Prognostic Factors for Immune Thrombocytopenic Purpura Remission after Laparoscopic Splenectomy: A Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Design
2.2. Definitions
2.3. Operative Technique
2.4. Ethics
2.5. Statistical Analysis
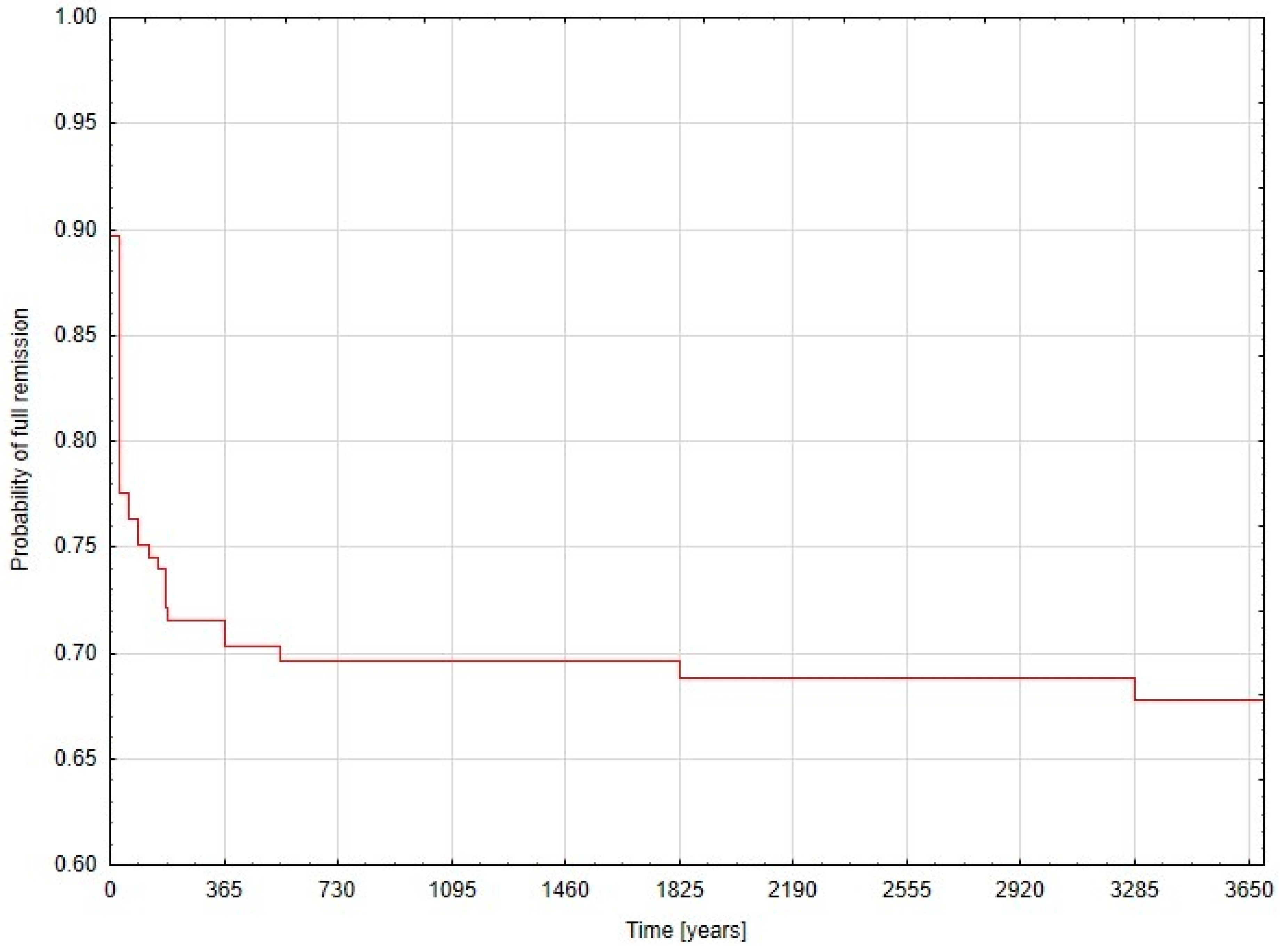
3. Results
Patients
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Akwari, O.E.; Itani, K.M.F.; Coleman, R.E.; Rosse, W.F. Splenectomy for primary and recurrent immune thrombocytopenic purpura (ITP). Ann. Surg. 1987, 206, 529–541. [Google Scholar] [CrossRef]
- George, J.N.; Woolf, S.H.; Raskob, G.E.; Wasser, J.S.; Aledort, L.M.; Ballem, P.J.; Blanchette, V.S.; Bussel, J.B.; Cines, D.B.; Kelton, J.G.; et al. Idiopathic thrombocytopenic purpura: A practice guideline developed by explicit methods for the American Society of Hematology. Blood 1996, 88, 3–40. [Google Scholar] [CrossRef] [PubMed]
- Ojima, H.; Kato, T.; Araki, K.; Okamura, K.; Manda, R.; Hirayama, I.; Hosouchi, Y.; Nishida, Y.; Kuwano, H. Factors predicting long-term responses to splenctomy in patients with idiopathic thrombocytopenic purpura. World J. Surg. 2006, 30, 553–559. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.M.; Lee, J.G.; Kim, K.S.; Choi, J.S.; Lee, W.J.; Kim, B.R.; Ko, Y.W.; Han, J.S.; Min, Y.H. Long-term follow-up of laparoscopic splenectomy in patients with immune thrombocytopenic purpura. J. Korean Med. Sci. 2007, 22, 420–424. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Delaitre, B.; Maignien, B. Splenectomy by the laparoscopic approach. Report of a case. Presse Med. 1991, 20, 2263. [Google Scholar] [PubMed]
- Rijcken, E.; Mees, S.T.; Bisping, G.; Krueger, K.; Bruewer, M.; Senninger, N.; Mennigen, R. Laparoscopic splenectomy for medically refractory immune thrombocytopenia (ITP): A retrospective cohort study on longtime response predicting factors based on consensus criteria. Int. J. Surg. 2014, 12, 1428–1433. [Google Scholar] [CrossRef]
- Delaitre, B.; Blezel, E.; Samama, G.; Barrat, C.; Gossot, D.; Bresler, L.; Meyer, C.; Heyd, B.; Collet, D.; Champault, G.; et al. Laparoscopic splenectomy for idiopathic thrombocytopenic purpura. Surg. Laparosc. Endosc. Percutan. Tech. 2002, 12, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Winslow, E.R.; Brunt, L.M. Perioperative outcomes of laparoscopic versus open splenectomy: A meta-analysis with an emphasis on complications. Surgery 2003, 134, 647–653. [Google Scholar] [CrossRef]
- Pattenden, C.J.; Mann, C.D.; Metcalfe, M.S.; Dyer, M.; Lloyd, D.M. Laparoscopic splenectomy: A personal series of 140 consecutive cases. Ann. R. Coll. Surg. Engl. 2010, 92, 398–402. [Google Scholar] [CrossRef]
- Zychowicz, A.; Radkowiak, D.; Lasek, A.; Małczak, P.; Witowski, J.; Major, P.; Strzałka, M.; Kulawik, J.; Budzyński, A.; Pędziwiatr, M.; et al. The safety of laparoscopic splenectomy for immune thrombocytopenia in patients with very low platelet count. Videosurgery Other Miniinvasive Tech. 2018, 13, 157–163. [Google Scholar] [CrossRef]
- Gutierrez, G.; Reines, H.D.; Wulf-Gutierrez, M.E. Clinical review: Hemorrhagic shock. Crit. Care 2004, 8, 373–381. [Google Scholar] [CrossRef]
- Kathariya, R.; Devanoorkar, A.; Jain, H. Intra-Operative Hemorrhage: A Review of Literature. J. Med. Diagn. Methods 2013, 2, 1–5. [Google Scholar] [CrossRef]
- Dindo, D.; Demartines, N.; Clavien, P.A. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Major, P.; Matłok, M.; Pȩdziwiatr, M.; Budzyński, A. Do we really need routine drainage after laparoscopic adrenalectomy and splenectomy? Videosurgery Other Miniinvasive Tech. 2012, 7, 33–39. [Google Scholar] [CrossRef]
- Radkowiak, D.; Zychowicz, A.; Lasek, A.; Wysocki, M.; Major, P.; Pędziwiatr, M.; Budzyński, P.; Kulawik, J.; Budzyński, A. 20 years’ experience with laparoscopic splenectomy. Single center outcomes of a cohort study of 500 cases. Int. J. Surg. 2018, 52, 285–292. [Google Scholar] [CrossRef]
- Radkowiak, D.; Zychowicz, A.; Wysocki, M.; Lasek, A.; Major, P.; Pędziwiatr, M.; Budzyński, P.; Dembiński, M.; Dworak, J.; Budzynski, A.; et al. Quest for the optimal technique of laparoscopic splenectomy—Vessels first or hilar transection? Videosurgery Other Miniinvasive Tech. 2018, 13, 460. [Google Scholar] [CrossRef]
- Motheral, B.; Brooks, J.; Clark, M.A.; Crown, W.H.; Davey, P.; Hutchins, D.; Martin, B.C.; Stang, P.A. A checklist for retrospective database studies—Report of the ISPOR task force on retrospective databases. Value Health 2003, 6, 90–97. [Google Scholar] [CrossRef]
- Yeh, C.C.; Liao, C.C.; Chang, Y.C.; Jeng, L.B.; Yang, H.R.; Shih, C.C.; Chen, T.L. Adverse outcomes after noncardiac surgery in patients with diabetes: A nationwide population-based retrospective cohort study. Diabetes Care 2013, 36, 3216–3221. [Google Scholar] [CrossRef] [PubMed]
- Neunert, C.; Lim, W.; Crowther, M.; Cohen, A.; Solberg, L.; Crowther, M.A. The American Society of Hematology 2011 evidence-based practice guideline for immune thrombocytopenia. Blood 2011, 117, 4190–4207. [Google Scholar] [CrossRef]
- Tastaldi, L.; Krpata, D.M.; Prabhu, A.S.; Petro, C.C.; Haskins, I.N.; Perez, A.J.; Alkhatib, H.; Colturato, I.; Tu, C.; Lichtin, A.; et al. Laparoscopic splenectomy for immune thrombocytopenia (ITP): Long-term outcomes of a modern cohort. Surg. Endosc. Other Interv. Tech. 2019, 33, 475–485. [Google Scholar] [CrossRef]
- Thai, L.H.; Mahévas, M.; Roudot-Thoraval, F.; Limal, N.; Languille, L.; Dumas, G.; Khellaf, M.; Bierling, P.; Michel, M.; Godeau, B.; et al. Long-term complications of splenectomy in adult immune thrombocytopenia. Medicine 2016, 95, e5098. [Google Scholar] [CrossRef] [PubMed]
- Cuker, A.; Cines, D.B.; Neunert, C.E. Controversies in the treatment of immune thrombocytopenia. Curr. Opin. Hematol. 2016, 23, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Liao, R.; Tang, P.-Y.; Song, J.-F.; Qin, K.-L.; Wang, X.; Yan, X. Platelet count on preoperative day 1 predicts the long-term responses to laparoscopic splenectomy for Chinese patients with medically refractory idiopathic thrombocytopenic purpura. BMC Surg. 2018, 18, 108. [Google Scholar] [CrossRef] [PubMed]
- Istl, A.C.; McCreery, G.; Allen, L.J.; Vogt, K.; Dubois, L.; Gray, D.K. Corticosteroid response predicts success of laparoscopic splenectomy in treating immune thrombocytopenia. Surgery 2018, 164, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Duperier, T.; Brody, F.; Felsher, J.; Walsh, R.M.; Rosen, M.; Ponsky, J. Predictive Factors for Successful Laparoscopic Splenectomy in Patients with Immune Thrombocytopenic Purpura. Arch. Surg. 2004, 139, 61–66. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Radaelli, F.; Faccini, P.; Goldaniga, M.; Guggiari, E.; Pozzoli, E.; Maiolo, A.T.; Ciani, A.; Pogliani, E.M. Factors predicting response to splenectomy in adult patients with idiopathic thrombocytopenic purpura. Haematologica 2000, 85, 1040–1044. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Huang, C.-S.; Huang, S.-B.; Zheng, C.-X. Laparoscopic splenectomy for primary immune thrombocytopenia: Current status and challenges. World J Gastrointest. Endosc. 2016, 8, 610–615. [Google Scholar] [CrossRef]
- Woo, J.H.; Park, S.H.; Park, Y.K.; Choi, C.B.; Choi, Y.Y.; Myung, J.A.; Kim, I.S. Postsplenectomy recurrence of thrombocytopenia with an accessory spleen. Korean J. Intern. Med. 2004, 19, 199–201. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.U.; Dominguez, E.P.; Sherman, V.; Sweeney, J.F. Laparoscopic accessory splenectomy for recurrent idiopathic thrombocytopenic purpura. JSLS 2008, 12, 314–317. [Google Scholar] [PubMed]
| All n = 165 (100%) | Group 1 n = 113 (68.48%) | Group 2 n = 52 (31.52%) | p-Value | |
|---|---|---|---|---|
| Gender, n (%) male female | 52 (32) 113 (68) | 34 (30) 79 (70) | 18 (35) 34 (65) | 0.561 |
| Age, median (IQR), years | 35 (25–52) | 31 (24–48) | 50 (36.5–60) | <0.001 |
| BMI, median (IQR), kg/m2 | 25.69 (21.76–29.30) | 24.30 (20.52–29.01) | 27.65 (25.50–30.62) | <0.001 |
| Spleen size, median (IQR), cm | 11 (10–12) | 11 (10–12) | 11 (10–12) | 0.599 |
| Lowest preoperative platelet count, median (IQR), ×103/mm3 | 8 (4–16) | 10 (5–18) | 7 (3–12) | 0.071 |
| Preoperative platelet count, median (IQR), ×103/mm3 | 90 (48–119) | 97 (50–125) | 68.5 (36.5–107) | 0.034 |
| Preoperative steroids administration, n (%) | 158 (95.76) | 107 (94.69) | 51 (98.08) | 0.293 |
| Immunoglobulin administration, n (%) | 37 (22.42) | 26 (23.01) | 11 (21.15) | 0.791 |
| Preoperative platelet transfusions, n (%) | 19 (11.52) | 9 (7.96) | 10 (19.23) | 0.065 |
| Accessory spleen, n (%) | 36 (21.82) | 25 (22.12) | 11 (21.15) | 0.950 |
| Perioperative complications, n (%) | 13 (7.88) | 9 (7.96) | 4 (7.69) | 0.610 |
| Blood transfusions, n (%) | 3 (1.82) | 1 (0.88) | 2 (2.85) | 0.234 |
| Time from diagnosis of ITP to procedures, median (IQR), months | 24 (6.75–57) | 18 (6.5–48) | 24 (9–84) | 0.241 |
| Symptomatic ITP, n (%) | 90 (54.55) | 59 (52.21) | 31 (59.62) | 0.375 |
| Preoperative time of conservative treatment, median (IQR), months | 9.5 (5–30) | 8.5 (4.5–24) | 12 (5–51) | 0.178 |
| All | Group 1 | Group 2 | ||
|---|---|---|---|---|
| Operative time, median (IQR), min | 85 (65–105) | 80 (60–100) | 90 (70–110) | |
| Blood loss, median (IQR), mL | 50 (20–100) | 30 (10–50) | 50 (20–100) | |
| LOS, median (IQR), days | 4 (3–4) | 3 (3–4) | 4 (3–5) | |
| Perioperative morbidity, n (%) | 13 (7.88) | 9 (7.96) | 4 (7.69) | |
| Clavien–Dindo | Morbidity | All | Remission | Non-remission |
| IIIb | Acute pancreatitis, sub-phrenic abscess | 1 | 1 | 0 |
| Gastric perforation, sub-phrenic abscesses | 1 | 1 | 0 | |
| Peritonitis, intra-abdominal abscesses | 1 | 1 | 0 | |
| Intra-abdominal bleeding | 5 | 2 | 3 | |
| IIIa | Pancreatitis | 1 | 0 | 1 |
| II | Pneumonia | 2 | 2 | 0 |
| Postoperative fever | 1 | 1 | 0 | |
| I | Sub-phrenic fluid collection | 1 | 1 | 0 |
| OR | 95% CI | p-Value | |
|---|---|---|---|
| Male/Female | 1.23 | 0.61–2.49 | 0.561 |
| Age, with every 1 year | 0.94 | 0.93–0.97 | <0.001 |
| BMI, with every 1 kg/m2 | 0.85 | 0.77–0.93 | <0.001 |
| LOS, with every 1 day | 0.82 | 0.59–1.16 | 0.263 |
| Ultrasound length of spleen, with every cm | 1.04 | 0.93–1.16 | 0.522 |
| Lowest preoperative platelet count, with every 1 × 103/mm3 | 1.03 | 0.99–1.07 | 0.080 |
| Preoperative platelet count, with every 1 × 103/mm3 | 1.01 | 1.00–1.01 | 0.025 |
| Preoperative steroids administration | 0.35 | 0.04–3.03 | 0.337 |
| Preoperative immunoglobulin administration | 1.11 | 0.50–2.49 | 0.791 |
| Platelet transfusions | 0.36 | 0.14–0.97 | 0.041 |
| Additional spleen | 1.06 | 0.47–2.37 | 0.889 |
| Perioperative complications | 1.03 | 0.30–3.56 | 0.952 |
| Blood transfusions | 0.22 | 0.02–2.57 | 0.223 |
| Operative time, with every 1 min | 0.99 | 0.98–1.01 | 0.671 |
| Blood loss, with every 1 mL | 0.99 | 0.98–1.01 | 0.696 |
| Time from diagnosis of ITP to procedures, with every 1 month | 0.99 | 0.99–1.00 | 0.396 |
| Symptoms | 0.74 | 0.38–1.45 | 0.376 |
| Preoperative time of conservative treatment, with every 1 month | 0.99 | 0.98–1.02 | 0.230 |
| OR | 95% CI | p-Value | |
|---|---|---|---|
| Age <41 years | 4.49 | 1.66–12.09 | 0.003 |
| BMI < 24.3 kg/m2 | 4.67 | 1.44–15.16 | 0.010 |
| Preoperative platelet count ≥97 × 103/mm3 | 3.50 | 1.30–9.47 | 0.012 |
| Platelet transfusions | 0.75 | 0.21–2.75 | 0.665 |
| OR | 95% CI | p-Value | |
|---|---|---|---|
| Male/Female | 1.44 | 0.49–4.23 | 0.505 |
| Age, with every 1 year | 0.95 | 0.92–0.98 | 0.001 |
| BMI, with every 1 kg/m2 | 0.92 | 0.82–1.03 | 0.131 |
| LOS, with every 1 day | 1.02 | 0.73–1.45 | 0.889 |
| Ultrasound length of spleen, with every cm | 1.15 | 0.90–1.47 | 0.254 |
| Lowest preoperative platelet count, with every 1 × 103/mm3 | 1.02 | 0.97–1.07 | 0.441 |
| Preoperative platelet count, with every 1 × 103/mm3 | 1.03 | 1.00–1.06 | 0.010 |
| Preoperative steroids administration | 0.51 | 0.20–1.32 | 0.162 |
| Preoperative immunoglobulin administration | 0.63 | 0.22–1.80 | 0.635 |
| Platelet transfusions | 0.43 | 0.27–0.71 | 0.048 |
| Additional spleen | 1.67 | 0.46–6.11 | 0.435 |
| Perioperative complications | 2.43 | 0.01–4.65 | 0.981 |
| Blood transfusions | 0.37 | 0.67–1.69 | 0.695 |
| Operative time, with every 1 min | 0.99 | 0.97–1.03 | 0.113 |
| Blood loss, with every 1 mL | 1.00 | 0.99–1.04 | 0.607 |
| Time from diagnosis of ITP to procedures, with every 1 month | 1.00 | 0.99–1.06 | 0.945 |
| Symptoms | 0.98 | 0.38–2.55 | 0.966 |
| Preoperative time of conservative treatment, with every 1 month | 0.99 | 0.98–1.02 | 0.248 |
| OR | 95% CI | p-Value | |
|---|---|---|---|
| Age <42 years | 4.93 | 1.49–16.34 | 0.009 |
| Preoperative platelet count ≥95 × 103/mm3 | 3.45 | 1.03–11.59 | 0.043 |
| Platelet transfusions | 0.69 | 0.23–2.07 | 0.501 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwiatkowska, A.; Radkowiak, D.; Wysocki, M.; Torbicz, G.; Gajewska, N.; Lasek, A.; Kulawik, J.; Budzyński, A.; Pędziwiatr, M. Prognostic Factors for Immune Thrombocytopenic Purpura Remission after Laparoscopic Splenectomy: A Cohort Study. Medicina 2019, 55, 112. https://doi.org/10.3390/medicina55040112
Kwiatkowska A, Radkowiak D, Wysocki M, Torbicz G, Gajewska N, Lasek A, Kulawik J, Budzyński A, Pędziwiatr M. Prognostic Factors for Immune Thrombocytopenic Purpura Remission after Laparoscopic Splenectomy: A Cohort Study. Medicina. 2019; 55(4):112. https://doi.org/10.3390/medicina55040112
Chicago/Turabian StyleKwiatkowska, Anna, Dorota Radkowiak, Michał Wysocki, Grzegorz Torbicz, Natalia Gajewska, Anna Lasek, Jan Kulawik, Andrzej Budzyński, and Michał Pędziwiatr. 2019. "Prognostic Factors for Immune Thrombocytopenic Purpura Remission after Laparoscopic Splenectomy: A Cohort Study" Medicina 55, no. 4: 112. https://doi.org/10.3390/medicina55040112
APA StyleKwiatkowska, A., Radkowiak, D., Wysocki, M., Torbicz, G., Gajewska, N., Lasek, A., Kulawik, J., Budzyński, A., & Pędziwiatr, M. (2019). Prognostic Factors for Immune Thrombocytopenic Purpura Remission after Laparoscopic Splenectomy: A Cohort Study. Medicina, 55(4), 112. https://doi.org/10.3390/medicina55040112





