Management of Direct Oral Anticoagulants in Patients with Atrial Fibrillation Undergoing Cardioversion
Abstract
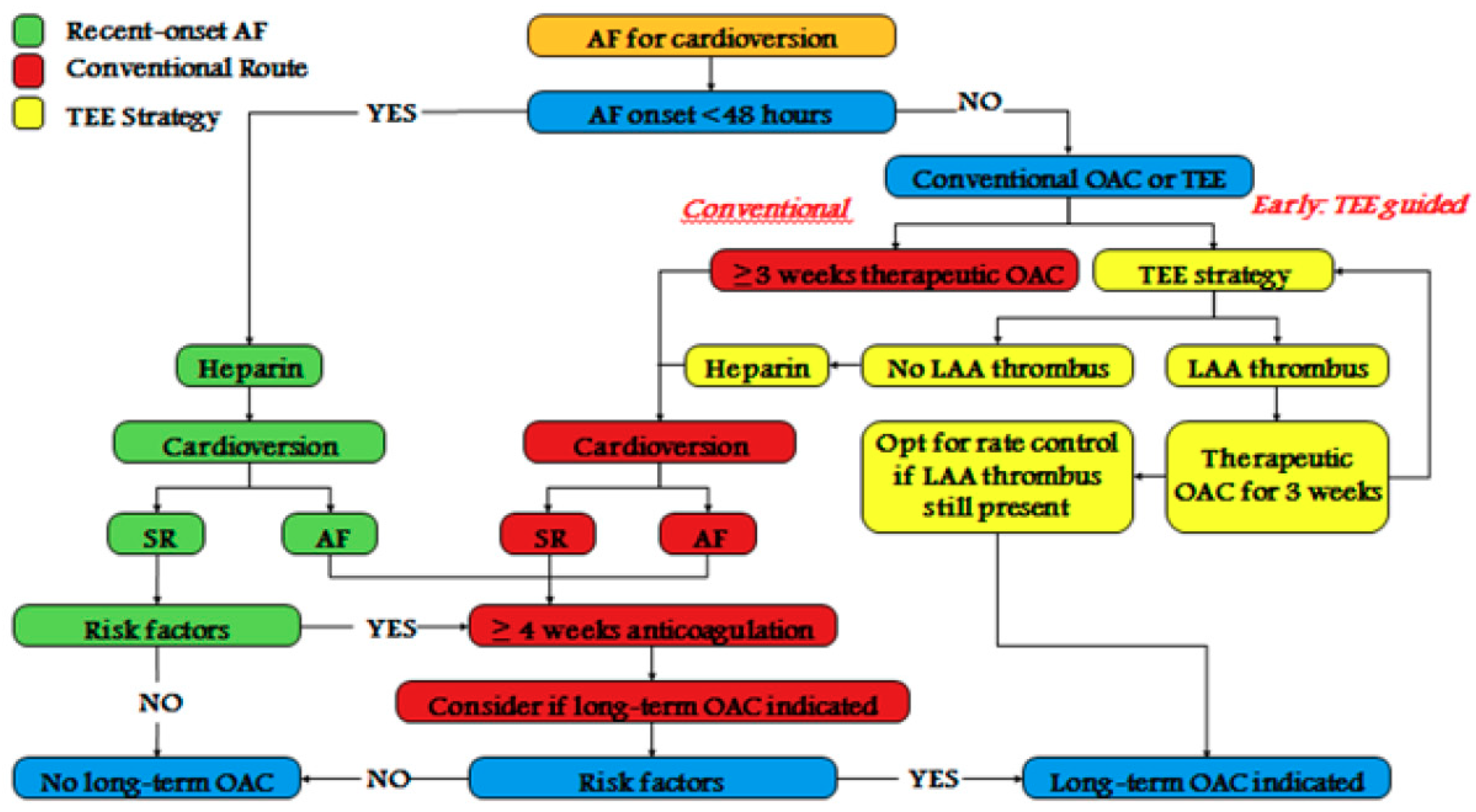
1. Introduction
2. DOACs for Cardioversion in Atrial Fibrillation
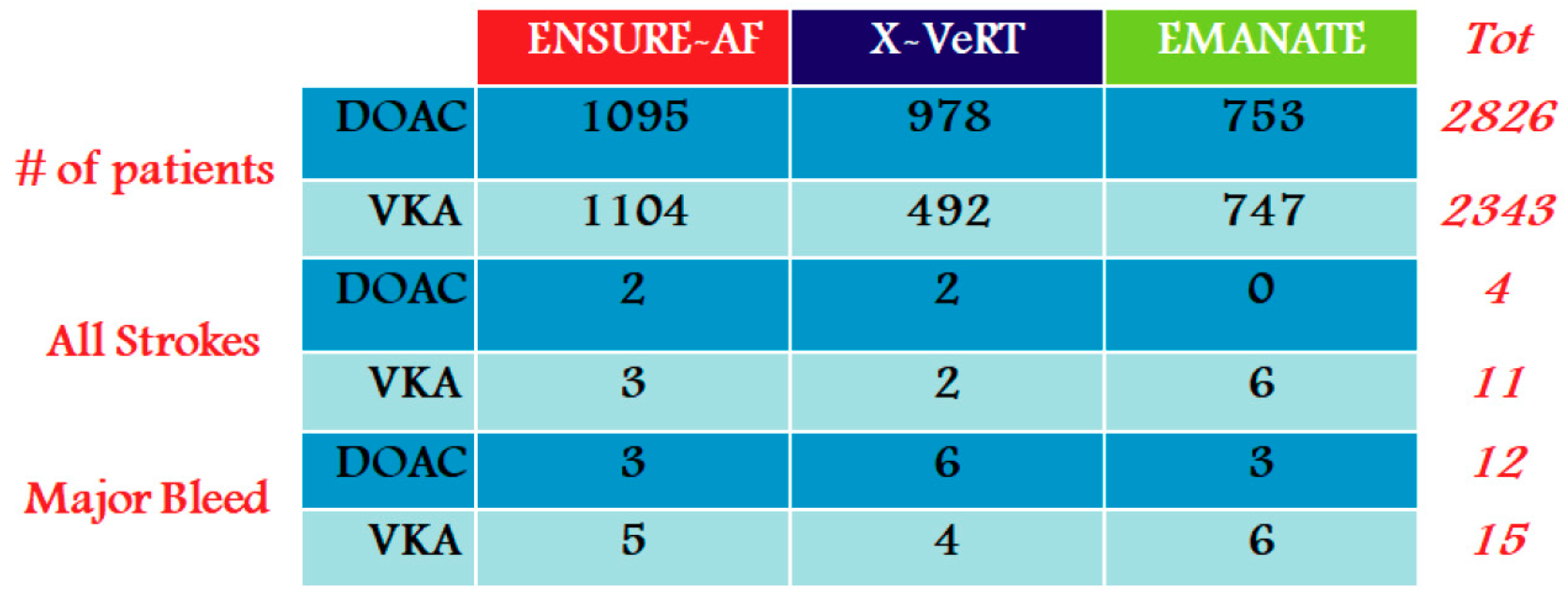
- Cardioversion of AF patient treated for >3 weeks with DOACs: we consider the subgroup analyses from RE-LY (dabigatran), ROCKET-AF (rivaroxaban), and ARISTOTLE (apixaban), including important news from the “eXplore the efficacy and safety of once-daily oral riVaroxaban for the prevention of caRdiovascular events in patients with nonvalvular aTrial fibrillation scheduled for cardioversion trial” (X-VeRT study) [20].
- Cardioversion of recent onset AF in an anticoagulation-naive patient: in this scenario, the results of the “Eliquis evaluated in acute cardioversion coMpared to usuAl treatmeNts for AnticoagulaTion in subjects with atrial fibrillation trial” (EMANATE trial) are very important [22].
- Patients with evidence of left atrial appendage (LAA) thrombus: we consider the few studies available in the literature on this scenario.
2.1. Cardioversion of AF Patient Treated for >3 Weeks with DOACs
2.2. Cardioversion of AF of >48 h in a Patient Not on DOACs Therapy
2.3. Cardioversion of Recent Onset AF, in an Anticoagulation-Naive Patient
2.4. Management of a Patient with Documented Left Atrial Appendage Thrombus
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Steffel, J.; Verhamme, P.; Potpara, T.S.; Albaladejo, P.; Antz, M.; Desteghe, L.; Georg Haeusler, K.; Oldgren, J.; Reinecke, H.; Roldan-Schilling, V.; et al. ESC Scientific Document Group. The 2018 European Heart Rhythm Association. Practical Guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Eur. Heart J. 2018, 39, 1330–1393. [Google Scholar] [CrossRef] [PubMed]
- Rietbrock, S.; Heeley, E.; Plumb, J.; van Sta, T. Chronic atrial fibrillation: Incidence, prevalence, and prediction of stroke using the Congestive heart failure, Hypertension, Age >75, Diabetes mellitus, and prior Stroke or transient ischemic attack (CHADS2) risk stratification scheme. Am. Heart J. 2008, 156, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Kulbertus, H.; Lancellotti, P. Fibrillation, an epidemic in the elderly? Rev. Med. Liege 2014, 69, 301–308. [Google Scholar] [PubMed]
- Puccio, D.; Novo, G.; Baiamonte, V.; Nuccio, A.; Fazio, G.; Corrado, E.; Coppola, G.; Muratori, I.; Vernuccio, L.; Novo, S. Atrial fibrillation and mild cognitive impairment: What correlation? Minerva Cardioangiol. 2009, 57, 143–150. [Google Scholar]
- Manno, G.; Novo, G.; Corrado, E.; Coppola, G.; Novo, S. Use of direct oral anticoagulants in very elderly patients: A case report of apixaban in an ultracentenary patient. J. Cardiovasc. Med. (Hagerstown) 2019, 20, 403–405. [Google Scholar] [CrossRef] [PubMed]
- Russo, V.; Carbone, A.; Rago, A.; Golino, P.; Nigro, G. Direct Oral Anticoagulants in octogenarians with atrial fibrillation: it’s never too late. J. Cardiovasc. Pharmacol. 2019, 73, 207–214. [Google Scholar] [CrossRef]
- McNamara, R.L.; Tamariz, L.J.; Segal, J.B.; Bass, E.B. Management of atrial fibrillation: Review of the evidence for the role of pharmacologic therapy, electrical cardioversion, and echocardiography. Ann. Intern. Med. 2003, 139, 1018–1033. [Google Scholar] [CrossRef]
- Naccarelli, G.V.; Dell’Orfano, J.T.; Wolbrette, D.L.; Patel, H.M.; Luck, J.C. Cost-effective management of acute atrial fibrillation: Role of rate control, spontaneous conversion, medical and direct current cardioversion, transesophageal echocardiography, and antiembolic therapy. Am. J. Cardiol. 2000, 85, 36D–45D. [Google Scholar] [CrossRef]
- Di Fusco, S.A.; Colivicchi, F.; Aspromonte, N.; Tubaro, M.; Aiello, A.; Santini, M. Direct oral anticoagulants in patients undergoing cardioversion: Insight from randomized clinical trials. Monaldi Arch. Chest Dis. 2017, 87, 805. [Google Scholar] [CrossRef]
- Russo, V.; Bottino, R.; Rago, A.; Micco, P.D.; D’Onofrio, A.; Liccardo, B.; Golino, P.; Nigro, G. Atrial Fibrillation and Malignancy: The Clinical Performance of Non-Vitamin K Oral Anticoagulants-A Systematic Review. Semin. Thromb. Hemost. 2019, 45, 205–214. [Google Scholar]
- Andò, G.; Trio, O. New oral anticoagulants versus Warfarin in patients undergoing cardioversion of atrial fibrillation. Int. J. Cardiol. 2016, 225, 244–246. [Google Scholar] [CrossRef] [PubMed]
- Andò, G.; Trio, O.; Carerj, S. New oral anticoagulants versus vitamin K antagonists before cardioversion of atrial fibrillation: A meta-analysis of data from 4 randomized trials. Expert Rev. Cardiovasc. Ther. 2015, 13, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Kirchhof, P.; Benussi, S.; Kotecha, D.; Ahlsson, A.; Atar, D.; Casadei, B.; Castella, M.; Diener, H.C.; Heidbuchel, H.; Hendriks, J.; et al. ESC Scientific Document Group. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur. Heart J. 2016, 37, 2893–2962. [Google Scholar] [CrossRef] [PubMed]
- January, C.T.; Wann, L.S.; Calkins, H.; Chen, L.Y.; Cigarroa, J.E.; Cleveland, J.C., Jr.; Ellinor, P.T.; Ezekowitz, M.D.; Field, M.E.; Furie, K.L.; et al. 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J. Am. Coll. Cardiol. 2019, 74, 104–132. [Google Scholar] [PubMed]
- Trujillo, T.C.; Dobesh, P.P.; Crossley, G.H.; Finks, S.W. Contemporary Management of Direct Oral Anticoagulants During Cardioversion and Ablation for Nonvalvular Atrial Fibrillation. Pharmacotherapy 2019, 39, 94–108. [Google Scholar] [CrossRef] [PubMed]
- Connolly, S.J.; Ezekowitz, M.D.; Yusuf, S.; Eikelboom, J.; Oldgren, J.; Parekh, A.; Pogue, J.; Reilly, P.A.; Themeles, E.; Varrone, J.; et al. for the RE-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N. Engl. J. Med. 2009, 361, 1139–1151. [Google Scholar] [CrossRef]
- Patel, M.R.; Mahaffey, K.W.; Garg, J.; Pan, G.; Singer, D.E.; Hacke, W.; Breithardt, G.; Halperin, J.L.; Hankey, G.J.; Piccini, J.P.; et al. ROCKET AF Investigators. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N. Engl. J. Med. 2011, 365, 883–891. [Google Scholar] [CrossRef]
- Granger, C.B.; Alexander, J.H.; McMurray, J.J.; Lopes, R.D.; Hylek, E.M.; Hanna, M.; Al-Khalidi, H.R.; Ansell, J.; Atar, D.; Avezum, A.; et al. Apixaban versus warfarin in patients with atrial fibrillation. N. Engl. J. Med. 2011, 0365, 981–992. [Google Scholar] [CrossRef] [PubMed]
- Reilly, P.A.; Lehr, T.; Haertter, S.; Connolly, S.J.; Yusuf, S.; Eikelboom, J.W.; Ezekowitz, M.D.; Nehmiz, G.; Wang, S.; Wallentin, L.; et al. The effect of dabigatran plasma concentrations and patient characteristics on the frequency of ischemic stroke and major bleeding in atrial fibrillation patients: The RE-LY Trial (Randomized Evaluation of Long-Term Anticoagulation Therapy). J. Am. Coll. Cardiol. 2014, 63, 321–328. [Google Scholar] [CrossRef]
- Cappato, R.; Ezekowitz, M.D.; Klein, A.L.; Camm, A.J.; Ma, C.S.; Le Heuzey, J.Y.; Talajic, M.; Scanavacca, M.; Vardas, P.E.; Kirchhof, P.; et al. X-VeRT Investigators. Rivaroxaban vs. vitamin K antagonists for cardioversion in atrial fibrillation. Eur. Heart J. 2014, 35, 3346–3355. [Google Scholar] [CrossRef]
- Goette, A.; Merino, J.L.; Ezekowitz, M.D.; Zamoryakhin, D.; Melino, M.; Jin, J.; Mercuri, M.F.; Grosso, M.A.; Fernandez, V.; Al-Saady, N.; et al. ENSURE-A investigators. Edoxaban versus enoxaparin-warfarin in patients undergoing cardioversion of atrial fibrillation (ENSURE-AF): A randomised, open-label, phase 3b trial. Lancet 2016, 388, 1995–2003. [Google Scholar] [CrossRef]
- Ezekowitz, M.D.; Pollack, C.V., Jr.; Halperin, J.L.; England, R.D.; VanPelt Nguyen, S.; Spahr, J.; Sudworth, M.; Cater, N.B.; Breazna, A.; Oldgren, J.; et al. Apixaban compared to heparin/vitamin K antagonist in patients with atrial fibrillation scheduled for cardioversion: The EMANATE trial. Eur. Heart J. 2018, 39, 2959–2971. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.D.; Lai, C.L.; Dong, Y.H.; Tu, Y.K.; Chan, K.A.; Suissa, S. Re-evaluating Safety and Effectiveness of Dabigatran Versus Warfarin in a Nationwide Data Environment: A Prevalent New-User Design Study. Drugs Real World Outcomes 2019, 6, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Nagarakanti, R.; Ezekowitz, M.D.; Oldgren, J.; Yang, S.; Chernick, M.; Aikens, T.H.; Flaker, G.; Brugada, J.; Kamensky, G.; Parekh, A.; et al. Dabigatran versus warfarin in patients with an analysis of patients undergoing cardioversion. Circulation 2011, 123, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Piccini, J.P.; Stevens, S.R.; Lokhnygina, Y.; Patel, M.R.; Halperin, J.L.; Singer, D.E.; Hankey, G.J.; Hacke, W.; Becker, R.C.; Nessel, C.C.; et al. Outcomes after cardioversion and atrial fibrillation ablation in patients treated with rivaroxaban and warfarin in the ROCKET AF trial. J. Am. Coll. Cardiol. 2013, 61, 1998–2006. [Google Scholar] [CrossRef] [PubMed]
- Lip, G.Y.H.; Khan, A.A.; Olshansky, B. Short-Term Outcomes of Apixaban Versus Warfarin in Patients With Atrial Fibrillation. Circulation 2019, 139, 2301–2303. [Google Scholar] [CrossRef] [PubMed]
- Flaker, G.; Lopes, R.D.; Al-Khatib, S.M.; Hermosillo, A.G.; Hohnloser, S.H.; Tinga, B.; Zhu, J.; Mohan, P.; Garcia, D.; Bartunek, J.; et al. Efficacy and safety of apixaban in patients after cardioversion for atrial fibrillation. J. Am. Coll. Cardiol. 2014, 63, 1082–1087. [Google Scholar] [CrossRef]
- Ruff, C.T.; Giugliano, R.P.; Braunwald, E.; Morrow, D.A.; Murphy, S.A.; Kuder, J.F.; Deenadayalu, N.; Jarolim, P.; Betcher, J.; Shi, M.; et al. Association between edoxaban dose, concentration, anti-Factor Xa activity, and outcomes: An analysis of data from the randomised, double-blind ENGAGE AF-TIMI 48 trial. Lancet 2015, 385, 2288–2295. [Google Scholar] [CrossRef]
- Masci, A.; Barone, L.; Dedè, L.; Fedele, M.; Tomasi, C.; Quarteroni, A.; Corsi, C. The Impact of Left Atrium Appendage Morphology on Stroke Risk Assessment in AtrialFibrillation: A Computational Fluid Dynamics Study. Front. Physiol. 2019, 9, 1938. [Google Scholar] [CrossRef]
- Stabile, G.; Russo, V.; Rapacciuolo, A.; De Divitiis, M.; De Simone, A.; Solimene, F.; D’Onofrio, A.; Iuliano, A.; Maresca, G.; Esposito, F.; et al. Transesophageal echocardiograpy in patients with persistent atrial fibrillation undergoing electrical cardioversion on new oral anticoagulants: A multi center registry. Int. J. Cardiol. 2015, 184, 283–284. [Google Scholar] [CrossRef]
- Lip, G.Y.; Hammerstingl, C.; Marin, F.; Cappato, R.; Meng, I.L.; Kirsch, B.; van Eickels, M.; Cohen, A. X-TRA study and CLOT-AF registry investigators. Left atrial thrombus resolution in atrial fibrillation or flutter: Results of a prospective study with rivaroxaban (X-TRA) and a retrospective observational registry providing baseline data (CLOT-AF). Am. Heart J. 2016, 178, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Xing, X.F.; Liu, N.N.; Han, Y.L.; Zhou, W.W.; Liang, M.; Wang, Z.L. Anticoagulation efficacy of dabigatran etexilate for left atrial appendage thrombus in patients with atrial fibrillation by transthoracic and transesophageal echocardiography. Medicine (Baltimore) 2018, 97, e11117. [Google Scholar] [CrossRef] [PubMed]
- Ferner, M.; Wachtlin, D.; Konrad, T.; Deuster, O.; Meinertz, T.; von Bardeleben, S.; Münzel, T.; Seibert-Grafe, M.; Breithardt, G.; Rostock, T. Rationale and design of the RE-LATED AF-AFNET 7 trial: Resolution of Left atrial-Appendage Thrombus-Effects of Dabigatran in patients with Atrial Fibrillation. Clin. Res. Cardiol. 2016, 105, 29–36. [Google Scholar] [CrossRef] [PubMed]
- European Atrial Fibrillation Trial Study. Optimal oral anticoagulant therapy in patients with non rheumatic atrial fibrillation and recent cerebral ischemia. N. Engl. J. Med. 1995, 333, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Rago, A.; Papa, A.A.; Cassese, A.; Arena, G.; Magliocca, M.C.G.; D’Onofrio, A.; Golino, P.; Nigro, G.; Russo, V. Clinical Performance of Apixaban vs. Vitamin K Antagonists in Patients with Atrial Fibrillation Undergoing Direct Electrical Current Cardioversion: A Prospective Propensity Score-Matched Cohort Study. Am. J. Cardiovasc. Drugs 2019, 19, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Russo, V.; Rago, A.; Proietti, R.; Di Meo, F.; Papa, A.; Calabrò, P.; D’Onofrio, A.; Nigro, G.; AlTurki, A. Efficacy and safety of the target specific oral anticoagulants for stroke prevention in atrial fibrillation: The real-life evidence. Ther. Adv. Drug Saf. 2017, 8, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Gibson, C.M.; Basto, A.N.; Howard, M.L. Direct Oral Anticoagulants in Cardioversion: A Review of Current Evidence. Ann. Pharmacother. 2018, 52, 277–284. [Google Scholar] [CrossRef]
- Brunetti, N.D.; Tarantino, N.; De Gennaro, L.; Correale, M.; Santoro, F.; Di Biase, M. Direct oral anti-coagulants compared to vitamin-K antagonists in cardioversion of atrial fibrillation: An updated meta-analysis. J. Thromb. Thromb. 2018, 45, 550–556. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coppola, G.; Manno, G.; Mignano, A.; Luparelli, M.; Zarcone, A.; Novo, G.; Corrado, E. Management of Direct Oral Anticoagulants in Patients with Atrial Fibrillation Undergoing Cardioversion. Medicina 2019, 55, 660. https://doi.org/10.3390/medicina55100660
Coppola G, Manno G, Mignano A, Luparelli M, Zarcone A, Novo G, Corrado E. Management of Direct Oral Anticoagulants in Patients with Atrial Fibrillation Undergoing Cardioversion. Medicina. 2019; 55(10):660. https://doi.org/10.3390/medicina55100660
Chicago/Turabian StyleCoppola, Giuseppe, Girolamo Manno, Antonino Mignano, Mirko Luparelli, Antonino Zarcone, Giuseppina Novo, and Egle Corrado. 2019. "Management of Direct Oral Anticoagulants in Patients with Atrial Fibrillation Undergoing Cardioversion" Medicina 55, no. 10: 660. https://doi.org/10.3390/medicina55100660
APA StyleCoppola, G., Manno, G., Mignano, A., Luparelli, M., Zarcone, A., Novo, G., & Corrado, E. (2019). Management of Direct Oral Anticoagulants in Patients with Atrial Fibrillation Undergoing Cardioversion. Medicina, 55(10), 660. https://doi.org/10.3390/medicina55100660




