Early Blood Biomarkers to Improve Sepsis/Bacteremia Diagnostics in Pediatric Emergency Settings
Abstract
:1. Introduction
2. Methods and Materials
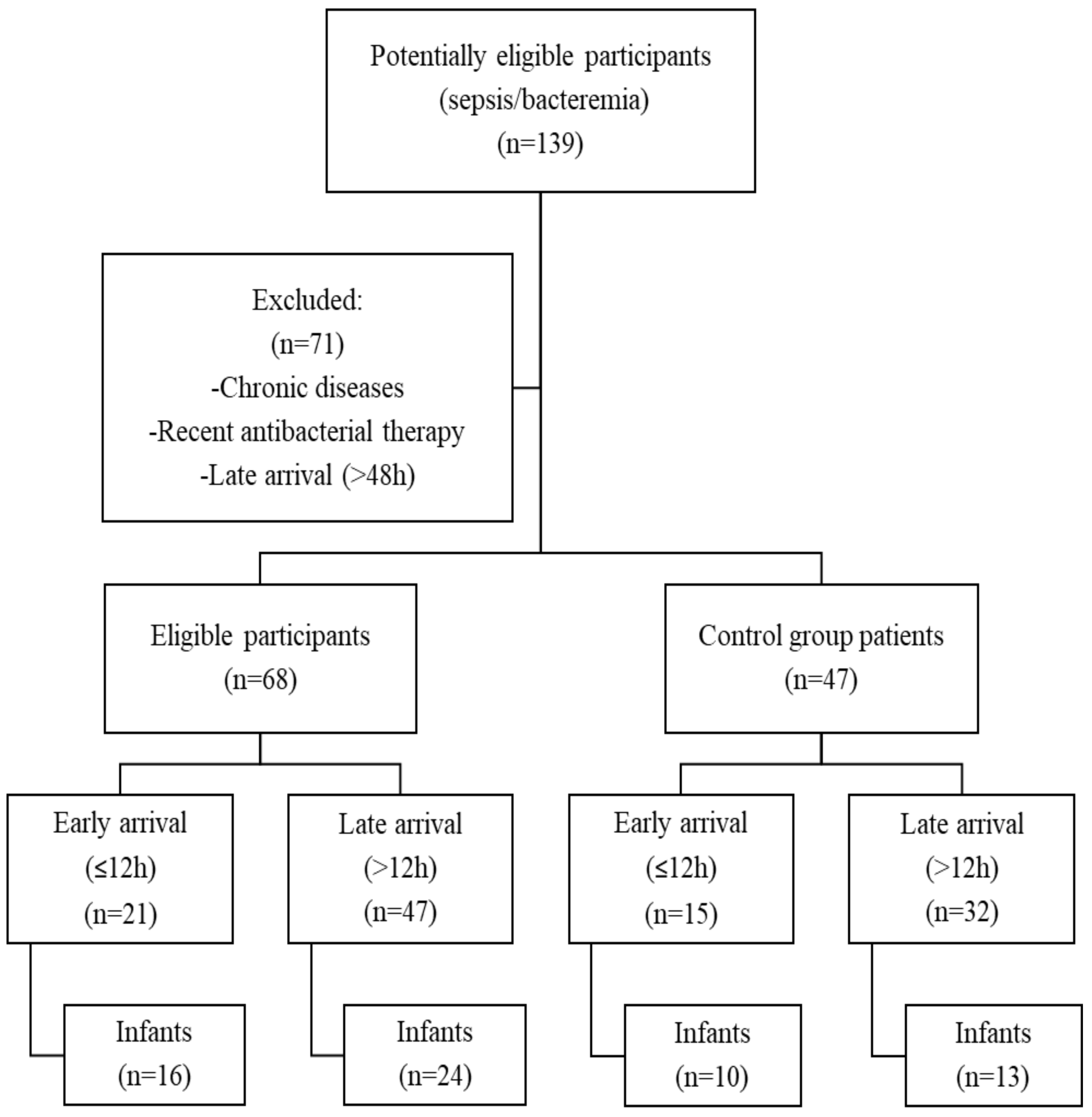
2.1. Study Design and Study Population
2.2. Laboratory Data
2.3. Statistical Analysis
3. Results
3.1. Early versus Late Arrival
3.2. Infants Population
3.3. Prediction of Sepsis/Bacteremia
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Schlapbach, L.J.; Straney, L.; Alexander, J.; MacLaren, G.; Festa, M.; Schibler, A.; Slater, A. Mortality related to invasive infections, sepsis, and septic shock in critically ill children in Australia and New Zealand, 2002–2013: A multicentre retrospective cohort study. Lancet Infect. Dis. 2015, 15, 46–54. [Google Scholar] [CrossRef]
- Balamuth, F.; Weiss, S.L.; Neuman, M.I.; Scott, H.; Brady, P.W.; Paul, R.; Farris, R.W.; McClead, R.; Hayes, K.; Gaieski, D.; et al. Pediatric severe sepsis in U.S. children’s hospitals. Pediatr. Crit. Care Med. J. Soc. Crit. Care Med. World Fed. Pediatr. Intensive Crit. Care Soc. 2014, 15, 798–805. [Google Scholar] [CrossRef]
- Hartman, M.E.; Linde-Zwirble, W.T.; Angus, D.C.; Watson, R.S. Trends in the epidemiology of pediatric severe sepsis. Pediatr. Crit. Care Med. J. Soc. Crit. Care Med. World Fed. Pediatr. Intensive Crit. Care Soc. 2013, 14, 686–693. [Google Scholar] [CrossRef]
- World Health Organization. Causes of Child Mortality. 2017. Available online: http://www.who.int/gho/child_health/mortality/causes/en/ (accessed on 1 February 2019).
- Weiss, S.L.; Fitzgerald, J.C.; Pappachan, J.; Wheeler, D.; Jaramillo-Bustamante, J.C.; Salloo, A.; Singhi, S.C.; Erickson, S.; Roy, J.A.; Bush, J.L.; et al. Global epidemiology of pediatric severe sepsis: The sepsis prevalence, outcomes, and therapies study. Am. J. Respir. Crit. Care Med. 2015, 191, 1147–1157. [Google Scholar] [CrossRef] [PubMed]
- Massin, M.M.; Montesanti, J.; Gerard, P.; Lepage, P. Spectrum and frequency of illness presenting to a pediatric emergency department. Acta Clin. Belg. 2006, 61, 161–165. [Google Scholar] [CrossRef]
- Claudius, I.; Baraff, L.J. Pediatric emergencies associated with fever. Emerg. Med. Clin. N. Am. 2010, 28, 67–84. [Google Scholar] [CrossRef]
- Hamilton, J.L.; John, S.P. Evaluation of fever in infants and young children. Am. Fam. Phys. 2013, 87, 254–260. [Google Scholar]
- Watt, K.; Waddle, E.; Jhaveri, R. Changing Epidemiology of Serious Bacterial Infections in Febrile Infants without Localizing Signs. PLoS ONE 2010, 5, e12448. [Google Scholar] [CrossRef] [PubMed]
- Baker, M.D.; Avner, J.R. The Febrile Infant: What’s New? Clin. Pediatr. Emerg. Med. 2008, 9, 213–220. [Google Scholar] [CrossRef]
- Simon, A.K.; Hollander, G.A.; McMichael, A. Evolution of the immune system in humans from infancy to old age. Proc. R. Soc. B Biol. Sci. 2015, 282, 20143085. [Google Scholar] [CrossRef] [PubMed]
- Harper, M.B. Update on the management of the febrile infant. Clin. Pediatr. Emerg. Med. 2004, 5, 5–12. [Google Scholar] [CrossRef]
- Woll, C.; Neuman, M.I.; Aronson, P.L. Management of the Febrile Young Infant: Update for the 21st Century. Pediatr. Emerg. Care 2017, 33, 748–753. [Google Scholar] [CrossRef]
- Foo, C.P.Z.; Sangha, G.; Seabrook, J.; Foster, J. Systemic inflammatory response in the pediatric emergency department: A common phenomenon that does not predict severe illness. Crit. Care 2014, 18, P38. [Google Scholar] [CrossRef]
- Han, M.; Fitzgerald, J.C.; Balamuth, F.; Keele, L.; Alpern, E.R.; Lavelle, J.; Chilutti, M.; Grundmeier, R.W.; Nadkarni, V.M.; Thomas, N.J.; et al. Association of Delayed Antimicrobial Therapy with One-Year Mortality in Pediatric Sepsis. Shock (Augustaga) 2017, 48, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Weiss, S.L.; Fitzgerald, J.C.; Balamuth, F.; Alpern, E.R.; Lavelle, J.; Chilutti, M.; Grundmeier, R.; Nadkarni, V.M.; Thomas, N.J. Delayed antimicrobial therapy increases mortality and organ dysfunction duration in pediatric sepsis. Crit. Care Med. 2014, 42, 2409–2417. [Google Scholar] [CrossRef]
- Weiss, S.L.; Balamuth, F.; Hensley, J.; Fitzgerald, J.C.; Bush, J.; Nadkarni, V.M.; Thomas, N.J.; Hall, M.; Muszynski, J. The Epidemiology of Hospital Death Following Pediatric Severe Sepsis: When, Why, and How Children With Sepsis Die. Pediatr. Crit. Care Med. J. Soc. Crit. Care Med. World Fed. Pediatr. Intensive Crit. Care Soc. 2017, 18, 823–830. [Google Scholar] [CrossRef] [PubMed]
- Burston, J.; Adhikari, S.; Hayen, A.; Doolan, H.; Kelly, M.L.; Fu, K.; Jensen, T.O.; Konecny, P. A Role for Antimicrobial Stewardship in Clinical Sepsis Pathways: A Prospective Interventional Study. Infect. Control Hosp. Epidemiol. 2017, 38, 1032–1038. [Google Scholar] [CrossRef]
- Arora, R.; Mahajan, P. Evaluation of child with fever without source: Review of literature and update. Pediatr. Clin. N. Am. 2013, 60, 1049–1062. [Google Scholar] [CrossRef]
- Lanziotti, V.S. Use of biomarkers in pediatric sepsis: Literature. Rev. Bras. Ter. Intensiva 2016, 28, 472–482. [Google Scholar] [CrossRef]
- Nathan, C. Points of control in inflammation. Nature 2002, 420, 846–852. [Google Scholar] [CrossRef]
- Basha, S.; Surendran, N.; Pichichero, M. Immune responses in neonates. Expert Rev. Clin. Immunol. 2014, 10, 1171–1184. [Google Scholar] [CrossRef] [PubMed]
- Marodi, L. Innate cellular immune responses in newborns. Clin. Immunol. (Orlandofla) 2006, 118, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Levy, O. Innate immunity of the newborn: Basic mechanisms and clinical correlates. Nat. Rev. Immunol. 2007, 7, 379–390. [Google Scholar] [CrossRef] [PubMed]
- Montero-Chacón, L.B.; Padilla-Cuadra, J.I.; Chiou, S.H.; Torrealba-Acosta, G. High-Density Lipoprotein, Mean Platelet Volume, and Uric Acid as Biomarkers for Outcomes in Patients With Sepsis: An Observational Study. J. Intensive Care Med. 2018. [Google Scholar] [CrossRef]
- Strunk, T.; Doherty, D.; Richmond, P.; Simmer, K.; Charles, A.; Levy, O.; Liyanage, K.; Smith, T.; Currie, A.; Burgner, D. Reduced levels of antimicrobial proteins and peptides in human cord blood plasma. Arch. Dis. Child. Fetal Neonatal Ed. 2009, 94, F230–F231. [Google Scholar] [CrossRef] [PubMed]
- Randolph, A.G.; McCulloh, R.J. Pediatric sepsis: Important considerations for diagnosing and managing severe infections in infants, children, and adolescents. Virulence 2014, 5, 179–189. [Google Scholar] [CrossRef]
- Reinhart, K.; Bauer, M.; Riedemann, N.C.; Hartog, C.S. New approaches to sepsis: Molecular diagnostics and biomarkers. Clin. Microbiol. Rev. 2012, 25, 609–634. [Google Scholar] [CrossRef]
- Pierrakos, C.; Vincent, J.-L. Sepsis biomarkers: A review. Crit. Care 2010, 14, R15. [Google Scholar] [CrossRef]
- Fan, S.L.; Miller, N.S.; Lee, J.; Remick, D.G. Diagnosing sepsis—The role of laboratory medicine. Clin. Chim. Acta Int. J. Clin. Chem. 2016, 460, 203–210. [Google Scholar] [CrossRef]
- Markic, J.; Kovacevic, T.; Krzelj, V.; Bosnjak, N.; Sapunar, A. Lab-Score Is a Valuable predictor of Serious Bacterial Infection in Infants Admitted to Hospital. Wien. Klin. Wochenschr. 2015, 127, 942–947. [Google Scholar]
- Cho, S.-Y.; Choi, J.-H. Biomarkers of Sepsis. Infect. Chemother. 2014, 46, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Standage, S.W.; Wong, H.R. Biomarkers for pediatric sepsis and septic shock. Expert Rev. Anti Infect. Ther. 2011, 9, 71–79. [Google Scholar] [CrossRef]
- Yang, Y.; Xie, J.; Guo, F.; Longhini, F.; Gao, Z.; Huang, Y.; Qiu, H. Combination of C-reactive protein, procalcitonin and sepsis-related organ failure score for the diagnosis of sepsis in critical patients. Ann. Intensive Care 2016, 6, 51. [Google Scholar] [CrossRef] [PubMed]
- Magrini, L.; Gagliano, G.; Travaglino, F.; Vetrone, F.; Marino, R.; Cardelli, P.; Salerno, G.; Di Somma, S. Comparison between white blood cell count, procalcitonin and C reactive protein as diagnostic and prognostic biomarkers of infection or sepsis in patients presenting to emergency department. Clin. Chem. Lab. Med. 2014, 52, 1465–1472. [Google Scholar] [CrossRef] [PubMed]
- Sônego, F.; Castanheira, F.V.e.S.; Ferreira, R.G.; Kanashiro, A.; Leite, C.A.V.G.; Nascimento, D.C.; Colón, D.F.; Borges, V.d.F.; Alves-Filho, J.C.; Cunha, F.Q. Paradoxical Roles of the Neutrophil in Sepsis: Protective and Deleterious. Front. Immunol. 2016, 7, 155. [Google Scholar] [CrossRef] [PubMed]
- Kovach, M.A.; Standiford, T.J. The function of neutrophils in sepsis. Curr. Opin. Infect. Dis. 2012, 25, 321–327. [Google Scholar] [CrossRef]
- Tang, H.; Li, B.; Zhang, A.; Lu, W.; Xiang, C.; Dong, J. Prognostic Significance of Neutrophil-to-Lymphocyte Ratio in Colorectal Liver Metastasis: A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0159447. [Google Scholar] [CrossRef]
- Pantzaris, N.-D.; Platanaki, C.; Pierrako, C.; Karamouzos, V.; Velissaris, D. Neutrophil-to-lymphocyte Ratio Relation to Sepsis Severity Scores and Inflammatory Biomarkers in Patients with Community-acquired Pneumonia: A Case Series. J. Transl. Intern. Med. 2018, 6, 43–46. [Google Scholar] [CrossRef]
- Djordjevic, D.; Rondovic, G.; Surbatovic, M. Neutrophil-to-Lymphocyte Ratio, Monocyte-to-Lymphocyte Ratio, Platelet-to-Lymphocyte Ratio, and Mean Platelet Volume-to-Platelet Count Ratio as Biomarkers in Critically Ill and Injured Patients: Which Ratio to Choose to Predict Outcome and Nature of Bacteremia? Mediat. Inflamm. 2018, 2018, 3758068. [Google Scholar]
- De Jager, C.P.C.; van Wijk, P.T.L.; Mathoera, R.B.; de Jongh-Leuvenink, J.; van der Poll, T.; Wever, P.C. Lymphocytopenia and neutrophil-lymphocyte count ratio predict bacteremia better than conventional infection markers in an emergency care unit. Crit. Care (Lond. Engl.) 2010, 14, R192. [Google Scholar] [CrossRef]
- Ljungstrom, L.; Pernestig, A.K.; Jacobsson, G.; Andersson, R.; Usener, B.; Tilevik, D. Diagnostic accuracy of procalcitonin, neutrophil-lymphocyte count ratio, C-reactive protein, and lactate in patients with suspected bacterial sepsis. PLoS ONE 2017, 12, e0181704. [Google Scholar] [CrossRef] [PubMed]
- Loonen, A.J.; de Jager, C.P.; Tosserams, J.; Kusters, R.; Hilbink, M.; Wever, P.C.; van den Brule, A.J. Biomarkers and molecular analysis to improve bloodstream infection diagnostics in an emergency care unit. PLoS ONE 2014, 9, e87315. [Google Scholar] [CrossRef] [PubMed]
- Alkan Ozdemir, S.; Arun Ozer, E.; Ilhan, O.; Sutcuoglu, S. Can neutrophil to lymphocyte ratio predict late-onset sepsis in preterm infants? J. Clin. Lab. Anal. 2018, 32, e22338. [Google Scholar] [CrossRef] [PubMed]
- Can, E.; Hamilcikan, S.; Can, C. The Value of Neutrophil to Lymphocyte Ratio and Platelet to Lymphocyte Ratio for Detecting Early-onset Neonatal Sepsis. J. Pediatr. Hematol./Oncol. 2018, 40, e229–e232. [Google Scholar] [CrossRef] [PubMed]
- Gurol, G.; Ciftci, I.H.; Terizi, H.A.; Atasoy, A.R.; Ozbek, A.; Koroglu, M. Are there standardized cutoff values for neutrophil-lymphocyte ratios in bacteremia or sepsis? J. Microbiol. Biotechnol. 2015, 25, 521–525. [Google Scholar] [CrossRef] [PubMed]
- Dewitte, A.; Lepreux, S.; Villeneuve, J.; Rigothier, C.; Combe, C.; Ouattara, A.; Ripoche, J. Blood platelets and sepsis pathophysiology: A new therapeutic prospect in critical ill patients? Ann. Intensive Care 2017, 7, 115. [Google Scholar] [CrossRef]
- Semple, J.W.; Italiano, J.E., Jr.; Freedman, J. Platelets and the immune continuum. Nat. Rev. Immunol. 2011, 11, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Morrell, C.N.; Aggrey, A.A.; Chapman, L.M.; Modjeski, K.L. Emerging roles for platelets as immune and inflammatory cells. Blood 2014, 123, 2759–2767. [Google Scholar] [CrossRef] [PubMed]
- Vieira-de-Abreu, A.; Campbell, R.A.; Weyrich, A.S.; Zimmerman, G.A. Platelets: Versatile effector cells in hemostasis, inflammation, and the immune continuum. Semin. Immunopathol. 2012, 34, 5–30. [Google Scholar] [CrossRef] [PubMed]
- Kraemer, B.F.; Campbell, R.A.; Schwertz, H.; Cody, M.J.; Franks, Z.; Tolley, N.D.; Kahr, W.H.; Lindemann, S.; Seizer, P.; Yost, C.C.; et al. Novel anti-bacterial activities of beta-defensin 1 in human platelets: Suppression of pathogen growth and signaling of neutrophil extracellular trap formation. PLoS Pathog. 2011, 7, e1002355. [Google Scholar] [CrossRef]
- Cognasse, F.; Hamzeh, H.; Chavarin, P.; Acquart, S.; Genin, C.; Garraud, O. Evidence of Toll-like receptor molecules on human platelets. Immunol. Cell Biol. 2005, 83, 196–198. [Google Scholar] [CrossRef]
- Claushuis, T.A.M.; Van Der Veen, A.I.P.; Horn, J.; Schultz, M.J.; Houtkooper, R.H.; Van’t Veer, C.; Van Der Poll, T. Platelet Toll-like receptor expression and activation induced by lipopolysaccharide and sepsis. Platelets 2018, 1–9. [Google Scholar] [CrossRef]
- Agrawal, S.; Sachdev, A.; Gupta, D.; Chugh, K. Platelet counts and outcome in the pediatric intensive care unit. Indian J. Crit. Care Med. 2008, 12, 102–108. [Google Scholar]
- Ree, I.M.C.; Fustolo-Gunnink, S.F.; Bekker, V.; Fijnvandraat, K.J.; Steggerda, S.J.; Lopriore, E. Thrombocytopenia in neonatal sepsis: Incidence, severity and risk factors. PLoS ONE 2017, 12, e0185581. [Google Scholar] [CrossRef] [PubMed]
- Yeaman, M.R. Platelets: At the nexus of antimicrobial defence. Nat. Rev. Microbiol. 2014, 12, 426–437. [Google Scholar] [CrossRef]
- Lindemann, S.; Tolley, N.D.; Dixon, D.A.; McIntyre, T.M.; Prescott, S.M.; Zimmerman, G.A.; Weyrich, A.S. Activated platelets mediate inflammatory signaling by regulated interleukin 1beta synthesis. J. Cell Biol. 2001, 154, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Rendu, F.; Brohard-Bohn, B. The platelet release reaction: Granules’ constituents, secretion and functions. Platelets 2001, 12, 261–273. [Google Scholar] [CrossRef] [PubMed]
- Koseoglu, S.; Flaumenhaft, R. Advances in platelet granule biology. Curr. Opin. Hematol. 2013, 20, 464–471. [Google Scholar] [CrossRef] [PubMed]
- Seyoum, M.; Enawgaw, B.; Melku, M. Human blood platelets and viruses: Defense mechanism and role in the removal of viral pathogens. Thromb. J. 2018, 16, 16. [Google Scholar] [CrossRef]
- Kim, C.H.; Kim, S.J.; Lee, M.J.; Kwon, Y.E.; Kim, Y.L.; Park, K.S.; Ryu, H.J.; Park, J.T.; Han, S.H.; Yoo, T.-H.; et al. An Increase in Mean Platelet Volume from Baseline Is Associated with Mortality in Patients with Severe Sepsis or Septic Shock. PLoS ONE 2015, 10, e0119437. [Google Scholar] [CrossRef]
- Aydemir, H.; Piskin, N.; Akduman, D.; Kokturk, F.; Aktas, E. Platelet and mean platelet volume kinetics in adult patients with sepsis. Platelets 2015, 26, 331–335. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.T.; Li, J.Y.; Cao, Z.G.; Li, Y. Mean platelet volume is decreased during an acute exacerbation of chronic obstructive pulmonary disease. Respirology 2013, 18, 1244–1248. [Google Scholar] [CrossRef] [PubMed]
- Canpolat, D.G.; Dogruel, F.; Gonen, Z.B.; Yilmaz, C.; Zararsiz, G.; Alkan, A. The role of platelet count, mean platelet volume, and the mean platelet volume/platelet count ratio in predicting postoperative vomiting in children after deep sedation. Saudi Med. J. 2016, 37, 1082–1088. [Google Scholar] [CrossRef] [PubMed]
- Doğan, K.; Guraslan, H.; Senturk, M.; Helvacioglu, C.; İdil, S.; Ekin, M. Can Platelet Count and Platelet Indices Predict the Risk and the Prognosis of Preeclampsia? Hypertens. Prenancy 2015, 34, 1–9. [Google Scholar]
- Oh, G.H.; Chung, S.P.; Park, Y.S.; Hong, J.H.; Lee, H.S.; Chung, H.S.; You, J.S.; Park, J.W.; Park, I. Mean Platelet Volume to Platelet Count Ratio as a Promising Predictor of Early Mortality in Severe Sepsis. Shock 2017, 47, 323–330. [Google Scholar] [CrossRef]
- Ates, S.; Oksuz, H.; Dogu, B.; Bozkus, F.; Ucmak, H.; Yanıt, F. Can mean platelet volume and mean platelet volume/platelet count ratio be used as a diagnostic marker for sepsis and systemic inflammatory response syndrome? Saudi Med. J. 2015, 36, 1186–1190. [Google Scholar] [CrossRef] [PubMed]
| Parameter | Bacterial (n = 68) | Viral (n = 47) | p-Value |
|---|---|---|---|
| Demographic Data | |||
| Age, months | 9 [3–24] | 12 [6–27] | 0.274 |
| Male gender, n (%) | 31 (45.6) | 23 (48.9) | 0.857 |
| Laboratory Markers | |||
| WBC, ×109/L | 17.94 ± 10.04 | 10.42 ± 4.21 | <0.001 |
| Neutrophils, ×109/L | 10.93 ± 8.03 | 5.08 ± 3.42 | <0.001 |
| PLT, ×109/L | 370.15 ± 134.65 | 288.91 ± 107.14 | 0.001 |
| CRP, mg/L | 64.86 [20.80–133.69] | 8.49 [2.11–20.85] | <0.001 |
| NLR | 2.69 ± 2.03 | 1.83 ± 1.70 | 0.006 |
| MPV | 9.03 ± 1.21 | 9.16 ± 1.33 | 0.717 |
| PLT/MPV | 41.42 ± 15.86 | 33.45 ± 17.97 | 0.001 |
| Laboratory Marker | Bacterial (n = 21) | Viral (n = 15) | p-Value |
|---|---|---|---|
| WBC, ×109/L | 16.11 ± 9.11 | 9.89 ± 4.65 | 0.007 |
| Neutrophils, ×109/L | 9.43 ± 8.59 | 5.06 ± 4.07 | 0.077 |
| PLT, ×109/L | 390.43 ± 88.11 | 282.29 ± 79.35 | 0.001 |
| CRP, mg/L | 20.00 (7.95–65.22) | 2.73 (1.00–13.89) | 0.008 |
| NLR | 2.58 ± 2.19 | 2.07 ± 2.00 | 0.547 |
| MPV | 9.02 ± 1.09 | 9.58 ± 0.82 | 0.202 |
| PLT/MPV | 43.86 ± 11.58 | 29.80 ± 8.69 | <0.001 |
| Laboratory Marker | Bacterial (n = 47) | Viral (n = 36) | p-Value |
|---|---|---|---|
| WBC, ×109/L | 18.70 ± 10.53 | 10.76 ± 4.08 | <0.001 |
| Neutrophils, ×109/L | 11.58 ± 7.85 | 5.17 ± 3.20 | <0.001 |
| PLT, ×109/L | 354.17 ± 144.81 | 291.41 ± 119.84 | 0.053 |
| CRP, mg/L | 99.58 (43.92–182.30) | 11.33 (6.11–23.40) | <0.001 |
| NLR | 2.77 ± 2.00 | 1.74 ± 1.60 | 0.003 |
| MPV | 9.03 ± 1.28 | 8.93 ± 1.46 | 0.640 |
| PLT/MPV | 40.31 ± 17.47 | 35.17 ± 20.87 | 0.106 |
| Laboratory Marker | Bacterial (n = 16) | Viral (n = 10) | p-Value |
|---|---|---|---|
| WBC, ×109/L | 12.80 ± 5.45 | 8.59 ± 3.62 | 0.037 |
| Neutrophils, ×109/L | 5.83 ± 4.09 | 3.49 ± 2.28 | 0.121 |
| PLT, ×109/L | 403.19 ± 84.03 | 304.50 ± 80.29 | 0.010 |
| CRP, mg/L | 15.86 (2.51–28.03) | 2.02 (1.22–15.89) | 0.120 |
| NLR | 1.64 ± 1.43 | 1.33 ± 1.20 | 0.598 |
| MPV | 9.44 ± 0.62 | 9.78 ± 0.44 | 0.152 |
| PLT/MPV | 42.70 ± 8.57 | 31.01 ± 8.21 | 0.008 |
| Laboratory Marker | Bacterial (n = 24) | Viral (n = 13) | p-Value |
|---|---|---|---|
| WBC, ×109/L | 19.74 ± 11.65 | 11.11 ± 4.41 | 0.015 |
| Neutrophils, ×109/L | 10.77 ± 7.67 | 4.45 ± 2.70 | 0.003 |
| PLT, ×109/L | 419.30 ± 155.35 | 338.49 ± 150.28 | 0.106 |
| CRP, mg/L | 63.71 (28.45–128.25) | 7.50 (4.57–14.05) | <0.001 |
| NLR | 1.90 ± 1.25 | 1.16 ± 1.06 | 0.025 |
| MPV | 9.44 ± 0.85 | 8.94 ± 0.95 | 0.046 |
| PLT/MPV | 44.80 ± 17.28 | 39.45 ± 22.10 | 0.316 |
| Laboratory Marker (Cut-Off Value) | Sensitivity, % | Specificity, % |
|---|---|---|
| WBC (>9.5 × 109/L) | 77.6 | 58.7 |
| Neutrophils (>5.0 × 109/L) | 71.6 | 43.5 |
| PLT (>300 × 109/L) | 71.7 | 54.5 |
| CRP (>20 mg/L) | 80.3 | 68.8 |
| NLR (1.58) | 73.0 | 57.7 |
| MPV (9.0) | 68.7 | 34.0 |
| PLT/MPV (30.0) | 76.1 | 46.8 |
| Laboratory Marker | AUC | 95% CI | p-Value |
|---|---|---|---|
| WBC | 0.739 | 0.647–0.830 | <0.001 |
| Neutrophils | 0.736 | 0.644–0.828 | <0.001 |
| PLT | 0.677 | 0.578–0.776 | 0.001 |
| CRP | 0.830 | 0.753–0.906 | <0.001 |
| NLR | 0.650 | 0.545–0.754 | 0.007 |
| MPV | 0.489 | 0.381–0.597 | 0.842 |
| PLT/MPV | 0.676 | 0.575–0.776 | 0.002 |
| Laboratory Marker (Cut-Off Value) | Sensitivity, % | Specificity, % |
|---|---|---|
| Infants arrived at ED within 12 h | ||
| NLR (1.35) | 50.0 | 77.8 |
| PLT/MPV (32.0) | 93.8 | 55.6 |
| Infants arrived at ED after 12 h | ||
| NLR (1.2) | 66.7 | 71.4 |
| PLT/MPV (40.0) | 56.5 | 64.3 |
| Laboratory Marker | AUC | 95% CI | p-Value |
|---|---|---|---|
| Infants arrived at ED within 12 h | |||
| NLR | 0.569 | 0.331–0.808 | 0.571 |
| PLT/MPV | 0.826 | 0.662–0.991 | 0.008 |
| Infants arrived at ED after 12 h | |||
| NLR | 0.720 | 0.550–0.890 | 0.025 |
| PLT/MPV | 0.599 | 0.405–0.794 | 0.316 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tamelytė, E.; Vaičekauskienė, G.; Dagys, A.; Lapinskas, T.; Jankauskaitė, L. Early Blood Biomarkers to Improve Sepsis/Bacteremia Diagnostics in Pediatric Emergency Settings. Medicina 2019, 55, 99. https://doi.org/10.3390/medicina55040099
Tamelytė E, Vaičekauskienė G, Dagys A, Lapinskas T, Jankauskaitė L. Early Blood Biomarkers to Improve Sepsis/Bacteremia Diagnostics in Pediatric Emergency Settings. Medicina. 2019; 55(4):99. https://doi.org/10.3390/medicina55040099
Chicago/Turabian StyleTamelytė, Emilija, Gineta Vaičekauskienė, Algirdas Dagys, Tomas Lapinskas, and Lina Jankauskaitė. 2019. "Early Blood Biomarkers to Improve Sepsis/Bacteremia Diagnostics in Pediatric Emergency Settings" Medicina 55, no. 4: 99. https://doi.org/10.3390/medicina55040099
APA StyleTamelytė, E., Vaičekauskienė, G., Dagys, A., Lapinskas, T., & Jankauskaitė, L. (2019). Early Blood Biomarkers to Improve Sepsis/Bacteremia Diagnostics in Pediatric Emergency Settings. Medicina, 55(4), 99. https://doi.org/10.3390/medicina55040099





