Midbody: From the Regulator of Cytokinesis to Postmitotic Signaling Organelle
Abstract
1. Introduction
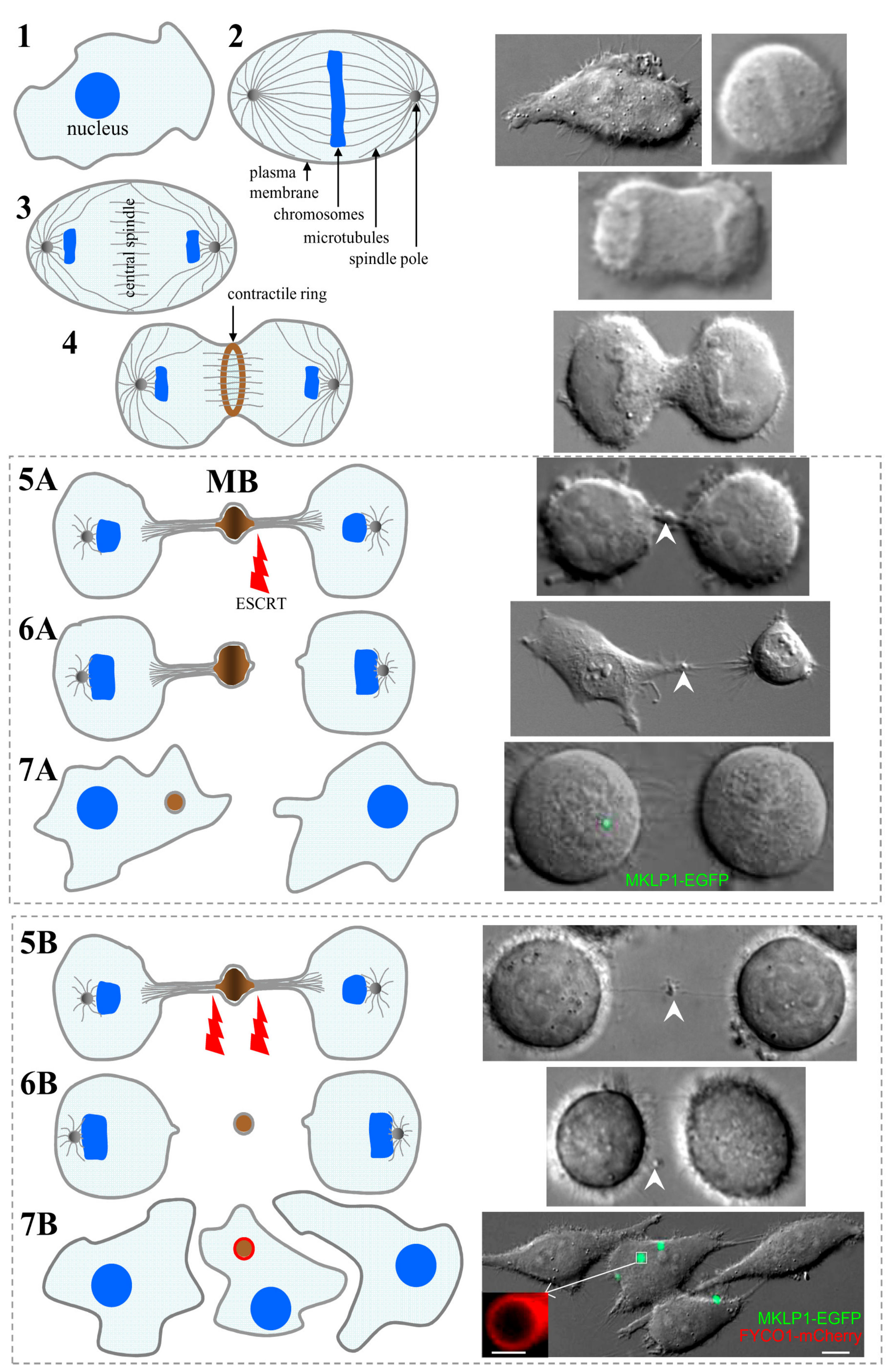
2. Midbody Formation and Inheritance
3. The Roles of Postmitotic MBs in Regulating Cell Polarity and Fate
4. Midbody Degradation
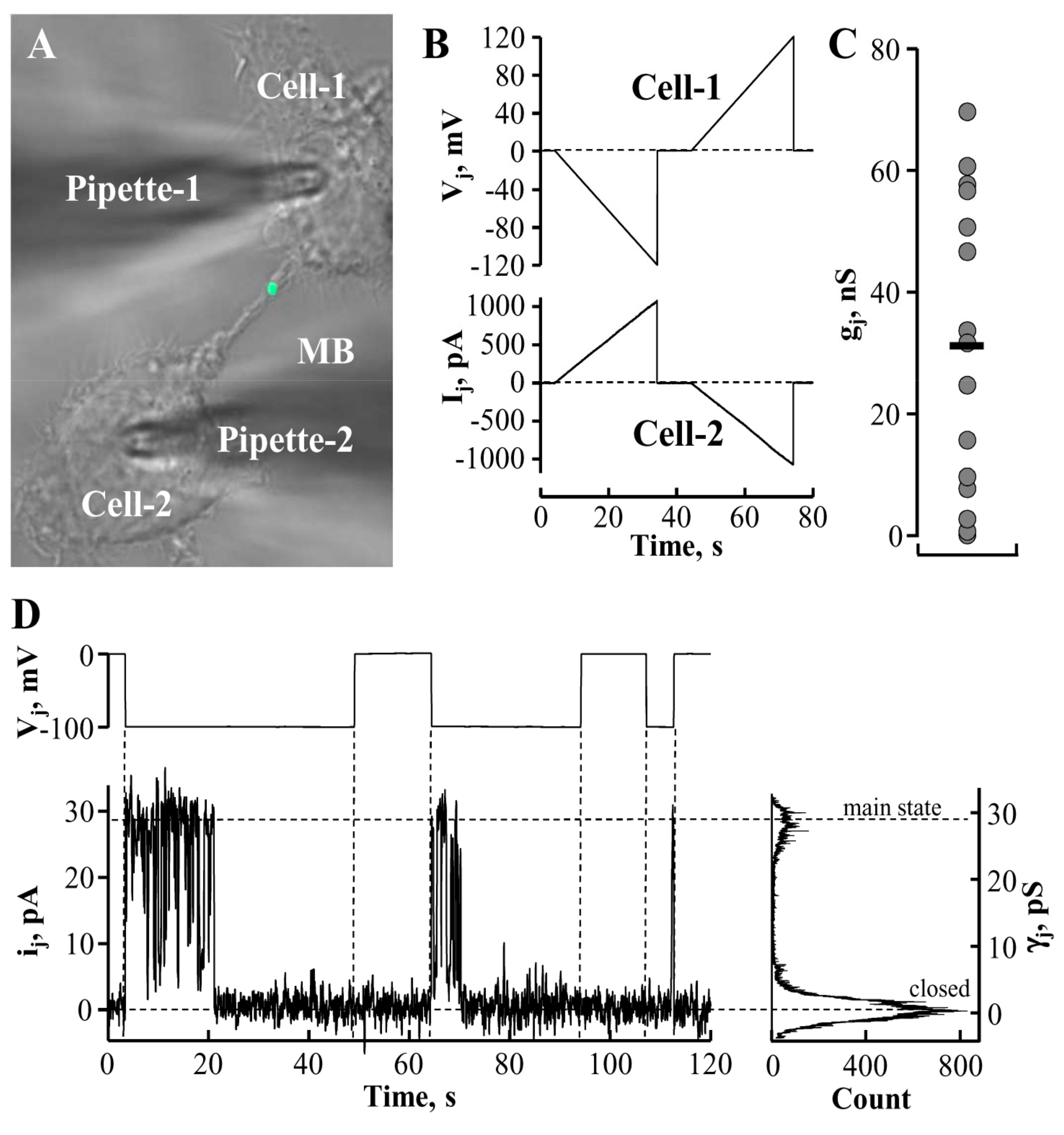
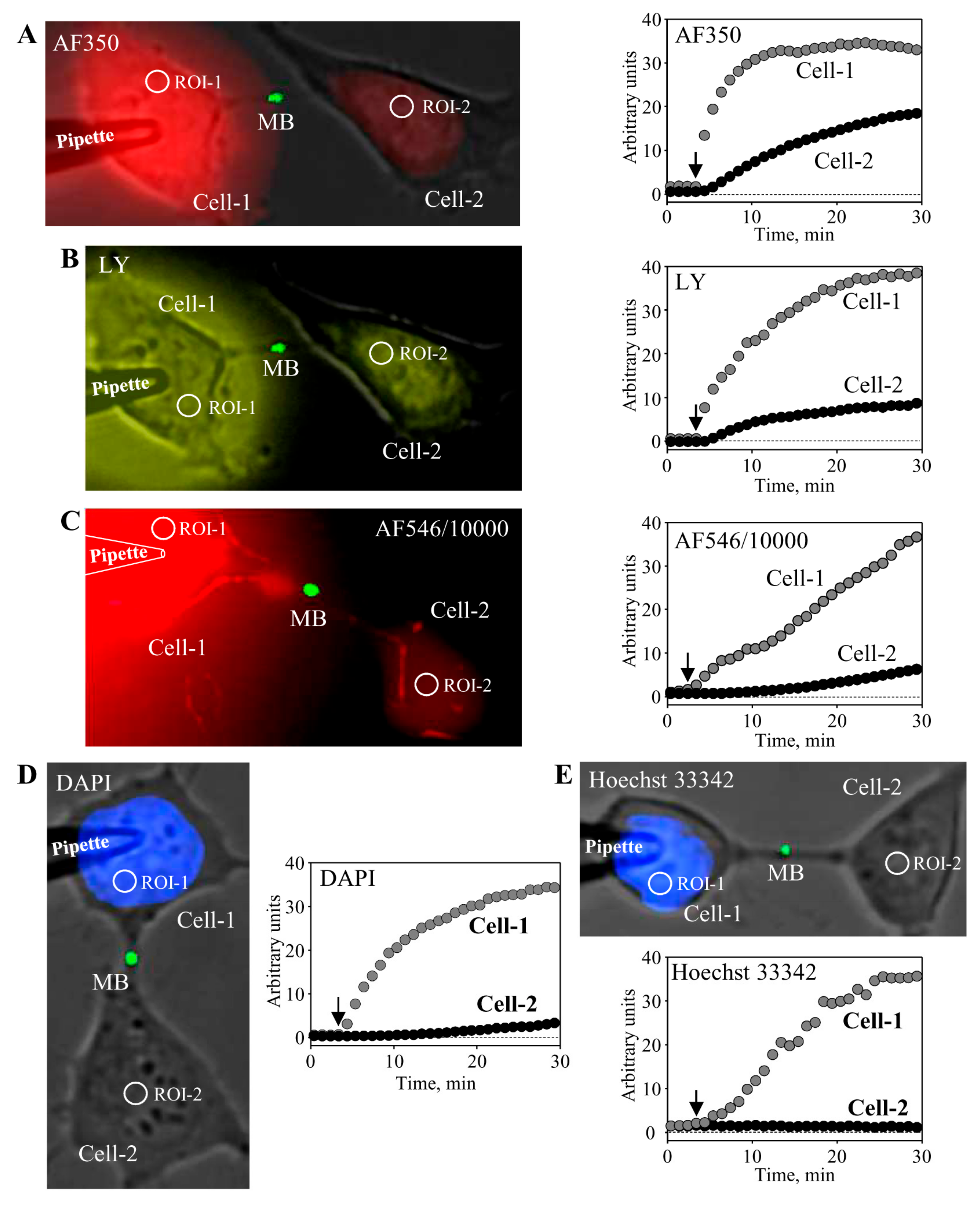
5. Conductance and Permeability of Midbody-Containing Intercellular Bridges
6. Concluding Remarks
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dionne, L.K.; Wang, X.J.; Prekeris, R. Midbody: From cellular junk to regulator of cell polarity and cell fate. Curr. Opin. Cell Biol. 2015, 35, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Pavicic-Kaltenbrunner, V.; Mishima, M.; Glotzer, M. Cooperative assembly of CYK-4/MgcRacGAP and ZEN-4/MKLP1 to form the centralspindlin complex. Mol. Biol. Cell 2007, 18, 4992–5003. [Google Scholar] [PubMed]
- Green, R.A.; Paluch, E.; Oegema, K. Cytokinesis in animal cells. Annu. Rev. Cell Dev. Biol. 2012, 28, 29–58. [Google Scholar] [CrossRef] [PubMed]
- Flemming, W. Neue beitrage zur kenntnis der zelle. Arch. Mikrosk Anat. 1891, 37, 685–751. [Google Scholar] [CrossRef]
- Chen, C.T.; Ettinger, A.W.; Huttner, W.B.; Doxsey, S.J. Resurrecting remnants: The lives of post-mitotic midbodies. Trends Cell Boil. 2013, 23, 118–128. [Google Scholar]
- Addi, C.; Bai, J.; Echard, A. Actin, microtubule, septin and ESCRT filament remodeling during late steps of cytokinesis. Curr. Opin. Cell Biol. 2018, 50, 27–34. [Google Scholar] [PubMed]
- Cauvin, C.; Rosendale, M.; Gupta-Rossi, N.; Rocancourt, M.; Larraufie, P.; Salomon, R.; Perrais, D.; Echard, A. Rab35 GTPase Triggers Switch-like Recruitment of the Lowe Syndrome Lipid Phosphatase OCRL on Newborn Endosomes. Curr. Biol. 2016, 26, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Schiel, J.A.; Park, K.; Morphew, M.K.; Reid, E.; Hoenger, A.; Prekeris, R. Endocytic membrane fusion and buckling-induced microtubule severing mediate cell abscission. J. Cell Sci. 2011, 124, 1411–1424. [Google Scholar] [CrossRef] [PubMed]
- Schiel, J.A.; Simon, G.C.; Zaharris, C.; Weisz, J.; Castle, D.; Wu, C.C.; Prekeris, R. FIP3-endosome-dependent formation of the secondary ingression mediates ESCRT-III recruitment during cytokinesis. Nat. Cell Biol. 2012, 14, 1068–1078. [Google Scholar] [CrossRef] [PubMed]
- Fremont, S.; Romet-Lemonne, G.; Houdusse, A.; Echard, A. Emerging roles of MICAL family proteins—From actin oxidation to membrane trafficking during cytokinesis. J. Cell Sci. 2017, 130, 1509–1517. [Google Scholar] [PubMed]
- Schoneberg, J.; Lee, I.H.; Iwasa, J.H.; Hurley, J.H. Reverse-topology membrane scission by the ESCRT proteins. Nat. Rev. 2017, 18, 5–17. [Google Scholar]
- Mierzwa, B.E.; Chiaruttini, N.; Redondo-Morata, L.; von Filseck, J.M.; Konig, J.; Larios, J.; Poser, I.; Müller-Reichert, T.; Scheuring, S.; Roux, A. Dynamic subunit turnover in ESCRT-III assemblies is regulated by Vps4 to mediate membrane remodelling during cytokinesis. Nat. Cell Biol. 2017, 19, 787–798. [Google Scholar] [PubMed]
- Carlton, J.G.; Martin-Serrano, J. Parallels between cytokinesis and retroviral budding: A role for the ESCRT machinery. Science 2007, 316, 1908–1912. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Rismanchi, N.; Renvoise, B.; Lippincott-Schwartz, J.; Blackstone, C.; Hurley, J.H. Structural basis for midbody targeting of spastin by the ESCRT-III protein CHMP1B. Nat. Struct. Mol. Biol. 2008, 15, 1278–1286. [Google Scholar] [CrossRef] [PubMed]
- Crowell, E.F.; Gaffuri, A.L.; Gayraud-Morel, B.; Tajbakhsh, S.; Echard, A. Engulfment of the midbody remnant after cytokinesis in mammalian cells. J. Cell Sci. 2014, 127, 3840–3851. [Google Scholar] [PubMed]
- Kuo, T.C.; Chen, C.T.; Baron, D.; Onder, T.T.; Loewer, S.; Almeida, S.; Weismann, C.M.; Xu, P.; Houghton, J.-M.; Gao, F.-B.; et al. Midbody accumulation through evasion of autophagy contributes to cellular reprogramming and tumorigenicity. Nat. Cell Biol. 2011, 13, 1214–1223. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Mangan, A.; Cicchini, L.; Margolis, B.; Prekeris, R. FIP5 phosphorylation during mitosis regulates apical trafficking and lumenogenesis. EMBO Rep. 2014, 15, 428–437. [Google Scholar] [CrossRef] [PubMed]
- Mangan, A.J.; Sietsema, D.V.; Li, D.; Moore, J.K.; Citi, S.; Prekeris, R. Cingulin and actin mediate midbody-dependent apical lumen formation during polarization of epithelial cells. Nat. Commun. 2016, 7, 12426. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Yanger, K.; Stanger, B.Z.; Cassio, D.; Bi, E. Cytokinesis defines a spatial landmark for hepatocyte polarization and apical lumen formation. J. Cell Sci. 2014, 127, 2483–2492. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Pohl, C. Coupling of rotational cortical flow, asymmetric midbody positioning, and spindle rotation mediates dorsoventral axis formation in C. elegans. Dev. Cell 2014, 28, 253–267. [Google Scholar] [CrossRef] [PubMed]
- Ou, G.; Gentili, C.; Gonczy, P. Stereotyped distribution of midbody remnants in early C. elegans embryos requires cell death genes and is dispensable for development. Cell Res. 2014, 24, 251–253. [Google Scholar] [CrossRef] [PubMed]
- Ettinger, A.W.; Wilsch-Brauninger, M.; Marzesco, A.M.; Bickle, M.; Lohmann, A.; Maliga, Z.; Karbanová, J.; Corbeil, D.; Hyman, A.A.; Huttner, W.B. Proliferating versus differentiating stem and cancer cells exhibit distinct midbody-release behaviour. Nat. Commun. 2011, 2, 503. [Google Scholar] [PubMed]
- Yu, L.; Chen, Y.; Tooze, S.A. Autophagy pathway: Cellular and molecular mechanisms. Autophagy 2018, 14, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Dionne, L.K.; Peterman, E.; Schiel, J.; Gibieza, P.; Skeberdis, V.A.; Jimeno, A.; Wang, X.-J.; Prekeris, R. FYCO1 regulates accumulation of post-mitotic midbodies by mediating LC3-dependent midbody degradation. J. Cell Sci. 2017, 130, 4051–4062. [Google Scholar] [CrossRef] [PubMed]
- Pankiv, S.; Johansen, T. FYCO1: Linking autophagosomes to microtubule plus end-directing molecular motors. Autophagy 2010, 6, 550–552. [Google Scholar] [CrossRef] [PubMed]
- Ariazi, J.; Benowitz, A.; De Biasi, V.; Den Boer, M.L.; Cherqui, S.; Cui, H.; Douillet, N.; Eugenin, E.A.; Favre, D.; Goodman, S.; et al. Tunneling Nanotubes and Gap Junctions-Their Role in Long-Range Intercellular Communication during Development, Health, and Disease Conditions. Front. Mol. Neurosci. 2017, 10, 333. [Google Scholar] [PubMed]
- Račkauskas, M.; Neverauskas, V.; Skeberdis, V.A. Diversity and properties of connexin gap junction channels. Medicina 2010, 46, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Antanavičiūtė, I.; Rysevaitė, K.; Liutkevičius, V.; Marandykina, A.; Rimkutė, L.; Sveikatienė, R.; Uloza, V.; Skeberdis, V.A. Long-distance communication between laryngeal carcinoma cells. PLoS ONE 2014, 9, e99196. [Google Scholar]
- Rimkute, L.; Jotautis, V.; Marandykina, A.; Sveikatiene, R.; Antanaviciute, I.; Skeberdis, V.A. The role of neural connexins in HeLa cell mobility and intercellular communication through tunneling tubes. BMC Cell Biol. 2016, 17, 3. [Google Scholar]
- González, D.; Gómez-Hernández, J.M.; Barrio, L.C. Molecular basis of voltage dependence of connexin channels: An integrative appraisal. Prog. Biophys. Mol. Biol. 2007, 94, 66–106. [Google Scholar] [CrossRef] [PubMed]
- Eckert, R.; Dunina-Barkovskaya, A.; Hulser, D.F. Biophysical characterization of gap-junction channels in HeLa cells. Pflugers Arch. 1993, 424, 335–342. [Google Scholar] [PubMed]
- Bukauskas, F.F. Neurons and beta-cells of the pancreas express connexin36, forming gap junction channels that exhibit strong cationic selectivity. J. Membr. Biol. 2012, 245, 243–253. [Google Scholar] [PubMed]
- Peracchia, C. Chemical gating of gap junction channels; roles of calcium, pH and calmodulin. Biochim. Biophys. Acta 2004, 1662, 61–80. [Google Scholar] [CrossRef] [PubMed]
- Palacios-Prado, N.; Hoge, G.; Marandykina, A.; Rimkute, L.; Chapuis, S.; Paulauskas, N.; Skeberdis, V.A.; O’Brien, J.; Pereda, A.E.; Bennett, M.V.L.; et al. Intracellular magnesium-dependent modulation of gap junction channels formed by neuronal connexin36. J. Neurosci. 2013, 33, 4741–4753. [Google Scholar] [CrossRef] [PubMed]
- Skeberdis, V.A.; Rimkutė, L.; Skeberdytė, A.; Paulauskas, N.; Bukauskas, F.F. pH-dependent modulation of connexin-based gap junctional uncouplers. J. Physiol. 2011, 589, 3495–3506. [Google Scholar] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antanavičiūtė, I.; Gibieža, P.; Prekeris, R.; Skeberdis, V.A. Midbody: From the Regulator of Cytokinesis to Postmitotic Signaling Organelle. Medicina 2018, 54, 53. https://doi.org/10.3390/medicina54040053
Antanavičiūtė I, Gibieža P, Prekeris R, Skeberdis VA. Midbody: From the Regulator of Cytokinesis to Postmitotic Signaling Organelle. Medicina. 2018; 54(4):53. https://doi.org/10.3390/medicina54040053
Chicago/Turabian StyleAntanavičiūtė, Ieva, Paulius Gibieža, Rytis Prekeris, and Vytenis Arvydas Skeberdis. 2018. "Midbody: From the Regulator of Cytokinesis to Postmitotic Signaling Organelle" Medicina 54, no. 4: 53. https://doi.org/10.3390/medicina54040053
APA StyleAntanavičiūtė, I., Gibieža, P., Prekeris, R., & Skeberdis, V. A. (2018). Midbody: From the Regulator of Cytokinesis to Postmitotic Signaling Organelle. Medicina, 54(4), 53. https://doi.org/10.3390/medicina54040053





