A View of Human Immunodeficiency Virus Infections in the North-West Region of Romania
Abstract
:1. Introduction
2. Materials and Methods
Statistical Analysis
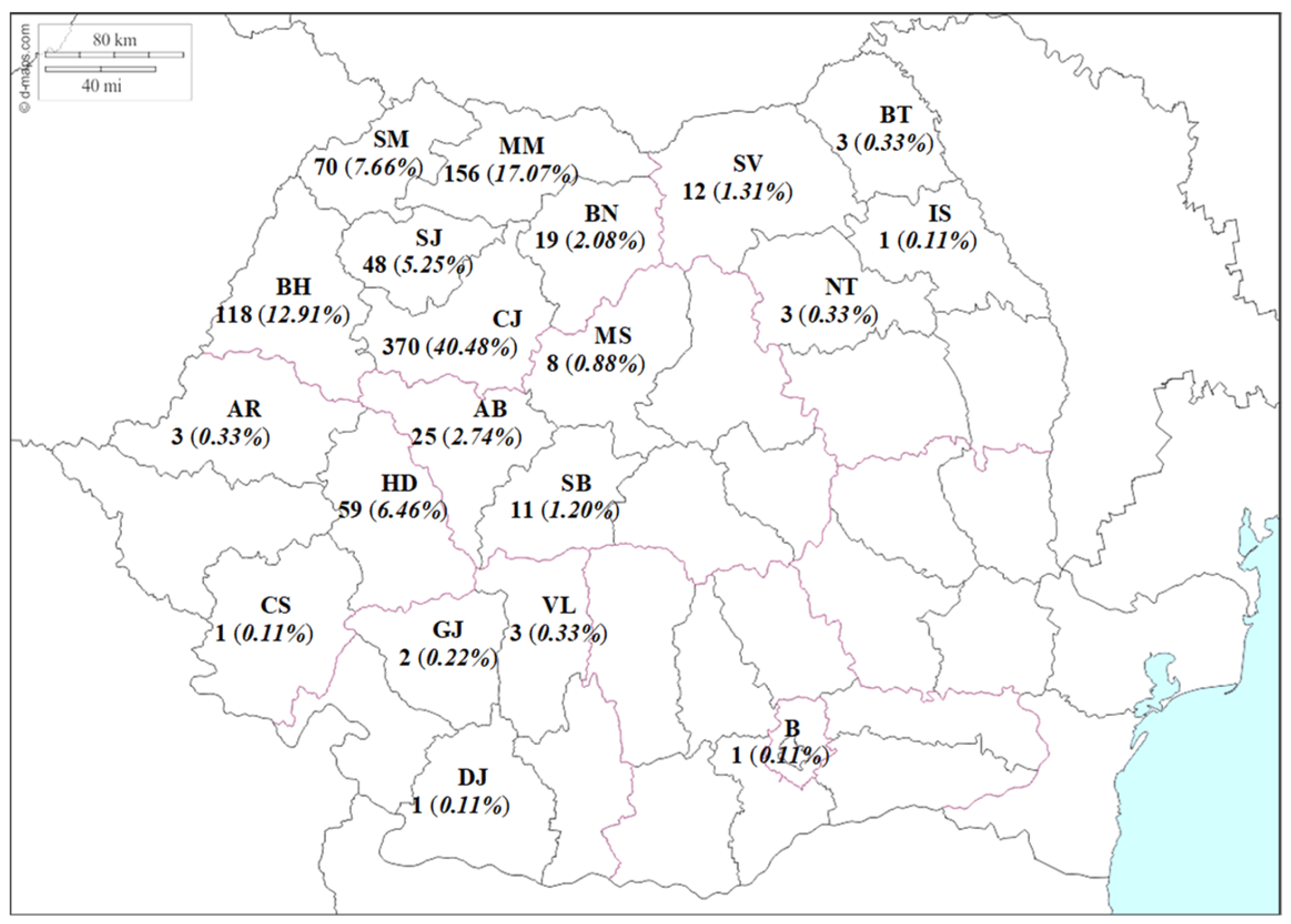
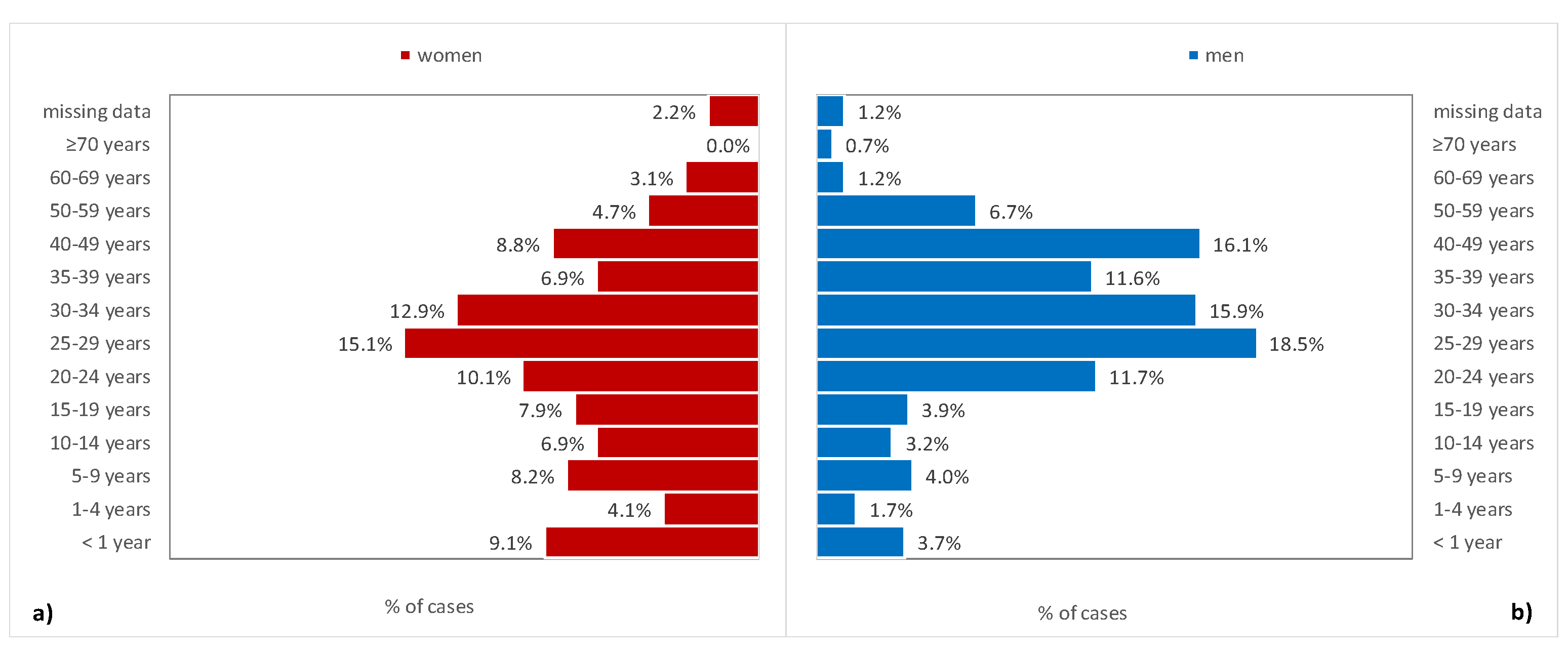
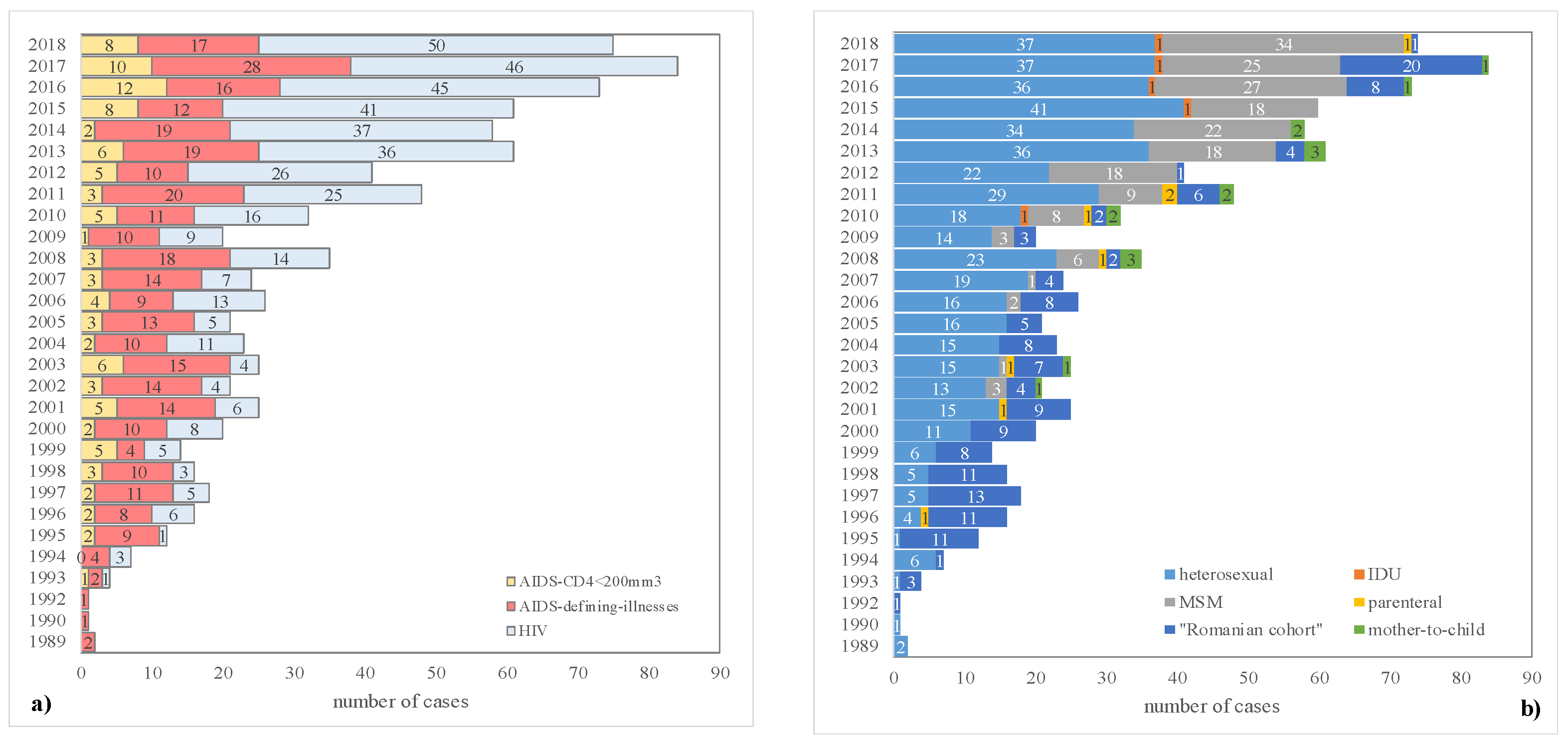
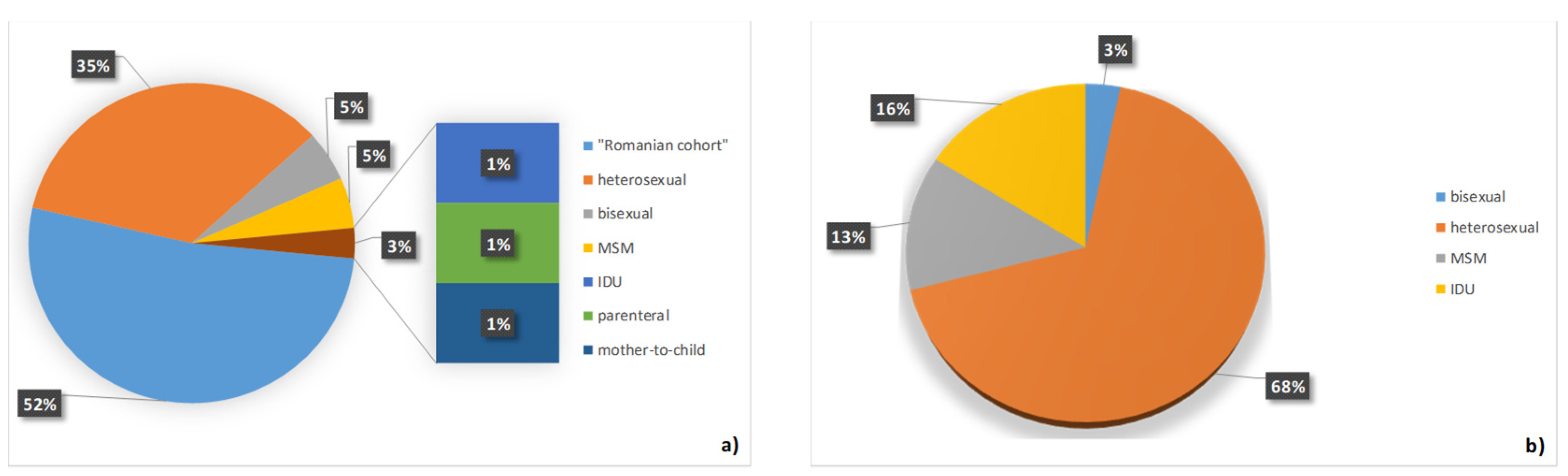
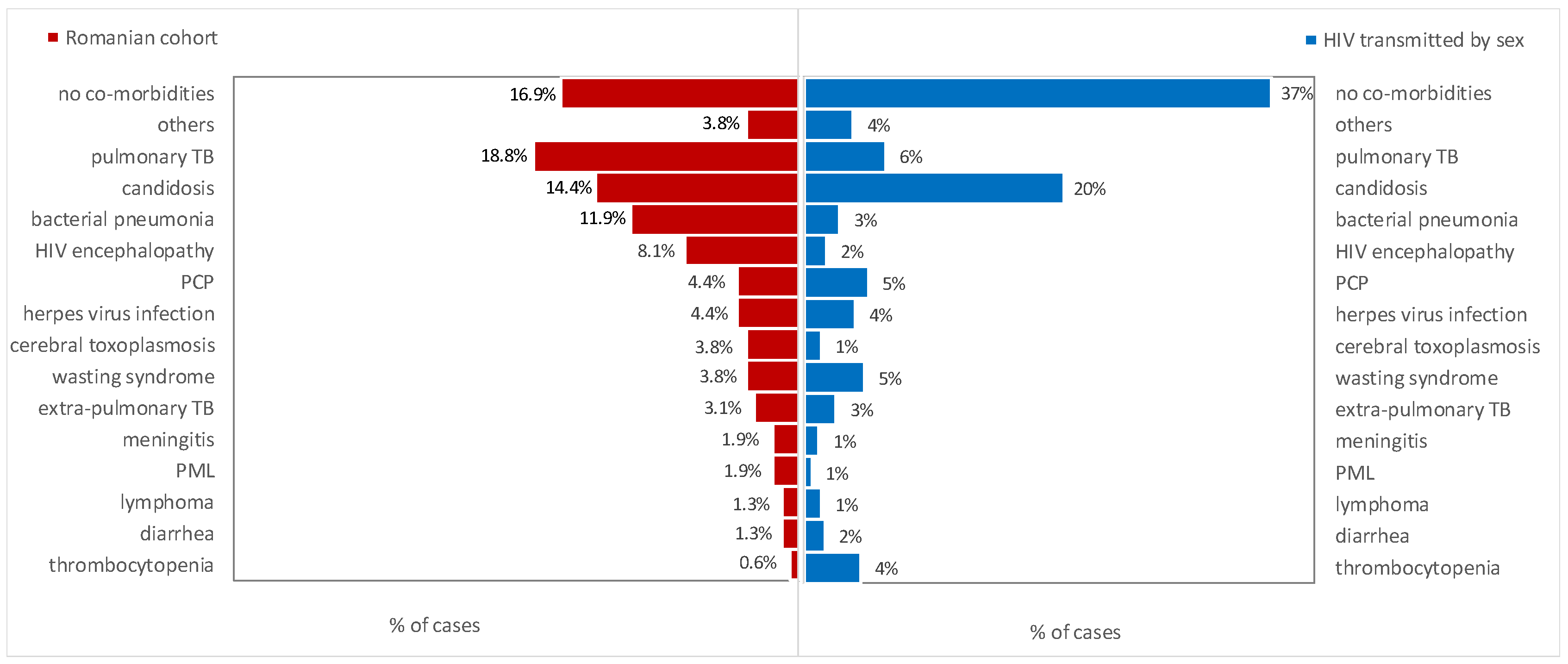
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- HIV Gov. Available online: https://www.hiv.gov/hiv-basics/overview/about-hiv-and-aids/what-are-hiv-and-aids (accessed on 26 January 2019).
- Cohen, J.; Powderly, W. Infectious Diseases, 2nd ed.; Butterworth-Heinemann: Oxford, UK, 2003; pp. 1197–1204. [Google Scholar]
- Laut, K.G.; Shepherd, L.; Gottfredsson, M.; Sedlacek, D.; Knysz, B.; Begovac, J.; Radoi, R.; Schmied, B.; Chkhartishvili, N.; Florence, E.; et al. Variation in antiretroviral treatment coverage and virological suppression among three HIV key populations. AIDS 2018, 32, 2807–2819. [Google Scholar] [CrossRef] [PubMed]
- Castro, K.G.; Ward, J.W.; Slutsker, L.; Buehler, J.W.; Jaffe, H.W.; Berkelman, R.L.; Curran, J.W. 1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR Recomm. Rep. 1992, 41, 1–19. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control, WHO Regional Office for Europe. HIV/AIDS Surveillance in Europe 2018–2017 Data; WHO Regional Office for Europe: Copenhagen, Denmark, 2018; Available online: https://ecdc.europa.eu/sites/portal/files/documents/hiv-aids-surveillance-europe-2018.pdf (accessed on 12 March 2019).
- Balayan, T.; Oprea, C.; Yurin, O.; Jevtovic, D.; Begovac, J.; Lakatos, B.; Sedlacek, D.; Karpov, I.; Horban, A.; Kowalska, J.D. Euro-guidelines in Central and Eastern Europe Network Group. Euro-guidelines in Central and Eastern Europe Network Group. People who inject drugs remain hard-to-reach population across all HIV continuum stages in Central, Eastern and South Eastern Europe—Data from Euro-guidelines in Central and Eastern Europe Network. Infect. Dis. 2019, 51, 277–286. [Google Scholar]
- ECDC Special Report Continuum of HIV Care Monitoring Implementation of the Dublin Declaration on Partnership to Fight HIV/AIDS in Europe and Central Asia: 2018 Progress Report; ECDC: Stockholm, Sweden, 2018; Available online: https://ecdc.europa.eu/sites/portal/files/documents/HIV-continuum-of-care-monitoring-dublin-declaration-progress-report-2018.pdf (accessed on 15 September 2019).
- Hersh, B.S.; Popovici, F.; Apetrei, R.C.; Zolotusca, L.; Beldescu, N.; Calomfirescu, A.; Jezek, Z.; Oxtoby, M.J.; Gromyko, A.; Heymann, D.L. Acquired immunodeficiency syndrome in Romania. Lancet 1991, 338, 645–649. [Google Scholar] [PubMed]
- Manolescu, L.S.; Temereanca, A.; Ruta, S. HIV-1 circulating subtypes in Romania. Roum. Arch. Microbiol. Immunol. 2013, 72, 121–134. [Google Scholar] [PubMed]
- Paraschiv, S.; Foley, B.; Otelea, D. Diversity of HIV-1 subtype C strains isolated in Romania. Infect. Genet. Evolut. 2011, 11, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Niculescu, I.; Paraschiv, S.; Paraskevis, D.; Abagiu, A.; Batan, I.; Banica, L.; Otelea, D. Recent HIV-1 Outbreak among Intravenous Drug Users in Romania: Evidence for Cocirculation of CRF14_BG and Subtype F1 Strains. AIDS Res. Hum. Retrovir. 2015, 31, 488–495. [Google Scholar] [CrossRef] [PubMed]
- De Wit, S.; Battegay, M.; D’Arminio Monforte, A.; Lundgren, J.D.; Oprea, C.; Antinori, A.; Bhagani, S.; Fätkenheuer, G.; Friis-Moller, N.; Furrer, H.; et al. European AIDS Clinical Society Second Standard of Care Meeting, Brussels 16–17 November 2016: A summary. HIV Med. 2018, 19, 77–80. [Google Scholar] [CrossRef] [PubMed]
- Schweitzer, A.M.; Bogdan, M.; Corduneanu, A.; Ciocea, I. Role of pretest counseling sessions on increasing subjective knowledge about HIV and hepatitis transmission among the beneficiaries of a free of charge, voluntary counseling and testing program in Constanta, Romania. HIV Med. 2018, 19, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Oprea, C.; Ianache, I.; Calistru, P.I.; Nica, M.; Ruta, S.; Smith, C.; Lipman, M. Increasing incidence of HIV—Associated tuberculosis in Romanian injecting drug users. HIV Med. 2018, 19, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Gheorghiță, V.; Conea, I.F.; Radu, A.M.C.; Ștefan, I.; Mărdărescu, M.; Petrea, S.; Streinu-Cercel, A. Epidemiological trends and therapeutic challenges of malignancies in adult HIV-1-infected patients receiving combination antiretroviral therapy in a tertiary hospital from Romania: An observational retrospective study. J. Infect. Public Health 2019, 12, 182–189. [Google Scholar] [CrossRef]
- HIV Infection and AIDS. Public Health Guidance on HIV, Hepatitis B and C Testing in the EU/EEA. Available online: https://ecdc.europa.eu/en/publications-data/public-health-guidance-hiv-hepatitis-b-and-c-testing-eueea (accessed on 10 January 2019).
- Jäntschi, L.; Bolboacă, S.D. Exact Probabilities and Confidence Limits for Binomial Samples: Applied to the Difference between Two Proportions. Sci. World J. 2010, 10, 865–878. [Google Scholar] [CrossRef]
- Greig, S.L.; Deeks, E.D. Abacavir/dolutegravir/lamivudine single-tablet regimen: A review of its use in HIV-1 infection. Drugs 2015, 75, 503–514. [Google Scholar] [CrossRef] [PubMed]
- Stellbrink, H.J.; Arribas, J.R.; Stephens, J.L.; Albrecht, H.; Sax, P.E.; Maggiolo, F.; Creticos, C.; Martorell, C.T.; Wei, X.; Acosta, R.; et al. Co-formulated bictegravir, emtricitabine, and tenofovir alafenamide versus dolutegravir with emtricitabine and tenofovir alafenamide for initial treatment of HIV-1 infection: Week 96 results from a randomised, double-blind, multicentre, phase 3, non-inferiority trial. Lancet HIV 2019, 6, 364–372. [Google Scholar]
- Kharsany, A.B.M.; Karim, Q.A. HIV Infection and AIDS in Sub-Saharan Africa: Current Status, Challenges and Opportunities. Open AIDS J. 2016, 10, 34–48. [Google Scholar] [CrossRef] [PubMed]
- Baugher, A.R.; Beer, L.; Fagan, J.L.; Mattson, C.L.; Freedman, M.; Skarbinski, J.; Shouse, R.L. Medical Monitoring Project. Prevalence of Internalized HIV-Related Stigma Among HIV-Infected Adults in Care, United States, 2011–2013. AIDS Behav. 2017, 21, 2600–2608. [Google Scholar] [CrossRef] [PubMed]
- Ragonnet-Cronin, M.; Hodcroft, E.B.; Wertheim, J.O. Understanding disclosed and cryptic HIV transmission risk via genetic analysis: What are we missing and when does it matter? Curr. Opin. HIV AIDS 2019, 14, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Kusejko, K.; Kadelka, C.; Marzel, A.; Battegay, M.; Bernasconi, E.; Calmy, A.; Cavassini, M.; Hoffmann, M.; Böni, J.; Yerly, S.; et al. Inferring the age difference in HIV transmission pairs by applying phylogenetic methods on the HIV transmission network of the Swiss HIV Cohort Study. Virus Evolut. 2018, 4. [Google Scholar] [CrossRef] [PubMed]
- Ragonnet-Cronin, M.; Hu, Y.W.; Morris, S.R.; Sheng, Z.; Poortinga, K.; Wertheim, J.O. HIV transmission networks among transgender women in Los Angeles County, CA, USA: A phylogenetic analysis of surveillance data. Lancet HIV 2019, 6, 164–172. [Google Scholar] [CrossRef]
- Juganariu, G.; Mihalache, D.; Miftode, E.; Teodor, A.; Teodor, D.; Manciuc, C.; Dorobat, C. Characteristics of co infection with hepatitis B virus among Romanian patients infected with human immunodeficiency virus. Rev. Med. Chir. Soc. Med. Nat. Iași 2014, 118, 339–345. [Google Scholar] [PubMed]
| Characteristic | Men (n = 596) | Women (n = 318) | p-value |
|---|---|---|---|
| Age, years* a | 26 (11 to 34) | 30 (23 to 39) | <0.0001 |
| HIV transmission, no (%) b | |||
| heterosexual | 291 (48.8) | 185 (58.2) | 0.0072 |
| MSM | 195 (32.6) | 0 (0.0) | <0.0001 |
| Romanian cohort | 100 (16.8) | 128 (40.3) | <0.0001 |
| parenteral non IDU | 5 (0.5) | 3 (0.9) | 0.4698 |
| IDU | 5 (0.8) | 1 (0.3) | 0.3446 |
| unknown | 1 (0.2) | 0 (0.0) | 0.4621 |
| Stage of HIV infection, no (%) c | <0.0001 | ||
| Seroconverters | 23 (3.9) | 28 (8.8) | |
| HIV | 318 (53.4) | 110 (34.6) | |
| AIDS | 255 (42.8) | 180 (56.6) | |
| Co-morbidities, no (%) b | |||
| candidosis | 98 (16.4) | 70 (22.0) | 0.0372 |
| pulmonary TB | 48 (8.1) | 38 (11.9) | 0.0611 |
| Pneumocystis jirovecii pneumonia | 29 (4.9) | 13 (4.1) | 0.5832 |
| bacterial pneumonia | 23 (3.9) | 18 (5.7) | 0.2124 |
| herpes virus infection | 27 (4.5) | 14 (4.4) | 0.9444 |
| thrombocytopenia | 23 (3.9) | 9 (2.8) | 0.3899 |
| HIV encephalopathy | 15 (2.5) | 11 (3.5) | 0.3866 |
| cerebral toxoplasmosis | 6 (1.0) | 9 (2.8) | 0.0404 |
| lymphoma | 6 (1.0) | 6 (1.9) | 0.2549 |
| diarrhea | 11 (1.8) | 1 (0.3) | 0.0545 |
| extra-pulmonary TB | 9 (1.5) | 3 (0.9) | 0.4441 |
| meningitis | 6 (1.0) | 5 (1.6) | 0.4291 |
| PML | 6 (1.0) | 1 (0.3) | 0.2447 |
| lymphadenopathy | 5 (0.8) | 2 (0.6) | 0.7352 |
| others | 19 (3.2) | 15 (4.7) | 0.7228 |
| Hepatitis co-infections, no (%) b | |||
| Hepatitis B | 50 (8.4) | 48 (15.1) | 0.0018 |
| Hepatitis C | 20 (3.4) | 11 (3.5) | 0.9370 |
| Status (December 2018), no (%) c | 0.0439 | ||
| Active follow-up | 455 (76.3) | 221 (69.5) | |
| Deceased | 91 (15.3) | 69 (21.7) | |
| Lost to follow-up | 50 (8.4) | 28 (8.8) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jianu, C.; Bolboacă, S.D.; Topan, A.V.; Filipescu, I.; Jianu, M.E.; Itu-Mureşan, C. A View of Human Immunodeficiency Virus Infections in the North-West Region of Romania. Medicina 2019, 55, 765. https://doi.org/10.3390/medicina55120765
Jianu C, Bolboacă SD, Topan AV, Filipescu I, Jianu ME, Itu-Mureşan C. A View of Human Immunodeficiency Virus Infections in the North-West Region of Romania. Medicina. 2019; 55(12):765. https://doi.org/10.3390/medicina55120765
Chicago/Turabian StyleJianu, Cristian, Sorana D. Bolboacă, Adriana Violeta Topan, Irina Filipescu, Mihaela Elena Jianu, and Corina Itu-Mureşan. 2019. "A View of Human Immunodeficiency Virus Infections in the North-West Region of Romania" Medicina 55, no. 12: 765. https://doi.org/10.3390/medicina55120765
APA StyleJianu, C., Bolboacă, S. D., Topan, A. V., Filipescu, I., Jianu, M. E., & Itu-Mureşan, C. (2019). A View of Human Immunodeficiency Virus Infections in the North-West Region of Romania. Medicina, 55(12), 765. https://doi.org/10.3390/medicina55120765






