Synergistic Effects of Plant Derivatives and Conventional Chemotherapeutic Agents: An Update on the Cancer Perspective
Abstract
:1. Introduction
2. Synergy
3. The Isobole Method
- (1)
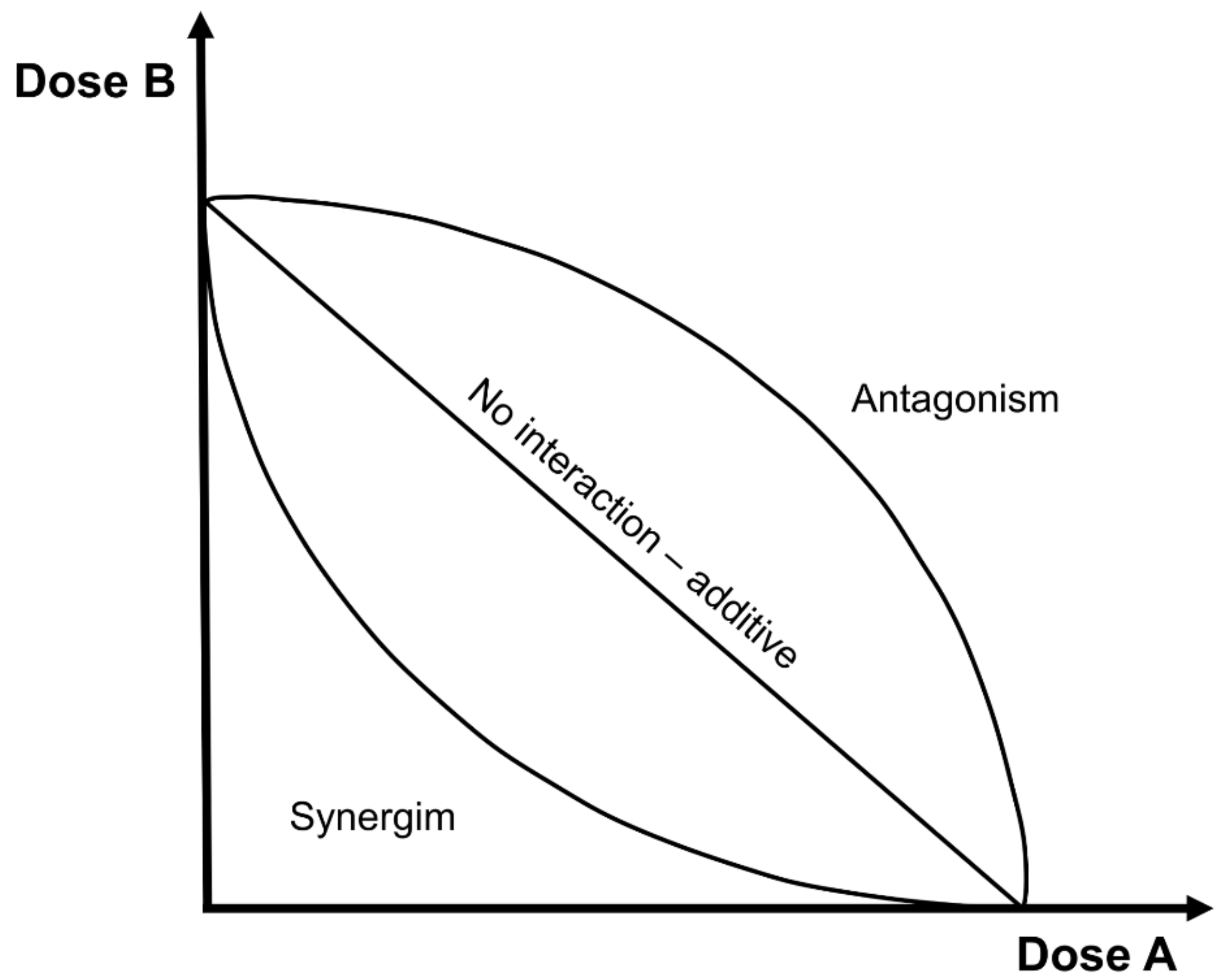
- No interaction curve: according to Berenbaum, this is an additive curve (straight line), in which the effects of 2 compounds are the simple sum of the single effects, and they do not interact.
- (2)
- Synergistic curve: this concave-shaped curve represents the real synergism (or potentiation), where the effects of 2 compounds are more than the simple sum of the single effects when the 2 compounds are given together.
- (3)
- Antagonistic curve: this convex shape curve represents the opposite effects of synergism; i.e., the effects of 2 compounds are less than the simple sum of the single effects.
4. Mechanical Bases of Synergistic Effects
4.1. Synergistic Multi-Target Effects
4.2. Modulation of Pharmacokinetic or Physicochemical Effects
4.3. Interference with Resistance Mechanisms
4.4. Elimination or Neutralization Potential
5. An Introduction to Generic Plant–Drug Interactions
5.1. The Social Problem
5.2. Most Common Examples of Detrimental Interactions
5.3. Most Common Examples of Beneficial Interactions
5.4. Controversial and Incomplete Data on Plant–Drug Interactions
6. Plant–Drug Interaction: Classical Chemotherapeutic Agents and Plant Derivatives
6.1. The Synergy between Classical Chemotherapeutic Agents and Plant Derivatives
6.1.1. Essential Oil Derivatives
6.1.2. Polyphenol Derivatives
6.2. Biochemical Plant Derivatives
7. Concluding Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Doos, L.; Roberts, E.O.; Corp, N.; Kadam, U.T. Multi-drug therapy in chronic condition multimorbidity: A systematic review. Fam. Pract. 2014, 31, 654–663. [Google Scholar] [CrossRef] [PubMed]
- Obodozie-Ofoegbu, O. Pharmacokinetics and Drug Interactions of Herbal Medicines: A Missing Critical Step in the Phytomedicine/Drug Development Process. In Readings in Advanced Pharmacokinetics—Theory, Methods and Applications; IntechOpen: London, UK, 2012. [Google Scholar]
- Katselou, M.G.; Matralis, A.N.; Kourounakis, A.P. Multi-target drug design approaches for multifactorial diseases: From neurodegenerative to cardiovascular applications. Curr. Med. Chem. 2014, 21, 2743–2787. [Google Scholar] [CrossRef]
- Petrovska, B.B. Historical review of medicinal plants’ usage. Pharmacogn. Rev. 2012, 6, 1–5. [Google Scholar] [CrossRef]
- Pautasso, M. Ten simple rules for writing a literature review. PLoS Comput. Biol. 2013, 9, e1003149. [Google Scholar] [CrossRef] [PubMed]
- Buckminster Fuller, R. Operating Manual for Spaceship Earth, 1st ed.; Lars Muller: Baden, Switzerland, 2008; p. 152. [Google Scholar]
- Tennakoon, P.L.K. Studies on Plant Growth Promoting Rhizomicroorganisms of Tea (Camellia sinensis (L.) kuntze) Plants. Master’s Thesis, University of Agriculture Sciences, Dharwad (Institute), Karnataka, India, 2007. [Google Scholar]
- Segen, J.C. Concise Dictionary of Modern Medicine; McGraw-Hill: New York, NY, USA, 2005. [Google Scholar]
- Jonas, W.B. Mosby’s Dictionary of Complementary & Alternative Medicine, 1st ed.; Elsevier Mosby: St. Louis, MO, USA, 2005. [Google Scholar]
- Berenbaum, M.C. Synergy, additivism and antagonism in immunosuppression. A critical review. Clin. Exp. Immunol. 1977, 28, 1–18. [Google Scholar] [PubMed]
- Berenbaum, M.C. Criteria for analyzing interactions between biologically active agents. Adv. Cancer Res. 1981, 35, 269–335. [Google Scholar]
- Berenbaum, M.C. What is synergy? Pharmacol. Rev. 1989, 41, 93–141. [Google Scholar] [PubMed]
- Loewe, S. The problem of synergism and antagonism of combined drugs. Arzneimittel-Forschung 1953, 3, 285–290. [Google Scholar]
- Leyden Webb, J. Enzyme and Metabolic Inhibitors; Academic Pres: New York, NY, USA; London, UK, 1963. [Google Scholar]
- Chou, T.C.; Talalay, P. Quantitative analysis of dose-effect relationships: The combined effects of multiple drugs or enzyme inhibitors. Adv. Enz. Regul. 1984, 22, 27–55. [Google Scholar] [CrossRef]
- Imming, P.; Sinning, C.; Meyer, A. Drugs, their targets and the nature and number of drug targets. Nat. Rev. Drug Discov. 2006, 5, 821–834. [Google Scholar] [CrossRef]
- Butterweck, V.; Jurgenliemk, G.; Nahrstedt, A.; Winterhoff, H. Flavonoids from hypericum perforatum show antidepressant activity in the forced swimming test. Planta Med. 2000, 66, 3–6. [Google Scholar] [CrossRef]
- Hemaiswarya, S.; Kruthiventi, A.K.; Doble, M. Synergism between natural products and antibiotics against infectious diseases. Phytomedicine 2008, 15, 639–652. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.Z.; Zheng, C.S.; Xu, X.J.; Wu, M.X.; Liu, X.X. Potential synergistic and multitarget effect of herbal pair chuanxiong rhizome-paeonia albifora pall on osteoarthritis disease: A computational pharmacology approach. Chin. J. Integr. Med. 2011, 17, 698–703. [Google Scholar] [CrossRef]
- Nagaprashantha, L.D.; Vatsyayan, R.; Singhal, J.; Fast, S.; Roby, R.; Awasthi, S.; Singhal, S.S. Anti-cancer effects of novel flavonoid vicenin-2 as a single agent and in synergistic combination with docetaxel in prostate cancer. Biochem. Pharmacol. 2011, 82, 1100–1109. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, J.D.; Whalley, B.J.; Baker, D.; Pryce, G.; Constanti, A.; Gibbons, S.; Williamson, E.M. Medicinal cannabis: Is Δ9-tetrahydrocannabinol necessary for all its effects? J. Pharm. Pharmacol. 2003, 55, 1687–1694. [Google Scholar] [CrossRef] [PubMed]
- Simmen, U.; Higelin, J.; Berger-Buter, K.; Schaffner, W.; Lundstrom, K. Neurochemical studies with St. John’s wort in vitro. Pharmacopsychiatry 2001, 34, S137–S142. [Google Scholar] [CrossRef]
- Zhang, A.; Sun, H.; Yuan, Y.; Sun, W.; Jiao, G.; Wang, X. An in vivo analysis of the therapeutic and synergistic properties of chinese medicinal formula yin-chen-hao-tang based on its active constituents. Fitoterapia 2011, 82, 1160–1168. [Google Scholar] [CrossRef]
- Haug, K.G.; Weber, B.; Hochhaus, G.; Butterweck, V. Pharmacokinetic evaluation of visnagin and ammi visnaga aqueous extract after oral administration in rats. Planta Med. 2012, 78, 1831–1836. [Google Scholar] [CrossRef]
- Banfield, C.; Gupta, S.; Marino, M.; Lim, J.; Affrime, M. Grapefruit juice reduces the oral bioavailability of fexofenadine but not desloratadine. Clin. Pharmacokinet. 2002, 41, 311–318. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, J.R.; Niu, T.; Gao, S.; Yin, T.; You, M.; Jiang, Z.H.; Hu, M. Inhibition of p-glycoprotein leads to improved oral bioavailability of compound k, an anticancer metabolite of red ginseng extract produced by gut microflora. Drug Metab. Dispos. Biol. Fate Chem. 2012, 40, 1538–1544. [Google Scholar] [CrossRef]
- Yoshida, N.; Takada, T.; Yamamura, Y.; Adachi, I.; Suzuki, H.; Kawakami, J. Inhibitory effects of terpenoids on multidrug resistance-associated protein 2- and breast cancer resistance protein-mediated transport. Drug Metab. Dispos. Biol. Fate Chem. 2008, 36, 1206–1211. [Google Scholar] [CrossRef] [PubMed]
- Eumkeb, G.; Chukrathok, S. Synergistic activity and mechanism of action of ceftazidime and apigenin combination against ceftazidime-resistant enterobacter cloacae. Phytomedicine 2013, 20, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Rosato, A.; Vitali, C.; de Laurentis, N.; Armenise, D.; Antonietta Milillo, M. Antibacterial effect of some essential oils administered alone or in combination with norfloxacin. Phytomedicine 2007, 14, 727–732. [Google Scholar] [CrossRef] [PubMed]
- Tamaki, H.; Satoh, H.; Hori, S.; Ohtani, H.; Sawada, Y. Inhibitory effects of herbal extracts on breast cancer resistance protein (BCRP) and structure-inhibitory potency relationship of isoflavonoids. Drug Metab. Pharmacokinet. 2010, 25, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Alhusainy, W.; Paini, A.; Punt, A.; Louisse, J.; Spenkelink, A.; Vervoort, J.; Delatour, T.; Scholz, G.; Schilter, B.; Adams, T.; et al. Identification of nevadensin as an important herb-based constituent inhibiting estragole bioactivation and physiology-based biokinetic modeling of its possible in vivo effect. Toxicol. Appl. Pharmacol. 2010, 245, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.H.; Cheng, Y.C. Old formula, new rx: The journey of phy906 as cancer adjuvant therapy. J. Ethnopharmacol. 2012, 140, 614–623. [Google Scholar] [CrossRef] [PubMed]
- Rasool, M.; Iqbal, J.; Malik, A.; Ramzan, H.S.; Qureshi, M.S.; Asif, M.; Qazi, M.H.; Kamal, M.A.; Chaudhary, A.G.; Al-Qahtani, M.H.; et al. Hepatoprotective effects of Silybum marianum (silymarin) and Glycyrrhiza glabra (glycyrrhizin) in combination: A possible synergy. eCAM 2014, 2014, 641597. [Google Scholar]
- Nahin, R.L.; Barnes, P.M.; Stussman, B.J.; Bloom, B. Costs of complementary and alternative medicine (cam) and frequency of visits to cam practitioners: United states, 2007. Natl. Health Stat. Rep. 2009, 18, 1–14. [Google Scholar]
- Izzo, A.A.; Hoon-Kim, S.; Radhakrishnan, R.; Williamson, E.M. A critical approach to evaluating clinical efficacy, adverse events and drug interactions of herbal remedies. Phytother. Res. 2016, 30, 691–700. [Google Scholar] [CrossRef]
- Zhou, S.; Gao, Y.; Jiang, W.; Huang, M.; Xu, A.; Paxton, J.W. Interactions of herbs with cytochrome p450. Drug Metab. Rev. 2003, 35, 35–98. [Google Scholar] [CrossRef]
- Ramos-Esquivel, A.; Viquez-Jaikel, A.; Fernandez, C. Potential drug-drug and herb-drug interactions in patients with cancer: A prospective study of medication surveillance. J. Oncol. Pract. 2017, 13, e613–e622. [Google Scholar] [CrossRef]
- Alsanad, S.M.; Williamson, E.M.; Howard, R.L. Cancer patients at risk of herb/food supplement-drug interactions: A systematic review. Phytother. Res. 2014, 28, 1749–1755. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Lee, S.L.; Yu, L.X. Mechanistic approaches to predicting oral drug absorption. AAPS J. 2009, 11, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Fung, W.T.; Subramaniam, G.; Lee, J.; Loh, H.M.; Leung, P.H. Assessment of extracts from red yeast rice for herb-drug interaction by in-vitro and in-vivo assays. Sci. Rep. 2012, 2, 298. [Google Scholar] [CrossRef] [PubMed]
- Awortwe, C.; Fasinu, P.S.; Rosenkranz, B. Application of caco-2 cell line in herb-drug interaction studies: Current approaches and challenges. J. Pharm. Pharma. Sci. 2014, 17, 1–19. [Google Scholar] [CrossRef]
- Hediger, M.A.; Romero, M.F.; Peng, J.B.; Rolfs, A.; Takanaga, H.; Bruford, E.A. The abcs of solute carriers: Physiological, pathological and therapeutic implications of human membrane transport proteinsintroduction. Eur. J. Physiol. 2004, 447, 465–468. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Sweet, D.H. Competitive inhibition of human organic anion transporters 1 (slc22a6), 3 (slc22a8) and 4 (slc22a11) by major components of the medicinal herb Salvia miltiorrhiza (danshen). Drug Metab. Pharmacokinet. 2013, 28, 220–228. [Google Scholar] [CrossRef]
- Mandery, K.; Bujok, K.; Schmidt, I.; Keiser, M.; Siegmund, W.; Balk, B.; Konig, J.; Fromm, M.F.; Glaeser, H. Influence of the flavonoids apigenin, kaempferol, and quercetin on the function of organic anion transporting polypeptides 1a2 and 2b1. Biochem. Pharmacol. 2010, 80, 1746–1753. [Google Scholar] [CrossRef]
- Zhou, S.; Chan, E.; Pan, S.Q.; Huang, M.; Lee, E.J. Pharmacokinetic interactions of drugs with st john’s wort. J. Psychopharmacol. 2004, 18, 262–276. [Google Scholar] [CrossRef]
- Diamond, B.J.; Bailey, M.R. Ginkgo biloba: Indications, mechanisms, and safety. Psychiatr. Clin. N. Am. 2013, 36, 73–83. [Google Scholar] [CrossRef]
- Naccarato, M.; Yoong, D.; Gough, K. A potential drug-herbal interaction between ginkgo biloba and efavirenz. J. Int. Assoc. Phys. AIDS Care 2012, 11, 98–100. [Google Scholar] [CrossRef] [PubMed]
- Dolton, M.J.; Roufogalis, B.D.; McLachlan, A.J. Fruit juices as perpetrators of drug interactions: The role of organic anion-transporting polypeptides. Clin. Pharmacol. Ther. 2012, 92, 622–630. [Google Scholar] [CrossRef]
- Wang, J.; Xu, R.; Jin, R.; Chen, Z.; Fidler, J.M. Immunosuppressive activity of the chinese medicinal plant tripterygium wilfordii. I. Prolongation of rat cardiac and renal allograft survival by the pg27 extract and immunosuppressive synergy in combination therapy with cyclosporine. Transplantation 2000, 70, 447–455. [Google Scholar] [CrossRef]
- Tung, Y.T.; Wu, J.H.; Huang, C.C.; Peng, H.C.; Chen, Y.L.; Yang, S.C.; Chang, S.T. Protective effect of acacia confusa bark extract and its active compound gallic acid against carbon tetrachloride-induced chronic liver injury in rats. Food Chem. Toxicol. 2009, 47, 1385–1392. [Google Scholar] [CrossRef]
- Bakare-Odunola, M.T.; Mustapha, K.B.; Garba, M.; Obodozie, O.O.; Enemali, I.S. The influence of nifadin, niprisan and niprd/92/001/1–1 (AM-1) on the pharmacokinetics of metronidazole in rats. Eur. J. Drug Metab. Pharmacokinet. 2010, 35, 55–58. [Google Scholar] [CrossRef] [PubMed]
- Asdaq, S.M.; Inamdar, M.N. Potential of garlic and its active constituent, s-allyl cysteine, as antihypertensive and cardioprotective in presence of captopril. Phytomedicine 2010, 17, 1016–1026. [Google Scholar] [CrossRef]
- Gharagozloo, M.; Moayedi, B.; Zakerinia, M.; Hamidi, M.; Karimi, M.; Maracy, M.; Amirghofran, Z. Combined therapy of silymarin and desferrioxamine in patients with beta-thalassemia major: A randomized double-blind clinical trial. Fundam. Clin. Pharmacol. 2009, 23, 359–365. [Google Scholar] [CrossRef]
- Obodozie, O.O.; Adelakun, T.A.; Tarfa, F.D.; Tijani, A.Y.; Busu, S.M.; Inyang, U.S. Evaluation of the effect of co-administration of selected first line antiretroviral agents with an investigational herbal immune booster in healthy rats. In Proceedings of the World Congress in Association with the AAPS Annual Meeting and Exposition, New Orleans, LA, USA, 15 November 2010. Abstract #3804. [Google Scholar]
- Sharifi-Rad, M.; Varoni, E.M.; Salehi, B.; Sharifi-Rad, J.; Matthews, K.R.; Ayatollahi, S.A.; Kobarfard, F.; Ibrahim, S.A.; Mnayer, D.; Zakaria, Z.A.; et al. Plants of the genus zingiber as a source of bioactive phytochemicals: From tradition to pharmacy. Molecules 2017, 22, 2145. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Williams, K.M.; Liauw, W.S.; Ammit, A.J.; Roufogalis, B.D.; Duke, C.C.; Day, R.O.; McLachlan, A.J. Effect of ginkgo and ginger on the pharmacokinetics and pharmacodynamics of warfarin in healthy subjects. Br. J. Clin. Pharmacol. 2005, 59, 425–432. [Google Scholar] [CrossRef]
- Vaes, L.P.; Chyka, P.A. Interactions of warfarin with garlic, ginger, ginkgo, or ginseng: Nature of the evidence. Ann. Pharmacother. 2000, 34, 1478–1482. [Google Scholar] [CrossRef]
- Liu, M.Z.; Zhang, Y.L.; Zeng, M.Z.; He, F.Z.; Luo, Z.Y.; Luo, J.Q.; Wen, J.G.; Chen, X.P.; Zhou, H.H.; Zhang, W. Pharmacogenomics and herb-drug interactions: Merge of future and tradition. ECAM 2015, 2015, 321091. [Google Scholar] [CrossRef] [PubMed]
- Izzo, A.A. Interactions between herbs and conventional drugs: Overview of the clinical data. Med. Princip. Pract. 2012, 21, 404–428. [Google Scholar] [CrossRef] [PubMed]
- Kufe, D.D.; Pollock, R.E.; Weichselbaum, R.R.; Bast, R.C.; Gansler, T.S.; Holland, J.F.; Frei, E. Holland-Frei Cancer Medicine, 6th ed.; B.C. Decker Publications: Hamilton, ON, Canada, 2003. [Google Scholar]
- American Cancer Society. Available online: http://www.cancer.org/research/cancerfactsstatistics/ (accessed on 31 December 2018).
- Gupta, D.; Lis, C.G.; Birdsall, T.C.; Grutsch, J.F. The use of dietary supplements in a community hospital comprehensive cancer center: Implications for conventional cancer care. Support. Care Cancer 2005, 13, 912–919. [Google Scholar] [CrossRef] [PubMed]
- Gautam, N.; Mantha, A.K.; Mittal, S. Essential oils and their constituents as anticancer agents: A mechanistic view. BioMed. Res. Int. 2014, 2014, 154106. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Bae, H.C.; Park, E.J.; Lee, C.R.; Kim, B.J.; Lee, S.; Park, H.H.; Kim, S.J.; So, I.; Kim, T.W.; et al. Geraniol inhibits prostate cancer growth by targeting cell cycle and apoptosis pathways. Biochem. Biophys. Res. Commun. 2011, 407, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Zou, B.; Li, Q.Q.; Zhao, J.; Li, J.M.; Cuff, C.F.; Reed, E. Beta-elemene and taxanes synergistically induce cytotoxicity and inhibit proliferation in ovarian cancer and other tumor cells. Anticancer Res. 2013, 33, 929–940. [Google Scholar]
- Liu, Y.; Jiang, Z.Y.; Zhou, Y.L.; Qiu, H.H.; Wang, G.; Luo, Y.; Liu, J.B.; Liu, X.W.; Bu, W.Q.; Song, J.; et al. β-elemene regulates endoplasmic reticulum stress to induce the apoptosis of NSCLC cells through PERK/IRE1α/ATF6 pathway. Biomed. Pharmacother. 2017, 93, 490–497. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Priyani, A.; Sadrieh, L.; Brahmbhatt, K.; Ahmed, M.; Sharma, C. Concurrent sulforaphane and eugenol induces differential effects on human cervical cancer cells. Integr. Cancer Ther. 2012, 11, 154–165. [Google Scholar] [CrossRef] [PubMed]
- Yi, J.L.; Shi, S.; Shen, Y.L.; Wang, L.; Chen, H.Y.; Zhu, J.; Ding, Y. Myricetin and methyl eugenol combination enhances the anticancer activity, cell cycle arrest and apoptosis induction of cis-platin against hela cervical cancer cell lines. Int. J. Clin. Exp. Pathol. 2015, 8, 1116–1127. [Google Scholar]
- Legault, J.; Pichette, A. Potentiating effect of beta-caryophyllene on anticancer activity of alpha-humulene, isocaryophyllene and paclitaxel. J. Pharm. Pharmacol. 2007, 59, 1643–1647. [Google Scholar] [CrossRef]
- Ambroz, M.; Matouskova, P.; Skarka, A.; Zajdlova, M.; Zakova, K.; Skalova, L. The effects of selected sesquiterpenes from myrica rubra essential oil on the efficacy of doxorubicin in sensitive and resistant cancer cell lines. Molecules 2017, 22, 1021. [Google Scholar] [CrossRef] [PubMed]
- Rabi, T.; Bishayee, A. d-limonene sensitizes docetaxel-induced cytotoxicity in human prostate cancer cells: Generation of reactive oxygen species and induction of apoptosis. J. Carcinog. 2009, 8, 9. [Google Scholar]
- Effenberger-Neidnicht, K.; Schobert, R. Combinatorial effects of thymoquinone on the anti-cancer activity of doxorubicin. Cancer Chemother. Pharmacol. 2011, 67, 867–874. [Google Scholar] [CrossRef]
- Sakalar, C.; Izgi, K.; Iskender, B.; Sezen, S.; Aksu, H.; Cakir, M.; Kurt, B.; Turan, A.; Canatan, H. The combination of thymoquinone and paclitaxel shows anti-tumor activity through the interplay with apoptosis network in triple-negative breast cancer. Tumour Biol. 2016, 37, 4467–4477. [Google Scholar] [CrossRef]
- Dirican, A.; Atmaca, H.; Bozkurt, E.; Erten, C.; Karaca, B.; Uslu, R. Novel combination of docetaxel and thymoquinone induces synergistic cytotoxicity and apoptosis in du-145 human prostate cancer cells by modulating pi3k-akt pathway. Clin. Trans. Oncol. 2015, 17, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Sutton, K.M.; Greenshields, A.L.; Hoskin, D.W. Thymoquinone, a bioactive component of black caraway seeds, causes g1 phase cell cycle arrest and apoptosis in triple-negative breast cancer cells with mutant p53. Nutr. Cancer 2014, 66, 408–418. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.C.; Lee, N.H.; Hsu, H.H.; Ho, T.J.; Tu, C.C.; Hsieh, D.J.; Lin, Y.M.; Chen, L.M.; Kuo, W.W.; Huang, C.Y. Thymoquinone induces caspase-independent, autophagic cell death in CPT-11-resistant lovo colon cancer via mitochondrial dysfunction and activation of JNK and p38. J. Agric. Food Chem. 2015, 63, 1540–1546. [Google Scholar] [CrossRef]
- Chen, M.C.; Lee, N.H.; Hsu, H.H.; Ho, T.J.; Tu, C.C.; Chen, R.J.; Lin, Y.M.; Viswanadha, V.P.; Kuo, W.W.; Huang, C.Y. Inhibition of nf-kappab and metastasis in irinotecan (CPT-11)-resistant lovo colon cancer cells by thymoquinone via JNK and p38. Environ. Toxicol. 2017, 32, 669–678. [Google Scholar] [CrossRef]
- Khalife, R.; El-Hayek, S.; Tarras, O.; Hodroj, M.H.; Rizk, S. Antiproliferative and proapoptotic effects of topotecan in combination with thymoquinone on acute myelogenous leukemia. Clin. Lymph. Myel. Leuk. 2014, 14, S46–S55. [Google Scholar] [CrossRef] [PubMed]
- Khalife, R.; Hodroj, M.H.; Fakhoury, R.; Rizk, S. Thymoquinone from nigella sativa seeds promotes the antitumor activity of noncytotoxic doses of topotecan in human colorectal cancer cells in vitro. Planta Med. 2016, 82, 312–321. [Google Scholar] [CrossRef] [PubMed]
- Mileo, A.M.; Miccadei, S. Polyphenols as modulator of oxidative stress in cancer disease: New therapeutic strategies. Oxid. Med. Cell. Longev. 2016, 2016, 6475624. [Google Scholar] [CrossRef]
- Burns, J.; Yokota, T.; Ashihara, H.; Lean, M.E.; Crozier, A. Plant foods and herbal sources of resveratrol. J. Agric. Food Chem. 2002, 50, 3337–3340. [Google Scholar] [CrossRef] [PubMed]
- Kweon, S.H.; Song, J.H.; Kim, T.S. Resveratrol-mediated reversal of doxorubicin resistance in acute myeloid leukemia cells via downregulation of MRP1 expression. Biochem. Biophys. Res. Commun. 2010, 395, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, A.; Sethi, G.; Vadhan-Raj, S.; Bueso-Ramos, C.; Takada, Y.; Gaur, U.; Nair, A.S.; Shishodia, S.; Aggarwal, B.B. Resveratrol inhibits proliferation, induces apoptosis, and overcomes chemoresistance through down-regulation of stat3 and nuclear Factor-κB-regulated antiapoptotic and cell survival gene products in human multiple myeloma cells. Blood 2007, 109, 2293–2302. [Google Scholar] [CrossRef]
- Salehi, B.; Mishra, A.P.; Nigam, M.; Sener, B.; Kilic, M.; Sharifi-Rad, M.; Fokou, P.V.T.; Martins, N.; Sharifi-Rad, J. Resveratrol: A double-edged sword in health benefits. Biomedicines 2018, 6, 91. [Google Scholar] [CrossRef]
- Spagnuolo, C.; Russo, G.L.; Orhan, I.E.; Habtemariam, S.; Daglia, M.; Sureda, A.; Nabavi, S.F.; Devi, K.P.; Loizzo, M.R.; Tundis, R.; et al. Genistein and cancer: Current status, challenges, and future directions. Adv. Nutr. 2015, 6, 408–419. [Google Scholar] [CrossRef]
- Mohammad, R.M.; Banerjee, S.; Li, Y.; Aboukameel, A.; Kucuk, O.; Sarkar, F.H. Cisplatin-induced antitumor activity is potentiated by the soy isoflavone genistein in BXPC-3 pancreatic tumor xenografts. Cancer 2006, 106, 1260–1268. [Google Scholar] [CrossRef]
- Papazisis, K.T.; Kalemi, T.G.; Zambouli, D.; Geromichalos, G.D.; Lambropoulos, A.F.; Kotsis, A.; Boutis, L.L.; Kortsaris, A.H. Synergistic effects of protein tyrosine kinase inhibitor genistein with camptothecins against three cell lines in vitro. Cancer Lett. 2006, 233, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Zhang, Y.; Ali, S.; Bhuiyan, M.; Wang, Z.; Chiao, P.J.; Philip, P.A.; Abbruzzese, J.; Sarkar, F.H. Molecular evidence for increased antitumor activity of gemcitabine by genistein in vitro and in vivo using an orthotopic model of pancreatic cancer. Cancer Res. 2005, 65, 9064–9072. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, H.; Zhang, W.; Shao, C.; Xu, P.; Shi, C.H.; Shi, J.G.; Li, Y.M.; Fu, Q.; Xue, W.; et al. Genistein sensitizes bladder cancer cells to HCPT treatment in vitro and in vivo via ATM/NF-κB/IKK pathway-induced apoptosis. PLoS ONE 2013, 8, e50175. [Google Scholar] [CrossRef] [PubMed]
- Sonaa, E.; Usha, S.; Ja In, J. An ex vivo study of selenium, genistein on the morphological and nuclear changes in anticancer drug-induced apoptosis in human peripheral blood lymphocytes. BioFactors 2013, 39, 279–293. [Google Scholar] [CrossRef]
- Chen, J.; He, Z.M.; Wang, F.L.; Zhang, Z.S.; Liu, X.Z.; Zhai, D.D.; Chen, W.D. Curcumin and its promise as an anticancer drug: An analysis of its anticancer and antifungal effects in cancer and associated complications from invasive fungal infections. Eur. J. Pharmacol. 2016, 772, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Stojanović-Radić, Z.; Matejić, J.; Sharifi-Rad, M.; Anil Kumar, N.V.; Martins, N.; Sharifi-Rad, J. The therapeutic potential of curcumin: A review of clinical trials. Eur. J. Med. Chem. 2019, 527–545. [Google Scholar] [CrossRef] [PubMed]
- Dhandapani, K.M.; Mahesh, V.B.; Brann, D.W. Curcumin suppresses growth and chemoresistance of human glioblastoma cells via ap-1 and nfkappab transcription factors. J. Neurochem. 2007, 102, 522–538. [Google Scholar] [CrossRef] [PubMed]
- Montopoli, M.; Ragazzi, E.; Froldi, G.; Caparrotta, L. Cell-cycle inhibition and apoptosis induced by curcumin and cisplatin or oxaliplatin in human ovarian carcinoma cells. Cell Prolif. 2009, 42, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Du, B.; Jiang, L.; Xia, Q.; Zhong, L. Synergistic inhibitory effects of curcumin and 5-fluorouracil on the growth of the human colon cancer cell line HT-29. Chemotherapy 2006, 52, 23–28. [Google Scholar] [CrossRef]
- Ke, C.S.; Liu, H.S.; Yen, C.H.; Huang, G.C.; Cheng, H.C.; Huang, C.Y.; Su, C.L. Curcumin-induced aurora-a suppression not only causes mitotic defect and cell cycle arrest but also alters chemosensitivity to anticancer drugs. J. Nutr. Biochem. 2014, 25, 526–539. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Shishodia, S.; Takada, Y.; Banerjee, S.; Newman, R.A.; Bueso-Ramos, C.E.; Price, J.E. Curcumin suppresses the paclitaxel-induced nuclear factor-kappab pathway in breast cancer cells and inhibits lung metastasis of human breast cancer in nude mice. Clin. Cancer Res. 2005, 11, 7490–7498. [Google Scholar] [CrossRef]
- D’Andrea, G. Quercetin: A flavonol with multifaceted therapeutic applications? Fitoterapia 2015, 106, 256–271. [Google Scholar] [CrossRef]
- Demiroglu-Zergeroglu, A.; Basara-Cigerim, B.; Kilic, E.; Yanikkaya-Demirel, G. The investigation of effects of quercetin and its combination with cisplatin on malignant mesothelioma cells in vitro. J. Biomed. Biotechnol. 2010, 2010, 851589. [Google Scholar] [CrossRef]
- Zanini, C.; Giribaldi, G.; Mandili, G.; Carta, F.; Crescenzio, N.; Bisaro, B.; Doria, A.; Foglia, L.; di Montezemolo, L.C.; Timeus, F.; et al. Inhibition of heat shock proteins (HSP) expression by quercetin and differential doxorubicin sensitization in neuroblastoma and ewing’s sarcoma cell lines. J. Neurochem. 2007, 103, 1344–1354. [Google Scholar] [CrossRef] [PubMed]
- Jakubowicz-Gil, J.; Langner, E.; Wertel, I.; Piersiak, T.; Rzeski, W. Temozolomide, quercetin and cell death in the moggccm astrocytoma cell line. Chem. Biol. Int. 2010, 188, 190–203. [Google Scholar] [CrossRef] [PubMed]
- Du, G.; Lin, H.; Yang, Y.; Zhang, S.; Wu, X.; Wang, M.; Ji, L.; Lu, L.; Yu, L.; Han, G. Dietary quercetin combining intratumoral doxorubicin injection synergistically induces rejection of established breast cancer in mice. Int. Immunopharmacol. 2010, 10, 819–826. [Google Scholar] [CrossRef]
- Nurcahyanti, A.D.; Wink, M. Cytotoxic potentiation of vinblastine and paclitaxel by l-canavanine in human cervical cancer and hepatocellular carcinoma cells. Phytomedicine 2015, 22, 1232–1237. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Chen, Q. Antitumor activities of rauwolfia vomitoria extract and potentiation of gemcitabine effects against pancreatic cancer. Integr. Cancer Ther. 2014, 13, 217–225. [Google Scholar] [CrossRef]
- Yu, J.; Ma, Y.; Drisko, J.; Chen, Q. Antitumor activities of rauwolfia vomitoria extract and potentiation of carboplatin effects against ovarian cancer. Curr. Ther. Res. Clin. Exp. 2013, 75, 8–14. [Google Scholar] [CrossRef]
- Einbond, L.S.; Mighty, J.; Kashiwazaki, R.; Figueroa, M.; Jalees, F.; Acuna, U.M.; le Gendre, O.; Foster, D.A.; Kennelly, E.J. Garcinia benzophenones inhibit the growth of human colon cancer cells and synergize with sulindac sulfide and turmeric. Anti Cancer Agents Med. Chem. 2013, 13, 1540–1550. [Google Scholar] [CrossRef]
- Kapadia, G.J.; Rao, G.S.; Ramachandran, C.; Iida, A.; Suzuki, N.; Tokuda, H. Synergistic cytotoxicity of red beetroot (Beta vulgaris L.) extract with doxorubicin in human pancreatic, breast and prostate cancer cell lines. J. Complement. Integr. Med. 2013, 10, 113–122. [Google Scholar] [CrossRef]
- Salehi, B.; Valussi, M.; Jugran, A.K.; Martorell, M.; Ramírez-Alarcón, K.; Stojanović-Radić, Z.Z.; Antolak, H.; Kręgiel, D.; Mileski, K.S.; Sharifi-Rad, M.; et al. Nepeta species: From farm to food applications and phytotherapy. Trends Food Sci. Technol. 2018, 80, 104–122. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Fokou, P.V.T.; Sharopov, F.; Martorell, M.; Ademiluyi, A.O.; Rajkovic, J.; Salehi, B.; Martins, N.; Iriti, M.; Sharifi-Rad, J. Antiulcer agents: From plant extracts to phytochemicals in healing promotion. Molecules 2018, 23, 1751. [Google Scholar] [CrossRef]
- Mishra, A.P.; Saklani, S.; Salehi, B.; Parcha, V.; Sharifi-Rad, M.; Milella, L.; Iriti, M.; Sharifi-Rad, J.; Srivastava, M. Satyrium nepalense, a high altitude medicinal orchid of indian himalayan region: Chemical profile and biological activities of tuber extracts. Cell. Mol. Biol. 2018, 64, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Sharopov, F.; Martorell, M.; Rajkovic, J.; Ademiluyi, A.O.; Sharifi-Rad, M.; Fokou, P.V.T.; Martins, N.; Iriti, M.; Sharifi-Rad, J. Phytochemicals in helicobacter pylori infections: What are we doing now? Int. J. Mol. Sci. 2018, 19, 2361. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Roberts, T.H.; Matthews, K.R.; Bezerra, C.F.; Morais-Braga, M.F.B.; Coutinho, H.D.M.; Sharopov, F.; Salehi, B.; Yousaf, Z.; Sharifi-Rad, M.; et al. Ethnobotany of the genus taraxacum—phytochemicals and antimicrobial activity. Phytother. Res. 2018, 32, 2131–2145. [Google Scholar] [CrossRef] [PubMed]
- U.S. National Institutes of Health. Available online: https://clinicaltrials.Gov/ (accessed on 31 December 2018).
| Mechanism | Plants Involved | References |
|---|---|---|
| Synergistic multi-target effects | Herbal pair Chuanxiong rhizome and Paeonia albifora | [19] |
| Ocimum sanctum flavonoid vicenin-2 | [20] | |
| Cannabis extract delta9-trans-tetrahydrocannabinol | [21] | |
| St. John’s wort (Hypericum perforatum) | [22] | |
| Pharmacokinetic or physicochemical effect modulations | Yin-Chen-Hao-Tang (YCHT), a Chinese herbal formula (Herba artemisiae Yinchenhao + fructus gardeniae gasminoidis + radix et rhizoma rhei) | [23] |
| Ammi visnaga aqueous extract | [24] | |
| Hypericum perforatum flavonoids | [17] | |
| Grapefruit juice (Citrus × paradise) | [25] | |
| Panax ginseng | [26] | |
| Interference with resistance mechanisms | Seven commercially available terpenoids | [27] |
| Three commercially available flavonoids (apigenin, quercetin, naringenin) | [28] | |
| Pelargonium graveolens essential oil | [29] | |
| Nine herbal extracts and 23 isoflavonoids | [30] | |
| Elimination or neutralization potential | Ocimum basilicum constituent nevadensin | [31] |
| PHY906, a mixture of Scutellaria baicalensis, Glycyrrhiza uralensis, Paeonia lactiflora, Ziziphus jujube | [32] | |
| Silybum marianum (Silymarin) and Glycyrrhiza glabra (Glycyrrhizin) extracts | [33] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pezzani, R.; Salehi, B.; Vitalini, S.; Iriti, M.; Zuñiga, F.A.; Sharifi-Rad, J.; Martorell, M.; Martins, N. Synergistic Effects of Plant Derivatives and Conventional Chemotherapeutic Agents: An Update on the Cancer Perspective. Medicina 2019, 55, 110. https://doi.org/10.3390/medicina55040110
Pezzani R, Salehi B, Vitalini S, Iriti M, Zuñiga FA, Sharifi-Rad J, Martorell M, Martins N. Synergistic Effects of Plant Derivatives and Conventional Chemotherapeutic Agents: An Update on the Cancer Perspective. Medicina. 2019; 55(4):110. https://doi.org/10.3390/medicina55040110
Chicago/Turabian StylePezzani, Raffaele, Bahare Salehi, Sara Vitalini, Marcello Iriti, Felipe Andrés Zuñiga, Javad Sharifi-Rad, Miquel Martorell, and Natália Martins. 2019. "Synergistic Effects of Plant Derivatives and Conventional Chemotherapeutic Agents: An Update on the Cancer Perspective" Medicina 55, no. 4: 110. https://doi.org/10.3390/medicina55040110
APA StylePezzani, R., Salehi, B., Vitalini, S., Iriti, M., Zuñiga, F. A., Sharifi-Rad, J., Martorell, M., & Martins, N. (2019). Synergistic Effects of Plant Derivatives and Conventional Chemotherapeutic Agents: An Update on the Cancer Perspective. Medicina, 55(4), 110. https://doi.org/10.3390/medicina55040110










