Magnetic Composite Scaffolds for Potential Applications in Radiochemotherapy of Malignant Bone Tumors
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
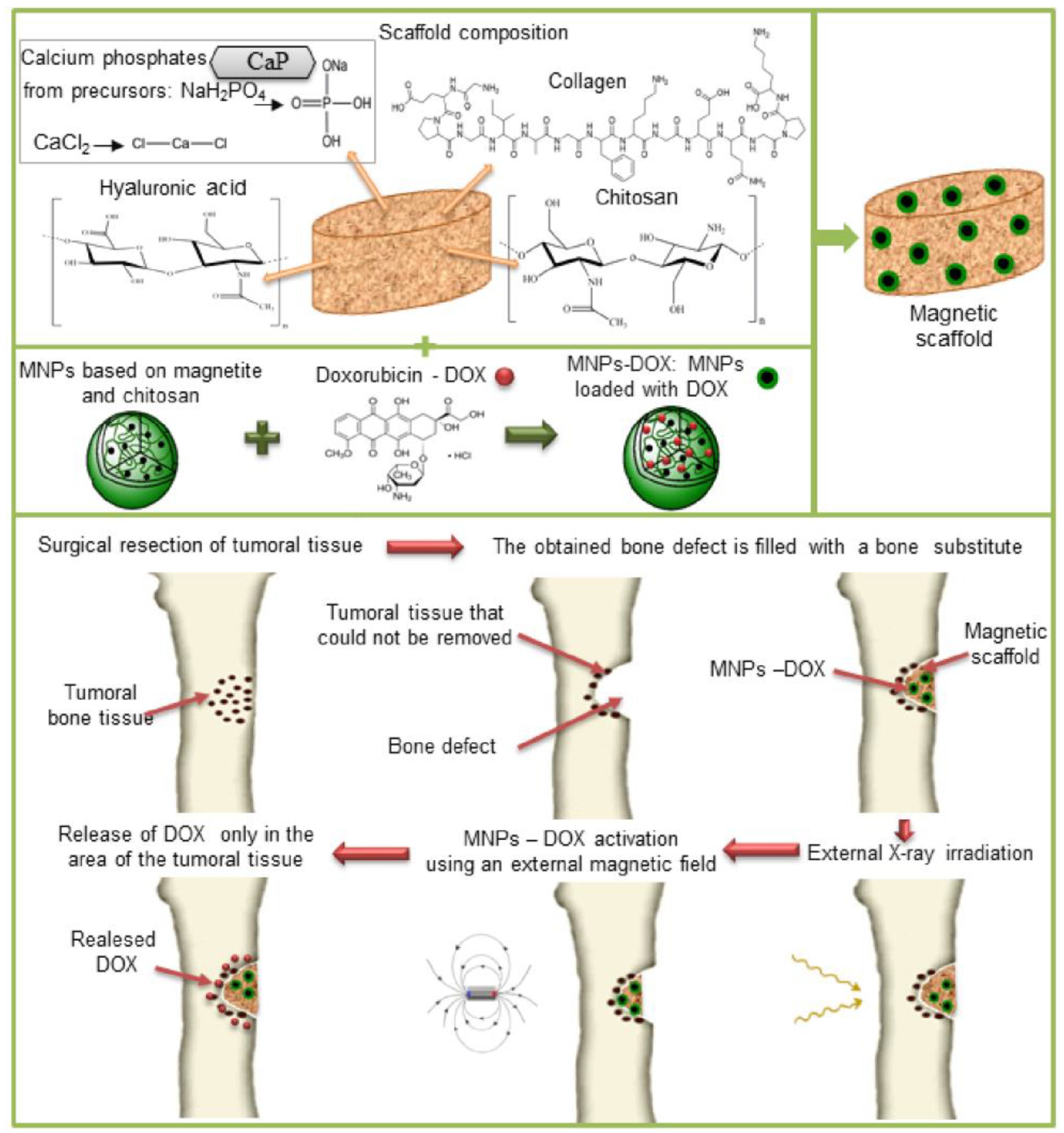
2.2. Preparation of the Magnetic Scaffolds
2.3. X-ray Irradiation of the Scaffolds
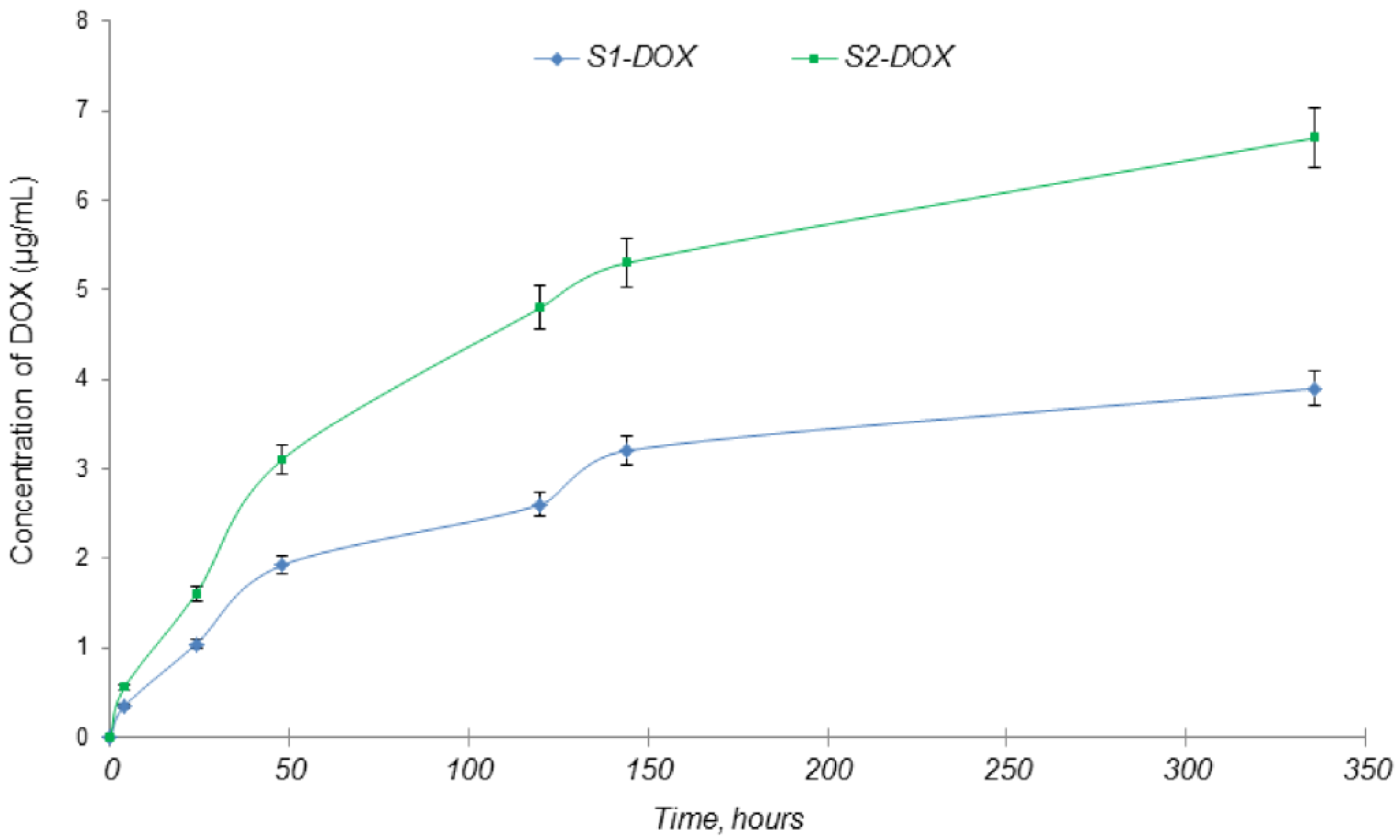
2.4. Drug Loading
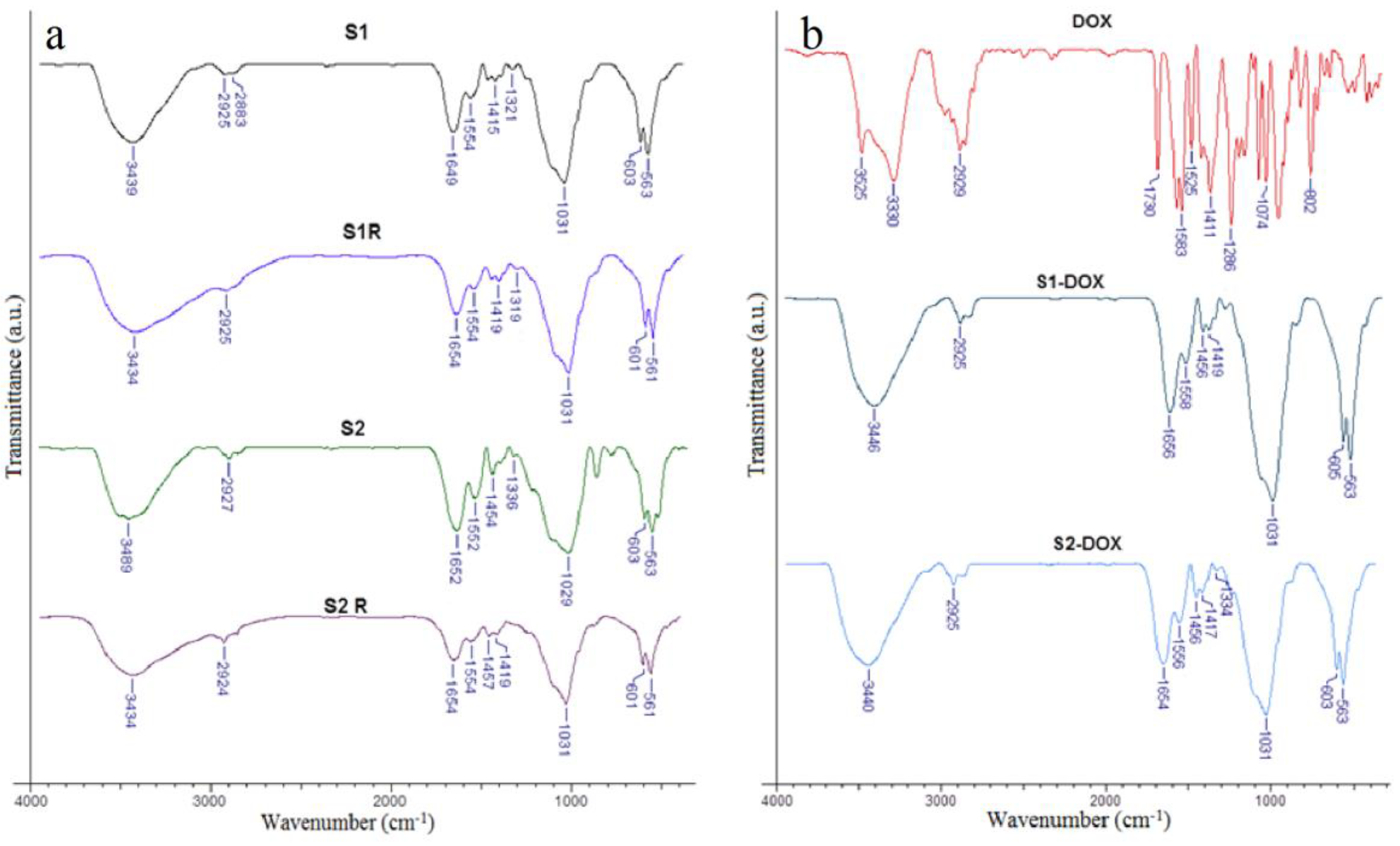
2.5. Characterization of the Magnetic Scaffolds
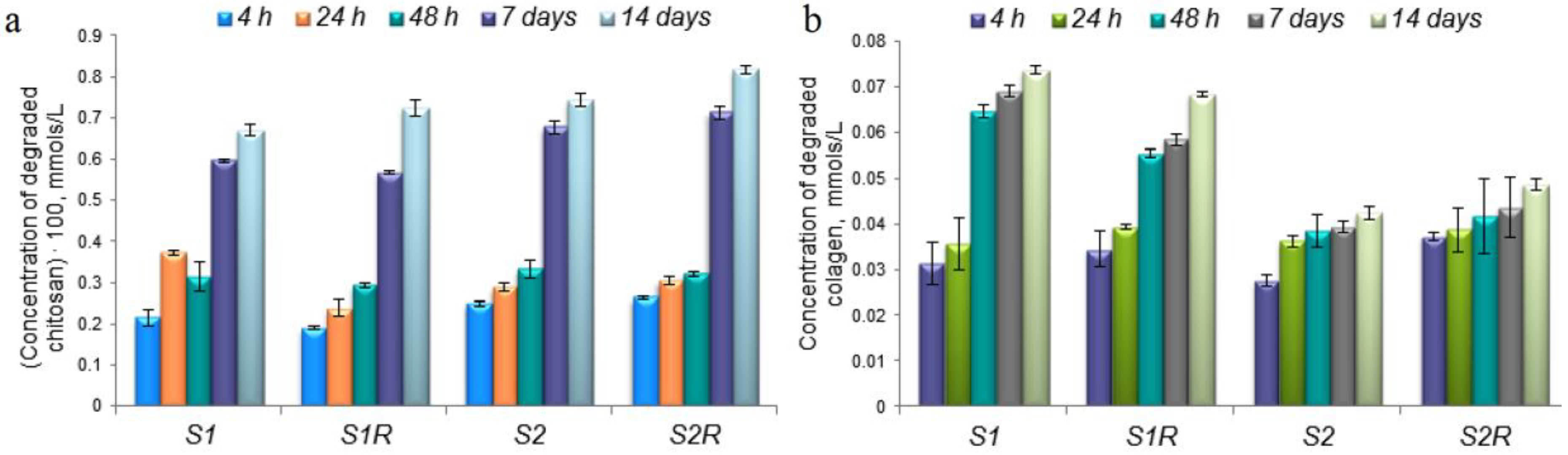
2.5.1. In Vitro Drug Release
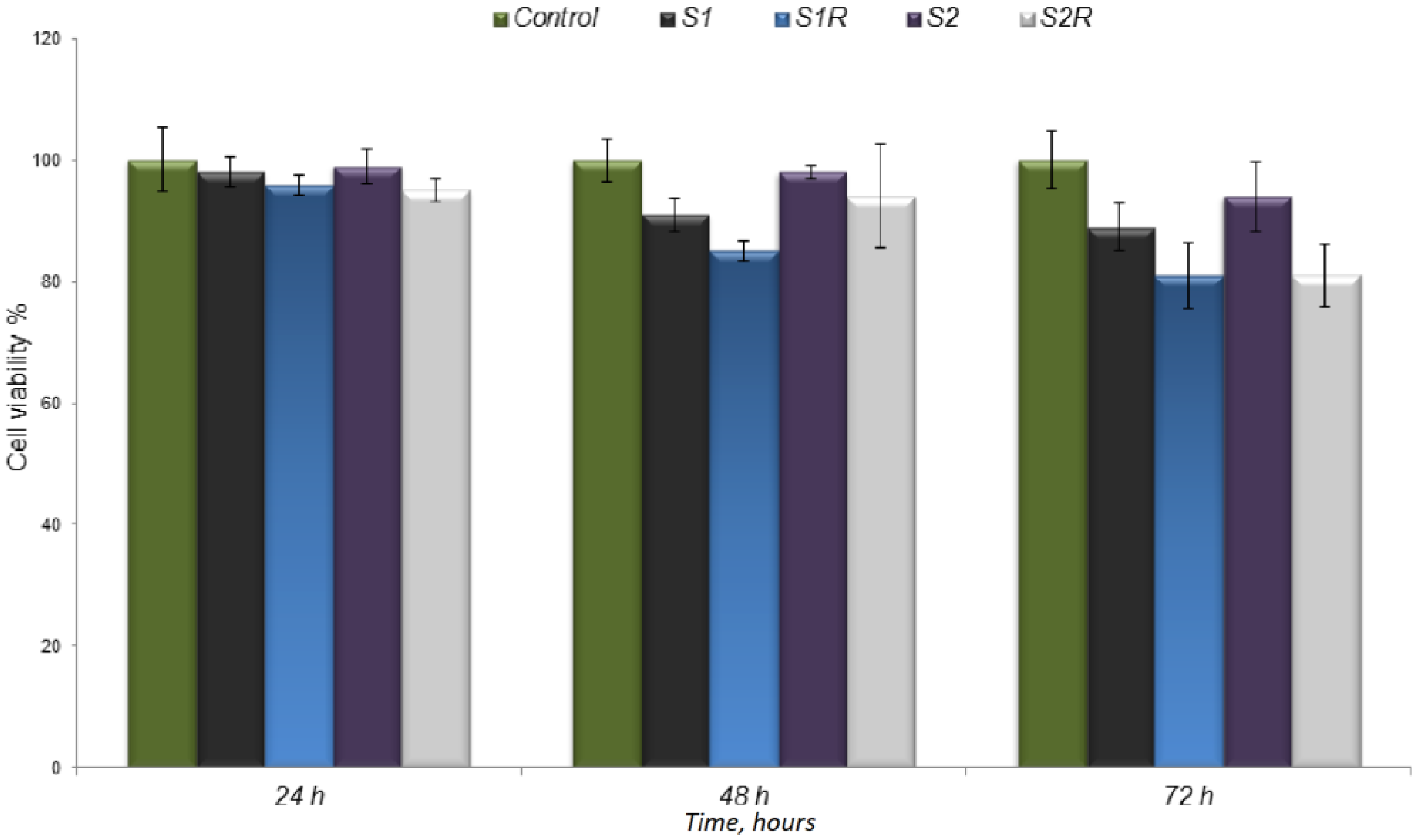
2.5.2. In Vitro Interaction Studies of Scaffolds with Cells
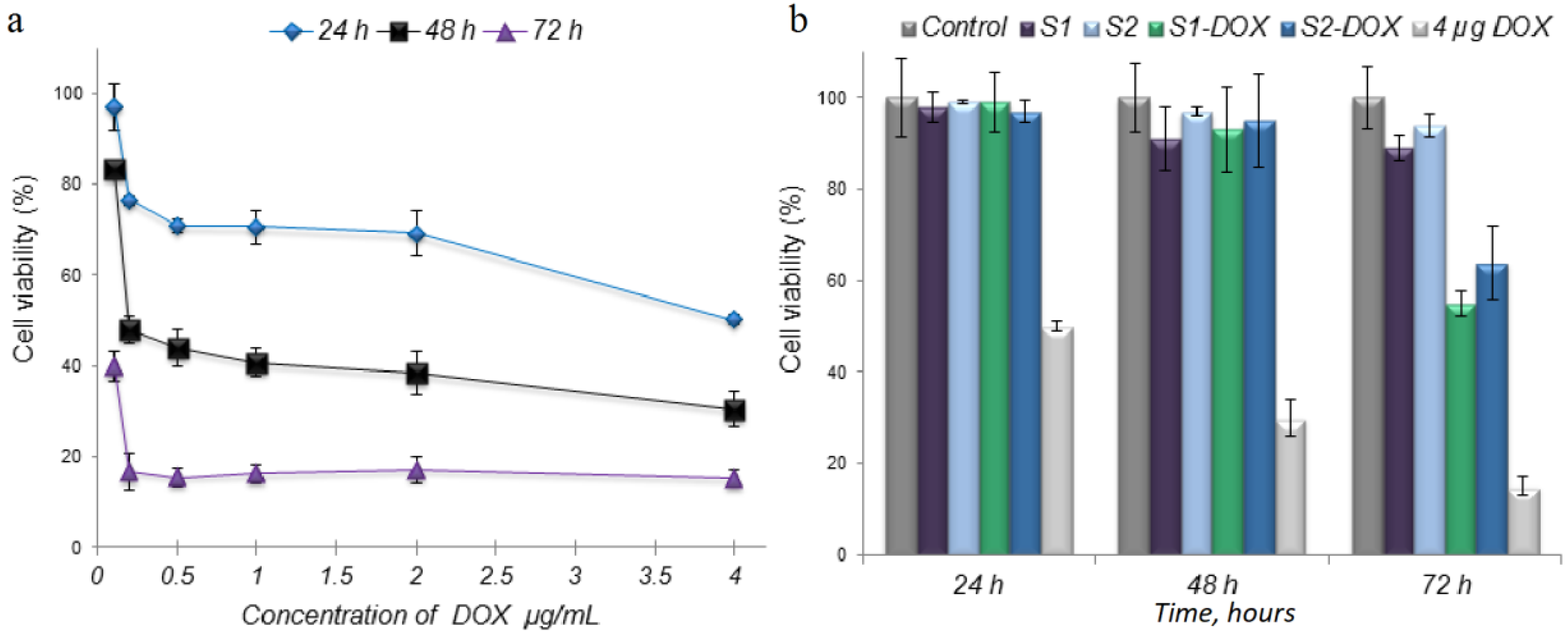
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Marques, C.; Ferreira, J.M.F.; Andronescu, E.; Ficai, D.; Sonmez, M.; Ficai, A. Multifunctional materials for bone cancer treatment. Int. J. Nanomed. 2014, 9, 2713–2725. [Google Scholar] [CrossRef]
- Freeman, A.K.; Sumathi, V.P.; Jeys, L. Primary malignant tumours of the bone. Surgery 2018, 36, 27–34. [Google Scholar] [CrossRef]
- Franchi, A. Epidemiology and classification of bone tumors. Clin. Cases. Miner. Bone Metab. 2012, 9, 92–95. [Google Scholar]
- Kindblom, L. Bone tumors: Epidemiology, classification, pathology. In Imaging of Bone Tumors and Tumor-Like Lesions. Medical Radiology; Davies, A., Sundaram, M., James, S., Eds.; Springer: Berlin, Germany, 2009; pp. 1–15. [Google Scholar]
- Hauben, E.I.; Hogendoorn, P.C.W. Epidemiology of primary bone tumors and economical aspects of bone metastases. In Bone Cancer. Primary Bone Cancers and Bone Metastases, 2nd ed.; Heymann, D., Ed.; Academic Press Elsevier: Amsterdam, The Netherlands, 2015; pp. 5–10. [Google Scholar]
- Wu, J.S.; Hochman, M.G. Bone Tumors a Practical Guide to Imaging; Springer: New York, NY, USA, 2012; pp. 1–9. [Google Scholar] [CrossRef]
- Zhou, Z.; Fan, T.; Yan, Y.; Zhang, S.; Zhou, Y.; Deng, H.; Cai, X.; Xiao, J.; Song, D.; Zhang, Q.; et al. One stone with two birds: Phytic acid-capped platinum nanoparticles for targeted combination therapy of bone tumor. Biomaterials 2019, 194, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Habash, R.W.Y. Therapeutic hyperthermia. In Handbook of Clinical Neurology, 3rd ed.; Romanovsky, A.A., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 853–868. [Google Scholar]
- Lee, S.; Son, B.; Park, G.; Kim, H.; Kang, H.; Jeon, J.; Youn, H.; Youn, B. Immunogenic effect of hyperthermia on enhancing radiotherapeutic efficacy. Int. J. Mol. Sci. 2018, 19, 2795. [Google Scholar] [CrossRef] [PubMed]
- Rajani, R.; Gibbs, C.P. Treatment of bone tumors. Surg. Pathol. Clin. 2012, 5, 301–318. [Google Scholar] [CrossRef] [PubMed]
- Andronescu, E.; Ficai, M.; Voicu, G.; Ficai, D.; Maganu, M.; Ficai, A. Synthesis and characterization of collagen/hydroxyapatite: Magnetite composite material for bone cancer treatment. J. Mater. Sci. Mater. Med. 2010, 21, 2237–2242. [Google Scholar] [CrossRef]
- Groenen, K.H.; Pouw, M.H.; Hannink, G.; Hosman, A.J.; van der Linden, Y.M.; Verdonschot, N.; Tanck, E. The effect of radiotherapy, and radiotherapy combined with bisphosphonates or RANK ligand inhibitors on bone quality in bone metastases. A systematic review. Radiother. Oncol. 2016, 119, 194–201. [Google Scholar] [CrossRef]
- Ezrahi, S.; Aserin, A.; Garti, N. Basic principles of drug delivery systems—The case of paclitaxel. Adv. Colloid Interface Sci. 2019, 263, 95–130. [Google Scholar] [CrossRef] [PubMed]
- Raavé, R.; van Kuppevelt, T.H.; Daamen, W.F. Chemotherapeutic drug delivery by tumoral extracellular matrix targeting. J. Control Release 2018, 274, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Yadav, H.K.S.; Almokdad, A.A.; Shaluf, S.I.M.; Debe, M.S. Polymer-based nanomaterials for drug-delivery carriers. In Nano-carriers for Drug Delivery: Nanoscience and Nanotechnology in Drug Delivery; Mohapatra, S.S., Ranjan, S., Dasgupta, N., Mishra, R.K., Sabu, T., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 531–556. [Google Scholar]
- Liechty, W.B.; Kryscio, D.R.; Slaughter, B.V.; Peppas, N.A. Polymers for drug delivery systems. Annu. Rev. Chem. Biomol. Eng. 2010, 1, 149–173. [Google Scholar] [CrossRef]
- Hajinasab, A.; Saber-Samandari, S.; Ahmadi, S.; Alamara, K. Preparation and characterization of a biocompatible magnetic scaffold for biomedical engineering. Mater. Chem. Phys. 2018, 204, 378–387. [Google Scholar] [CrossRef]
- Akbarzadeh, A.; Mikaeili, H.; Zarghami, N.; Mohammad, R.; Barkhordari, A.; Davaran, S. Preparation and in vitro evaluation of doxorubicin-loaded Fe3O4 magnetic nanoparticles modified with biocompatible copolymers. Int. J. Nanomed. 2012, 7, 511–526. [Google Scholar]
- Bianchi, M.; Cauci, S.; Marcacci, M.; Russo, T. Magnetic scaffolds for bone tissue engineering. In Biomimetic Approaches for Tissue Healing; Panseri, S., Taraballi, F., Cunha, C., Eds.; OMICS Group eBooks: Hearthrow, UK, 2015; pp. 1–9. [Google Scholar]
- Iafisco, M.; Drouet, C.; Adamiano, A.; Pascaud, P.; Montesi, M.; Panseri, S.; Sarda, S.; Tampieri, A. Superparamagnetic iron-doped nanocrystalline apatite as a delivery system for doxorubicin. J. Mater. Chem. B 2016, 4, 57–70. [Google Scholar] [CrossRef]
- Balan, V.; Petrache, I.A.; Popa, M.I.; Butnaru, M.; Barbu, E.; Tsibouklis, J.; Verestiuc, L. Biotinylated chitosan-based SPIONs with potential in blood-contacting applications. J. Nanopart. Res. 2012, 14, 1–14. [Google Scholar] [CrossRef]
- Ivan, F.D.; Balan, V.; Butnaru, M.; Popa, I.M.; Verestiuc, L. Magnetic nanoparticles inclusion into scaffolds based on calcium phosphates and biopolymers for bone regeneration. Key Eng. Mater. 2017, 745, 16–25. [Google Scholar] [CrossRef]
- Tanase, C.E.; Popa, M.I.; Verestiuc, L. Biomimetic bone scaffolds based on chitosan and calcium phosphates. Mater. Lett. 2011, 65, 1681–1683. [Google Scholar] [CrossRef]
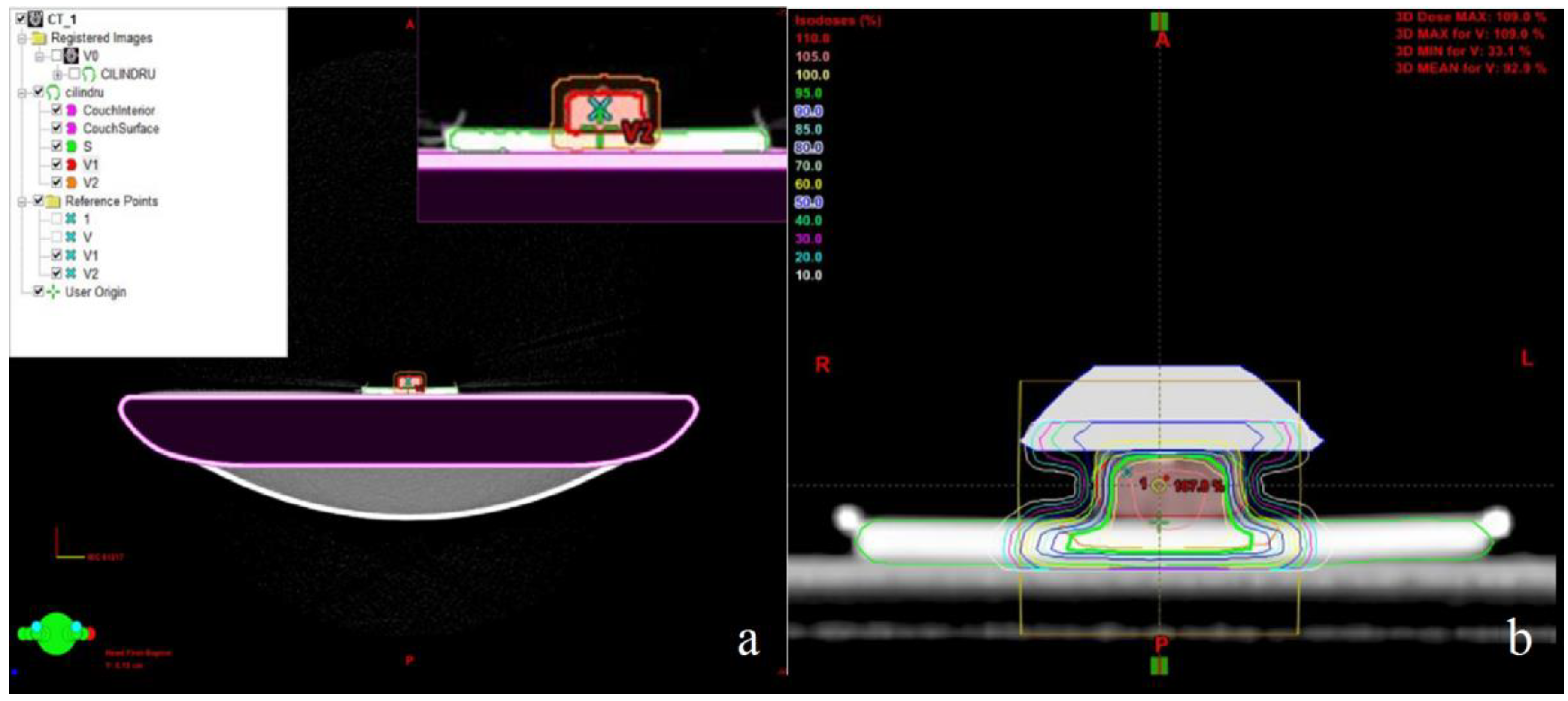
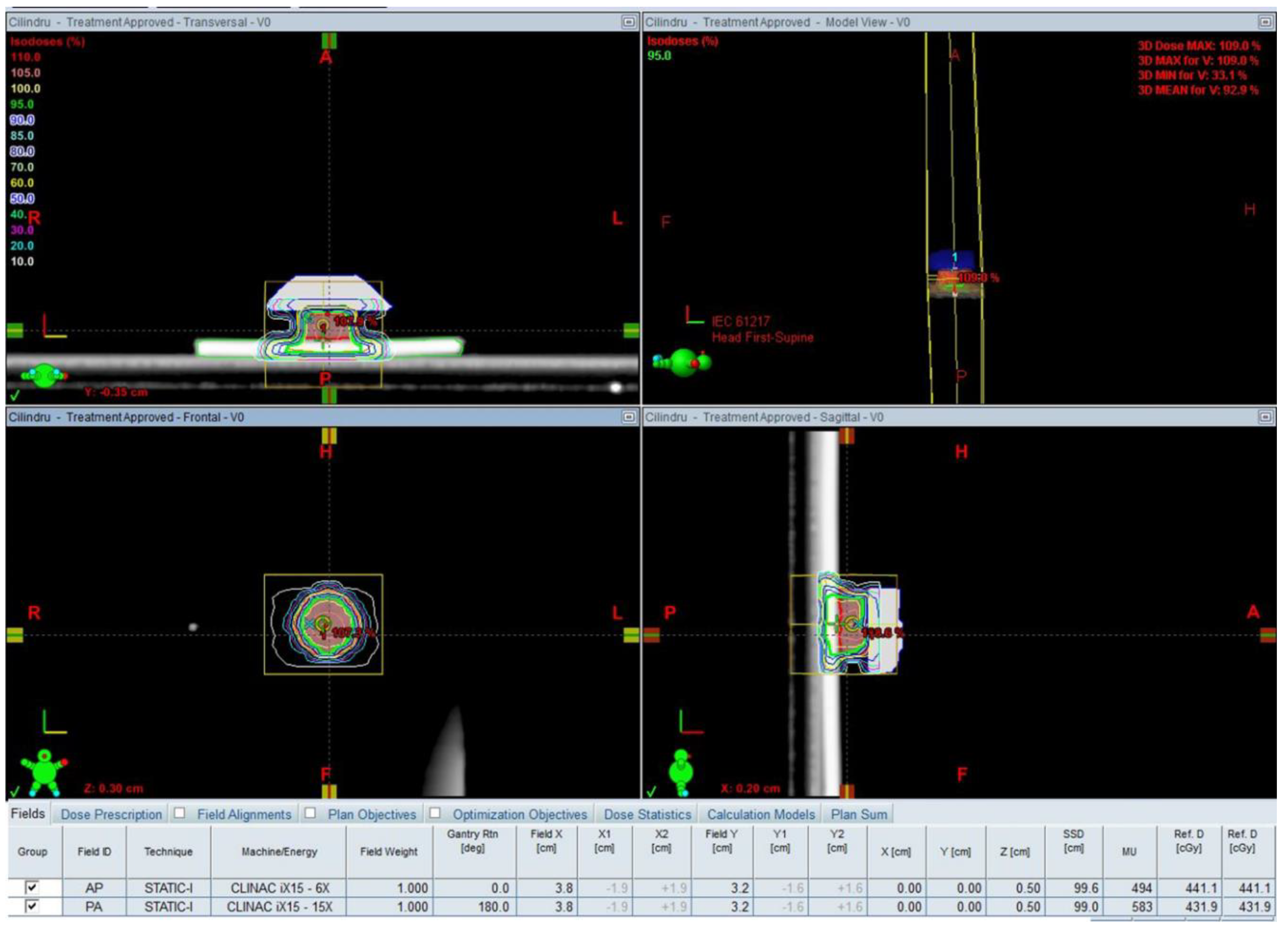
- Munteanu, A.; Ivan, F.D.; Patrascu, A.; Balan, V.; Ursache, C.; Verestiuc, L. Treatment planning optimization in radiotherapy using the bolus. Mater. Plast. 2017, 54, 731–734. [Google Scholar]
- Cojocaru, F.D.; Balan, V.; Popa, M.I.; Lobiuc, A.; Antoniac, A.; Antoniac, I.V.; Verestiuc, L. Biopolymers—Calcium phosphates composites with inclusions of magnetic nanoparticles for bone tissue engineering. Int. J. Biol. Macromol. 2019, 125, 612–620. [Google Scholar] [CrossRef]
- Panseri, S.; Russo, A.; Sartori, M.; Giavaresi, M.; Sandri, M.; Fini, M.; Maltarello, M.C.; Shelyakova, T.; Ortolani, A.; Visani, A.; et al. Modifying bone scaffold architecture in vivo with permanent magnets to facilitate fixation of magnetic scaffolds. Bone 2013, 56, 432–439. [Google Scholar] [CrossRef]
- Nurgali, K.; Jagoe, R.T.; Abalo, R. Editorial: Adverse effects of cancer chemotherapy: Anything new to improve tolerance and reduce sequelae? Front Pharmacol. 2018, 9, 245. [Google Scholar] [CrossRef]
- Meeuse, J.J.; Van Der Linden, Y.M.; Van Tienhoven, G.; Gans, R.O.B.; Leer, J.W.H.; Reyners, A.K.L. Efficacy of radiotherapy for painful bone metastases during the last 12 weeks of life: Results from the Dutch Bone Metastasis Study. Cancer 2010, 116, 2716–2725. [Google Scholar] [CrossRef]
- Chow, E.; Zeng, L.; Salvo, N.; Dennis, K.; Tsao, M.; Lutz, S. Update on the systematic review of palliative radiotherapy trials for bone metastases. Clin. Oncol. R. Coll. Radiol. 2012, 24, 112–124. [Google Scholar] [CrossRef]
- Lutz, S.; Balboni, T.; Jones, J.; Lo, S.; Petit, J.; Rich, S.E.; Wong, E.; Hahn, C. Palliative radiation therapy for bone metastases: Update of an ASTRO Evidence-Based Guideline. Pract. Radiat. Oncol. 2017, 7, 4–12. [Google Scholar] [CrossRef]
- Xia, Z.; Yu, X.; Jiang, X.; Brody, H.D.; Rowe, D.W.; Wei, M. Fabrication and characterization of biomimetic collagen–apatite scaffolds with tunable structures for bone tissue engineering. Acta Biomater. 2013, 9, 7308–7319. [Google Scholar] [CrossRef]
- Heidari, F.; Bahrololoom, M.E.; Vashaee, D.; Tayebi, L. In situ preparation of iron oxide nanoparticles in natural hydroxyapatite/chitosanmatrix for bone tissue engineering application. Ceram. Int. 2015, 41, 3094–3100. [Google Scholar] [CrossRef]
- Weng, X.; Ma, L.; Guo, M.; Su, Y.; Dharmarajan, R.; Chen, Z. Removal of doxorubicin hydrochloride using Fe3O4 nanoparticles synthesized by euphorbia cochinchinensis extract. Chem. Eng. J. 2018, 353, 482–489. [Google Scholar] [CrossRef]
- Loh, Q.L.; Choong, C. Three-dimensional scaffolds for tissue engineering applications: Role of porosity and pore size. Tissue Eng. Part. B Rev. 2013, 19, 485–502. [Google Scholar] [CrossRef]
- Brouwer, J.; van Leeuwen-Herberts, T.; Otting-van de Ruit, M. Determination of lysozyme in serum, urine, cerebrospinal fluid and feces by enzyme immunoassay. Clin. Chim. Acta 1984, 142, 21–30. [Google Scholar] [CrossRef]
- Daniel, M.P.; Gaikwad, V.; Verghese, M.; Abraham, R.; Kapoor, R. Serum lysozyme (muramidase) levels in intra-abdominal abscesses: An experimental study. Indian J. Surg. 2012, 77, 117–119. [Google Scholar] [CrossRef][Green Version]
- Firkin, F. Diagnostic value of the serum lyzozyme (Muramidase) level. Pathology 1971, 3, 76. [Google Scholar] [CrossRef]
- Partridge, N.C.; Walling, H.W.; Bloch, S.R.; Omura, T.H.; Chan, P.T.; Pearman, A.T.; Chou, W.Y. The regulation and regulatory role of collagenase in bone. Crit. Rev. Eukaryot. Gene Expr. 1996, 6, 15–27. [Google Scholar] [CrossRef]
- Wilhelm, S.; Eisen, A.; Teter, M.; Clark, S.; Kronberger, A.; Goldberg, G. Human fibroblast collagenase: Glycosylation and tissue-specific levels of enzyme synthesis. Proc. Nat. Acad. Sci. USA 1986, 83, 3756–3760. [Google Scholar] [CrossRef]
- Kozlu, S.; Sahin, A.; Ultav, G.; Yerlikaya, F.; Calis, S.; Capan, Y. Development and in vitro evaluation of doxorubicin and celecoxib co-loaded bone targeted nanoparticles. J. Drug Deliv. Sci. Technol. 2018, 45, 213–219. [Google Scholar] [CrossRef]
- Ghosh, S.; Kumar, R.S.; Ghosh, N.; Chaudhury, K.; Ghosh, S.; Banerjee, I.; Pramanik, N. Development and physicochemical characterization of doxorubicin-encapsulated hydroxyapatite–polyvinyl alcohol nanocomposite for repair of osteosarcoma-affected bone tissues. Comptes Rendus Chim. 2019, 22, 46–57. [Google Scholar] [CrossRef]
- Pelšs, J.; Loča, D.; Bērziņa-Cimdiņa, L.; Ločs, J.; Lakevičs, V. Release of anticancer drug doxorubicin from biodegradable polymer coated porous hydroxyapatite scaffolds. Adv. Mat. Res. 2011, 284–286, 1770–1773. [Google Scholar] [CrossRef]
- Yang, C.L.; Chen, J.P.; Wei, K.C.; Chen, J.Y.; Huang, C.W.; Liao, Z.X. Release of doxorubicin by a folate-grafted, chitosan-coated magnetic nanoparticle. Nanomaterials 2017, 7, 85. [Google Scholar] [CrossRef]
- Souza, J.; Silva, M.; Costa, M. Potential doxorubicin delivery system based on magnetic gelatin microspheres crosslinked with sugars. Polímeros 2018, 28, 131–138. [Google Scholar] [CrossRef]
- Yang, F.; Lu, J.; Ke, Q.; Peng, X.; Guo, Y.; Xie, X. Magnetic mesoporous calcium sillicate/chitosan porous scaffolds for enhanced bone regeneration and photothermal-chemotherapy of osteosarcoma. Nat. Sci. Rep. 2018, 8, 7345. [Google Scholar] [CrossRef]
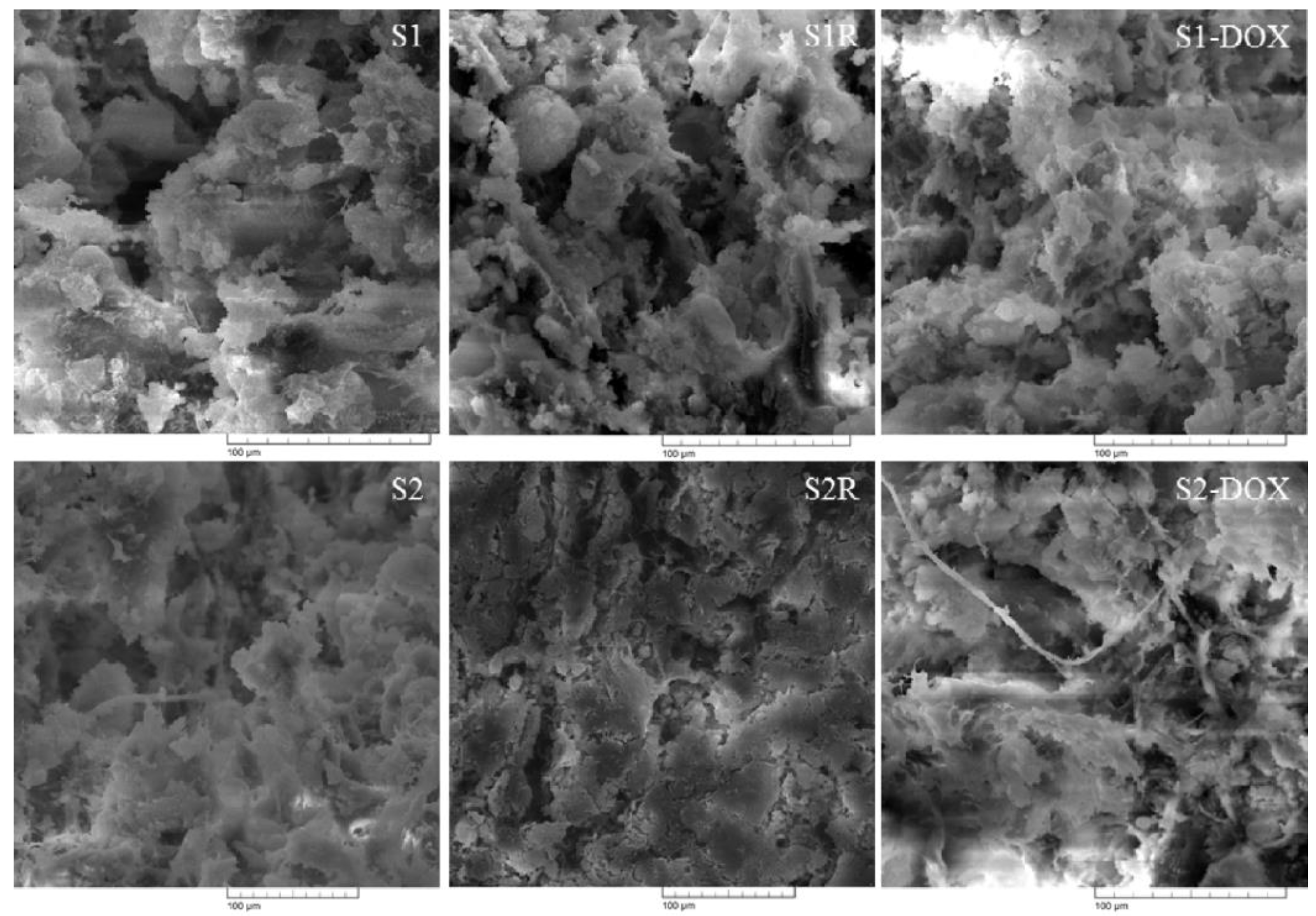
| Magnetic Scaffold | Biopolymer Composition | Ca/P (Initial) | Ca/P (Final) | MNPs Concentration | PBS RD% | Magnetization (emu/g) | Distinctive Features |
|---|---|---|---|---|---|---|---|
| S1 | 28.79% Col, | 1.65 | 1.63 | 5% MNPs | 995 ± 30 | 22.41 ± 1.41 | Used as control |
| 71.21% Cs | |||||||
| S2 | 50% Cs, | 1.65 | 1.64 | 5% MNPs | 1040 ± 35 | 44.42 ± 0.92 | Used as control |
| 50% Col | |||||||
| S1R | 28.79% Col, | 1.65 | 1.63 | 5% MNPs | 990 ± 50 | n.a. | X-ray irradiated |
| 71.21% Cs | |||||||
| S2R | 50% Cs, | 1.65 | 1.64 | 5% MNPs | 987 ± 19 | n.a. | X-ray irradiated |
| 50% Col | |||||||
| S1-DOX | 28.79% Col, | 1.65 | 1.62 | 5% MNPs-DOX | n.a. | n.a. | MNPs loaded with DOX |
| 71.21% Cs | |||||||
| S2-DOX | 50% Cs, | 1.65 | 1.63 | 5% MNPs-DOX | n.a. | n.a. | MNPs loaded with DOX |
| 50% Col |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cojocaru, F.D.; Balan, V.; Popa, I.M.; Munteanu, A.; Anghelache, A.; Verestiuc, L. Magnetic Composite Scaffolds for Potential Applications in Radiochemotherapy of Malignant Bone Tumors. Medicina 2019, 55, 153. https://doi.org/10.3390/medicina55050153
Cojocaru FD, Balan V, Popa IM, Munteanu A, Anghelache A, Verestiuc L. Magnetic Composite Scaffolds for Potential Applications in Radiochemotherapy of Malignant Bone Tumors. Medicina. 2019; 55(5):153. https://doi.org/10.3390/medicina55050153
Chicago/Turabian StyleCojocaru, Florina Daniela, Vera Balan, Ionel Marcel Popa, Anca Munteanu, Anisoara Anghelache, and Liliana Verestiuc. 2019. "Magnetic Composite Scaffolds for Potential Applications in Radiochemotherapy of Malignant Bone Tumors" Medicina 55, no. 5: 153. https://doi.org/10.3390/medicina55050153
APA StyleCojocaru, F. D., Balan, V., Popa, I. M., Munteanu, A., Anghelache, A., & Verestiuc, L. (2019). Magnetic Composite Scaffolds for Potential Applications in Radiochemotherapy of Malignant Bone Tumors. Medicina, 55(5), 153. https://doi.org/10.3390/medicina55050153








