Topical Anti-Inflammatory and Analgesic Activities of Citrullus colocynthis Extract Cream in Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Plant Material
2.3. Hydroalcoholic Extraction
2.4. Formulation of Topical Preparation
2.5. Inflammation and Nociception Assessment
2.5.1. Animal
2.5.2. Carrageenan-Induced Acute Inflammatory Model
2.5.3. Formalin Test
2.5.4. Involvement of Opioid Receptors in the Peripheral Antinociceptive Effect of CC Cream
2.6. Cytokine Assay
2.7. Skin Irritation Test
2.8. Statistical Analysis
3. Results
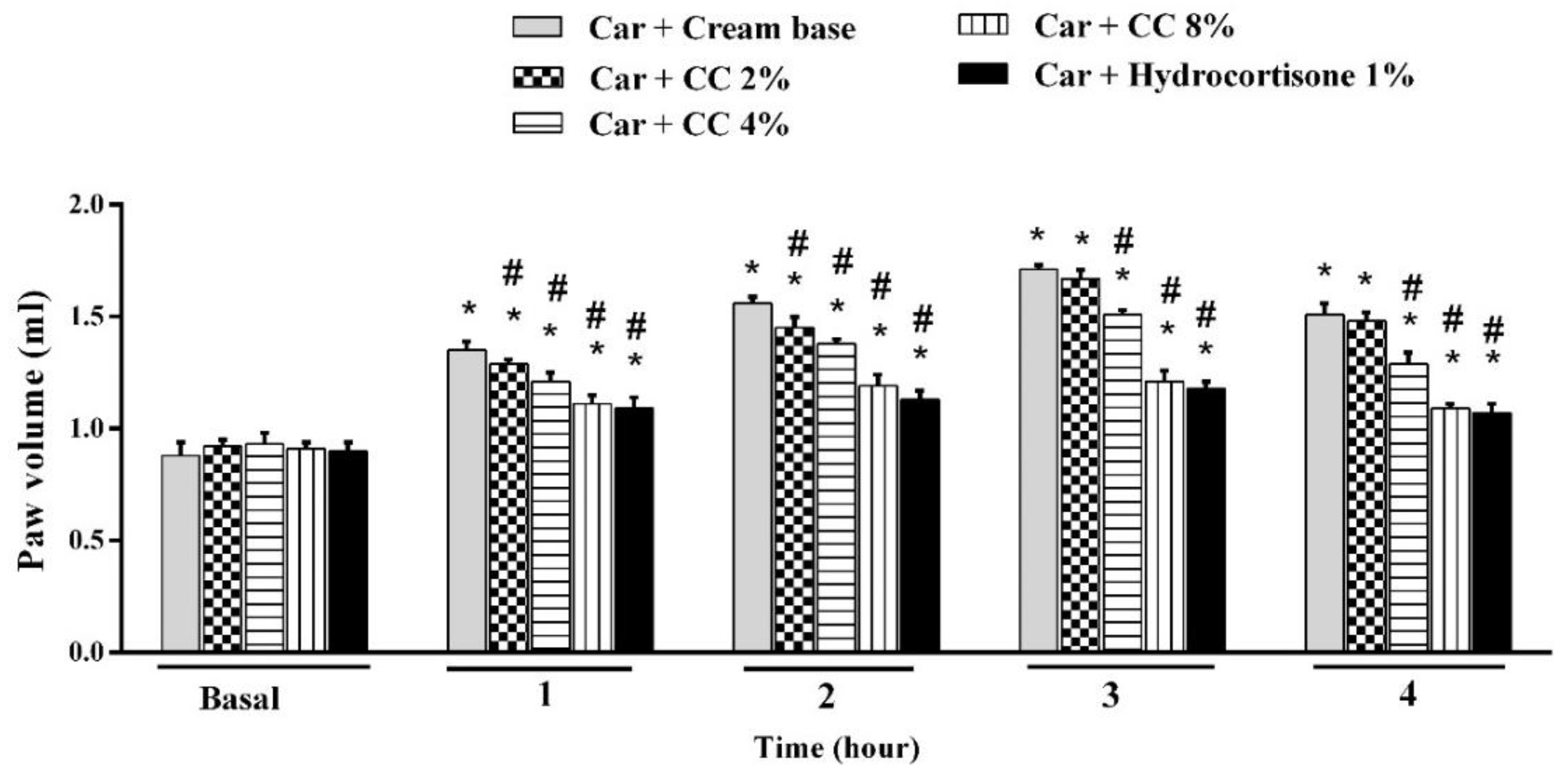
3.1. Effect of Topical CC Cream on Carrageenan-Induced Inflammation
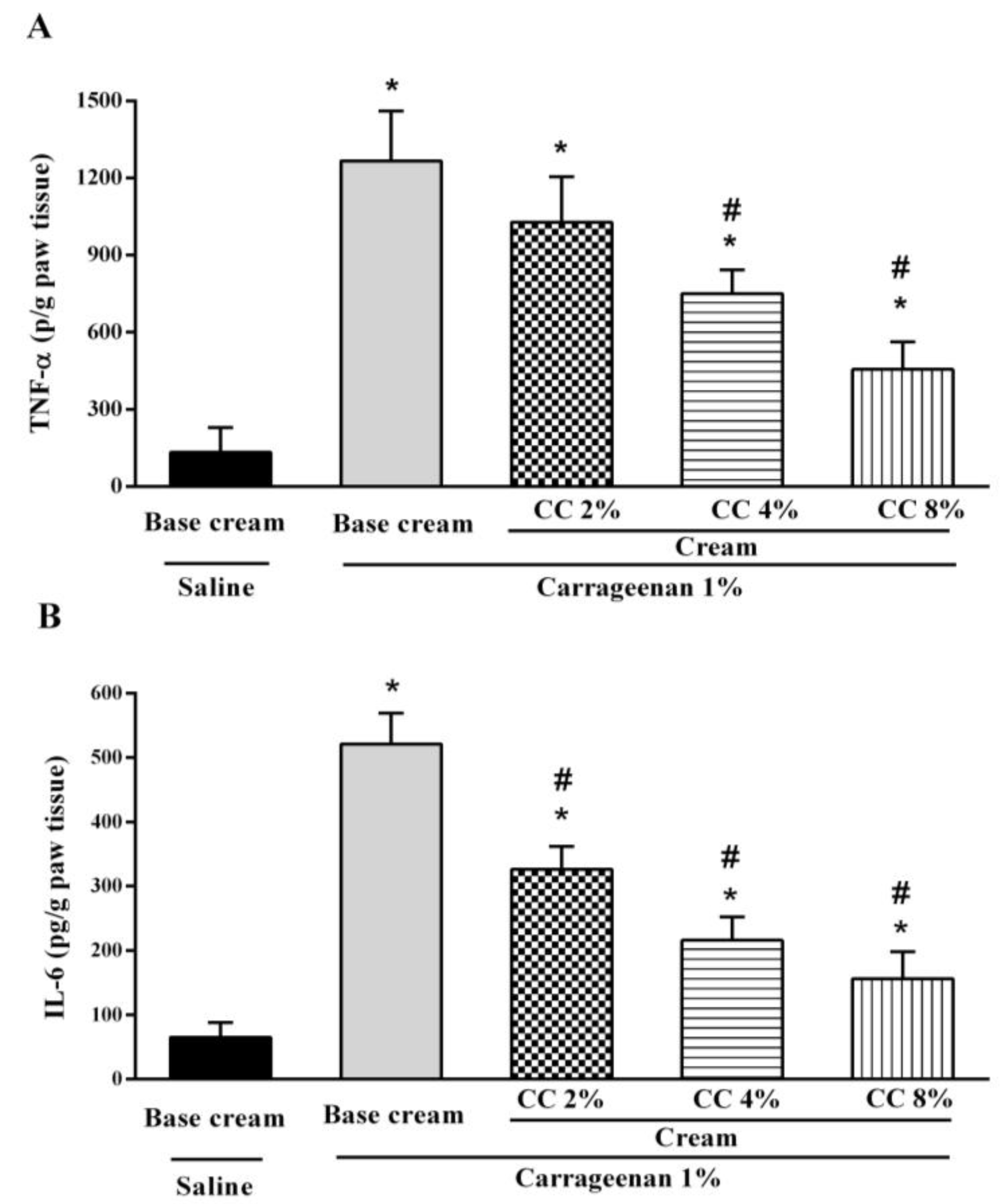
3.2. Effect of Topical CC Cream on Cytokines Level
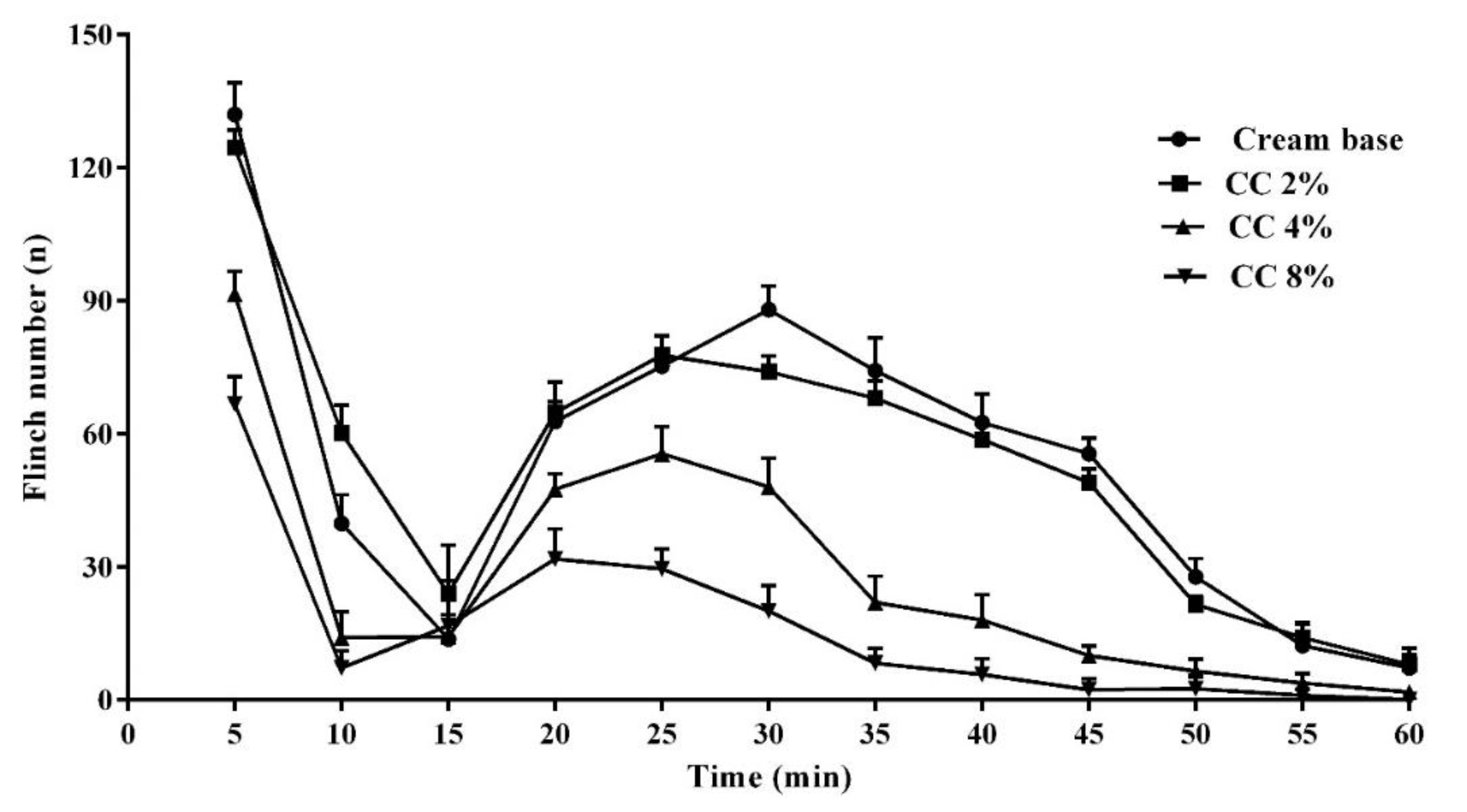
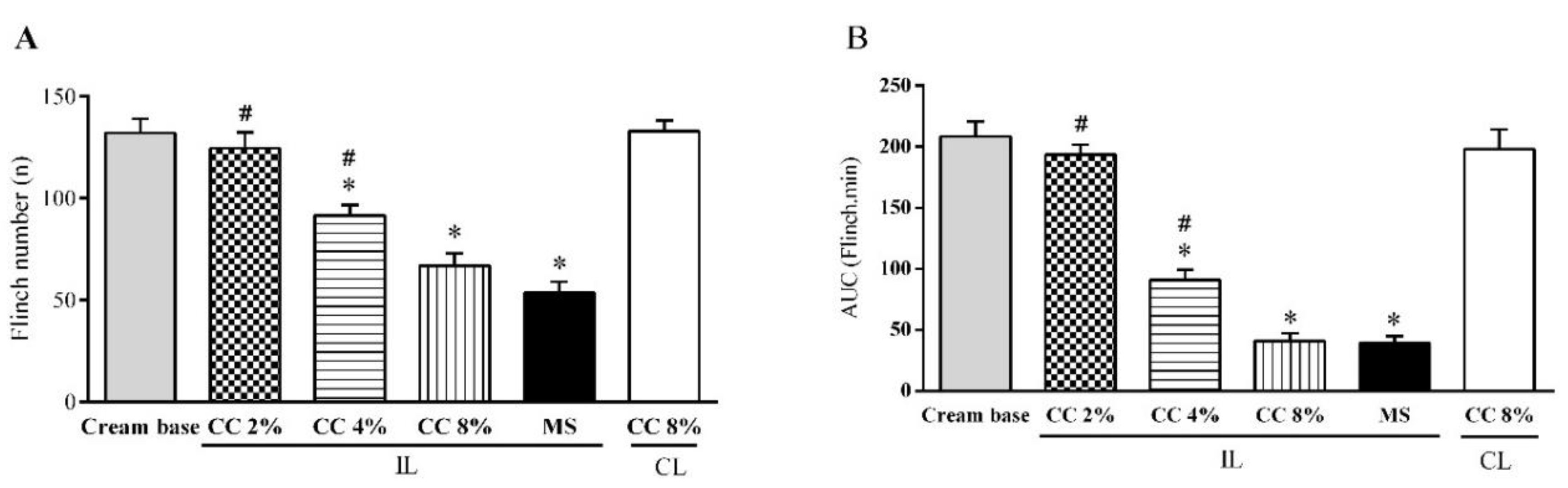
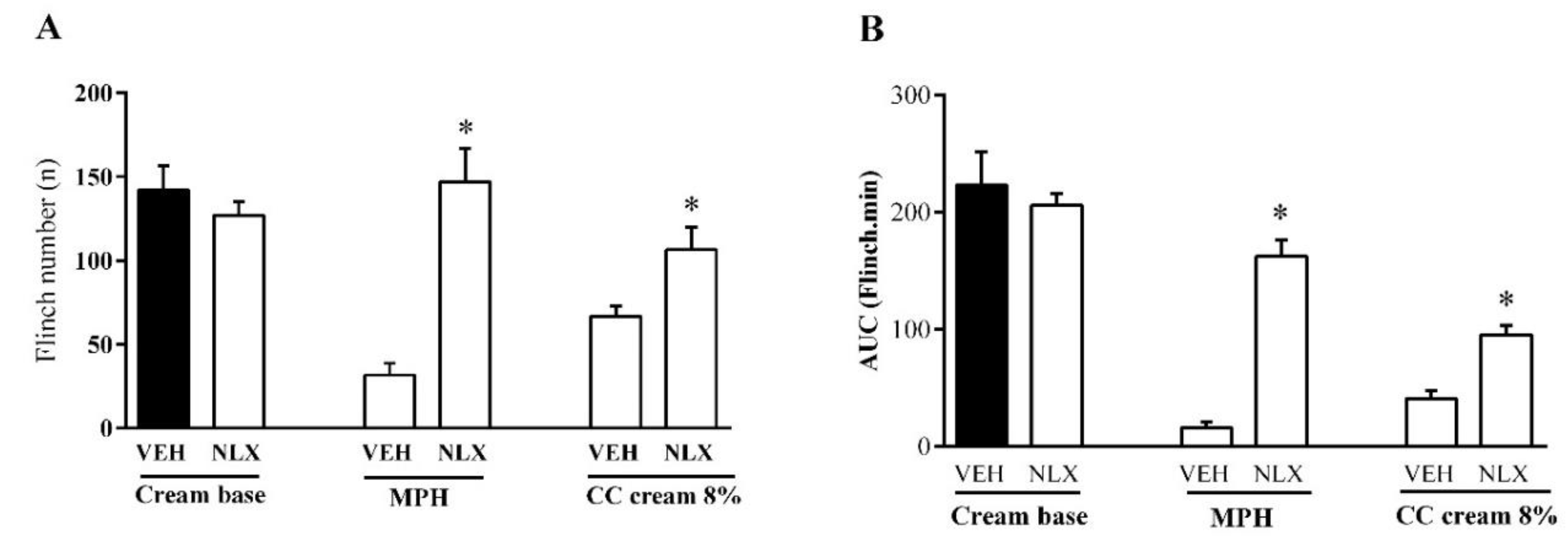
3.3. Effect of Topical CC Cream on Formalin-Induced Nociception
3.4. Skin Irritation Test
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Finch, C.E. Developmental origins of aging in brain and blood vessels: An overview. Neurobiol. Aging 2005, 26, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Marzouk, B.; Marzouk, Z.; Fenina, N.; Bouraoui, A.; Aouni, M. Anti-inflammatory and analgesic activities of Tunisian Citrullus colocynthis Schrad. Immature fruit and seed organic extracts. Eur. Rev. Med. Pharmacol. Sci. 2011, 15, 665–672. [Google Scholar] [PubMed]
- Pietrovski, E.F.; Magina, M.D.A.; Gomig, F.; Pietrovski, C.F.; Micke, G.A.; Barcellos, M.; Pizzolatti, M.G.; Cabrini, D.A.; Brighente, I.M.C.; Otuki, M.F. Topical anti-inflammatory activity of Eugenia brasiliensis Lam. (myrtaceae) leaves. J. Pharm. Pharmacol. 2008, 60, 479–487. [Google Scholar] [PubMed]
- Rus, H.; Niculescu, F.I. Inflammation, aspirin, and the risk of cardiovascular disease. N. Engl. J. Med. 1997, 337, 423. [Google Scholar] [PubMed]
- Sanadgol, N.; Najafi, S.; Ghasemi, L.V.; Motalleb, G.; Estakhr, J. A study of the inhibitory effects of Citrullus colocynthis (CCT) using hydro-alcoholic extract on the expression of cytokines: Tnf-and il-6 in high fat diet-fed mice towards a cure for diabetes mellitus. J. Pharm. Phytother. 2011, 3, 81–88. [Google Scholar]
- Price, D.D.; Mao, J.; Lu, J.; Caruso, F.S.; Frenk, H.; Mayer, D.J. Effects of the combined oral administration of nsaids and dextromethorphan on behavioral symptoms indicative of arthritic pain in rats. Pain 1996, 68, 119–127. [Google Scholar] [CrossRef]
- Koehn, F.E.; Carter, G.T. The evolving role of natural products in drug discovery. Nat. Rev. Drug Discov. 2005, 4, 206–220. [Google Scholar] [PubMed]
- Heydari, M.; Homayouni, K.; Hashempur, M.H.; Shams, M. Topical Citrullus colocynthis (bitter apple) extract oil in painful diabetic neuropathy: A double-blind randomized placebo-controlled clinical trial. J. Diabetes 2016, 8, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Rajamanickam, E.; Gurudeeban, S.; Ramanathan, T.; Satyavani, K. Evaluation of anti inflammatory activity of Citrullus colocynthis. Int. J. Curr. Res. 2010, 2, 67–69. [Google Scholar]
- Marzouk, B.; Marzouk, Z.; Haloui, E.; Fenina, N.; Bouraoui, A.; Aouni, M. Screening of analgesic and anti-inflammatory activities of Citrullus colocynthis from Southern Tunisia. J. Ethnopharmacol. 2010, 128, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Tannin-Spitz, T.; Grossman, S.; Dovrat, S.; Gottlieb, H.E.; Bergman, M. Growth inhibitory activity of cucurbitacin glucosides isolated from Citrullus colocynthis on human breast cancer cells. Biochem. Pharmacol. 2007, 73, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Rahbar, A.R.; Nabipour, I. The hypolipidemic effect of Citrullus colocynthis on patients with hyperlipidemia. Pak. J. Biol. Sci. 2010, 13, 1202–1207. [Google Scholar] [CrossRef] [PubMed]
- Bendjeddou, D.; Lalaoui, K.; Satta, D. Immunostimulating activity of the hot water-soluble polysaccharide extracts of Anacyclus pyrethrum, Alpinia galanga and Citrullus colocynthis. J. Ethnopharmacol. 2003, 88, 155–160. [Google Scholar] [CrossRef]
- Rizvi, T.S.; Khan, A.L.; Ali, L.; Al-Mawali, N.; Mabood, F.; Hussain, J.; Adnan, M.; Al-Harrasi, A. In vitro oxidative stress regulatory potential of Citrullus colocynthis and Tephrosia apollinea. Acta Pharm. 2018, 68, 235–242. [Google Scholar] [CrossRef] [PubMed]
- James, A. Duke’s Hand Book of Medicinal Plants of the Bible; Taylor and Francis Group: Boca Raton, FL, USA, 2008. [Google Scholar]
- Alhawiti, N.M. Antiplatelets and profibrinolytic activity of Citrullus colocynthis in control and high-fat diet-induced obese rats: Mechanisms of action. Arch. Physiol. Biochem. 2018, 124, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Akhzari, M.; Mirghiasi, S.; Vassaf, M.; Bidgoli, M.; Tari, Z. The effect of Citrullus colocynthis on the reduction of inflammatory agents in osteoarthritis. J. Mol. Biol. 2015. [Google Scholar] [CrossRef]
- Yun, K.-J.; Kim, J.-Y.; Kim, J.-B.; Lee, K.-W.; Jeong, S.-Y.; Park, H.-J.; Jung, H.-J.; Cho, Y.-W.; Yun, K.; Lee, K.-T. Inhibition of LPS-induced no and PGE2 production by Asiatic acid via NF-κb inactivation in RAW 264.7 macrophages: Possible involvement of the IKK and MAPK pathways. Int. Immunopharmacol. 2008, 8, 431–441. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Kumar, D.; Manjusha; Saroha, K.; Singh, N.; Vashishta, B. Antioxidant and free radical scavenging potential of Citrullus colocynthis (L.) schrad. Methanolic fruit extract. Acta Pharm. 2008, 58, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Adam, S.; Al-Yahya, M.; Al-Farhan, A. Combined toxicity of cassia senna and Citrullus colocynthis in rats. Vet. Hum. Toxicol. 2001, 43, 70–72. [Google Scholar] [PubMed]
- Javadzadeh, H.R.; Davoudi, A.; Davoudi, F.; Valizadegan, G.; Goodarzi, H.; Mahmoodi, S.; Ghane, M.R.; Faraji, M. Citrullus colocynthis as the cause of acute rectorrhagia. Case Rep. Emerg. Med. 2013, 2013, 652192. [Google Scholar] [PubMed]
- Dehghani, F.; Panjehshahin, M.R. The toxic effect of alcoholic extract of Citrullus colocynthis on rat liver. Iranian J. Pharmacol. Ther. 2006, 5, 117–119. [Google Scholar]
- Sawynok, J. Topical and peripherally acting analgesics. Pharmacol. Rev. 2003, 55, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Winter, C.A.; Risley, E.A.; Nuss, G.W. Carrageenin-induced edema in hind paw of the rat as an assay for antiinflammatory drugs. Proc. Soc. Exp. Boil. Med. 1962, 111, 544–547. [Google Scholar] [CrossRef]
- Dubuisson, D.; Dennis, S.G. The formalin test: A quantitative study of the analgesic effects of morphine, meperidine, and brain stem stimulation in rats and cats. Pain 1977, 4, 161–174. [Google Scholar] [CrossRef]
- Sakurada, T.; Mizoguchi, H.; Kuwahata, H.; Katsuyama, S.; Komatsu, T.; Morrone, L.A.; Corasaniti, M.T.; Bagetta, G.; Sakurada, S. Intraplantar injection of bergamot essential oil induces peripheral antinociception mediated by opioid mechanism. Pharmacol. Biochem. Behav. 2011, 97, 436–443. [Google Scholar] [CrossRef] [PubMed]
- Mutalik, S.; Udupa, N. Glibenclamide transdermal patches: Physicochemical, pharmacodynamic, and pharmacokinetic evaluations. J. Pharm. Sci. 2004, 93, 1577–1594. [Google Scholar] [CrossRef] [PubMed]
- Sosa, S.; Balick, M.; Arvigo, R.; Esposito, R.; Pizza, C.; Altinier, G.; Tubaro, A. Screening of the topical anti-inflammatory activity of some Central American plants. J. Ethnopharmacol. 2002, 81, 211–215. [Google Scholar] [CrossRef]
- Schaible, H.G.; Ebersberger, A.; Banchet, G.S. Mechanisms of pain in arthritis. Ann. N. Y. Acad. Sci. 2002, 966, 343–354. [Google Scholar] [CrossRef] [PubMed]
- Woolf, C.; Allchorne, A.; Safieh-Garabedian, B.; Poole, S. Cytokines, nerve growth factor and inflammatory hyperalgesia: The contribution of tumour necrosis factor α. Br. J. Pharmacol. 1997, 121, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Cunha, T.M.; Verri, W.; Silva, J.; Poole, S.; Cunha, F.; Ferreira, S. A cascade of cytokines mediates mechanical inflammatory hypernociception in mice. Proc. Natl. Acad. Sci. USA 2005, 102, 1755–1760. [Google Scholar] [CrossRef] [PubMed]
- Murray, A.; Kisin, E.; Castranova, V.; Kommineni, C.; Gunther, M.; Shvedova, A. Phenol-induced in vivo oxidative stress in skin: Evidence for enhanced free radical generation, thiol oxidation, and antioxidant depletion. Chem. Res. Toxicol. 2007, 20, 1769–1777. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.-H.; Huang, S.-S.; Wang, B.-S.; Wu, C.-H.; Sheu, M.-J.; Hou, W.-C.; Lin, S.-S.; Huang, G.-J. Antioxidant and anti-inflammatory properties of Cardiospermum halicacabum and its reference compounds ex vivo and in vivo. J. Ethnopharmacol. 2011, 133, 743–750. [Google Scholar] [PubMed]
- Yu, M.; Zheng, X.; Witschi, H.; Pinkerton, K.E. The role of interleukin-6 in pulmonary inflammation and injury induced by exposure to environmental air pollutants. Toxicol. Sci. 2002, 68, 488–497. [Google Scholar] [CrossRef] [PubMed]
- Martindale, J.; Bland-Ward, P.; Chessell, I. Inhibition of C-fibre mediated sensory transmission in the rat following intraplantar formalin. Neurosci. Lett. 2001, 316, 33–36. [Google Scholar] [CrossRef]
- Abbott, F.V.; Hellemans, K.G. Phenacetin, acetaminophen and dipyrone: Analgesic and rewarding effects. Behav. Brain Res. 2000, 112, 177–186. [Google Scholar] [CrossRef]
- Gendron, L.; Lucido, A.L.; Mennicken, F.; O’Donnell, D.; Vincent, J.-P.; Stroh, T.; Beaudet, A. Morphine and pain-related stimuli enhance cell surface availability of somatic δ-opioid receptors in rat dorsal root ganglia. Neurosci. Res. 2006, 26, 953–962. [Google Scholar] [CrossRef] [PubMed]
- Stein, C.; Zöllner, C. Opioids and sensory nerves. In Sensory Nerves; Springer: Berlin/Heidelberg, Germany, 2009; pp. 495–518. [Google Scholar]
- Mandegary, A.; Pournamdari, M.; Sharififar, F.; Pournourmohammadi, S.; Fardiar, R.; Shooli, S. Alkaloid and flavonoid rich fractions of fenugreek seeds (Trigonella foenum-graecum L.) with antinociceptive and anti-inflammatory effects. Food Chem. Toxicol. 2012, 50, 2503–2507. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pashmforosh, M.; Rajabi Vardanjani, H.; Rajabi Vardanjani, H.; Pashmforosh, M.; Khodayar, M.J. Topical Anti-Inflammatory and Analgesic Activities of Citrullus colocynthis Extract Cream in Rats. Medicina 2018, 54, 51. https://doi.org/10.3390/medicina54040051
Pashmforosh M, Rajabi Vardanjani H, Rajabi Vardanjani H, Pashmforosh M, Khodayar MJ. Topical Anti-Inflammatory and Analgesic Activities of Citrullus colocynthis Extract Cream in Rats. Medicina. 2018; 54(4):51. https://doi.org/10.3390/medicina54040051
Chicago/Turabian StylePashmforosh, Marzieh, Hossein Rajabi Vardanjani, Hassan Rajabi Vardanjani, Mahdi Pashmforosh, and Mohammad Javad Khodayar. 2018. "Topical Anti-Inflammatory and Analgesic Activities of Citrullus colocynthis Extract Cream in Rats" Medicina 54, no. 4: 51. https://doi.org/10.3390/medicina54040051
APA StylePashmforosh, M., Rajabi Vardanjani, H., Rajabi Vardanjani, H., Pashmforosh, M., & Khodayar, M. J. (2018). Topical Anti-Inflammatory and Analgesic Activities of Citrullus colocynthis Extract Cream in Rats. Medicina, 54(4), 51. https://doi.org/10.3390/medicina54040051



