Amelioration of Ovalbumin-Induced Food Allergy in Mice by Targeted Rectal and Colonic Delivery of Cyanidin-3-O-Glucoside
Abstract
:1. Introduction
2. Materials and Methods
2.1. Regents and Materials
2.2. Preparation of Samples
2.3. Animals
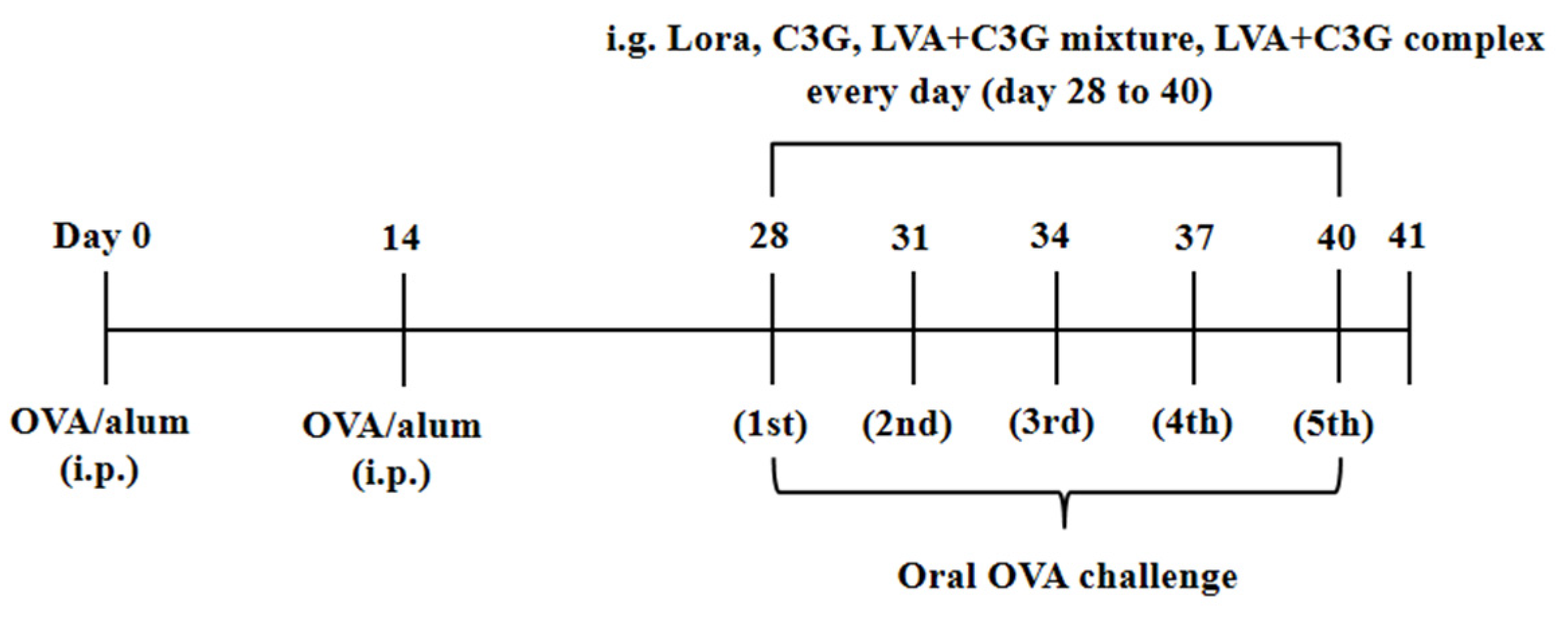
2.4. Protocol of the FA Mouse Model
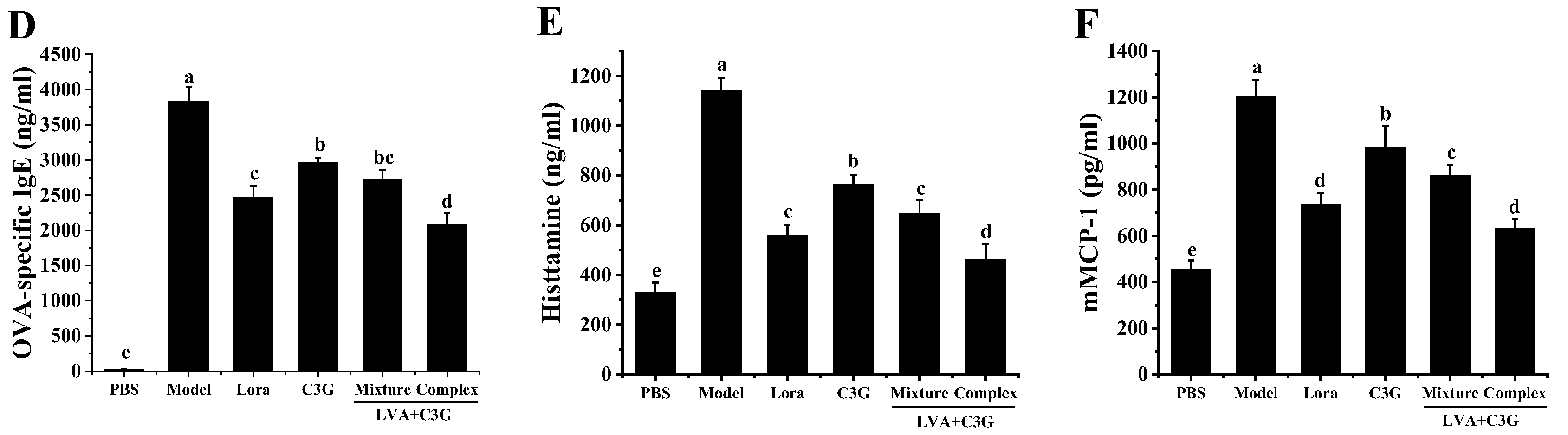
2.5. Allergy-Related Factors in the Serum
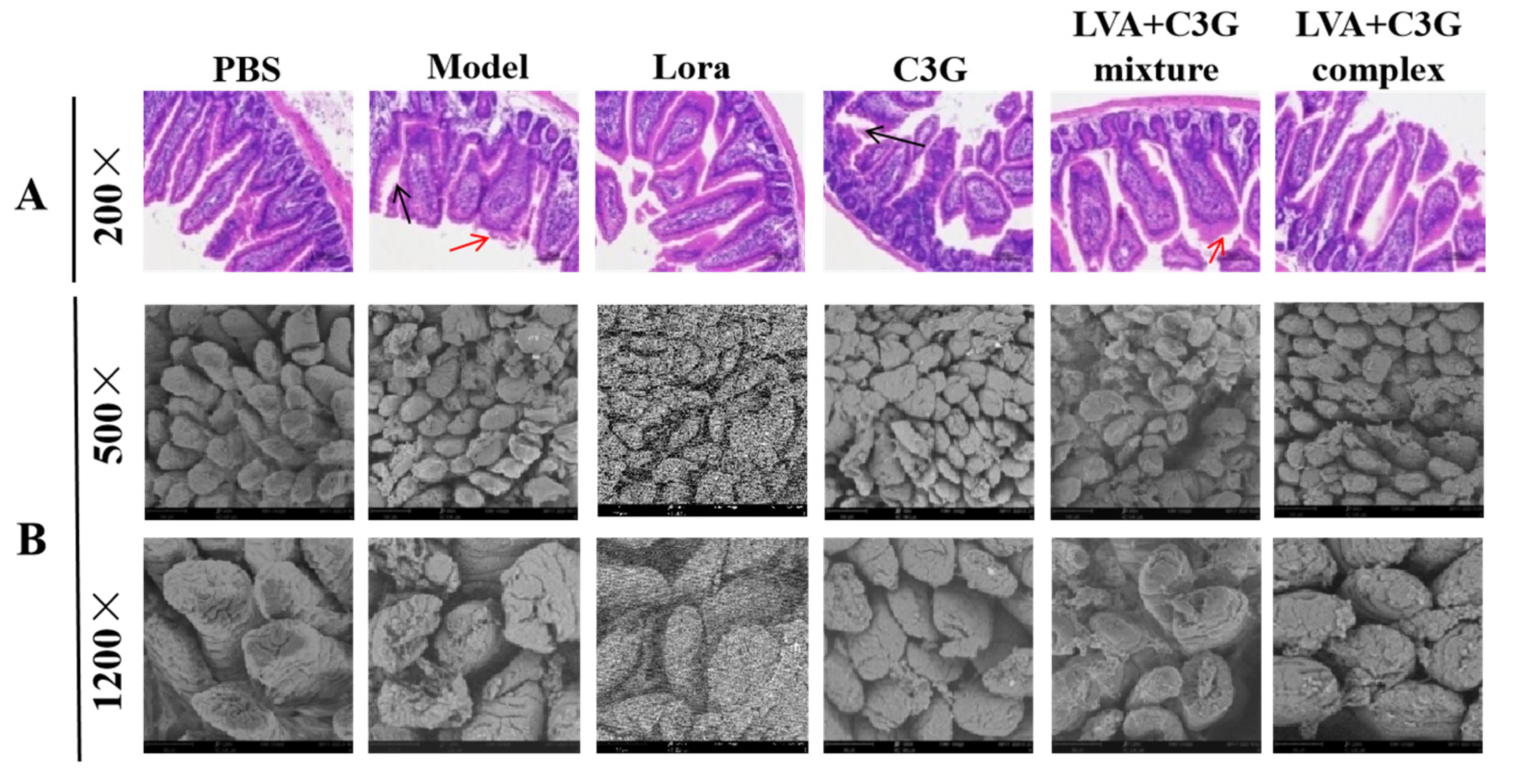
2.6. Histological Examination
2.7. Determination of the Na+-K+-ATPase Activity
2.8. Analysis of the Intestinal Villi Integrity
2.9. Quantification of the Cytokine and TJ Protein Expression in the Intestinal Tissue
2.10. 16S rDNA Gene Sequencing of the Gut Microbiota
2.11. Statistical Analysis
3. Results
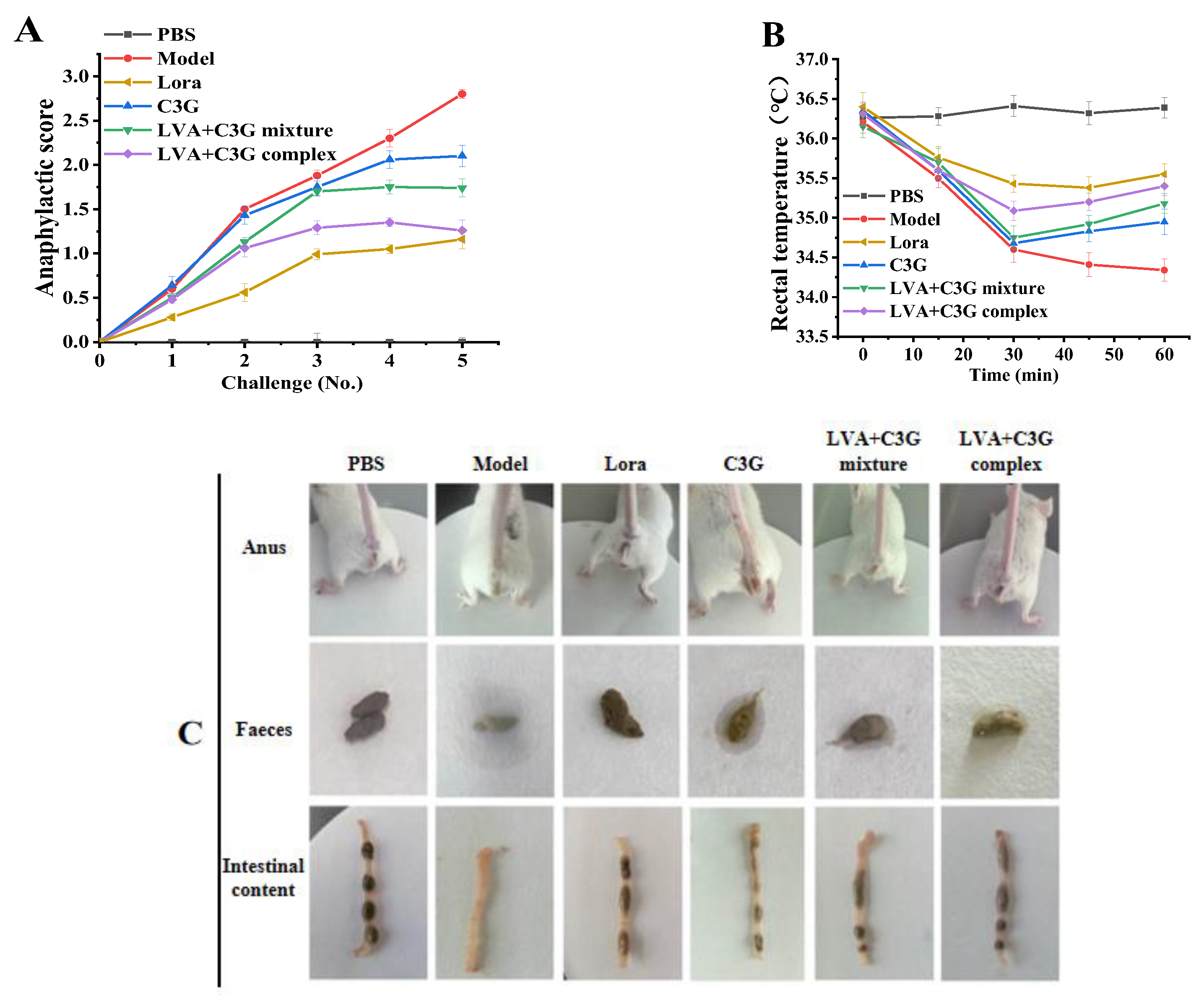
3.1. Clinical Symptoms and Anatomic Observations of the Allergic Mice
3.2. Observation of the Physical Barrier Function of the Intestinal Epithelium
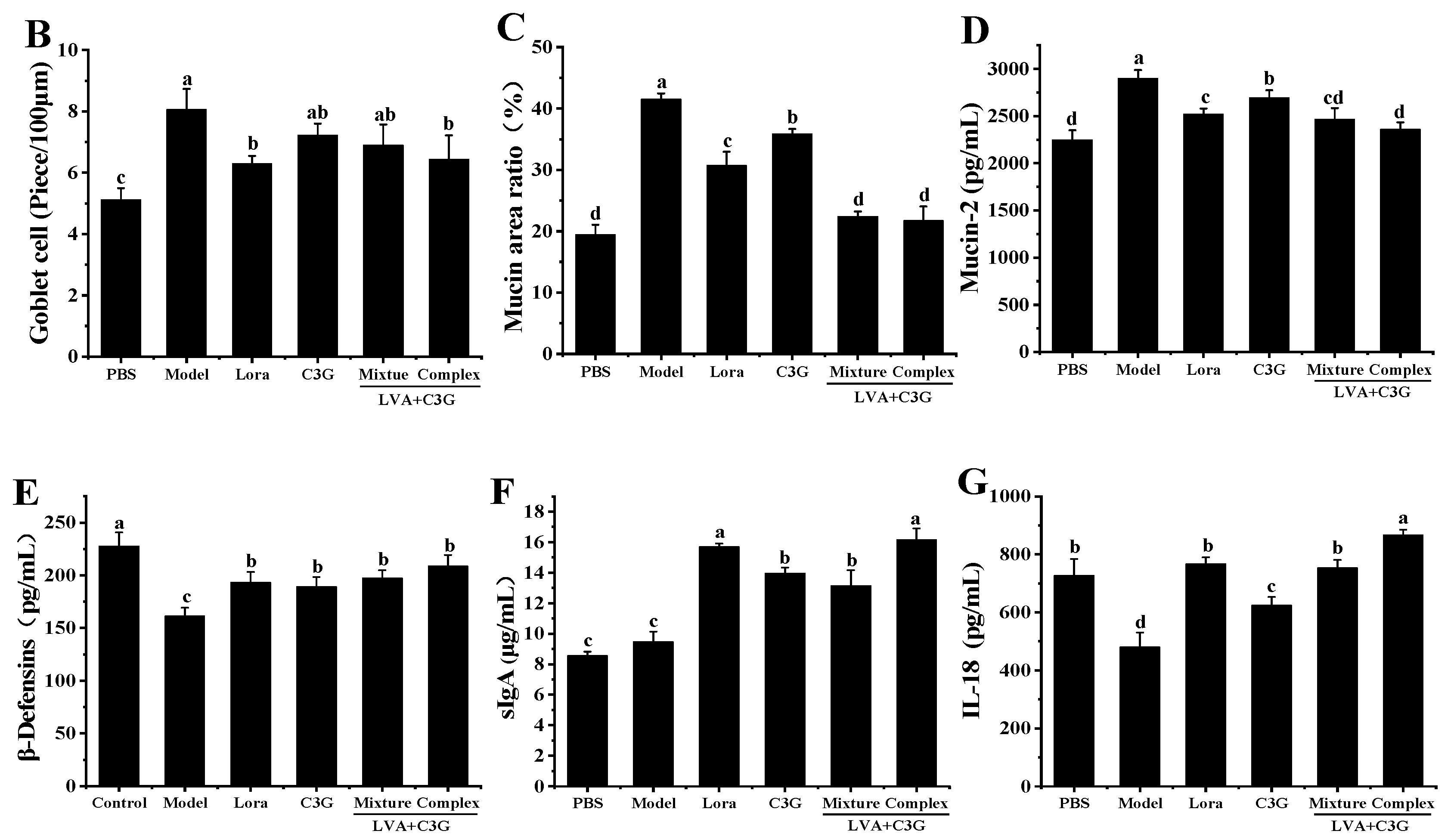
3.3. Observation of the Chemical Barrier Function of the Intestinal Epithelium
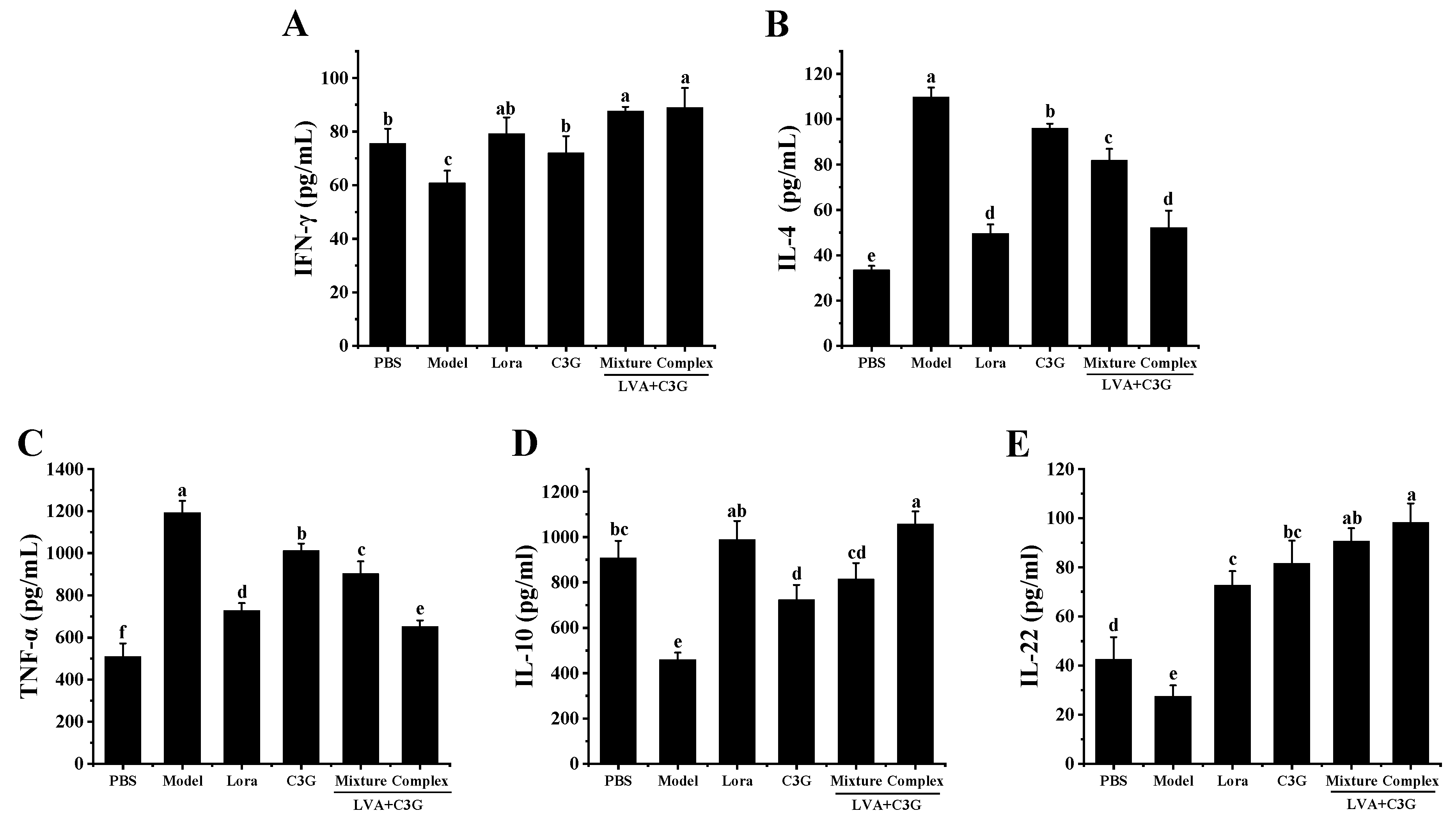
3.4. Regulation of the Intestinal Immune-Related Cytokines
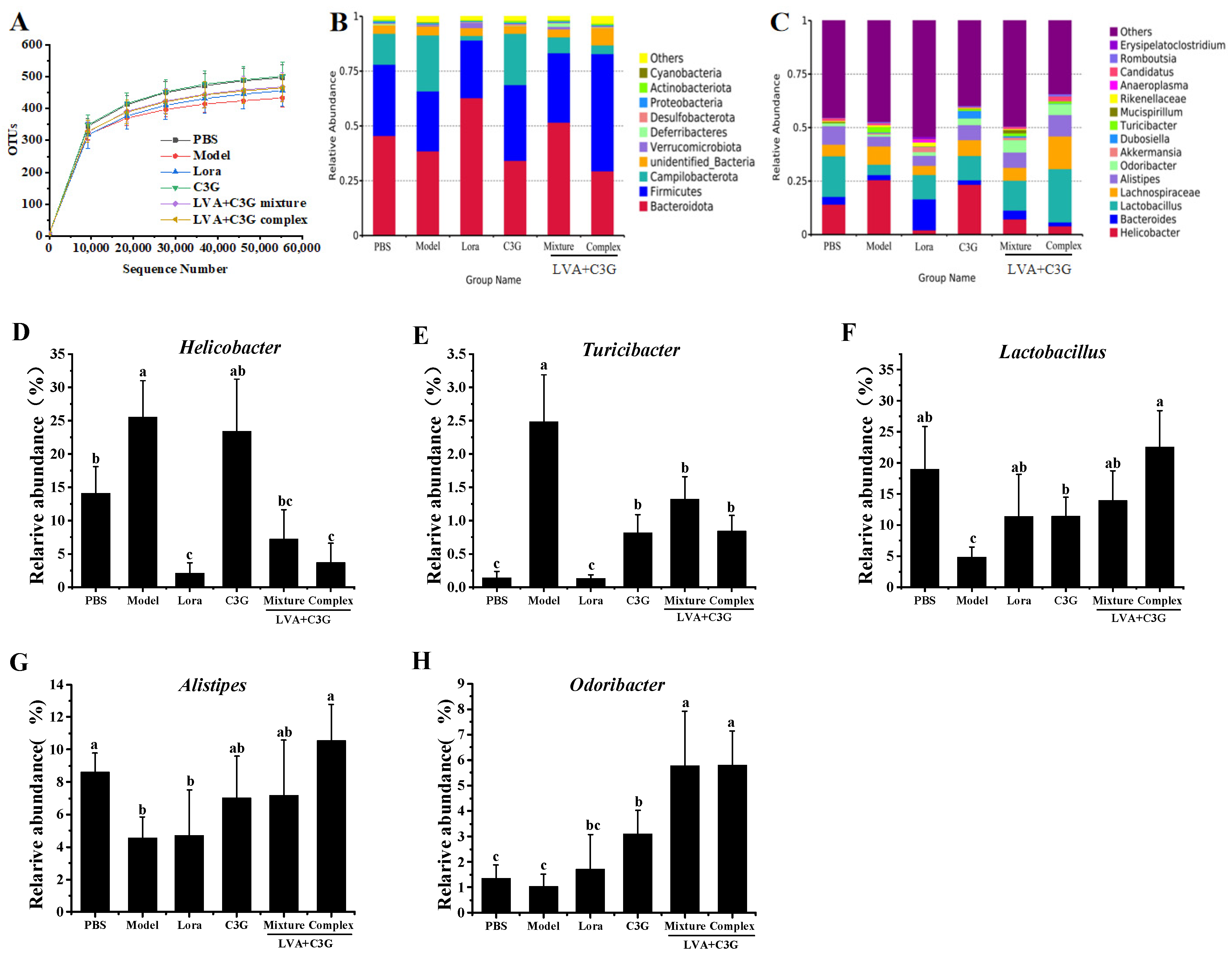
3.5. Analysis of the Composition of the Flora
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, Z.; Zhong, J.; Meng, X.; Gao, J.; Li, H.; Sun, J.; Li, X.; Chen, H. The gut microbiome-immune axis as a target for nutrition-mediated modulation of food allergy. Trends Food Sci. Technol. 2021, 114, 116–132. [Google Scholar] [CrossRef]
- Boyce, J.A.; Assa’ad, A.; Burks, A.W.; Jones, S.M.; Sampson, H.A.; Wood, R.A.; Schwaninger, J.M.; Luccioli, S.; McCall, K.M.; Schwaninger, J.M.; et al. Guidelines for the Diagnosis and Management of Food Allergy in the United States: Summary of the NIAID-Sponsored Expert Panel Report. J. Allergy Clin. Immunol. 2010, 126, S1–S58. [Google Scholar] [CrossRef] [PubMed]
- Loh, W.; Tang, M.L. The epidemiology of food allergy in the global context. Int. J. Environ. Res. Public Health 2018, 15, 2043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.; Tan, X.; Fu, Y.; Dai, H.; Wang, H.; Zhao, G.; Zhang, Y. The development of natural and designed protein nanocages for encapsulation and delivery of active compounds. Food Hydrocoll. 2021, 121, 107004. [Google Scholar] [CrossRef]
- Ali, A.; Tan, H.; Kaiko, G.E. Role of the intestinal epithelium and its interaction with the microbiota in food allergy. Front. Immunol. 2020, 11, 3222. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Yan, J.; Xiang, Q.; Wang, F.; Dai, H.; Huang, K.; Zhang, W.; Fang, L.; Yao, H.; Wang, L. Fructooligosaccharides protect against OVA-induced food allergy in mice by regulating the Th17/Treg cell balance using tryptophan metabolites. Food Funct. 2021, 12, 3191–3205. [Google Scholar] [CrossRef] [PubMed]
- Kasote, D.M.; Jayaprakasha, G.K.; Patil, B.S. Encapsulation of polyphenols: An effective way to enhance their bioavailability for gut health. In Advances in Plant Phenolics: From Chemistry to Human Health; Jayaprakasha, G.K., Patil, B.S., Gattuso, G., Eds.; ACS Symposium Series: Washington, DC, USA, 2018; Volume 1286, pp. 239–259. [Google Scholar] [CrossRef]
- He, B.; Ge, J.; Yue, P.; Yue, X.; Fu, R.; Liang, J.; Gao, X. Loading of anthocyanins on chitosan nanoparticles influences anthocyanin degradation in gastrointestinal fluids and stability in a beverage. Food Chem. 2017, 221, 1671–1677. [Google Scholar] [CrossRef]
- Pasukamonset, P.; Kwon, O.; Adisakwattana, S. Alginate-based encapsulation of polyphenols from Clitoria ternatea petal flower extract enhances stability and biological activity under simulated gastrointestinal conditions. Food Hydrocoll. 2016, 61, 772–779. [Google Scholar] [CrossRef]
- Tang, H.Y.; Fang, Z.; Ng, K. Dietary fiber-based colon-targeted delivery systems for polyphenols. Trends Food Sci. Tech. 2020, 100, 333–348. [Google Scholar] [CrossRef]
- Rabišková, M.; Bautzová, T.; Gajdziok, J.; Dvořáčková, K.; Lamprecht, A.; Pellequer, Y.; Spilková, J. Coated chitosan pellets containing rutin intended for the treatment of inflammatory bowel disease: In vitro characteristics and in vivo evaluation. Int. J. Pharmaceut. 2012, 422, 151–159. [Google Scholar] [CrossRef]
- Caddeo, C.; Nácher, A.; Díez-Sales, O.; Merino-Sanjuán, M.; Fadda, A.M.; Manconi, M. Chitosan–xanthan gum microparticle-based oral tablet for colon-targeted and sustained delivery of quercetin. J. Microencapsul. 2014, 31, 694–699. [Google Scholar] [CrossRef] [PubMed]
- West, C.E.; Dzidic, M.; Prescott, S.L.; Jenmalm, M.C. Bugging allergy; role of pre-, pro-and synbiotics in allergy prevention. Allergol. Int. 2017, 66, 529–538. [Google Scholar] [CrossRef] [PubMed]
- Kunisawa, J.; Kiyono, H. Aberrant interaction of the gut immune system with environmental factors in the development of food allergies. Curr. Allergy Asthma Rep. 2010, 10, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Tu, Y.; Bourgeois, J.; Di Leo, V.; Irvine, E.J.; Marshall, J.K.; Perdue, M.H. CD23-mediated IgE transport across human intestinal epithelium: Inhibition by blocking sites of translation or binding. Gastroenterology 2005, 129, 928–940. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ma, Y.; Chen, Z.; Zou, C.; Liu, W.; Yang, L.; Fu, L.; Wang, Y.; Liu, G.; Cao, M. Depolymerized sulfated galactans from Eucheuma serra ameliorate allergic response and intestinal flora in food allergic mouse model. Int. J. Biol. Macromol. 2021, 166, 977–985. [Google Scholar] [CrossRef] [PubMed]
- Nowak-Wegrzyn, A.; Szajewska, H.; Lack, G. Food allergy and the gut. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 241–257. [Google Scholar] [CrossRef] [PubMed]
- Shu, S.A.; Yuen, A.W.; Woo, E.; Chu, K.H.; Kwan, H.S.; Yang, G.X.; Yang, Y.; Leung, P.S.C. Microbiota and food allergy. Clin. Rev. Allergy Immunol. 2019, 57, 83–97. [Google Scholar] [CrossRef]
- Stefka, A.T.; Feehley, T.; Tripathi, P.; Qiu, J.; McCoy, K.; Mazmanian, S.K.; Tjota, M.Y.; Seo, G.-Y.; Cao, S.; Theriault, B.R.; et al. Commensal bacteria protect against food allergen sensitization. Proc. Natl. Acad. Sci. USA 2014, 111, 13145–13150. [Google Scholar] [CrossRef] [Green Version]
- Zou, C.; Huang, L.; Li, D.; Ma, Y.; Liu, Y.; Wang, Y.; Cao, M.; Liu, G.; Sun, L. Assembling cyanidin-3-O-glucoside by using low-viscosity alginate to improve its in vitro bioaccessibility and in vivo bioavailability. Food Chem. 2021, 355, 129681. [Google Scholar] [CrossRef]
- Liu, Q.M.; Xie, C.L.; Gao, Y.Y.; Liu, B.; Lin, W.X.; Liu, H.; Cao, M.; Su, W.; Yang, X.; Liu, G. Deep-sea-derived butyrolactone I suppresses ovalbumin-induced anaphylaxis by regulating mast cell function in a murine model. J. Agri. Food Chem. 2018, 66, 5581–5592. [Google Scholar] [CrossRef]
- Chung, M.Y.; Shin, H.S.; Choi, D.W.; Shon, D.H. Citrus Tachibana leaf extract mitigates symptoms of food allergy by inhibiting Th2-associated responses. J. Food Sci. 2016, 81, H1537–H1545. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Liu, B.; Liu, Q.M.; Zhang, Y.F.; Liu, Y.X.; Liu, H.; Cao, M.; Liu, G. Red algae sulfated polysaccharides effervescent tablets attenuated ovalbumin-induced anaphylaxis by upregulating regulatory T cells in mouse models. J. Agri. Food Chem. 2019, 67, 11911–11921. [Google Scholar] [CrossRef] [PubMed]
- Azumi, R.; Morita, K.; Mizutani, Y.; Hayatsu, M.; Terai, S.; Ushiki, T. Dynamics of basal lamina fenestrations in the rat intestinal villous epithelium in response to dietary conditions. Biomed. Res. 2018, 39, 65–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Lozupone, C.A.; Turnbaugh, P.J.; Fierer, N. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc. Natl. Acad. Sci. USA 2011, 108, 4516–4522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cole, J.R.; Chai, B.; Farris, R.J.; Wang, Q.; Kulam, S.A.; McGarrell, D.M.; Garrity, G.M.; Tiedje, J.M. The Ribosomal Database Project (RDP-II): Sequences and tools for high-throughput rRNA analysis. Nucleic Acids Res. 2005, 33, D294–D296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mine, Y.; Majumder, K.; Jin, Y.; Zeng, Y. Chinese sweet tea (Rubus suavissimus) polyphenols attenuate the allergic responses in a Balb/c mouse model of egg allergy. J. Funct. Foods 2020, 67, 103827. [Google Scholar] [CrossRef]
- Liu, Q.M.; Yang, Y.; Maleki, S.J.; Alcocer, M.; Xu, S.S.; Shi, C.L.; Cao, M.; Liu, G. Anti-food allergic activity of sulfated polysaccharide from Gracilaria lemaneiformis is dependent on immunosuppression and inhibition of p38 MAPK. J. Agri. Food Chem. 2016, 64, 4536–4544. [Google Scholar] [CrossRef]
- Yu, B.; Bi, D.; Yao, L.; Li, T.; Gu, L.; Xu, H.; Li, X.; Li, H.; Hu, Z.; Xu, X. The inhibitory activity of alginate against allergic reactions in an ovalbumin-induced mouse model. Food Funct. 2020, 11, 2704–2713. [Google Scholar] [CrossRef]
- Wang, M.; Han, J.; Domenico, J.; Shin, Y.S.; Jia, Y.; Gelfand, E.W. Combined blockade of the histamine H1 and H4 receptor suppresses peanut-induced intestinal anaphylaxis by regulating dendritic cell function. Allergy 2016, 71, 1561–1574. [Google Scholar] [CrossRef] [Green Version]
- Groschwitz, K.R.; Hogan, S.P. Intestinal barrier function: Molecular regulation and disease pathogenesis. J. Allergy Clin. Immun. 2009, 124, 3–20. [Google Scholar] [CrossRef] [Green Version]
- Feng, Z.; Peng, S.; Wu, Z.; Jiao, L.; Xu, S.; Wu, Y.; Liu, Z.; Hu, Y.; Liu, J.; Wu, Y.; et al. Ramulus mori polysaccharide-loaded PLGA nanoparticles and their anti-inflammatory effects in vivo. Int. J. Biol. Macromol. 2021, 81, 2024–2036. [Google Scholar] [CrossRef] [PubMed]
- Tian, B.; Zhang, Z.; Zhao, J.; Ma, Q.; Liu, H.; Nie, C.; Ma, Z.; An, W.; Li, J. Dietary whole Goji berry (Lycium barbarum) intake improves colonic barrier function by altering gut microbiota composition in mice. Int. J. Food Sci. Tech. 2021, 56, 103–114. [Google Scholar] [CrossRef]
- Xie, S.Z.; Liu, B.; Ye, H.Y.; Li, Q.M.; Pan, L.H.; Zha, X.Q.; Liu, J.; Duan, J.; Luo, J.-P. Dendrobium huoshanense polysaccharide regionally regulates intestinal mucosal barrier function and intestinal microbiota in mice. Carbohyd. Polym. 2019, 206, 149–162. [Google Scholar] [CrossRef] [PubMed]
- Bae, M.J.; Shin, H.S.; See, H.J.; Jung, S.Y.; Kwon, D.A.; Shon, D.H. Baicalein induces CD4+ Foxp3+ T cells and enhances intestinal barrier function in a mouse model of food allergy. Sci. Rep. 2016, 6, 470. [Google Scholar] [CrossRef] [Green Version]
- Perrier, C.; Corthesy, B. Gut permeability and food allergies. Clin. Exp. Allergy 2011, 41, 20–28. [Google Scholar] [CrossRef]
- Mahapatro, M.; Erkert, L.; Becker, C. Cytokine-mediated crosstalk between immune cells and epithelial cells in the gut. Cells 2021, 10, 111. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, J.; Xue, L.; Liu, J.; Nie, C.; Fan, M.; Qian, H.; Zhang, D.; Ying, H.; Li, Y.; et al. l-Arabinose attenuates gliadin-induced food allergy via regulation of Th1/Th2 balance and upregulation of regulatory T cells in mice. J. Agri. Food Chem. 2021, 69, 3638–3646. [Google Scholar] [CrossRef]
- Shin, H.S.; Bae, M.J.; Jung, S.Y.; Shon, D.H. Preventive effects of skullcap (Scutellaria baicalensis) extract in a mouse model of food allergy. J. Ethnopharmacol. 2014, 153, 667–673. [Google Scholar] [CrossRef]
- Han, R.; Wang, L.; Zhao, Z.; You, L.; Pedisić, S.; Kulikouskaya, V.; Lin, Z. Polysaccharide from Gracilaria Lemaneiformis prevents colitis in Balb/c mice via enhancing intestinal barrier function and attenuating intestinal inflammation. Food Hydrocolloid. 2020, 109, 106048. [Google Scholar] [CrossRef]
- Ouyang, W.; Rutz, S.; Crellin, N.K.; Valdez, P.A.; Hymowitz, S.G. Regulation and functions of the IL-10 family of cytokines in inflammation and disease. Annu. Rev. Immunol. 2011, 29, 71–109. [Google Scholar] [CrossRef]
- Lee, D.; Kim, H.S.; Shin, E.; Do, S.G.; Lee, C.K.; Kim, Y.M.; Lee, M.B.; Min, K.Y.; Koo, J.; Kim, S.J.; et al. Polysaccharide isolated from Aloe vera gel suppresses ovalbumin-induced food allergy through inhibition of Th2 immunity in mice. Biomed. Pharmacother. 2018, 101, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zhang, Y.; Shu, Z.; Liu, M.; Zeng, R.; Wang, Y.; Liu, H.; Cao, M.; Su, W.; Liu, G. Sulfated oligosaccharide of Gracilaria lemaneiformis protect against food allergic response in mice by up-regulating immunosuppression. Carbohyd. Polym. 2020, 230, 115567. [Google Scholar] [CrossRef] [PubMed]
- Remely, M.; Hippe, B.; Zanner, J.; Aumueller, E.; Brath, H.G.; Haslberger, A. Gut microbiota of obese, type 2 diabetic individuals is enriched in Faecalibacterium prausnitzii, Akkermansia muciniphila and Peptostreptococcus anaerobius after weight loss. Endocr. Metab Immune 2016, 16, 99–106. [Google Scholar] [CrossRef]
- Ni, Y.; Yang, X.; Zheng, L.; Wang, Z.; Wu, L.; Jiang, J.; Yang, T.; Ma, L.; Fu, Z. Lactobacillus and Bifidobacterium improves physiological function and cognitive ability in aged mice by the regulation of gut microbiota. Mol. Nutr. Food Res. 2019, 63, 1900603. [Google Scholar] [CrossRef] [PubMed]
- Hiippala, K.; Barreto, G.; Burrello, C.; Diaz-Basabe, A.; Suutarinen, M.; Kainulainen, V.; Bowers, J.R.; Lemmer, D.; Engelthaler, D.M.; Eklund, K.K.; et al. Novel odoribacter splanchnicus strain and its outer membrane vesicles exert immunoregulatory effects in vitro. Front Microbiol. 2020, 11, 2906. [Google Scholar] [CrossRef]
- Ni, W.W.; Zhang, Q.M.; Zhang, X.; Li, Y.; Yu, S.S.; Wu, H.Y.; Chen, Z.; Li, A.-I.; Du, P.; Li, C. Modulation effect of Lactobacillus acidophilus KLDS 1.0738 on gut microbiota and TLR4 expression in β-lactoglobulin-induced allergic mice model. Allergol. Immunopath. 2020, 48, 149–157. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, C.; Dong, X.; Hu, T.; Wang, L.; Zhao, W.; Zhu, S.; Li, G.; Hu, Y.; Gao, Q.; et al. Chronic alcohol exposure induced gut microbiota dysbiosis and its correlations with neuropsychic behaviors and brain BDNF/Gabra1 changes in mice. Biofactors 2019, 45, 187–199. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Zou, C.; Liu, Y. Amelioration of Ovalbumin-Induced Food Allergy in Mice by Targeted Rectal and Colonic Delivery of Cyanidin-3-O-Glucoside. Foods 2022, 11, 1542. https://doi.org/10.3390/foods11111542
Li J, Zou C, Liu Y. Amelioration of Ovalbumin-Induced Food Allergy in Mice by Targeted Rectal and Colonic Delivery of Cyanidin-3-O-Glucoside. Foods. 2022; 11(11):1542. https://doi.org/10.3390/foods11111542
Chicago/Turabian StyleLi, Jie, Chao Zou, and Yixiang Liu. 2022. "Amelioration of Ovalbumin-Induced Food Allergy in Mice by Targeted Rectal and Colonic Delivery of Cyanidin-3-O-Glucoside" Foods 11, no. 11: 1542. https://doi.org/10.3390/foods11111542
APA StyleLi, J., Zou, C., & Liu, Y. (2022). Amelioration of Ovalbumin-Induced Food Allergy in Mice by Targeted Rectal and Colonic Delivery of Cyanidin-3-O-Glucoside. Foods, 11(11), 1542. https://doi.org/10.3390/foods11111542






