Epidemiological Study of Lung Cancer Incidence in Lebanon
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Overview
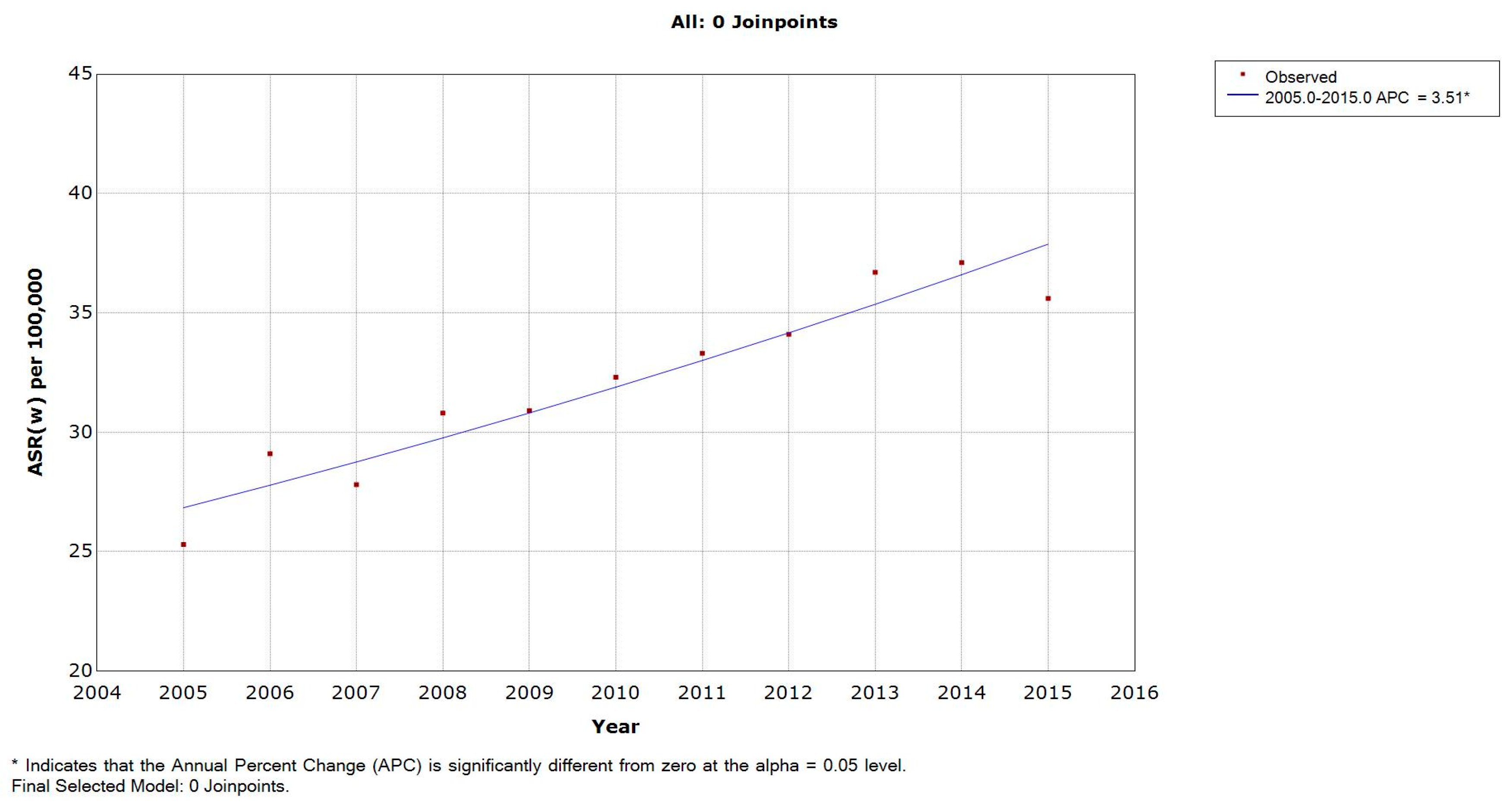
3.2. Incidence Rates in Lebanese Males
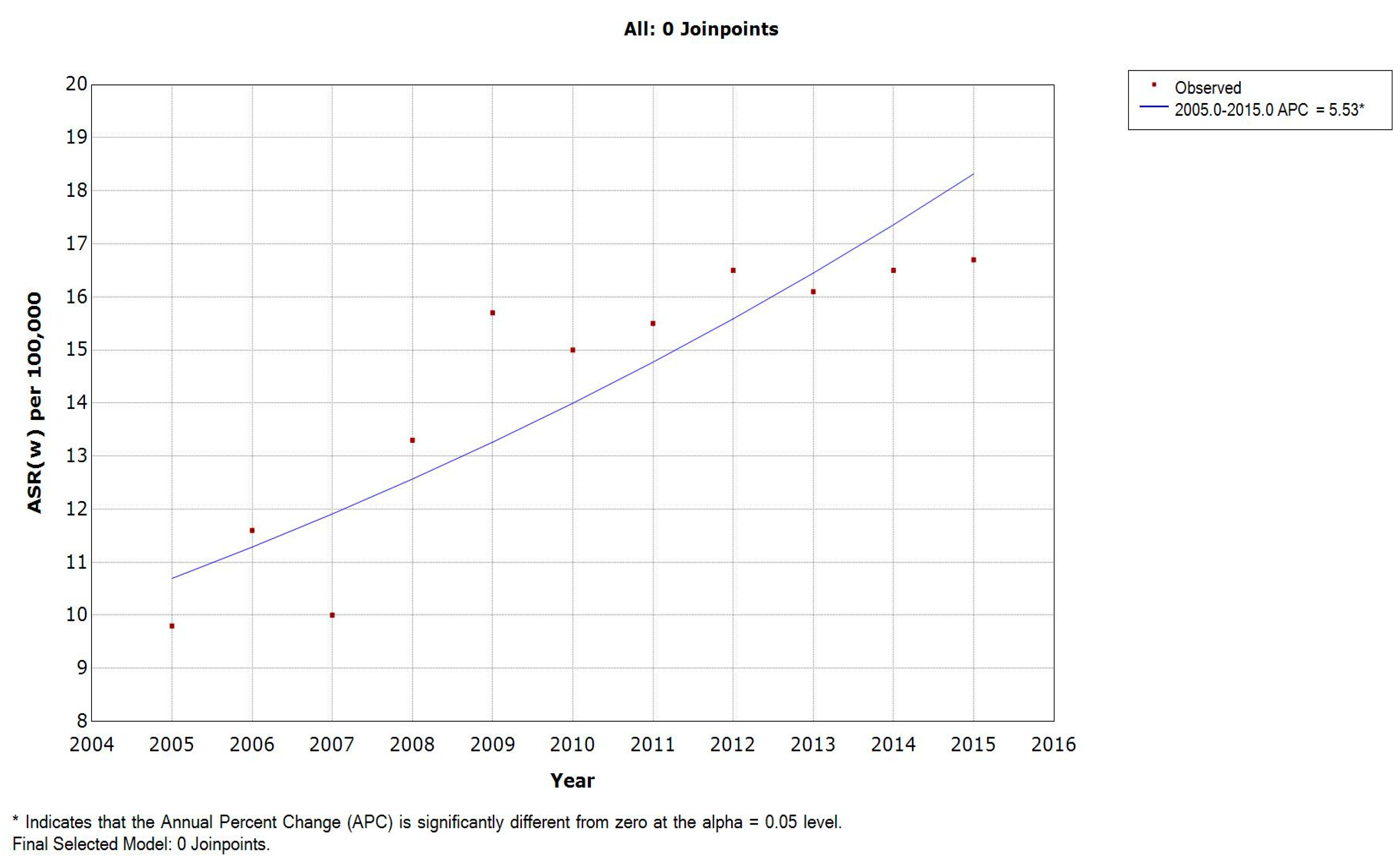
3.3. Incidence Rates in Lebanese Females
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Weir, H.K.; White, M.C. cancer incidence and mortality through 2020. Prev. Chronic Dis. 2016, 13, E48. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Khachfe, H.H.; Refaat, M.M. Bibliometric analysis of cardiovascular disease research activity in the Arab world. Int. Cardiovasc. Forum J. 2019, 15. [Google Scholar] [CrossRef]
- Shamseddine, A.; Saleh, A.; Charafeddine, M.; Seoud, M.; Mukherji, D.; Temraz, S.; Sibai, A.M. Cancer trends in Lebanon: A review of incidence rates for the period of 2003–2008 and projections until 2018. Popul. Health Metr. 2014, 12, 4. [Google Scholar] [CrossRef]
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef] [PubMed]
- Dela Cruz, C.S.; Tanoue, L.T.; Matthay, R.A. Lung cancer: Epidemiology, etiology, and prevention. Clin. Chest Med. 2011, 32, 605–644. [Google Scholar] [CrossRef] [PubMed]
- UN. World Statistics Pocketbook; UN: New York, NY, USA, 2016. (In Lebanese) [Google Scholar]
- Adib, S.; Daniel, J. Cancer in Lebanon 2003; Ministry of Public Health, National Cancer Registry Lebanon: Beirut, Lebanon, 2006. [Google Scholar]
- MOPH. National Cancer Registry (NCR) of Lebanon; MOPH: Beirut, Lebanon, 2018. [Google Scholar]
- Freddie Bray, J.F. Age Standardization. Cancer Incidence in Five Continents XI; IARC Press: Lyon, France, 2002. [Google Scholar]
- IARC. CI5 XI: Cancer Incidence in Five Continents Volume XI; IARC: Lyon, France, 2019. [Google Scholar]
- IARC. CI5PLUS: Cancer Incidence in Five Continents Time Trends; IARC: Lyon, France, 2019. [Google Scholar]
- Osann, K.E.; Anton-Culver, H.; Kurosaki, T.; Taylor, T. Sex differences in lung-cancer risk associated with cigarette smoking. Int. J. Cancer 1993, 54, 44–48. [Google Scholar] [CrossRef]
- Toyooka, S.; Tsuda, T.; Gazdar, A.F. The TP53 gene, tobacco exposure, and lung cancer. Hum. Mutat. 2003, 21, 229–239. [Google Scholar] [CrossRef]
- Patel, J.D. Lung cancer in women. J. Clin. Oncol. 2005, 23, 3212–3218. [Google Scholar] [CrossRef]
- Yu, Y.; Liu, H.; Zheng, S.; Ding, Z.; Chen, Z.; Jin, W.; Wang, L.; Wang, Z.; Fei, Y.; Zhang, S.; et al. Gender susceptibility for cigarette smoking-attributable lung cancer: A systematic review and meta-analysis. Lung Cancer 2014, 85, 351–360. [Google Scholar] [CrossRef]
- Salti, N.; Chaaban, J.; Naamani, N. The economics of tobacco in Lebanon: An estimation of the social costs of tobacco consumption. Subst. Use Misuse 2014, 49, 735–742. [Google Scholar] [CrossRef]
- Rahal, Z.; El Nemr, S.; Sinjab, A.; Chami, H.; Tfayli, A.; Kadara, H. Smoking and lung cancer: A geo-regional perspective. Front. Oncol. 2017, 7, 194. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.; Ward, E.; Brawley, O.; Jemal, A. Cancer statistics, 2011: The impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J. Clin. 2011, 61, 212–236. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, F.L. Cancer and Smoking Habits. Ann. Surg. 1931, 93, 50–67. [Google Scholar] [CrossRef] [PubMed]
- Wynder, E.L.; Graham, E.A. Tobacco smoking as a possible etiologic factor in bronchiogenic carcinoma; a study of 684 proved cases. J. Am. Med. Assoc. 1950, 143, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Doll, R.; Hill, A.B. Smoking and carcinoma of the lung; preliminary report. Br. Med. J. 1950, 2, 739–748. [Google Scholar] [CrossRef] [PubMed]
- Saade, G.; Seidenberg, A.B.; Rees, V.W.; Otrock, Z.; Connolly, G.N. Indoor secondhand tobacco smoke emission levels in six Lebanese cities. Tob. Control 2010, 19, 138–142. [Google Scholar] [CrossRef] [PubMed]
- Sibai, A.M.; Iskandarani, M.; Darzi, A.; Nakkash, R.; Saleh, S.; Fares, S.; Hwalla, N. Cigarette smoking in a Middle Eastern country and its association with hospitalisation use: A nationwide cross-sectional study. BMJ Open 2016, 6, e009881. [Google Scholar] [CrossRef] [PubMed]
- WHO, W.H.O. Tobacco Control Country Profiles. Available online: https://www.who.int/tobacco/surveillance/policy/country_profile/en/ (accessed on 10 November 2018).
- Burger, M.; Catto, J.W.; Dalbagni, G.; Grossman, H.B.; Herr, H.; Karakiewicz, P.; Kassouf, W.; Kiemeney, L.A.; La Vecchia, C.; Shariat, S.; et al. Epidemiology and risk factors of urothelial bladder cancer. Eur. Urol. 2013, 63, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Lakkis, N.A.; Adib, S.M.; Hamadeh, G.N.; El-Jarrah, R.T.; Osman, M.H. Bladder cancer in Lebanon: Incidence and comparison to regional and western countries. Cancer Control 2018, 25. [Google Scholar] [CrossRef] [PubMed]
- Hyland, A.; Travers, M.J.; Dresler, C.; Higbee, C.; Cummings, K.M. A 32-country comparison of tobacco smoke derived particle levels in indoor public places. Tob. Control 2008, 17, 159–165. [Google Scholar] [CrossRef]
- Temraz, S.; Charafeddine, M.; Mukherji, D.; Shamseddine, A. Trends in lung cancer incidence in Lebanon by gender and histological type over the period 2005–2008. J. Epidemiol. Glob. Health 2017, 7, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Li, W.W.; Li, V.W.; Hutnik, M.; Chiou, A.S. Tumor angiogenesis as a target for dietary cancer prevention. J. Oncol. 2012, 2012, 879623. [Google Scholar] [CrossRef] [PubMed]
- Salhab, H.A.; Salameh, P.; Hajj, H.; Hosseini, H. Stroke in the Arab World: A bibliometric analysis of research activity (2002–2016). eNeurologicalSci 2018, 13, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Fares, M.Y.; Fares, J.; Baydoun, H.; Fares, Y. Sport and exercise medicine research activity in the Arab world: A 15-year bibliometric analysis. BMJ Open Sport Exerc. Med. 2017, 3, e000292. [Google Scholar] [CrossRef] [PubMed][Green Version]
- de Groot, P.; Munden, R.F. Lung cancer epidemiology, risk factors, and prevention. Radiol. Clin. N. Am. 2012, 50, 863–876. [Google Scholar] [CrossRef] [PubMed]
- Nakhle, M.M.; Farah, W.; Ziade, N.; Abboud, M.; Coussa-Koniski, M.L.; Annesi-Maesano, I. Beirut Air Pollution and Health Effects—BAPHE study protocol and objectives. Multidiscip. Respir. Med. 2015, 10, 21. [Google Scholar] [CrossRef] [PubMed]
- Nasser, Z.; Salameh, P.; Dakik, H.; Elias, E.; Abou Abbas, L.; Leveque, A. Outdoor air pollution and cardiovascular diseases in Lebanon: A case-control study. J. Environ. Public Health 2015, 2015, 810846. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, R.; Lopipero, P.; Smith, A.H. Diesel exhaust exposure and lung cancer. Epidemiology 1998, 9, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Lipsett, M.; Campleman, S. Occupational exposure to diesel exhaust and lung cancer: A meta-analysis. Am. J. Public Health 1999, 89, 1009–1017. [Google Scholar] [CrossRef]
- Yokota, J.; Shiraishi, K.; Kohno, T. Genetic basis for susceptibility to lung cancer: Recent progress and future directions. Adv. Cancer Res. 2010, 109, 51–72. [Google Scholar] [CrossRef]
- Rahal, Z.; Abdulhai, F.; Kadara, H.; Saab, R. Genomics of adult and pediatric solid tumors. Am. J. Cancer Res. 2018, 8, 1356–1386. [Google Scholar] [PubMed]
- Alavanja, M.C. Biologic damage resulting from exposure to tobacco smoke and from radon: Implication for preventive interventions. Oncogene 2002, 21, 7365–7375. [Google Scholar] [CrossRef] [PubMed]
- Masjedi, M.; Ainy, E.; Zayeri, F.; Paydar, R. Assessing the prevalence and incidence of asthma and chronic obstructive pulmonary disease in the Eastern Mediterranean region. Turk. Thorac. J. 2018, 19, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Waked, M.; Khayat, G.; Salameh, P. Chronic obstructive pulmonary disease prevalence in Lebanon: A cross-sectional descriptive study. Clin. Epidemiol. 2011, 3, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Mannino, D.M.; Buist, A.S. Global burden of COPD: Risk factors, prevalence, and future trends. Lancet 2007, 370, 765–773. [Google Scholar] [CrossRef]
- Klein, F.; Amin Kotb, W.F.; Petersen, I. Incidence of human papilloma virus in lung cancer. Lung Cancer 2009, 65, 13–18. [Google Scholar] [CrossRef]
- Brenner, D.R.; McLaughlin, J.R.; Hung, R.J. Previous lung diseases and lung cancer risk: A systematic review and meta-analysis. PLoS ONE 2011, 6, e17479. [Google Scholar] [CrossRef]
- Araj, G.F.; Saade, A.; Itani, L.Y.; Avedissian, A.Z. Tuberculosis burden in Lebanon: Evolution and current status. J. Med. Leban. 2016, 64, 1–7. [Google Scholar] [CrossRef]
- Pakkala, S.; Ramalingam, S.S. Lung cancer in HIV-positive patients. J. Thorac. Oncol. 2010, 5, 1864–1871. [Google Scholar] [CrossRef]
- Pakkala, S.; Chen, Z.; Rimland, D.; Owonikoko, T.K.; Gunthel, C.; Brandes, J.R.; Saba, N.R.; Shin, D.M.; Curran, W.J., Jr.; Khuri, F.R.; et al. Human immunodeficiency virus-associated lung cancer in the era of highly active antiretroviral therapy. Cancer 2012, 118, 164–172. [Google Scholar] [CrossRef]
- Guiguet, M.; Boue, F.; Cadranel, J.; Lang, J.M.; Rosenthal, E.; Costagliola, D. Clinical Epidemiology Group of the FHDH-ANRS CO4 Cohort. Effect of immunodeficiency, HIV viral load, and antiretroviral therapy on the risk of individual malignancies (FHDH-ANRS CO4): A prospective cohort study. Lancet Oncol. 2009, 10, 1152–1159. [Google Scholar] [CrossRef]
- Grulich, A.E.; van Leeuwen, M.T.; Falster, M.O.; Vajdic, C.M. Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: A meta-analysis. Lancet 2007, 370, 59–67. [Google Scholar] [CrossRef]
- UNAIDS. UNAIDS DATA 2017. Available online: http://www.unaids.org/sites/default/files/media_asset/2017_data-book_en.pdf (accessed on 20 November 2018).
- Abu-Raddad, L.J.; Hilmi, N.; Mumtaz, G.; Benkirane, M.; Akala, F.A.; Riedner, G.; Tawil, O.; Wilson, D. Epidemiology of HIV infection in the Middle East and North Africa. AIDS 2010, 24 (Suppl. 2), S5–S23. [Google Scholar] [CrossRef] [PubMed]
- Azar, M.; Kroll, T.; Bradbury-Jones, C. Lebanese women and sexuality: A qualitative inquiry. Sex. Reprod. Healthc. 2016, 8, 13–18. [Google Scholar] [CrossRef] [PubMed]

| Year | ASR(w) | Age-Specific Rate | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0–4 y | 5–9 y | 10–14 y | 15–19 y | 20–24 y | 25–29 y | 30–34 y | 35–39 y | 40–44 y | 45–49 y | 50–54 y | 55–59 y | 60–64 y | 65–69 y | 70–74 y | 75+ y | ||
| 2005 | 25.3 | 0.6 | 0 | 0 | 0.5 | 1 | 0.7 | 2.8 | 4.8 | 14.1 | 35.5 | 38.4 | 69.6 | 115 | 162.4 | 147.1 | 222 |
| 2006 | 29.1 | 0 | 0 | 0 | 0.5 | 1 | 0.6 | 2 | 3.2 | 11.4 | 24 | 53.4 | 113.4 | 145.4 | 179.6 | 191.9 | 212 |
| 2007 | 27.8 | 0.7 | 0 | 0 | 0.5 | 0.5 | 0.6 | 2.8 | 1.7 | 9.3 | 29.6 | 48 | 106.3 | 124.7 | 189.3 | 197.3 | 188 |
| 2008 | 30.8 | 0 | 0 | 0 | 0 | 0 | 2.4 | 3.2 | 9.7 | 21.5 | 28.5 | 63.7 | 98.2 | 116.3 | 199.3 | 259.3 | 197 |
| 2009 | 30.9 | 0 | 0 | 0 | 0 | 0.5 | 1.8 | 1.9 | 1.6 | 15.3 | 29.2 | 38.8 | 112.8 | 140.4 | 217.7 | 227.4 | 235 |
| 2010 | 32.3 | 0 | 0 | 0 | 0 | 0 | 0 | 3.1 | 2.3 | 15.1 | 35.6 | 55.3 | 90.8 | 140.8 | 220.3 | 282.3 | 234 |
| 2011 | 33.3 | 0 | 0 | 0 | 0 | 0 | 1.1 | 0.1 | 9.2 | 9.3 | 41.8 | 60.5 | 122.2 | 113.3 | 246.2 | 280.2 | 209 |
| 2012 | 34.1 | 0 | 0 | 0 | 0 | 0.5 | 1.1 | 1.8 | 3 | 7.3 | 29.9 | 79.6 | 87.8 | 142.9 | 241.9 | 270.5 | 274 |
| 2013 | 36.7 | 0 | 0 | 0 | 0.4 | 0 | 0 | 1.6 | 2 | 7.3 | 30.8 | 70.9 | 134 | 172 | 253.1 | 295 | 247 |
| 2014 | 37.1 | 0.3 | 1.2 | 1 | 0.4 | 0.8 | 1.8 | 6 | 4.1 | 14 | 36.4 | 87.3 | 115.9 | 148.7 | 227.1 | 260.7 | 297 |
| 2015 | 35.6 | 0 | 0 | 0 | 0.3 | 0 | 0.4 | 1.3 | 4 | 14.1 | 28 | 52.2 | 99.8 | 142.9 | 268.9 | 321.4 | 297 |
| APC | 3.51 * | - | - | - | - | - | - | - | −0.03 | −2.3 | 0.96 | 5.19 * | 2.24 | 2.15 | 4.5 * | 6.22 * | 3.86 * |
| Country | Years | ASR(w) | Annual Incidence per 100,000 by Age Group: Males | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0–4 y | 5–9 y | 10–14 y | 15–19 y | 20–24 y | 25–29 y | 30–34 y | 35–39 y | 40–44 y | 45–49 y | 50–54 y | 55–59 y | 60–64 y | 65–69 y | 70–74 y | 75+ y | ||||
| MENA Countries | Algeria (setif) | 2008–2011 | 19.8 | - | - | - | 0.9 | 0.3 | 0.6 | 3.9 | 9.5 | 5.1 | 19.1 | 42 | 50.7 | 116.7 | 130.7 | 142.3 | 93.4 |
| Algeria (Batna) | 2008–2012 | 11.8 | - | - | - | - | - | 0.4 | 0.5 | 1.1 | 5.8 | 8 | 20.9 | 39.5 | 69.2 | 76.5 | 88.2 | 70 | |
| Bahrain | 2005–2012 | 19.5 | 0.4 | 0 | 0 | 0 | 0 | 0 | 0.6 | 1.5 | 0.8 | 7.7 | 9.6 | 31 | 67.2 | 118 | 195 | 314.3 | |
| Egypt (Damietta) | 2009–2012 | 10 | 0 | 0 | 0 | 0.4 | 0 | 0.5 | 0.6 | 1.2 | 6.3 | 10.7 | 17 | 41.1 | 52.2 | 35 | 78.6 | 77.7 | |
| Iran (Golestan) | 2008–2011 | 15.1 | - | - | - | - | 0.5 | 0.5 | 1 | 2.5 | 8.6 | 10.5 | 32.1 | 44 | 73.5 | 75 | 137.3 | 126.1 | |
| Jordan | 2008–2012 | 18.1 | 0.1 | - | - | 0.1 | 0.3 | 0.3 | 0.5 | 2.1 | 8.9 | 21.2 | 38.1 | 46.8 | 74.5 | 118.1 | 155.6 | 133.6 | |
| Kuwait | 2005–2012 | 13.1 | 0.2 | 0 | 0 | 0 | 0 | 0 | 0.7 | 1.6 | 1.9 | 6.6 | 13.7 | 26.6 | 53.1 | 102.1 | 138.7 | 135.5 | |
| Lebanon | 2005–2012 | 30.5 | 0.2 | 0 | 0 | 0.3 | 0.2 | 0.3 | 1.1 | 2.8 | 5.3 | 12 | 24.8 | 40 | 72.3 | 93.6 | 133.7 | 135.8 | |
| Malta | 2005–2012 | 35.2 | - | - | - | - | - | 1.6 | 0.8 | 0.9 | 11.4 | 11.2 | 32.6 | 74.4 | 143.9 | 217.2 | 343.3 | 503.4 | |
| Qatar | 2008–2012 | 16.7 | - | - | - | - | - | - | - | - | 6.6 | 20 | 35.2 | 21.4 | 70.2 | 105 | 146.3 | 181.4 | |
| Saudi Arabia | 2008–2012 | 5.8 | - | - | 0.1 | - | 0.3 | 0.2 | 0.4 | 0.5 | 2.2 | 4.1 | 6 | 12.5 | 23.1 | 45.1 | 52.8 | 59.1 | |
| Non-MENA Countries | Turkey | 2005–2012 | 74.9 | 0.1 | - | 0.1 | 0.2 | 0.4 | 0.5 | 1.9 | 7.1 | 26.5 | 71.3 | 143 | 247 | 371.2 | 490.8 | 577.6 | 455.2 |
| Cyprus | 2005–2012 | 31 | - | - | - | 0.4 | 0.4 | 0.4 | 2.6 | 1.9 | 8.6 | 18.8 | 40.2 | 76.8 | 142.7 | 218.7 | 288.7 | 293 | |
| Canada * | 2005–2012 | 39.6 | 0.2 | 0.1 | 0 | 0.2 | 0.2 | 0.5 | 0.9 | 2 | 6.2 | 17.6 | 42.5 | 88 | 162.4 | 276.7 | 387.4 | 485 | |
| Brazil, Goiania | 2005–2012 | 23.5 | - | - | - | 0.2 | 0.2 | 1.2 | 0.5 | 2.1 | 6.3 | 12.5 | 30.7 | 51.9 | 108.2 | 165.8 | 238.7 | 216.3 | |
| Thailand | 2005–2012 | 28.7 | 0.1 | - | - | 0.1 | 0.6 | 1.7 | 2.9 | 6.4 | 11 | 19.4 | 45.9 | 71.8 | 113.2 | 193.6 | 273.5 | 256.5 | |
| Denmark | 2005–2012 | 43.4 | - | - | 0.1 | 0.1 | 0.3 | 0.2 | 1 | 3.2 | 8.1 | 21.1 | 51.9 | 111.3 | 187.2 | 288.3 | 413.1 | 481 | |
| Germany | 2005–2012 | 50.2 | - | - | - | 0.2 | 0.6 | 0.5 | 0.4 | 3.1 | 10.7 | 31.3 | 92 | 161 | 239.2 | 340.8 | 395.2 | 429.5 | |
| Switzerland | 2005–2012 | 38.8 | - | - | 0.2 | 0.2 | 0.2 | 0.8 | 2.1 | 1.6 | 9.1 | 24.1 | 55.9 | 108.7 | 180.1 | 258.5 | 335.8 | 374.9 | |
| Japan | 2005–2010 | 45.9 | - | - | - | 0.2 | 0.3 | 0.8 | 1.8 | 5 | 9.4 | 23.8 | 49.7 | 100.3 | 168.4 | 271.4 | 400.3 | 734.8 | |
| Poland, Kielce | 2005–2012 | 61 | - | - | - | 0.5 | 0.2 | 0.9 | 0.8 | 3.8 | 13.4 | 42.5 | 103.6 | 196.4 | 325 | 429 | 492.8 | 391 | |
| Italy | 2005–2010 | 46.7 | 0.1 | - | - | 0.2 | 0.4 | 0.8 | 0.8 | 2.7 | 7.5 | 21.9 | 48.8 | 109.3 | 209.4 | 302.6 | 426.4 | 592.6 | |
| Costa Rica | 2005–2011 | 9.4 | 0.1 | - | 0.1 | - | 0.5 | 0.4 | 0.4 | 0.6 | 1.7 | 5.2 | 9.1 | 18.1 | 33.8 | 66.6 | 83 | 132.8 | |
| Year | ASR(w) | Age-Specific Rates | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0–4 y | 5–9 y | 10–14 y | 15–19 y | 20–24 y | 25–29 y | 30–34 y | 35–39 y | 40–44 y | 45–49 y | 50–54 y | 55–59 y | 60–64 y | 65–69 y | 70–74 y | 75+ y | ||
| 2005 | 9.8 | 0 | 0 | 0 | 0 | 0 | 1.2 | 1.3 | 5.4 | 6 | 13.8 | 20.3 | 30.1 | 55.2 | 46.4 | 33.9 | 78.7 |
| 2006 | 11.6 | 0 | 0 | 0 | 0 | 0 | 1.2 | 2.6 | 2 | 9.3 | 18.1 | 18.9 | 34.8 | 47.6 | 80 | 57.9 | 87.8 |
| 2007 | 10 | 0 | 0 | 0 | 1.1 | 0 | 0 | 0.7 | 6.1 | 9.3 | 8.4 | 27.3 | 39.1 | 37.9 | 35.7 | 74.5 | 69.2 |
| 2008 | 13.3 | 0 | 0 | 0 | 0 | 0.5 | 0.5 | 0 | 2.9 | 14 | 23.4 | 27.4 | 35.1 | 61.8 | 72 | 85.4 | 86.8 |
| 2009 | 15.7 | 0 | 0 | 0 | 0 | 0 | 2.2 | 1.7 | 3.5 | 10.3 | 29.5 | 29.3 | 52.4 | 59.3 | 76.6 | 108 | 121.1 |
| 2010 | 15 | 0 | 0 | 0 | 0.5 | 0 | 0.5 | 2.7 | 2.8 | 5.9 | 17.2 | 42 | 40.2 | 65.2 | 81.1 | 110.8 | 111.1 |
| 2011 | 15.5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 9.2 | 24.3 | 39 | 53.2 | 71 | 104.5 | 95.1 | 72.9 |
| 2012 | 16.5 | 0 | 0 | 0 | 0 | 0 | 0.5 | 1.1 | 6 | 9 | 37.4 | 56.9 | 46.2 | 68.4 | 95.2 | 70.6 | 77.3 |
| 2013 | 16.1 | 0 | 0 | 0 | 0 | 0 | 0.9 | 1.9 | 1.8 | 3.7 | 25.3 | 45.6 | 53.9 | 61.6 | 91.7 | 103.3 | 112.2 |
| 2014 | 16.5 | 0 | 0.3 | 0.7 | 0 | 1.9 | 1.2 | 2.1 | 3.7 | 6.7 | 26 | 40.5 | 58.2 | 73.1 | 88.7 | 91.9 | 99.7 |
| 2015 | 16.7 | 0 | 0 | 0 | 0.4 | 0.4 | 0 | 0.8 | 1.6 | 8.5 | 14.8 | 44.5 | 66.4 | 62.6 | 92 | 108.3 | 139.3 |
| APC | 5.53 * | - | - | - | - | - | - | - | −5.64 | −2.98 | 5.46 | 9.78 * | 7.10 * | 3.88 * | 7.09 * | 7.67 * | 3.77 |
| Country | Year | ASR(w) | Annual Incidence per 100,000 by Age Group: Females | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0–4 y | 5–9 y | 10–14 y | 15–19 y | 20–24 y | 25–29 y | 30–34 y | 35–39 y | 40–44 y | 45–49 y | 50–54 y | 55–59 y | 60–64 y | 65–69 y | 70–74 y | 75+ y | ||||
| MENA Countries | Algeria (setif) | 2008–2011 | 4.6 | - | - | - | 0.3 | - | - | 1.1 | 3.3 | 2.9 | 5.3 | 9.9 | 10.7 | 25.7 | 30.6 | 21.8 | 25.9 |
| Algeria (Batna) | 2008–2012 | 1.9 | - | - | - | - | - | - | - | - | 0.6 | 2.1 | 4.5 | 6 | 11.1 | 12.1 | 12.1 | 12.2 | |
| Bahrain | 2005–2012 | 7.6 | 0 | 0 | 0 | 0 | 0 | 0.6 | 0.6 | 0 | 0.7 | 1.7 | 10.7 | 11.2 | 23.6 | 48.6 | 77.6 | 104.2 | |
| Egypt (Damietta) | 2009–2012 | 4.7 | 0.4 | 0 | 0 | 0 | 0.4 | 0.5 | 0 | 0.6 | 2 | 4.9 | 8.8 | 15.7 | 15.3 | 13.6 | 41.7 | 60.4 | |
| Iran (Golestan) | 2008–2011 | 6.5 | 0.3 | - | - | 0.3 | 0.5 | 1 | 1.7 | 2.1 | 4.1 | 3.6 | 12.6 | 17.9 | 17.8 | 49.3 | 37.9 | 61.8 | |
| Jordan | 2008–2012 | 3.8 | 0.1 | - | - | - | 0.1 | 0.1 | 0.8 | 1.1 | 1.7 | 5.8 | 7.6 | 8.9 | 14 | 24.6 | 30.1 | 29.6 | |
| Kuwait | 2005–2012 | 4 | 0 | 0.2 | 0 | 0 | 0.3 | 0 | 0 | 1.4 | 0.8 | 2.5 | 2.5 | 5 | 18.1 | 30.1 | 40.8 | 44.1 | |
| Lebanon | 2005–2012 | 13.4 | 0.2 | 0.1 | 0 | 0.1 | 0.3 | 0.4 | 0.7 | 1.4 | 1.8 | 3.7 | 8.1 | 10.8 | 17.9 | 29.8 | 37.4 | 48.3 | |
| Malta | 2005–2012 | 9.6 | - | - | - | - | - | 0.8 | - | 3.9 | 7.9 | 13.1 | 22.7 | 31.9 | 41.9 | 46.2 | 61.5 | 67.4 | |
| Qatar | 2008–2012 | 2.5 | - | - | - | - | - | - | - | - | - | - | - | 6.4 | 20.2 | 12.7 | 53.3 | - | |
| Saudi Arabia | 2008–2012 | 2.4 | - | - | - | - | 0.1 | 0.1 | 0.1 | 0.4 | 1 | 0.7 | 3.7 | 7 | 15.6 | 14 | 17 | 19.2 | |
| Non-MENA Countries | Turkey | 2005–2012 | 2 | - | 0.1 | 0.1 | 0.1 | 0.3 | 0.2 | 0.9 | 2.5 | 6.3 | 13.1 | 21.2 | 27 | 39.2 | 55.2 | 60.3 | 61.7 |
| Cyprus | 2005–2012 | 8.6 | 0.6 | - | - | 0.4 | 0.4 | 0.7 | 2.3 | 2.4 | 6.3 | 8.6 | 11.5 | 27 | 34.1 | 58.4 | 56.9 | 62.5 | |
| Canada * | 2005–2012 | 31.9 | 0.1 | - | 0 | 0.2 | 0.4 | 0.5 | 1 | 2.5 | 7.9 | 23.1 | 48.4 | 82.5 | 141.3 | 222.1 | 288.1 | 284.7 | |
| Brazil, Goiania | 2005–2012 | 12.6 | - | - | - | 0.2 | 0.2 | 0.4 | 1.6 | 1.2 | 7 | 12 | 23.3 | 32.1 | 44.1 | 94.9 | 96.6 | 114.3 | |
| Thailand | 2005–2012 | 14.4 | - | - | 0.1 | 0.1 | 0.4 | 0.8 | 2.7 | 3.3 | 6.7 | 12.1 | 22.4 | 39.2 | 58.1 | 96.3 | 121 | 122.2 | |
| Denmark | 2005–2012 | 36.5 | - | - | - | 0.2 | 0.2 | 0.4 | 1.4 | 3 | 9.9 | 28.6 | 70.5 | 108.7 | 169.8 | 234.7 | 310.4 | 272.7 | |
| Germany | 2005–2012 | 24.3 | - | - | - | - | 0.4 | 0.4 | 1.6 | 3.3 | 12.7 | 30.7 | 60 | 90.4 | 124.9 | 143.6 | 131.3 | 139.2 | |
| Switzerland | 2005–2012 | 20.2 | 0.2 | 0.2 | - | 0.2 | 0.5 | 1.4 | 1 | 3 | 7.3 | 20.8 | 41.5 | 65.3 | 97.3 | 127.8 | 148.2 | 127.1 | |
| Japan | 2005–2010 | 16.1 | - | - | 0.1 | 0.1 | 0 | 0.6 | 1.1 | 3.2 | 6.5 | 14.1 | 24.6 | 43.3 | 62 | 92.1 | 126.6 | 202.1 | |
| Poland, Kielce | 2005–2012 | 13.6 | - | - | 0.3 | - | 0.2 | 0.8 | 1.1 | 0.9 | 5.5 | 14.7 | 37.1 | 56.1 | 79.5 | 65.5 | 77.5 | 66.3 | |
| Italy | 2005–2010 | 14.1 | 0.1 | - | - | 0.2 | 0.1 | 0.9 | 1.5 | 2.6 | 5.4 | 15.9 | 32.8 | 41 | 62.8 | 73.7 | 96.9 | 128.9 | |
| Costa Rica | 2005–2011 | 4.5 | 0.1 | - | - | - | - | 0.4 | 0.4 | 1.1 | 1.3 | 3 | 5.9 | 10.9 | 17.9 | 31.1 | 36.1 | 48.7 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salhab, H.A.; Fares, M.Y.; Khachfe, H.H.; Khachfe, H.M. Epidemiological Study of Lung Cancer Incidence in Lebanon. Medicina 2019, 55, 217. https://doi.org/10.3390/medicina55060217
Salhab HA, Fares MY, Khachfe HH, Khachfe HM. Epidemiological Study of Lung Cancer Incidence in Lebanon. Medicina. 2019; 55(6):217. https://doi.org/10.3390/medicina55060217
Chicago/Turabian StyleSalhab, Hamza A., Mohamad Y. Fares, Hussein H. Khachfe, and Hassan M. Khachfe. 2019. "Epidemiological Study of Lung Cancer Incidence in Lebanon" Medicina 55, no. 6: 217. https://doi.org/10.3390/medicina55060217
APA StyleSalhab, H. A., Fares, M. Y., Khachfe, H. H., & Khachfe, H. M. (2019). Epidemiological Study of Lung Cancer Incidence in Lebanon. Medicina, 55(6), 217. https://doi.org/10.3390/medicina55060217





