The Effects of High-Intensity Interval Exercise and Hypoxia on Cognition in Sedentary Young Adults
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Experimental Procedure
2.3. Maximal Graded Exercise Test
2.4. High-Intensity Interval Exercise
2.5. Cognitive Task Measurement
2.6. Habitual Physical Activity Assessment
2.7. Statistical Analyses
3. Results
3.1. Exercise Data and Habitual Physical Activity
3.2. Physiological Parameters
3.3. Cognitive Task
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhang, Y.; Li, C.; Zou, L.; Liu, X.; Song, W. The Effects of Mind-Body Exercise on Cognitive Performance in Elderly: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2018, 15, 2791. [Google Scholar] [CrossRef] [PubMed]
- Loprinzi, D.; Frith, E.; Edwards, K.; Sng, E.; Ashpole, N. The effects of exercise on memory function among young to middle-aged adults: Systematic review and recommendations for future research. Am. J. Health Promot. 2018, 32, 691–704. [Google Scholar] [CrossRef] [PubMed]
- Loprinzi, D.; Blough, J.; Ryu, S.; Kang, M. Experimental effects of exercise on memory function among mild cognitive impairment: Systematic review and meta-analysis. Phys. Sportsmed. 2018, 22, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Akatsuka, K.; Yamashiro, K.; Nakazawa, S.; Mitsuzono, R.; Maruyama, A. Acute aerobic exercise influences the inhibitory process in the go/no-go task in humans. Neurosci. Lett. 2015, 600, 80–84. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.; Huang, T.; Tsang, T.; Pan, Z.; Wang, C.; Liu, Y.; Sun, L.; Wang, H. Hard martial arts for cognitive function across the lifespan: A systematic review. Arch. Budo 2018, 14, 41–58. [Google Scholar]
- Frith, E.; Sng, E.; Loprinzi, D. Randomized controlled trial evaluating the temporal effects of high-intensity exercise on learning, short-term and long-term memory, and prospective memory. Eur. J. Neurosci. 2017, 46, 2557–2564. [Google Scholar] [CrossRef] [PubMed]
- Erickson, K.I.; Voss, M.W.; Prakash, R.S.; Basak, C.; Szabo, A.; Chaddock, L.; White, S.M. Exercise training increases size of hippocampus and improves memory. Proc. Natl. Acad. Sci. USA 2011, 108, 3017–3022. [Google Scholar] [CrossRef] [PubMed]
- Winter, B.; Breitenstein, C.; Mooren, F.C.; Voelker, K.; Fobker, M.; Lechtermann, A.; Floel, A. High impact running improves learning. Neurobiol. Learn. Mem. 2007, 87, 597–609. [Google Scholar] [CrossRef] [PubMed]
- Carro, E.; Trejo, J.L.; Busiguina, S.; Torres-Aleman, I. Circulating insulin-like growth factor I mediates the protective effects of physical exercise against brain insults of different etiology and anatomy. J. Neurosci. 2001, 21, 5678–5684. [Google Scholar] [CrossRef]
- Weston, M.; Taylor, K.L.; Batterham, A.M.; Hopkins, W.G. Effects of low-volume high-intensity interval training (HIT) on fitness in adults: A meta-analysis of controlled and non-controlled trials. Sports Med. 2014, 44, 1005–1017. [Google Scholar] [CrossRef]
- Kong, Z.; Sun, S.; Liu, M.; Shi, Q. Short-Term High-Intensity Interval Training on Body Composition and Blood Glucose in Overweight and Obese Young Women. J. Diabetes Res. 2016, 2, 1–9. [Google Scholar] [CrossRef]
- Trapp, E.G.; Chisholm, D.J.; Freund, J.; Boutcher, S.H. The effects of high-intensity intermittent exercise training on fat loss and fasting insulin levels of young women. Int. J. Obes. 2008, 32, 684–691. [Google Scholar] [CrossRef]
- Alves, C.R.; Tessaro, V.H.; Teixeira, L.A.; Murakava, K.; Roschel, H.; Gualano, B.; Takito, M.Y. Influence of acute high-intensity aerobic interval exercise bout on selective attention and short-term memory tasks. Percept. Mot. Skills 2014, 118, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, S.T.; Takenaka, S.; Tanaka, D.; Takeuchi, T.; Hamaoka, T.; Hashimoto, T. Greater impact of acute high-intensity interval exercise on post-exercise executive function compared to moderate-intensity continuous exercise. Physiol. Behav. 2016, 155, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Kashihara, K.; Maruyama, T.; Murota, M.; Nakahara, Y. Positive effects of acute and moderate physical exercise on cognitive function. J. Physiol. Anthropol. 2009, 28, 155–164. [Google Scholar] [CrossRef]
- Loprinzi, D. Intensity-specific effects of acute exercise on human memory function: Considerations for the timing of exercise and the type of memory. Health Promot. Perspect. 2018, 8, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Kong, Z.; Zang, Y.; Hu, Y. Normobaric hypoxia training causes more weight loss than normoxia training after a 4-week residential camp for obese young adults. Sleep Breath. 2014, 18, 591–597. [Google Scholar] [CrossRef] [PubMed]
- Kong, Z.; Shi, Q.; Nie, J.; Tong, T.K.; Song, L.; Yi, L.; Hu, Y. High-intensity interval training in normobaric hypoxia improves cardiorespiratory fitness in overweight chinese young women. Front. Physiol. 2017, 8, 175. [Google Scholar] [CrossRef] [PubMed]
- Navarrete-Opazo, A.; Mitchell, G.S. Therapeutic potential of intermittent hypoxia: A matter of dose. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2014, 307, R1181–R1197. [Google Scholar] [CrossRef]
- Ochi, G.; Yamada, Y.; Hyodo, K.; Suwabe, K.; Fukuie, T.; Byun, K.; Soya, H. Neural basis for reduced executive performance with hypoxic exercise. NeuroImage 2018, 171, 75–83. [Google Scholar] [CrossRef]
- Willis, S.J.; Alvarez, L.; Millet, G.P.; Borrani, F. Changes in Muscle and Cerebral Deoxygenation and Perfusion during Repeated Sprints in Hypoxia to Exhaustion. Front. Physiol. 2017, 8, 846. [Google Scholar] [CrossRef] [PubMed]
- De Aquino-Lemos, V.; Santos, R.V.; Antunes, H.K.M.; Lira, F.S.; Bittar, I.G.L.; Caris, A.V.; Mello, M.T. Acute physical exercise under hypoxia improves sleep, mood and reaction time. Physiol. Behav. 2016, 154, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Turner, C.E.; Barker-Collo, S.L.; Connell, C.J.; Gant, N. Acute hypoxic gas breathing severely impairs cognition and task learning in humans. Physiol. Behav. 2015, 142, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Ando, S.; Hatamoto, Y.; Sudo, M.; Kiyonaga, A.; Tanaka, H.; Higaki, Y. The effects of exercise under hypoxia on cognitive function. PLoS ONE 2013, 8, e63630. [Google Scholar] [CrossRef] [PubMed]
- Seo, Y.; Burns, K.; Fennell, C.; Kim, J.H.; Gunstad, J.; Glickman, E.; McDaniel, J. The influence of exercise on cognitive performance in normobaric hypoxia. High Alt. Med. Biol. 2015, 16, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Czuba, M.; Waskiewicz, Z.; Zajac, A.; Poprzecki, S.; Cholewa, J.; Roczniok, R. The effects of intermittent hypoxic training on aerobic capacity and endurance performance in cyclists. J. Sports Sci. Med. 2011, 10, 175. [Google Scholar] [CrossRef] [PubMed]
- Meeuwsen, T.; Hendriksen, I.J.; Holewijn, M. Training-induced increases in sea-level performance are enhanced by acute intermittent hypobaric hypoxia. Eur. J. Appl. Physiol. 2001, 84, 283–290. [Google Scholar] [CrossRef]
- Komiyama, T.; Sudo, M.; Higaki, Y.; Kiyonaga, A.; Tanaka, H.; Ando, S. Does moderate hypoxia alter working memory and executive function during prolonged exercise? Physiol. Behav. 2015, 139, 290–296. [Google Scholar] [CrossRef]
- Smith, M.; Tallis, J.; Miller, A.; Clarke, N.D.; Guimaraes-Ferreira, L.; Duncan, M.J. The effect of exercise intensity on cognitive performance during short duration treadmill Running. J. Hum. Kinet. 2016, 51, 27–35. [Google Scholar] [CrossRef]
- Hester, R.; Fassbender, C.; Garavan, H. Individual differences in error processing: A review and reanalysis of three event-related fMRI studies using the GO/NOGO task. Cereb. Cortex 2004, 14, 986–994. [Google Scholar] [CrossRef]
- Heatherton, T.F. Neuroscience of self and self-regulation. Annu. Rev. Psychol. 2011, 62, 363–390. [Google Scholar] [CrossRef] [PubMed]
- Langenecker, S.A.; Zubieta, J.K.; Young, E.A.; Akil, H.; Nielson, K.A. A task to manipulate attentional load, set-shifting, and inhibitory control: Convergent validity and test-retest reliability of the Parametric Go/No-Go Test. J. Clin. Exp. Neuropsychol. 2007, 29, 842–853. [Google Scholar] [CrossRef] [PubMed]
- Duncan, S.; Schofield, G.; Duncan, K.; Hinkckson, A. Effects of age, walking speed, and body composition on pedometer accuracy in children. Res. Q. Exerc. Sport 2007, 78, 420–428. [Google Scholar] [CrossRef]
- Gomez-Pinilla, F.; Hillman, C. The influence of exercise on cognitive abilities. Compr. Physiol. 2013, 3, 403–428. [Google Scholar] [CrossRef]
- McMorris, T.; Hale, J. Differential effects of differing intensities of acute exercise on speed and accuracy of cognition: Meta-analytical investigation. Brain Cogn. 2012, 80, 338–351. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.K.; Labban, J.; Gapin, J.; Etnier, J.L. The effects of acute exercise on cognitive performance: A meta-analysis. Brain Res. 2012, 1453, 87–101. [Google Scholar] [CrossRef] [PubMed]
- Byun, K.; Hyodo, K.; Suwabe, K.; Ochi, G.; Sakairi, Y.; Kato, M.; Soya, H. Positive effect of acute mild exercise on executive function via arousal-related prefrontal activations: An fNIRS study. NeuroImage 2014, 98, 336–345. [Google Scholar] [CrossRef] [PubMed]
- Kamijo, K.; Nishihira, Y.; Hatta, A.; Kaneda, T.; Kida, T.; Higashiura, T.; Kuroiwa, K. Changes in arousal level by differential exercise intensity. Clin. Neurophysiol. 2004, 115, 2693–2698. [Google Scholar] [CrossRef]
- Komiyama, T.; Katayama, K.; Sudo, M.; Ishida, K.; Higaki, Y.; Ando, S. Cognitive function during exercise under severe hypoxia. Sci. Rep. 2017, 7, 10000. [Google Scholar] [CrossRef]
- Bärtsch, P.; Swenson, E.R. Acute high-altitude illnesses. N. Engl. J. Med. 2013, 368, 2294–2302. [Google Scholar] [CrossRef]
- Virués-Ortega, J.; Buela-Casal, G.; Garrido, E.; Alcázar, B. Neuropsychological functioning associated with high-altitude exposure. Neuropsychol. Rev. 2004, 14, 197–224. [Google Scholar] [CrossRef] [PubMed]
- Curtelin, D.; Morales-Alamo, D.; Torres-Peralta, R.; Rasmussen, P.; Martin-Rincon, M.; Perez-Valera, M.; Sheel, A.W. Cerebral blood flow, frontal lobe oxygenation and intra-arterial blood pressure during sprint exercise in normoxia and severe acute hypoxia in humans. J. Cereb. Blood Flow Metab. 2018, 38, 136–150. [Google Scholar] [CrossRef] [PubMed]
- Moraine, J.; Lamotte, M.; Berré, J.; Niset, G.; Leduc, A.; Naeijel, R. Relationship of middle cerebral artery blood flow velocity to intensity during dynamic exercise in normal subjects. Eur. J. Appl. Physiol. Occup. Physiol. 1993, 67, 35–38. [Google Scholar] [CrossRef] [PubMed]
| Male | Female | All | ||||
|---|---|---|---|---|---|---|
| Age (year) | 24.1 | ±1.4 | 23.6 | ±3.3 | 23.9 | ±2.5 |
| Height (cm) | 175.2 | ±6.8 | 159.0 | ±3.8 * | 167.1 | ±9.8 |
| Weight (kg) | 69.9 | ±13.5 | 51.2 | ±5.0 * | 60.5 | ±13.8 |
| BMI (kg·m−2) | 22.7 | ±3.9 | 20.2 | ±1.5 | 21.5 | ±3.2 |
| VO2peak (mL·kg−1·min−1) | 36.5 | ±7.0 | 27.4 | ±3.9 * | 31.9 | ±7.2 |
| NOR | HYP | |||
|---|---|---|---|---|
| Peak Power (w/kg) | 6.8 | ±2.2 | 6.8 | ±2.4 |
| Average Power (w/kg) | 4.5 | ±0.6 | 4.4 | ±0.6 |
| Fatigue Index (%) | 45.8 | ±16.4 | 43.4 | ±17.7 |
| Exercise Effect | Condition Effect | Interaction Effect | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pre | Exe | p Partial η2 | p Partial η2 | p Partial η2 | ||||||
| HR (bpm) | ||||||||||
| NOR | 72 | ±6 * | 138 | ±12 | <0.001 | 0.974 | 0.085 | 0.156 | 0.275 | 0.066 |
| HYP | 73 | ±7 * | 141 | ±12 | ||||||
| RPE | ||||||||||
| NOR | 6 | ±1 * | 14 | ±2 | <0.001 | 0.953 | 0.250 | 0.069 | 0.837 | 0.002 |
| HYP | 7 | ±1 * | 14 | ±2 | ||||||
| SaO2 (%) | ||||||||||
| NOR | 98 | ±1 | 98 | ±2 | <0.001 | 0.724 | <0.001 | 0.550 | <0.001 | 0.450 |
| HYP | 96 | ±3 *† | 92 | ±3 † | ||||||
| Exercise Effect | Condition Effect | Interaction Effect | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pre-CON | Exe-CON | p Partial η2 | p Partial η2 | p Partial η2 | ||||||
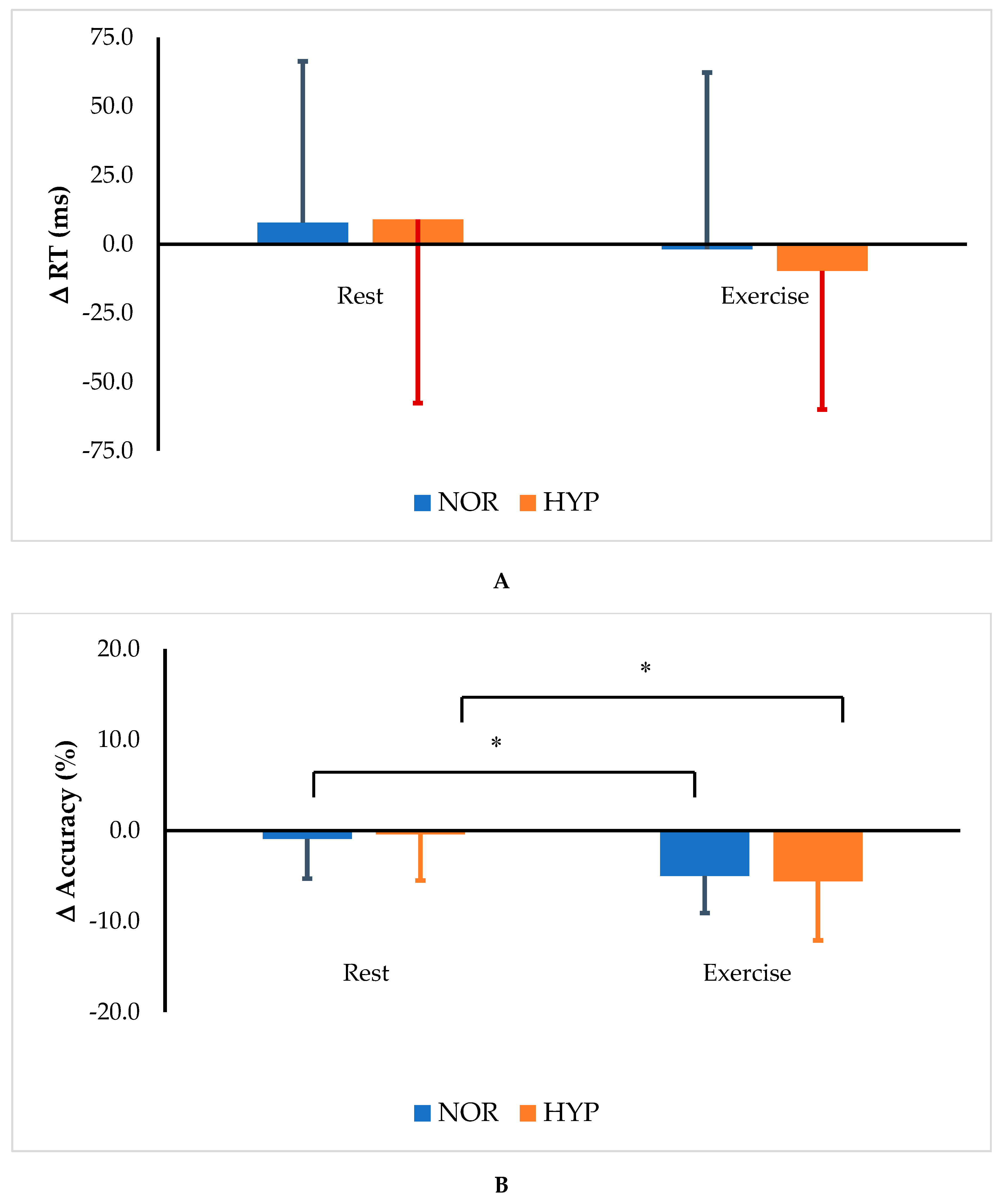
| Δ RT (ms) | ||||||||||
| NOR | 7.8 | ±58.5 | −1.8 | ±64.0 | 0.204 | 0.083 | 0.782 | 0.004 | 0.514 | 0.023 |
| HYP | 9.0 | ±66.7 | −9.7 | ±50.3 | ||||||
| Δ Accuracy (%) | ||||||||||
| NOR | −0.9 | ±4.4 * | −5.0 | ±4.1 | 0.001 | 0.467 | 0.972 | 0.000 | 0.537 | 0.020 |
| HYP | −0.4 | ±5.1 * | −5.6 | ±6.5 | ||||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, S.; Loprinzi, P.D.; Guan, H.; Zou, L.; Kong, Z.; Hu, Y.; Shi, Q.; Nie, J. The Effects of High-Intensity Interval Exercise and Hypoxia on Cognition in Sedentary Young Adults. Medicina 2019, 55, 43. https://doi.org/10.3390/medicina55020043
Sun S, Loprinzi PD, Guan H, Zou L, Kong Z, Hu Y, Shi Q, Nie J. The Effects of High-Intensity Interval Exercise and Hypoxia on Cognition in Sedentary Young Adults. Medicina. 2019; 55(2):43. https://doi.org/10.3390/medicina55020043
Chicago/Turabian StyleSun, Shengyan, Paul D. Loprinzi, Hongwei Guan, Liye Zou, Zhaowei Kong, Yang Hu, Qingde Shi, and Jinlei Nie. 2019. "The Effects of High-Intensity Interval Exercise and Hypoxia on Cognition in Sedentary Young Adults" Medicina 55, no. 2: 43. https://doi.org/10.3390/medicina55020043
APA StyleSun, S., Loprinzi, P. D., Guan, H., Zou, L., Kong, Z., Hu, Y., Shi, Q., & Nie, J. (2019). The Effects of High-Intensity Interval Exercise and Hypoxia on Cognition in Sedentary Young Adults. Medicina, 55(2), 43. https://doi.org/10.3390/medicina55020043





