IL28B Genetic Variations in Patients with Recurrent Herpes Simplex Keratitis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Setting
2.2. Inclusion and Exclusion Criteria
2.3. Immunological Analyses
2.4. Determination of IL28B Genotype
2.5. Ethical Approval
2.6. Statistical Analysis
3. Results
3.1. Clinical Characteristics
3.2. Distribution of IL28B Genotype Polymorphisms
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Boppana, S.B.; Fowler, K.B. Persistence in the population: Epidemiology and transmisson. In Human Herpesviruses: Biology, Therapy, and Immunoprophylaxis; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Smith, J.S.; Robinson, N.J. Age-specific prevalence of infection with herpes simplex virus types 2 and 1: A global review. J. Infect. Dis. 2002, 186, S3–S28. [Google Scholar] [CrossRef] [PubMed]
- Liesegang, T.J. Herpes simplex virus epidemiology and ocular importance. Cornea 2001, 20, 1–13. [Google Scholar] [CrossRef]
- Biswas, P.S.; Rouse, B.T. Early events in HSV keratitis—Setting the stage for a blinding disease. Microbes Infect. 2005, 7, 799–810. [Google Scholar] [CrossRef] [PubMed]
- Miranda-Saksena, M.; Denes, C.E.; Diefenbach, R.J.; Cunningham, A.L. Infection and transport of herpes simplex virus type 1 in neurons: Role of the cytoskeleton. Viruses 2018, 10, 92. [Google Scholar] [CrossRef] [PubMed]
- Shimeld, C.; Hill, T.; Blyth, B.; Easty, D. An improved model of recurrent herpetic eye disease in mice. Curr. Eye Res. 1989, 8, 1193–1205. [Google Scholar] [CrossRef] [PubMed]
- Abdelfattah, N.S.; Amgad, M.; Zayed, A.A. Host immune cellular reactions in corneal neovascularization. Int. J. Ophthalmol. 2016, 9, 625–633. [Google Scholar] [PubMed]
- Hochrein, H.; Schlatter, B.; O’Keeffe, M.; Wagner, C.; Schmitz, F.; Schiemann, M.; Bauer, S.; Suter, M.; Wagner, H. Herpes simplex virus type-1 induces IFN-α production via Toll-like receptor 9-dependent and-independent pathways. Proc. Natl. Acad. Sci. USA 2004, 101, 11416–11421. [Google Scholar] [CrossRef] [PubMed]
- Leib, D.A. Counteraction of interferon-induced antiviral responses by herpes simplex viruses. In Viral Proteins Counteracting Host Defenses; Springer: Berlin/Heidelberg, Germany, 2002; pp. 171–185. [Google Scholar]
- Kotenko, S.V.; Gallagher, G.; Baurin, V.V.; Lewis-Antes, A.; Shen, M.; Shah, N.K.; Langer, J.A.; Sheikh, F.; Dickensheets, H.; Donnelly, R.P. IFN-λs mediate antiviral protection through a distinct class II cytokine receptor complex. Nat. Immunol. 2003, 4, 69–77. [Google Scholar] [CrossRef]
- Uzé, G.; Monneron, D. IL-28 and IL-29: Newcomers to the interferon family. Biochimie 2007, 89, 729–734. [Google Scholar] [CrossRef]
- Srinivas, S.; Dai, J.; Eskdale, J.; Gallagher, G.E.; Megjugorac, N.J.; Gallagher, G. Interferon-λ1 (interleukin-29) preferentially down-regulates interleukin-13 over other T helper type 2 cytokine responses in vitro. Immunology 2008, 125, 492–502. [Google Scholar] [CrossRef]
- Li, J.; Hu, S.; Zhou, L.; Ye, L.; Wang, X.; Ho, J.; Ho, W. Interferon lambda inhibits herpes simplex virus type I infection of human astrocytes and neurons. Glia 2011, 59, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Sheppard, P.; Kindsvogel, W.; Xu, W.; Henderson, K.; Schlutsmeyer, S.; Whitmore, T.E.; Kuestner, R.; Garrigues, U.; Birks, C.; Roraback, J.; et al. IL-28, IL-29 and their class II cytokine receptor IL-28R. Nat. Immunol. 2003, 4, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Dumoutier, L.; Tounsi, A.; Michiels, T.; Sommereyns, C.; Kotenko, S.V.; Renauld, J.C. Role of the Interleukin (IL)-28 Receptor Tyrosine Residues for Antiviral and Antiproliferative Activity of IL-29/Interferon-λ1 similarities with type I interferon signaling. J. Biol. Chem. 2004, 279, 32269–32274. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Hamming, O.J.; Ank, N.; Paludan, S.R.; Nielsen, A.L.; Hartmann, R. Type III interferon (IFN) induces a type I IFN-like response in a restricted subset of cells through signaling pathways involving both the Jak-STAT pathway and the mitogen-activated protein kinases. J. Virol. 2007, 81, 7749–7758. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, S.J.; Koegl, M.; Boutell, C.; Zenner, H.L.; Crump, C.M.; Pica, F.; Gonzalez, O.; Friedel, C.C.; Barry, G.; Martin, K.; et al. A systematic analysis of host factors reveals a Med23-interferon-λ regulatory axis against herpes simplex virus type 1 replication. PLoS Pathog. 2013, 9, e1003514. [Google Scholar] [CrossRef] [PubMed]
- Ge, D.; Fellay, J.; Thompson, A.J.; Simon, J.S.; Shianna, K.V.; Urban, T.J.; Heinzen, E.L.; Qiu, P.; Bertelsen, A.H.; Muir, A.J.; et al. Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance. Nature 2009, 461, 399–401. [Google Scholar] [CrossRef] [PubMed]
- Langhans, B.; Kupfer, B.; Braunschweiger, I.; Arndt, S.; Schulte, W.; Nischalke, H.D.; Nattermann, J.; Oldenburg, J.; Sauerbruch, T.; Spengler, U. Interferon-lambda serum levels in hepatitis C. J. Hepatol. 2011, 54, 859–865. [Google Scholar] [CrossRef]
- Jaggi, U.; Bhela, S.; Rouse, B.T. Role of Interferon lambda (IL-28A) in Herpes Stromal Keratitis. J. Immunol. Res. Ther. 2018, 3, 135–144. [Google Scholar]
- Hori, K.; Shin, W.S.; Hemmi, C.; Toyo-oka, T.; Makino, T. High fidelity SNP genotyping using sequence-specific primer elongation and fluorescence correlation spectroscopy. Curr. Pharm. Biotechnol. 2003, 4, 477–484. [Google Scholar] [CrossRef]
- Rolinski, J.; Hus, I. Immunological aspects of acute and recurrent herpes simplex keratitis. J. Immunol. Res. 2014, 2014, 513560. [Google Scholar] [CrossRef]
- Azher, T.N.; Yin, X.T.; Tajfirouz, D.; Huang, A.J.; Stuart, P.M. Herpes simplex keratitis: Challenges in diagnosis and clinical management. Clin. Ophthalmol. 2017, 11, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Metcalf, J.F.; Michaelis, B.A. Herpetic keratitis in inbred mice. Investig. Ophthalmol. Vis. Sci. 1984, 25, 1222–1225. [Google Scholar]
- Brandt, C.R. The role of viral and host genes in corneal infection with herpes simplex virus type 1. Exp. Eye Res. 2005, 80, 607–621. [Google Scholar] [CrossRef]
- Moraru, M.; Cisneros, E.; Gómez-Lozano, N.; de Pablo, R.; Portero, F.; Cañizares, M.; Vaquero, M.; Roustán, G.; Millán, I.; López-Botet, M.; et al. Host genetic factors in susceptibility to herpes simplex type 1 virus infection: Contribution of polymorphic genes at the interface of innate and adaptive immunity. J. Immunol. 2012, 188, 4412–4420. [Google Scholar] [CrossRef] [PubMed]
- Russell, C.D.; Griffiths, S.J.; Haas, J. Interferon lambda genetic polymorphisms and viral infection: The tip of the iceberg? DNA Cell Biol. 2014, 33, 60–63. [Google Scholar] [CrossRef]
- Abe, H.; Hayes, C.N.; Ochi, H.; Maekawa, T.; Tsuge, M.; Miki, D.; Mitsui, F.; Hiraga, N.; Imamura, M.; Takahashi, S.; et al. IL28 variation affects expression of interferon stimulated genes and peg-interferon and ribavirin therapy. J. Hepatol. 2011, 54, 1094–1101. [Google Scholar] [CrossRef] [PubMed]
- Honda, M.; Sakai, A.; Yamashita, T.; Nakamoto, Y.; Mizukoshi, E.; Sakai, Y.; Yamashita, T.; Nakamura, M.; Shirasaki, T.; Horimoto, K.; et al. Hepatic ISG expression is associated with genetic variation in interleukin 28B and the outcome of IFN therapy for chronic hepatitis C. Gastroenterology 2010, 139, 499–509. [Google Scholar] [CrossRef] [PubMed]
- Ank, N.; West, H.; Bartholdy, C.; Eriksson, K.; Thomsen, A.R.; Paludan, S.R. Lambda interferon (IFN-λ), a type III IFN, is induced by viruses and IFNs and displays potent antiviral activity against select virus infections in vivo. J. Virol. 2006, 80, 4501–4509. [Google Scholar] [CrossRef]
- Melchjorsen, J.; Sirén, J.; Julkunen, I.; Paludan, S.R.; Matikainen, S. Induction of cytokine expression by herpes simplex virus in human monocyte-derived macrophages and dendritic cells is dependent on virus replication and is counteracted by ICP27 targeting NF-κB and IRF-3. J. Gen. Virol. 2006, 87, 1099–1108. [Google Scholar] [CrossRef]
- Pica, F.; Volpi, A.; Gaziano, R.; Garaci, E. Interferon-λ in immunocompetent individuals with a history of recurrent herpes labialis. Antivir. Ther. 2010, 15, 737. [Google Scholar] [CrossRef]
- Sainz, B.; Loutsch, J.M.; Marquart, M.E.; Hill, J.M. Stress-associated immunomodulation and herpes simplex virus infections. Med. Hypotheses 2001, 56, 348–356. [Google Scholar] [CrossRef] [PubMed]
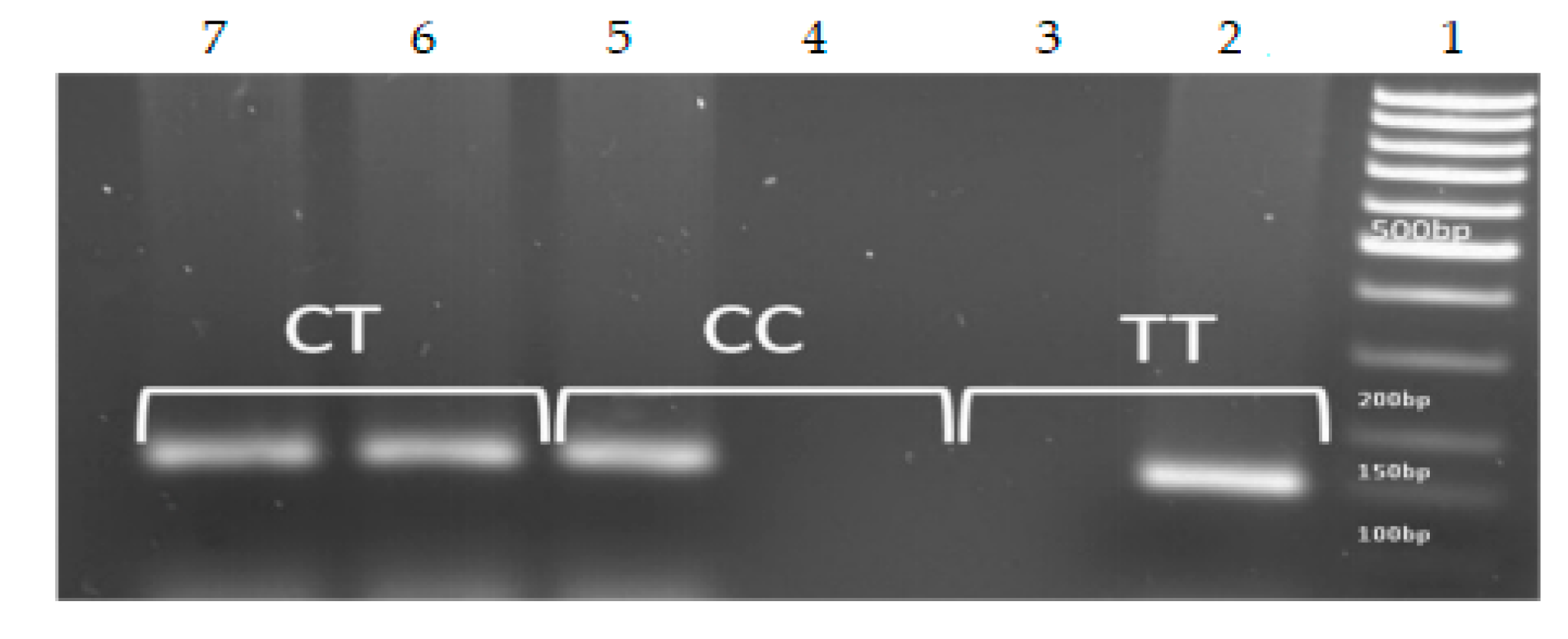


| IL28B SNP | Nucleotide Sequence | Size of PCR Product |
|---|---|---|
| rs12979860 | Gen (sense) 5′-TTATCGCATACGG CTAGGC-3′ | 153 bp |
| C (antisense) 5′TGCAATTCAACCCTGGTTC G-3′ | ||
| T (antisense) 5′ TGCAATTCAACCCTGGTTC A-3 |
| Characteristics | All Patients |
|---|---|
| n | 75 |
| Gender (M/F) | 36:39 |
| Age, y | 44.95 ± 11.294 |
| Have keratitis (M) | 5 (6.7%) |
| Do not have keratitis (M) | 31 (41.3%) |
| Have keratitis (F) | 19 (25.3%) |
| Do not have keratitis (F) | 20 (26.7%) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borivoje, S.; Svetlana, S.; Milan, H.-M.; Nela, Đ.; Olivera, M.-Đ.; Filip, M.; Milenko, S.; Srbislav, P. IL28B Genetic Variations in Patients with Recurrent Herpes Simplex Keratitis. Medicina 2019, 55, 642. https://doi.org/10.3390/medicina55100642
Borivoje S, Svetlana S, Milan H-M, Nela Đ, Olivera M-Đ, Filip M, Milenko S, Srbislav P. IL28B Genetic Variations in Patients with Recurrent Herpes Simplex Keratitis. Medicina. 2019; 55(10):642. https://doi.org/10.3390/medicina55100642
Chicago/Turabian StyleBorivoje, Savić, Stanojlović Svetlana, Hadži-Milić Milan, Đonović Nela, Milošević-Đorđević Olivera, Milisavljević Filip, Stojković Milenko, and Pajić Srbislav. 2019. "IL28B Genetic Variations in Patients with Recurrent Herpes Simplex Keratitis" Medicina 55, no. 10: 642. https://doi.org/10.3390/medicina55100642
APA StyleBorivoje, S., Svetlana, S., Milan, H.-M., Nela, Đ., Olivera, M.-Đ., Filip, M., Milenko, S., & Srbislav, P. (2019). IL28B Genetic Variations in Patients with Recurrent Herpes Simplex Keratitis. Medicina, 55(10), 642. https://doi.org/10.3390/medicina55100642





