–148. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, Y.M.; Chen, Y.; Zhao, D.; Chen, J.T.; Liu, Y. Construction of a graphene oxide based noncovalent multiple nanosupramolecular assembly as a scaffold for drug delivery. Chem. Eur. J. 2012, 18, 4208–4215. [Google Scholar] [CrossRef]
- Chung, C.; Kim, Y.K.; Shin, D.; Ryoo, S.R.; Hong, B.H.; Min, D.H. Biomedical applications of graphene and graphene oxide. Acc. Chem. Res. 2013, 46, 2211–2224. [Google Scholar] [CrossRef]
- Zhou, X.; Liang, F. Application of graphene/graphene oxide in biomedicine and biotechnology. Curr. Med. Chem. 2014, 21, 855–869. [Google Scholar] [CrossRef]
- Morales-Narvaez, E.; Merkoci, A. Graphene oxide as an optical biosensing platform: A progress report. Adv. Mater. 2019, 31, 1805043. [Google Scholar] [CrossRef] [PubMed]
- Bianco, A.; Cheng, H.M.; Enoki, T.; Gogotsi, Y.; Hurt, R.H.; Koratkar, N.; Kyotani, T.; Monthioux, M.; Park, C.R.; Tascon, J.M.D.; et al. All in the graphene family-A recommended nomenclature for two-dimensional carbon materials. Carbon 2013, 65, 1–6. [Google Scholar] [CrossRef]
- Wick, P.; Louw-Gaume, A.E.; Kucki, M.; Krug, H.F.; Kostarelos, K.; Fadeel, B.; Dawson, K.A.; Salvati, A.; Vazquez, E.; Ballerini, L.; et al. Classification framework for graphene-based materials. Angew. Chem. Int. Ed. 2014, 53, 7714–7718. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.S.; Das Modac, M.; Paik, P. Graphene oxide for biomedical applications. J. Nanomed. Res. 2017, 5, 1–6. [Google Scholar]
- Singh, D.P.; Herrera, C.E.; Singh, B.; Singh, S.; Singh, R.K.; Kumar, R. Graphene oxide: An efficient material and recent approach for biotechnological and biomedical applications. Mater. Sci. Eng. C 2018, 86, 173–197. [Google Scholar] [CrossRef]
- Singh, S.K.; Singh, M.K.; Kulkarni, P.P.; Sonkar, V.K.; Gracio, J.J.; Dash, D. Amine-modified graphene: Thrombo-protective safer alternative to graphene oxide for biomedical applications. ACS Nano 2012, 6, 2731–2740. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.Y.; An, S.S.; Hulme, J. Current applications of graphene oxide in nanomedicine. Int. J. Nanomed. 2015, 10, 9–24. [Google Scholar]
- Wang, K.; Ruan, J.; Song, H.; Zhang, J.; Wo, Y.; Guo, S.; Cui, D. Biocompatibility of graphene oxide. Nanoscale Res. Lett. 2011, 6, 8. [Google Scholar] [CrossRef]
- Dreyer, D.R.; Park, S.; Bielawski, C.W.; Ruoff, R.S. The chemistry of graphene oxide. Chem. Soc. Rev. 2010, 39, 228–240. [Google Scholar] [CrossRef]
- Wu, S.; Zhao, X.; Cui, Z.; Zhao, C.; Wang, Y.; Du, L.; Li, Y. Cytotoxicity of graphene oxide and graphene oxide loaded with doxorubicin on human multiple myeloma cells. Int. J. Nanomed. 2014, 9, 1413–1421. [Google Scholar]
- Han, W.; Niu, W.Y.; Sun, B.; Shi, G.C.; Cui, X.Q. Biofabrication of polyphenols stabilized reduced graphene oxide and its anti-tuberculosis activity. J. Photochem. Photobiol. B Biol. 2016, 165, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Jianchang, L.; Xiangqiong, Z.; Tianhui, R.; Emile, H. The preparation of graphene oxide and its derivatives and their application in bio-tribological systems. Lubricants 2014, 2, 137–161. [Google Scholar]
- Sebe, I.; Kallai-Szabo, B.; Zelko, R.; Szabo, D. Polymers and formulation strategies of nanofibrous systems for drug delivery application and tissue engineering. Curr. Med. Chem. 2015, 22, 604–617. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Q.; Hao, L.Y.; Zhang, C.L.; Chen, J.; Zhang, P. High efficient anti-cancer drug delivery systems using tea polyphenols reduced and functionalized graphene oxide. J. Biomater. Appl. 2017, 31, 1108–1122. [Google Scholar] [CrossRef] [PubMed]
- Chanwitheesuk, A.; Teerawutgulrag, A.; Kilburn, J.D.; Rakariyatham, N. Antimicrobial gallic acid from Caesalpinia mimosoides Lamk. Food Chem. 2007, 100, 1044–1048. [Google Scholar] [CrossRef]
- Santos, A.; Barros, L.; Calhelha, R.C.; Duenas, M.; Carvalho, A.M.; Santos-Buelga, C.; Ferreira, I.C.F.R. Leaves and decoction of Juglans regia L.: Different performances regarding bioactive compounds and in vitro antioxidant and antitumor effects. Ind. Crop. Prod. 2013, 51, 430–436. [Google Scholar] [CrossRef]
- Cosmulescu, S.; Trandafir, I.; Nour, V. Seasonal variation of the main individual phenolics and juglone in walnut (Juglans regia) leaves. Pharm. Biol. 2014, 52, 575–580. [Google Scholar] [CrossRef][Green Version]
- Fernandez-Agullo, A.; Pereira, E.; Freire, M.S.; Valentao, P.; Andrade, P.B.; Gonzalez-Alvarez, J.; Pereira, J.A. Influence of solvent on the antioxidant and antimicrobial properties of walnut (Juglans regia L.) green husk extracts. Ind. Crop. Prod. 2013, 42, 126–132. [Google Scholar] [CrossRef]
- Zhang, X.B.; Zou, C.L.; Duan, Y.X.; Wu, F.; Li, G. Activity guided isolation and modification of juglone from Juglans regia as potent cytotoxic agent against lung cancer cell lines. Bmc Complement. Altern. Med. 2015, 15, 396. [Google Scholar] [CrossRef]
- Martinez Paino, I.M.; Santos, F.; Zucolotto, V. Biocompatibility and toxicology effects of graphene oxide in cancer, normal, and primary immune cells. J. Biomed. Mater. Res. Part A 2017, 105, 728–736. [Google Scholar] [CrossRef]
- Zaaba, N.I.; Foo, K.L.; Hashim, U.; Tan, S.J.; Liu, W.W.; Voon, C.H. Synthesis of graphene oxide using modified hummers method: Solvent influence. Proced. Eng. 2017, 184, 469–477. [Google Scholar] [CrossRef]
- Weng, Y.P.; Hung, P.F.; Ku, W.Y.; Chang, C.Y.; Wu, B.H.; Wu, M.H.; Yao, J.Y.; Yang, J.R.; Lee, C.H. The inhibitory activity of gallic acid against DNA methylation: Application of gallic acid on epigenetic therapy of human cancers. Oncotarget 2018, 9, 361–374. [Google Scholar] [CrossRef] [PubMed]
- Dorniani, D.; Saifullah, B.; Barahuie, F.; Arulselvan, P.; Hussein, M.Z.; Fakurazi, S.; Twyman, L.J. Graphene Oxide-Gallic Acid Nanodelivery System for Cancer Therapy. Nanoscale Res. Lett. 2016, 11, 491. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Y.G.; Chen, H.; Deng, Y.W. Simultaneous determination of catechins, caffeine and gallic acids in green, Oolong, black and pu-erh teas using HPLC with a photodiode array detector. Talanta 2002, 57, 307–316. [Google Scholar] [CrossRef]
- Mota, F.L.; Queimada, A.J.; Pinho, S.P.; Macedo, E.A. Aqueous solubility of some natural phenolic compounds. Ind. Eng. Chem. Res. 2008, 47, 5182–5189. [Google Scholar] [CrossRef]
- Ji, W.Q.; Meng, Q.Q.; Ding, L.; Wang, F.; Dong, J.J.; Zhou, G.Y.; Wang, B.H. Measurement and correlation of the solubility of caffeic acid in eight mono and water plus ethanol mixed solvents at temperatures from (293.15 to 333.15) K. J. Mol. Liq. 2016, 224, 1275–1281. [Google Scholar] [CrossRef]
- Zahi, M.R.; Liang, H.; Yuan, Q.P. Improving the antimicrobial activity of D-limonene using a novel organogel-based nanoemulsion. Food Control 2015, 50, 554–559. [Google Scholar] [CrossRef]
- Yang, C.; Chen, H.; Chen, H.L.; Zhong, B.L.; Luo, X.Z.; Chun, J. Antioxidant and anticancer activities of essential oil from gannan navel orange peel. Molecules 2017, 22, 1391. [Google Scholar] [CrossRef]
- De Doz, M.B.G.; Cases, A.M.; Solimo, H.N. (Liquid plus liquid) equilibria of (water plus linalool plus limonene) ternary system at T = (298.15, 308.15, and 318.15) K. J. Chem. Thermodyn. 2008, 40, 1575–1579. [Google Scholar] [CrossRef]
- Mendanha, S.A.; Marquezin, C.A.; Ito, A.S.; Alonso, A. Effects of nerolidol and limonene on stratum corneum membranes: A probe EPR and fluorescence spectroscopy study. Int. J. Pharm. 2017, 532, 547–554. [Google Scholar] [CrossRef]
- Swamy, M.K.; Akhtar, M.S.; Sinniah, U.R. Antimicrobial properties of plant essential oils against human pathogens and their mode of action: An updated review. Evid. Based Complement. Altern. Med. 2016. [Google Scholar] [CrossRef] [PubMed]
- Al-Rawi, S.S.; Ibrahim, A.H.; Ab Rahman, N.N.N.; Nama, M.M.B.; Majid, A.M.A.; Ab Kadir, M.O. The effect of supercritical fluid extraction parameters on the nutmeg oil extraction and its cytotoxic and antiangiogenic properties. Proced. Food Sci. 2011, 1, 1946–1952. [Google Scholar] [CrossRef]
- Inouye, S.; Takizawa, T.; Yamaguchi, H. Antibacterial activity of essential oils and their major constituents against respiratory tract pathogens by gaseous contact. J. Antimicrob. Chemother. 2001, 47, 565–573. [Google Scholar] [CrossRef] [PubMed]
- Saifullah, B.; Hussein, M.Z.; Hussein-Al-Ali, S.H.; Arulselvan, P.; Fakurazi, S. Sustained release formulation of an anti-tuberculosis drug based on para-amino salicylic acid-zinc layered hydroxide nanocomposite. Chem. Cent. J. 2013, 7, 72. [Google Scholar] [CrossRef] [PubMed]
- Dorniani, D.; Kura, A.U.; Bin Hussein, M.Z.; Fakurazi, S.; Shaari, A.H.; Ahmad, Z. Controlled-release formulation of perindopril erbumine loaded PEG-coated magnetite nanoparticles for biomedical applications. J. Mater. Sci. 2014, 49, 8487–8497. [Google Scholar] [CrossRef][Green Version]
- Nekahi, A.; Marashi, S.P.H.; Fatmesari, D.H. Modified structure of graphene oxide by investigation of structure evolution. Bull. Mater. Sci. 2015, 38, 1717–1722. [Google Scholar] [CrossRef]
- Kumar, N.; Srivastava, V.C. Simple synthesis of large graphene oxide sheets via electrochemical method coupled with oxidation process. ACS Omega 2018, 3, 10233–10242. [Google Scholar] [CrossRef]
- Schonherr, J.; Buchheim, J.R.; Scholz, P.; Adelhelm, P. Boehm titration revisited (Part I): Practical aspects for achieving a high precision in quantifying oxygen-containing surface groups on carbon materials. J. Carbon Res. 2018, 4, 1–13. [Google Scholar]
- Amaro-Gahete, J.; Benitez, A.; Otero, R.; Esquivel, D.; Jimenez-Sanchidrian, C.; Morales, J.; Caballero, A.; Romero-Salguero, F.J. A comparative study of particle size distribution of graphene nanosheets synthesized by an ultrasound-assisted method. Nanomaterials 2019, 9, 152. [Google Scholar] [CrossRef]
- Mohammed-Ziegler, I.; Billes, F. Vibrational spectroscopic calculations on pyrogallol and gallic acid. J. Mol. Struct. Theochem 2002, 618, 259–265. [Google Scholar] [CrossRef]
- Dimitric-Markovic, J.M.; Mioc, U.B.; Baranac, J.M.; Nedic, Z.P. A study of the IR spectra of the copigments of malvin chloride with organic acids. J. Serb. Chem. Soc. 2001, 66, 451–462. [Google Scholar] [CrossRef]
- Urena, F.P.; Moreno, J.R.A.; Gonzalez, J.J.L. Conformational study of (R)-(+)-limonene in the liquid phase using vibrational spectroscopy (IR, Raman, and VCD) and DFT calculations. Tetrahedron Asymmetry 2009, 20, 89–97. [Google Scholar] [CrossRef]
- Jukic, M.; Politeo, O.; Milos, M. Chemical composition and antioxidant effect of free volatile aglycones from nutmeg (Myristica fragrans Houtt.) compared to its essential oil. Croat. Chem. Acta 2006, 79, 209–214. [Google Scholar]
- Subarnas, A.; Apriyantono, A.; Mustarichie, R. Identification of compounds in the essential oil of nutmeg seeds (Myristica fragrans Houtt.) that inhibit locomotor activity in mice. Int. J. Mol. Sci. 2010, 11, 4771–4781. [Google Scholar]
- Shafiq, M.I.; Ahmed, M.; Rasul, A.; Samra, Z.Q.; Qadir, M.A.; Mazhar, S.; Ali, A. Chemical Composition of the Essential Oils of Nutmeg and Mace by GC-FID/MS Indigenous to Pakistan and Evaluation of their Biological Activities. Lat. Am. J. Pharm. 2016, 35, 2176–2184. [Google Scholar]
- Salem, M.Z.M.; Ali, H.M.; Basalah, M.O. Essential oils from wood, bark, and needles of Pinus roxburghii Sarg. from Alexandria, Egypt: Antibacterial and antioxidant activities. Bioresources 2014, 9, 7454–7466. [Google Scholar] [CrossRef]
- Eremina, E.A.; Kaplin, A.V.; Eliseev, A.A.; Sidorov, A.V.; Radzhabzoda, S.S.; Grigor’eva, A.V.; Gudilin, E.A. Multifunctional composites based on graphite oxide, doxorubicin, and magnetic nanoparticles for targeted drug delivery. Nanotechnol. Russ. 2018, 13, 152–160. [Google Scholar] [CrossRef]
- Sabbaghan, M.; Charkhan, H.; Ghalkhani, M.; Beheshtian, J. Ultrasonic route synthesis, characterization and electrochemical study of graphene oxide and reduced graphene oxide. Res. Chem. Intermed. 2019, 45, 487–505. [Google Scholar] [CrossRef]
- Hashemi, M.; Yadegari, A.; Yazdanpanah, G.; Omidi, M.; Jabbehdari, S.; Haghiralsadat, F.; Yazdian, F.; Tayebi, L. Normalization of doxorubicin release from graphene oxide: New approach for optimization of effective parameters on drug loading. Biotechnol. Appl. Biochem. 2017, 64, 433–442. [Google Scholar] [CrossRef]
- Gurunathan, S.; Han, J.W.; Dayem, A.A.; Eppakayala, V.; Kim, J.H. Oxidative stress-mediated antibacterial activity of graphene oxide and reduced graphene oxide in Pseudomonas aeruginosa. Int. J. Nanomed. 2012, 7, 5901–5914. [Google Scholar] [CrossRef]
- Dresselhaus, M.S.; Jorio, A.; Hofmann, M.; Dresselhaus, G.; Saito, R. Perspectives on carbon nanotubes and graphene Raman spectroscopy. Nano Lett. 2010, 10, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Krishnamoorthy, K.; Veerapandian, M.; Mohan, R.; Kim, S.J. Investigation of Raman and photoluminescence studies of reduced graphene oxide sheets. Appl. Phys. Mater. 2012, 106, 501–506. [Google Scholar] [CrossRef]
- Cao, L.; Li, Z.; Su, K.; Cheng, B. Hydrophilic Graphene preparation from gallic acid modified graphene oxide in magnesium self-propagating high temperature synthesis process. Sci. Rep. 2016, 6, 35184. [Google Scholar] [CrossRef] [PubMed]
- Kostiuk, D.; Bodik, M.; Siffalovic, P.; Jergel, M.; Halahovets, Y.; Hodas, M.; Pelletta, M.; Pelach, M.; Hulman, M.; Spitalsky, Z.; et al. Reliable determination of the few-layer graphene oxide thickness using Raman spectroscopy. J. Raman Spectrosc. 2016, 47, 391–394. [Google Scholar] [CrossRef]
- Muzyka, R.; Drewniak, S.; Pustelny, T.; Chrubasik, M.; Gryglewicz, G. Characterization of graphite oxide and reduced graphene oxide obtained from different graphite precursors and oxidized by different methods using Raman spectroscopy. Materials 2018, 11, 1050. [Google Scholar] [CrossRef] [PubMed]
- Chang, B.Y.S.; Huang, N.M.; An’amt, M.N.; Marlinda, A.R.; Norazriena, Y.; Muhamad, M.R.; Harrison, I.; Lim, H.N.; Chia, C.H. Facile hydrothermal preparation of titanium dioxide decorated reduced graphene oxide nanocomposite. Int. J. Nanomed. 2012, 7, 3379–3387. [Google Scholar]
- Kuila, T.; Mishra, A.K.; Khanra, P.; Kim, N.H.; Lee, J.H. Recent advances in the efficient reduction of graphene oxide and its application as energy storage electrode materials. Nanoscale 2013, 5, 52–71. [Google Scholar] [CrossRef] [PubMed]
- Kampars, V.; Legzdina, M. Thermal Deoxygenation of Graphite Oxide at Low Temperature; IOP Publishing: London, UK, 2015; p. 77. [Google Scholar]
- Niu, Y.A.; Fang, Q.H.; Zhang, X.; Zhang, P.P.; Li, Y. Reduction and structural evolution of graphene oxide sheets under hydrothermal treatment. Phys. Lett. A 2016, 380, 3128–3132. [Google Scholar] [CrossRef]
- Zhang, H.; Yu, H.M.; Xu, C.H.; Zhang, M.H.; Pan, X.H.; Gao, Y.F. A study of graphene oxidation using thermal analysis-mass spectrometry combined with pulse thermal analysis. Acta Phys. Chim. Sin. 2016, 32, 1634–1638. [Google Scholar]
- Dorniani, D.; Bin Hussein, M.Z.; Kura, A.U.; Fakurazi, S.; Shaari, A.H.; Ahmad, Z. Preparation of Fe3O4 magnetic nanoparticles coated with gallic acid for drug delivery. Int. J. Nanomed. 2012, 7, 5745–5756. [Google Scholar] [CrossRef] [PubMed]
- Stathi, P.; Gournis, D.; Deligiannakis, Y.; Rudolf, P. Stabilization of phenolic radicals on graphene oxide: An XPS and EPR study. Langmuir 2015, 31, 10508–10516. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Brazel, C.S. On the importance and mechanisms of burst release in matrix-controlled drug delivery systems. J. Control Release 2001, 73, 121–136. [Google Scholar] [CrossRef]
- Gurunathan, S.; Han, J.W.; Eppakayala, V.; Kim, J.H. Green synthesis of graphene and its cytotoxic effects in human breast cancer cells. Int. J. Nanomed. 2013, 8, 1015–1027. [Google Scholar] [CrossRef] [PubMed]
- Li, J.D.; Wei, L.F.; Yu, C.L.; Fang, W.; Xie, Y.; Zhou, W.Q.; Zhu, L.H. Preparation and characterization of graphene oxide/Ag2CO3 photocatalyst and its visible light photocatalytic activity. Appl. Surf. Sci. 2015, 358, 168–174. [Google Scholar] [CrossRef]
- Alam, S.N.; Sharma, N.; Kumar, L. Synthesis of graphene oxide (GO) by modified hummers method and its thermal reduction to obtain reduced graphene oxide (rGO)*. Graphene 2017, 6, 1–18. [Google Scholar] [CrossRef]
- Bengtson, S.; Kling, K.; Madsen, A.M.; Noergaard, A.W.; Jacobsen, N.R.; Clausen, P.A.; Alonso, B.; Pesquera, A.; Zurutuza, A.; Ramos, R.; et al. No cytotoxicity or genotoxicity of graphene and graphene oxide in murine lung epithelial FE1 cells in vitro. Environ. Mol. Mutagen. 2016, 57, 469–482. [Google Scholar] [CrossRef] [PubMed]
- Stobinski, L.; Lesiaka, B.; Malolepszyc, A.; Mazurkiewiczc, M.; Mierzwaa, B.; Zemek, J.; Jiricek, P.; Bieloshapka, I. Graphene oxide and reduced graphene oxide studied by the XRD, TEM and electron spectroscopy methods. J. Electron Spectrosc. Relat. Phenom. 2014, 195, 145–154. [Google Scholar] [CrossRef]
- Pooresmaeil, M.; Namazi, H. Surface modification of graphene oxide with stimuli-responsive polymer brush containing beta-cyclodextrin as a pendant group: Preparation, characterization, and evaluation as controlled drug delivery agent. Colloid Surf. B Biointerfaces 2018, 172, 17–25. [Google Scholar] [CrossRef]
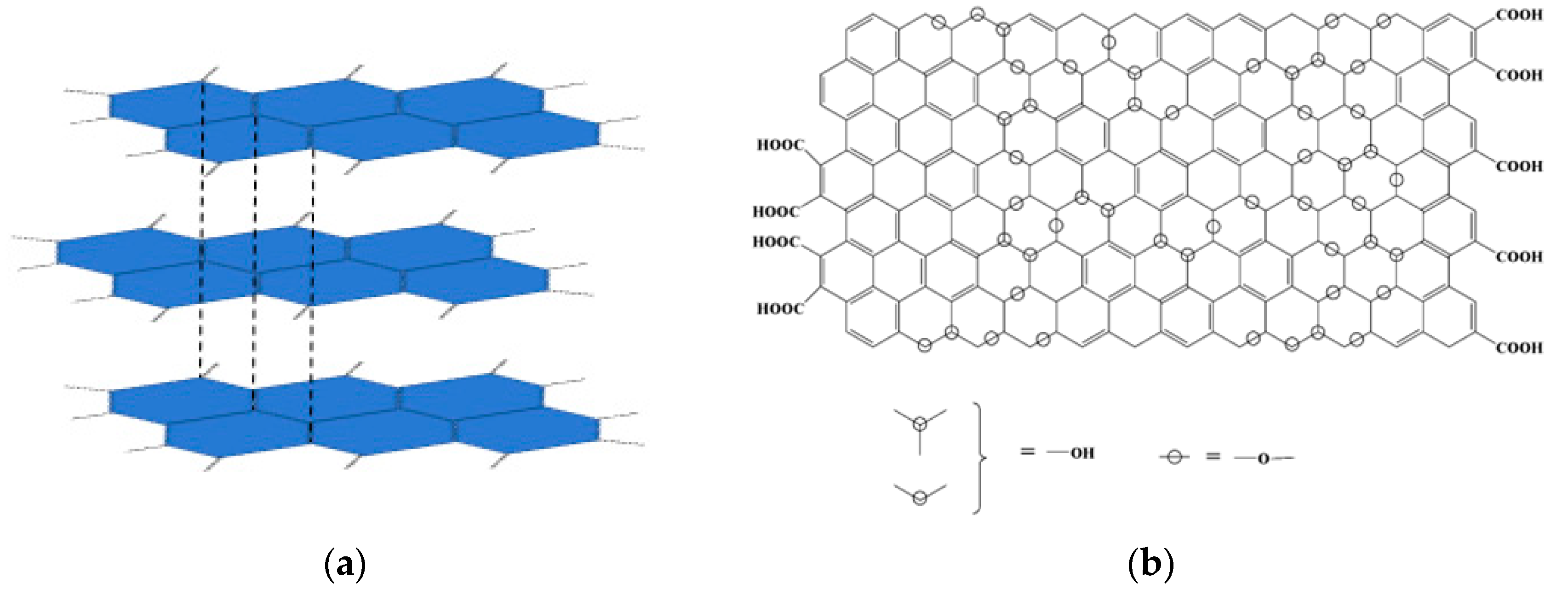


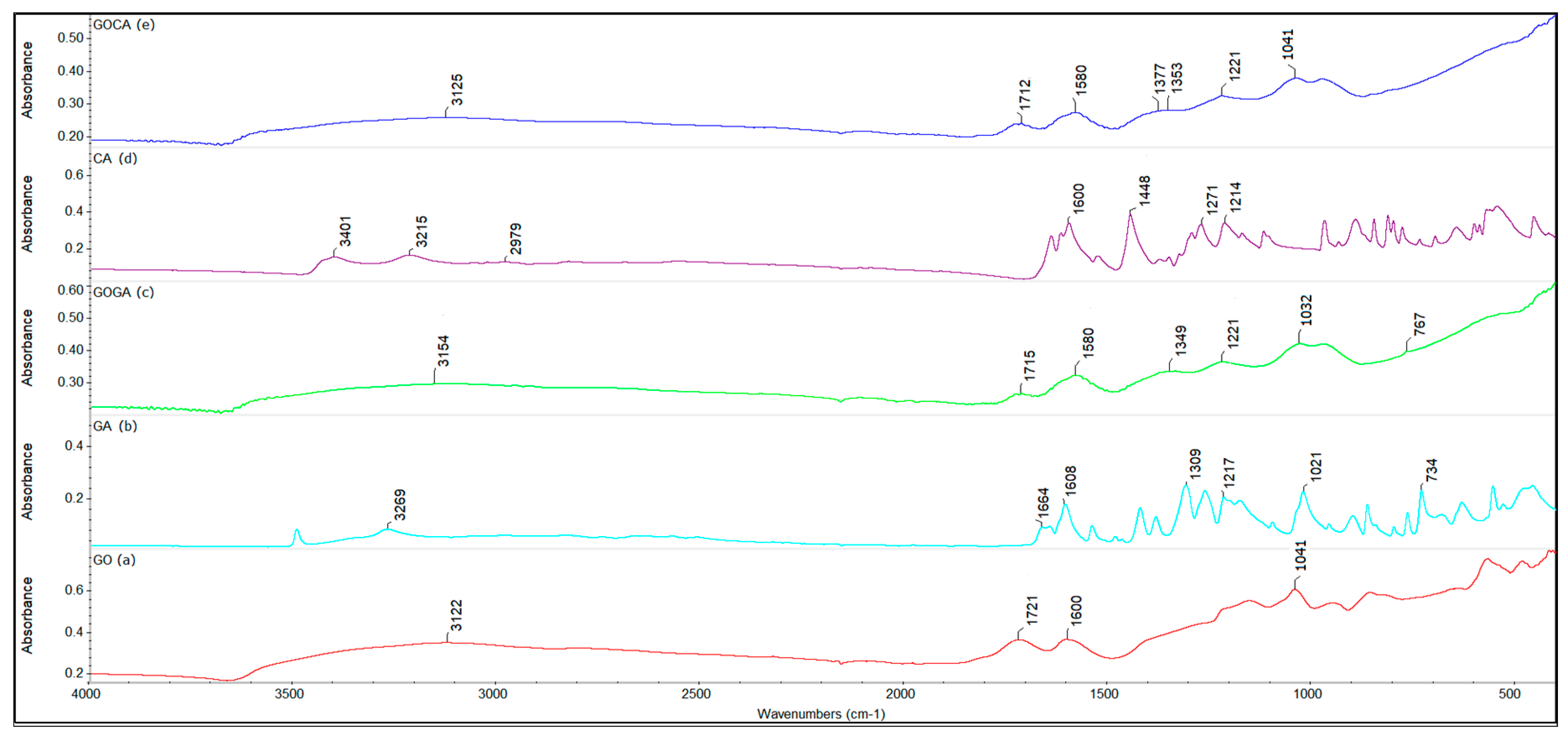



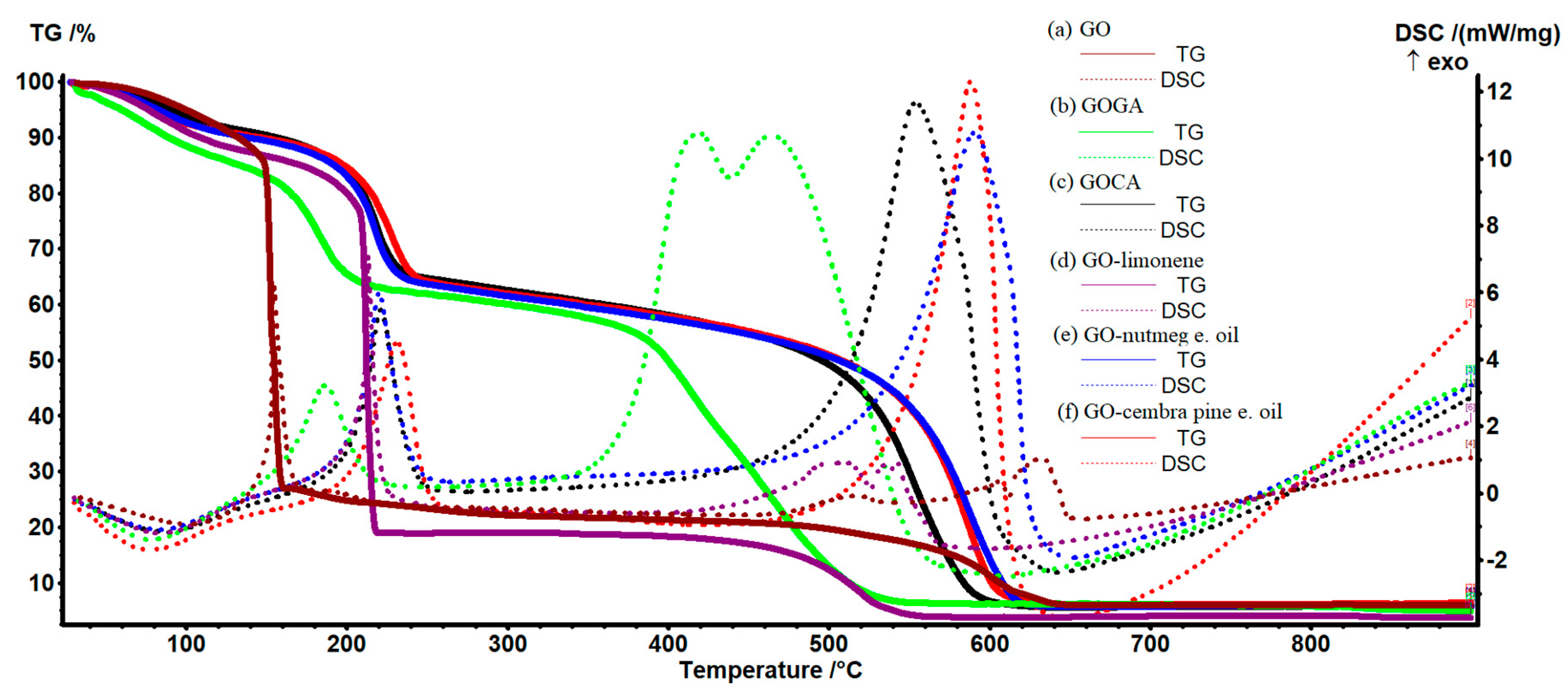
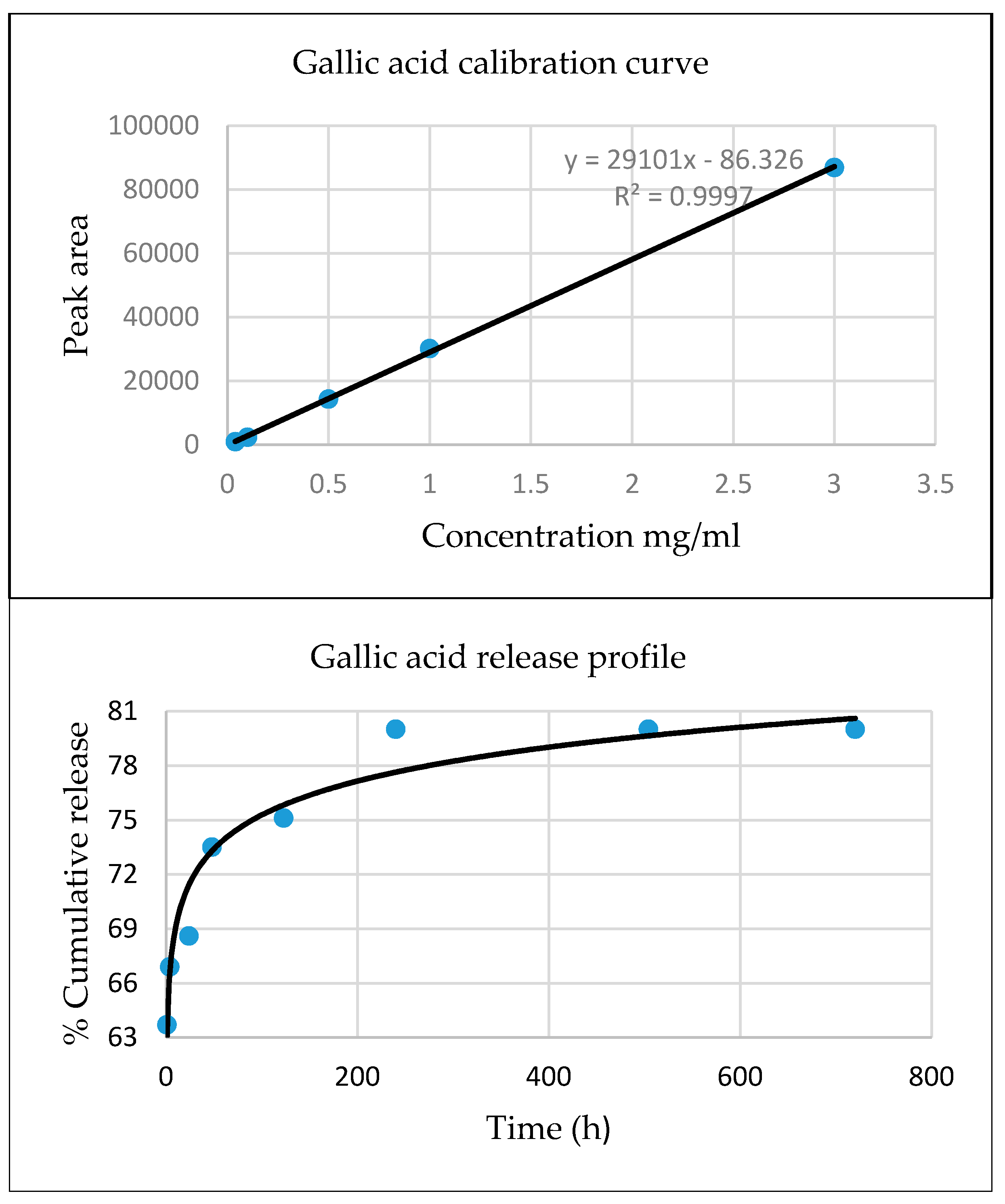



| Active Substance | Biological Activity | Ref. |
|---|---|---|
| 3,4,5-trihydroxybenzoic acid (gallic acid) | -antioxidant -anti-inflammatory -antibacterial -antimutagenic -antiviral | [35,42,43,44] |
| 3- (3,4-dihydroxyphenyl) -2-propenoic acid (caffeic acid) | -antioxidant -antibacterial -cardiovascular -antimutagenic -anticancer -anti-leukemic -immunomodulatory | [45,46] |
| 4-isopropenyl-1-methylcyclohexane (D-limonene) | -antioxidant -antimicrobial -anti-tumor -antidiabetic | [47,48,49,50] |
| Essential oils | -antimicrobial -anticancer | [51,52,53] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Croitoru, A.; Oprea, O.; Nicoara, A.; Trusca, R.; Radu, M.; Neacsu, I.; Ficai, D.; Ficai, A.; Andronescu, E. Multifunctional Platforms Based on Graphene Oxide and Natural Products. Medicina 2019, 55, 230. https://doi.org/10.3390/medicina55060230
Croitoru A, Oprea O, Nicoara A, Trusca R, Radu M, Neacsu I, Ficai D, Ficai A, Andronescu E. Multifunctional Platforms Based on Graphene Oxide and Natural Products. Medicina. 2019; 55(6):230. https://doi.org/10.3390/medicina55060230
Chicago/Turabian StyleCroitoru, Alexa, Ovidiu Oprea, Adrian Nicoara, Roxana Trusca, Mihai Radu, Ionela Neacsu, Denisa Ficai, Anton Ficai, and Ecaterina Andronescu. 2019. "Multifunctional Platforms Based on Graphene Oxide and Natural Products" Medicina 55, no. 6: 230. https://doi.org/10.3390/medicina55060230
APA StyleCroitoru, A., Oprea, O., Nicoara, A., Trusca, R., Radu, M., Neacsu, I., Ficai, D., Ficai, A., & Andronescu, E. (2019). Multifunctional Platforms Based on Graphene Oxide and Natural Products. Medicina, 55(6), 230. https://doi.org/10.3390/medicina55060230










