One of the Primary Functions of Tissue-Resident Pluripotent Pericytes Cells May Be to Regulate Normal Organ Growth and Maturation: Implications for Attempts to Repair Tissues Later in Life
Abstract
1. Background
2. Introduction
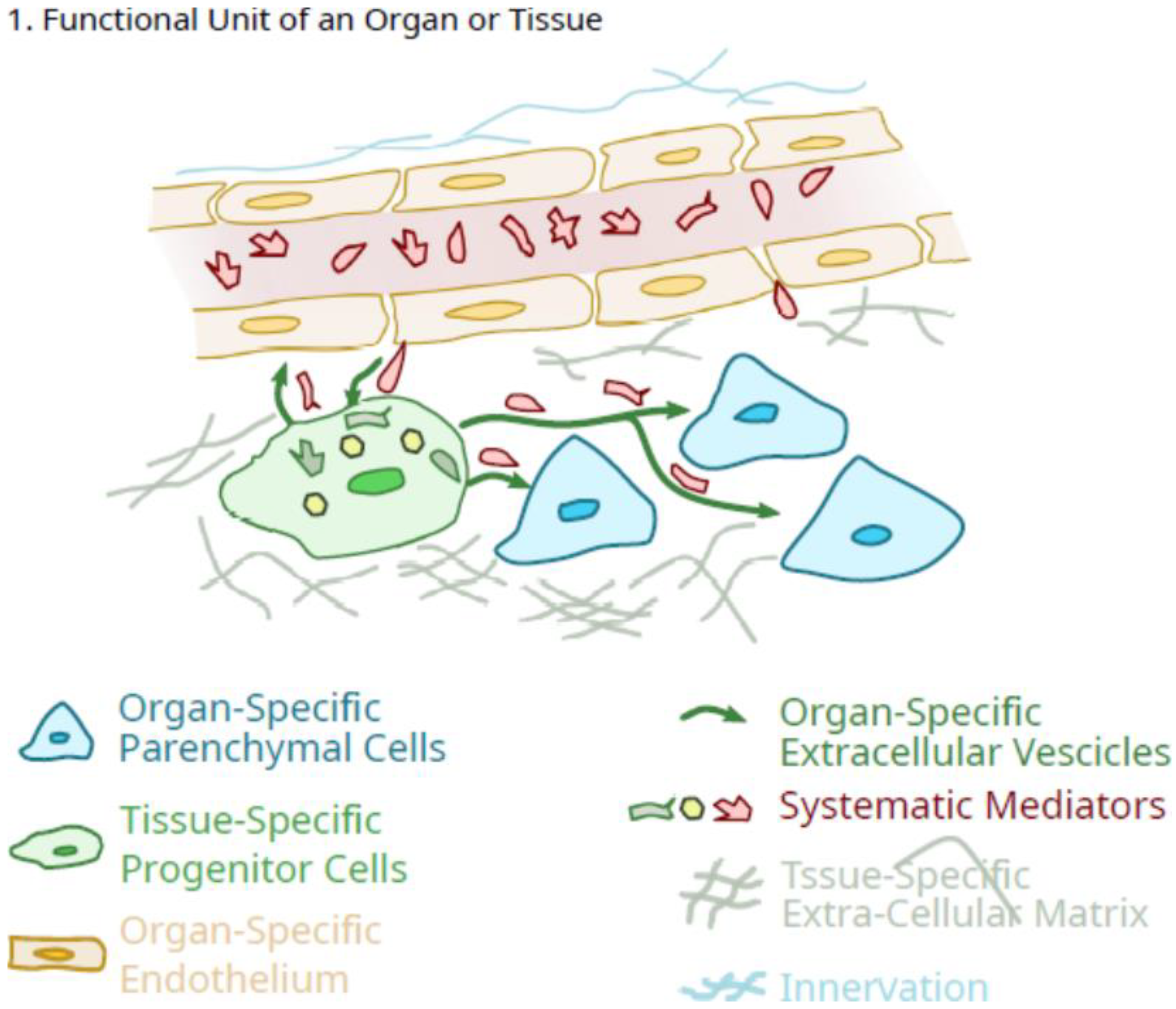
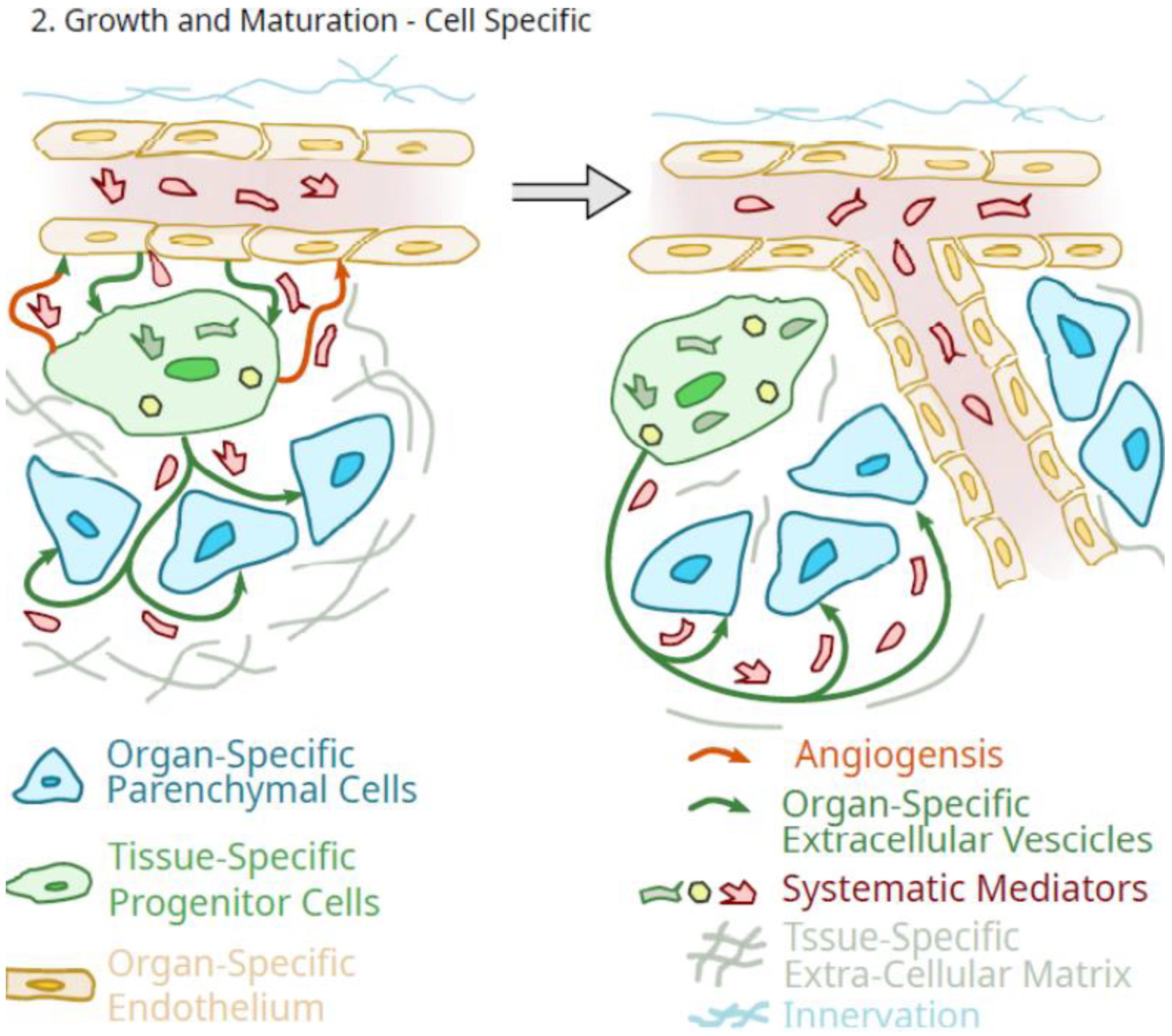
3. Potential Role of Pluripotent Organ-Specific Pericytes in Growth and Maturation, as Well as Senescence and Decline in Organ and Tissue Integrity
4. What Is the Role of Pluripotent Progenitor Cells That Circulate or Are Detected in the Free State?
5. Does the Decline in Tissue-Specific Pericyte Function with Age Have Implications for a Potential Role in Loss of Health?
6. Is There Potential to Reverse the Decline in Tissue-Specific Pericytes Function with Aging?
7. How Realistic Are the Expectations of Using Pluripotent Progenitor Cells to Facilitate Tissue/Organ Regeneration?
8. Focus on the Pluripotency of the Cells Rather Than Their “Stemness”
9. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- da Silva Meirelles, L.; Chagastelles, P.C.; Nardi, N.B. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J. Cell Sci. 2006, 119, 2204–2213. [Google Scholar] [CrossRef] [PubMed]
- Pittenger, M.F.; Discher, D.E.; Péault, B.M.; Phinney, D.G.; Hare, J.M.; Caplan, A.I. Mesenchymal stem cell perspective: Cell biology to clinical progress. NPJ Regen. Med. 2019, 4, 22. [Google Scholar] [CrossRef] [PubMed]
- Mabuchi, Y.; Okawara, C.; Méndez-Ferrer, S.; Akazawa, C. Cellular heterogeneity of mesenchymal stem/stromal cells in the bone marrow. Front. Cell Dev. Biol. 2021, 9, 689366. [Google Scholar] [CrossRef] [PubMed]
- Ong, W.; Chakraborty, S.; Sugii, S. Adipose tissue: Understanding the heterogeneity of stem cells for regenerative medicine. Biomolecules 2021, 11, 918. [Google Scholar] [CrossRef]
- McLeod, C.; Mauck, R. On the origin and impact of mesenchymal stem cell heterogeneity: New insights and emerging tools for single cell analysis. Eur. Cells Mater. 2017, 34, 217–231. [Google Scholar] [CrossRef]
- Hart, D.A. Why Mesenchymal Stem/Progenitor Cell Heterogeneity in Specific Environments? —Implications for Tissue Engineering Applications Following Injury or Degeneration of Connective Tissues. J. Biomed. Eng. Sci. 2014, 7, 526–532. [Google Scholar] [CrossRef][Green Version]
- Talaei-Khozani, T.; Aleahmad, F.; Bazrafshan, A.; Aliabadi, E.; Vojdani, Z. Lectin Profile Variation in Mesenchymal Stem Cells Derived from Different Sources. Cell Tissues Organs 2019, 208, 101–112. [Google Scholar] [CrossRef]
- Ando, W.; Kutcher, J.J.; Krawetz, R.; Sen, A.; Nakamura, N.; Frank, C.B.; Hart, D.A. Clonal analysis of synovial fluid stem cells to characterize and identify stable mesenchymal stromal cell/mesenchymal progenitor cell phenotypes in a porcine model: A cell source with enhanced commitment to the chondrogenic linages. Cytotherapy. 2014, 16, 776–788. [Google Scholar] [CrossRef]
- Hermann, A.; List, C.; Habisch, H.-J.; Vukicevic, V.; Ehrhart-Bornstein, M.; Brenner, R.; Bernstein, P.; Fickert, S.; Storch, A. Age-dependent neuroectodermal differentiation capacity of human mesenchymal stromal cells: Limitations for autologous cell replacement strategies. Cytotherapy 2010, 12, 17–30. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, T.; Han, J.; Yang, Z.; Xue, X.; Jiang, H.; Wang, H. Advanced Age Impairs Cardioprotective Function of Mesenchymal Stem Cell Transplantation from Patients to Myocardially Infarcted Rats. Cardiology 2014, 128, 209–219. [Google Scholar] [CrossRef]
- Doshida, Y.; Sano, H.; Iwabuchi, S.; Aigaki, T.; Yoshida, M.; Hashimoto, S.; Ishigami, A. Age-associated changes in the transcriptomes of non-cultured adipose-derived stem cells from young and old mice assessed via single-cell transcriptome analysis. PLoS ONE 2010, 15, e0242171. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.-M.; Rong, Y.-X.; Liang, Z.-J.; Hunag, D.-L.; Ma, Y.-F.; Luo, Z.-Z.; Wu, F.-X.; Liu, X.-H.; Liu, Y.; Mo, S.; et al. Landscape of transcription and expression regulated by DNA methylation related to age of donor and cell passage in adipose-derived mesenchymal stem cells. Aging 2020, 12, 21186–21201. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Liu, O.; Chen, S.; Zhou, Y. Aging and Mesenchymal Stem Cells: Therapeutic Opportunities and Challenges in the Older Group. Gerontology 2021, 68, 339–352. [Google Scholar] [CrossRef] [PubMed]
- Peffers, M.J.; Goljanek-Whysall, K.; Collins, J.; Fang, Y.; Rushton, M.; Loughlin, J.; Proctor, C.; Clegg, P.D. Decoding the Regulatory Landscape of Ageing in Musculoskeletal Engineered Tissues Using Genome-Wide DNA Methylation and RNASeq. PLoS ONE 2016, 11, e0160517. [Google Scholar] [CrossRef] [PubMed]
- Roforth, M.M.; Farr, J.N.; Fugita, K.; McCready, L.K.; Atkinson, E.J.; Therneau, T.M.; Cunningham, J.M.; Drake, M.T.; Monroe, D.G.; Khosla, S. Global transcriptional profiling using RNA sequencing and DNA methylation patterns in highly enriched mesenchymal cells from young versus elderly women. Bone 2015, 76, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Di Giuseppe, F.; Pierdomenico, L.; Eleuterio, E.; Sulpizio, M.; Lanuti, P.; Riviello, A.; Bologna, G.; Gesi, M.; Di Ilio, C.; Miscia, S.; et al. Cryopreservation effects on wharton’s jelly stem cells proteome. Stem Cells Rev. Rep. 2014, 10, 429–446. [Google Scholar] [CrossRef]
- Kalaszczynska, I.; Ferdyn, K. Wharton’s Jelly Derived Mesenchymal Stem Cells: Future of Regenerative Medicine? Recent Findings and Clinical Significance. BioMed Res. Int. 2015, 2015, 430847. [Google Scholar] [CrossRef]
- Shimomura, K.; Yasui, Y.; Koisumi, K.; Chijimatsu, R.; Hart, D.A.; Yonetani, Y.; Ando, W.; Nishi, T.; Kanamoto, T.; Horibe, S.; et al. First-in-human pilot study of implantation of a scaffold-free tissue-engineered construct generated from autologous synovial mesenchymal stem cells for repair of chondral lesions. Am. J. Sports Med. 2018, 46, 2384–2393. [Google Scholar] [CrossRef]
- Shimomura, K.; Hamada, H.; Hart, D.A.; Ando, W.; Nishii, T.; Trattnig, S.; Nehrer, S.; Nakamura, N. Histological Analysis of Cartilage Defects Repaired with an Autologous Human Stem Cell Construct 48 Weeks Postimplantation Reveals Structural Details Not Detected by T2-Mapping MRI. Cartilage 2021, 13 (Suppl. 1), 694S–706S. [Google Scholar] [CrossRef]
- Galderisi, U.; Peluso, G.; Di Bernardo, G. Clinical Trials Based on Mesenchymal Stromal Cells are Exponentially Increasing: Where are We in Recent Years? Stem Cell Rev. Rep. 2022, 18, 23–36. [Google Scholar] [CrossRef]
- Caplan, A.I. Mesenchymal Stem Cells: Time to Change the Name! Stem Cells Transl. Med. 2017, 6, 1445–1451. [Google Scholar] [CrossRef] [PubMed]
- Caplan, A.I. There Is No “Stem Cell Mess”. Tissue Eng. Part B 2019, 25, 291–293. [Google Scholar] [CrossRef] [PubMed]
- Capomaccio, S.; Cappelli, K.; Bazzucchi, C.; Coletti, M.; Gialletti, R.; Moriconi, F.; Passamonti, F.; Pepe, M.; Petrini, S.; Mecocci, S.; et al. Equine adipose-derived mesenchymal stem cells release extracellular vesicles enclosing different subsets of small RNAs. Stem Cells Int. 2019, 2019, 4957806. [Google Scholar] [CrossRef] [PubMed]
- Asgarpour, K.; Shojaei, Z.; Amiri, F.; Ai, J.; Mahjoubin-Tehran, M.; Ghasemi, F.; ArefNezhad, R.; Hamblin, M.R.; Mirzaei, H. Exosomal microRNAs derived from mesenchymal stem cells: Cell-to-cell messages. Cell Commun. Signal. 2020, 18, 149. [Google Scholar] [CrossRef]
- Hur, Y.H.; Cerione, R.A.; Antonyak, M.A. Extracellular vesicles and their roles in stem cell biology. Stem Cells 2020, 38, 469–476. [Google Scholar] [CrossRef]
- Shi, J.; Zhao, Y.-C.; Niu, Z.-F.; Fan, H.-J.; Hou, S.-K.; Guo, X.-Q.; Sang, L.; Lv, Q. Mesenchymal stem cell-derived small extracellular vesicles in the treatment of human diseases. World J. Stem Cells 2021, 13, 49–63. [Google Scholar] [CrossRef]
- Jin, T.; Gu, J.; Li, Z.; Xu, Z.; Gui, Y. Recent Advances in Extracellular Vesicles in Central Nervous System Diseases. Clin. Interv. Aging 2021, 16, 257–274. [Google Scholar] [CrossRef]
- Racchetti, G.; Meldolesi, J. Extracellular Vesicles of Mesenchymal Stem Cells: Therapeutic Properties Discovered with Extraordinary Success. Biomedicines 2021, 9, 667. [Google Scholar] [CrossRef]
- Caplan, A.I. MSCs: The Sentinel and Safe-Guards of Injury. J. Cell. Physiol. 2016, 231, 1413–1416. [Google Scholar] [CrossRef]
- Zha, K.; Li, X.; Yang, Z.; Tian, G.; Sun, Z.; Sui, X.; Dai, Y.; Liu, S.; Guo, Q. Heterogeneity of mesenchymal stem cells in cartilage regeneration: From characterization to application. NPJ Regen. Med. 2021, 6, 14. [Google Scholar] [CrossRef]
- James, S.; Fox, J.; Afsari, F.; Lee, J.; Clough, S.; Knight, C.; Ashmore, J.; Ashton, P.; Preham, O.; Hoogduijn, M.; et al. Multiparameter Analysis of Human Bone Marrow Stromal Cells Identifies Distinct Immunomodulatory and Differentiation-Competent Subtypes. Stem Cell Rep. 2015, 4, 1004–1015. [Google Scholar] [CrossRef] [PubMed]
- Hart, D.A. Perspective: Is it time to rename MSC [mesenchymal Stem cells/medicinal signaling cells] with a name that reflects their in vivo functions and in vitro abilities?-Possibly “Pluripotent mesenchymal regulatory cells (PMRC)”. J. Biomed. Sci. Eng. 2021, 14, 317–324. [Google Scholar] [CrossRef]
- Augustin, H.G.; Koh, G.Y. Organotypic vasculature: From descriptive heterogeneity to functional pathophysiology. Science 2017, 357, eaal2379. [Google Scholar] [CrossRef] [PubMed]
- Nguyen-Lefebvre, A.T.; Selzner, N.; Wrana, J.L.; Bhat, M. The hippo pathway: A master regulator of liver metabolism, regeneration, and disease. FASEB J. 2021, 35, e21570. [Google Scholar] [CrossRef] [PubMed]
- Lobo, D.; Beane, W.S.; Levin, M. Modeling Planarian Regeneration: A Primer for Reverse-Engineering the Worm. PLOS Comput. Biol. 2012, 8, e1002481. [Google Scholar] [CrossRef]
- Yamamoto, S.; Kashimoto, R.; Furukawa, S.; Sakamoto, H.; Satoh, A. Nerve-mediated FGF-signaling in the early phase of various organ regeneration. J. Exp. Zool. B Mol. Dev. Evol. 2021, 336, 529–539. [Google Scholar] [CrossRef]
- Kawakami, A. Stem cells in tissue regeneration in fish. Dev. Growth Differ. 2010, 52, 77–87. [Google Scholar] [CrossRef]
- Maden, M. The evolution of regeneration–where does that leave mammals? Int. J. Dev. Biol. 2018, 62, 369–372. [Google Scholar] [CrossRef]
- Hart, D.A.; Natsu-ume, T.; Sciore, P.; Tasevski, V.; Frank, C.B.; Shrive, N.G. Mechanobiology: Similarities and differences between in vivo and in vitro analysis at the functional and molecular levels. Recent Res. Dev. Biophys. Biochem. 2002, 2, 153–177. [Google Scholar]
- Gifre-Renom, L.; Daems, M.; Luttun, A.; Jones, E.A.V. Organ-Specific Endothelial Cell Differentiation and Impact of Microenvironmental Cues on Endothelial Heterogeneity. Int. J. Mol. Sci. 2022, 23, 1477. [Google Scholar] [CrossRef]
- McDougall, J.; Giles, R.W.; Bray, R.C.; Hart, D.A. Pregnancy-induced changes in rabbit medial collateral ligament vasoregulation. Am. J. Physiol. 1998, 275, R1380–R1385. [Google Scholar] [CrossRef] [PubMed]
- McDougall, J.J.; Bray, R.C.; Hart, D.A. Late gestational changes in sympathomimetic sensitivity in primigravid rabbit ligaments. Can. J. Physiol. Pharmacol. 2000, 78, 528–534. [Google Scholar] [CrossRef] [PubMed]
- Yianni, V.; Sharpe, P.T. Perivascular-Derived Mesenchymal Stem Cells. J. Dent. Res. 2019, 98, 1066–1072. [Google Scholar] [CrossRef] [PubMed]
- Sbierski-Kind, J.; Mroz, N.; Molofsky, A.B. Perivascular stromal cells: Directors of tissue immune niches. Immunol. Rev. 2021, 302, 10–31. [Google Scholar] [CrossRef]
- Colle, I.; Van Vlierberghe, H.; Troisi, R.; De Hemptinne, B. Transplanted liver: Consequences of denervation for liver functions. Anat. Rec. A Discov. Mol. Cell Evol. Biol. 2004, 280, 924–931. [Google Scholar] [CrossRef] [PubMed]
- Ando, W.; Heard, B.J.; Chung, M.; Nakamura, N.; Frank, C.B.; Hart, D.A. Ovine synovial membrane-derived mesenchymal progenitor cells retain the phenotype of the original tissue that was exposed to an in vivo inflammation: Evidence for a suppressed chondrogenic differentiation potential of the cells. Inflam. Res. 2012, 61, 599–608. [Google Scholar] [CrossRef]
- Cucu, I.; Nicolescu, M.I. A Synopsis of Signaling Crosstalk of Pericytes and Endothelial Cells in Salivary Gland. Dent. J. 2021, 9, 144. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Dang, H.; Xu, Y. Recent advancement of decellularization extracellular matrix for tissue engineering and biomedical application. Artif. Organs 2022, 46, 549–567. [Google Scholar] [CrossRef]
- Carvalho, M.S.; Alves, L.; Bogalho, I.; Cabral, J.M.S.; da Silva, C.L. Impact of Donor Age on the Osteogenic Supportive Capacity of Mesenchymal Stromal Cell-Derived Extracellular Matrix. Front. Cell Del. Biol. 2021, 9, 747521. [Google Scholar] [CrossRef]
- Porter, G.A.; Palade, G.E.; Milici, A.J. Differential bonding of the lectins Griffonia simplicifolia I and Lycopersicon esculentum to microvascular endothelium: Organ-specific localization and partial glycoprotein characterization. Eur. J. Cell Biol. 1990, 5, 85–95. [Google Scholar]
- Hart, D.A. What Molecular Recognition Systems Do Mesenchymal Stem Cells/Medicinal Signaling Cells (MSC) Use to Facilitate Cell-Cell and Cell Matrix Interactions? A Review of Evidence and Options. Int. J. Mol. Sci. 2021, 22, 8637. [Google Scholar] [CrossRef] [PubMed]
- Dallas, S.L.; Rosser, J.L.; Mundy, G.R.; Bonewald, L.F. Proteolysis of latent transforming growth factor-beta (TGF-B)-binding protein-1 by osteoclasts. A cellular mechanism for release of TGF-beta from bone matrix. J. Biol. Chem. 2002, 277, 21352–21360. [Google Scholar] [CrossRef] [PubMed]
- E Scott, J. The first and second ’laws’ of chemical morphology, exemplified in mammalian extracellular matrices. Eur. J. Histochem. 2002, 46, 111–124. [Google Scholar] [CrossRef]
- Ewald, C.Y. The Matrisome during Aging and Longevity: A Systems-Level Approach Defining Matreotypes Promoting Healthy Aging. Gerontology 2020, 66, 266–274. [Google Scholar] [CrossRef]
- Kim, B.S.; Das, S.; Jang, J.; Cho, D.-W. Decellularized Extracellular Matrix-based Bioinks for Engineering Tissue- and Organ-specific Microenvironments. Chem. Rev. 2020, 120, 10608–10661. [Google Scholar] [CrossRef] [PubMed]
- Yianni, V.; Sharpe, P.T. Epigenetic mechanisms driving lineage commitment in mesenchymal stem cells. Bone 2020, 134, 115309. [Google Scholar] [CrossRef] [PubMed]
- Huh, S.J.; Clement, K.; Jee, D.; Merlini, A.; Choudhury, S.; Maruyama, R.; Yoo, R.; Chytil, A.; Boyle, P.; Ran, F.A.; et al. Age- and Pregnancy-Associated DNA Methylation Changes in Mammary Epithelial Cells. Stem Cell Rep. 2015, 4, 297–311. [Google Scholar] [CrossRef]
- Shalev, D.; Melamed, P. The role of the hypothalamus and pituitary epigenomes in central activation of the reproductive axis at puberty. Mol. Cell. Endocrinol. 2020, 518, 111031. [Google Scholar] [CrossRef]
- Vazquez, M.J.; Daza-Dueñas, S.; Tena-Sempere, M. Emerging Roles of Epigenetics in the Control of Reproductive Function: Focus on Central Neuroendocrine Mechanisms. J. Endocr. Soc. 2021, 5, bvab152. [Google Scholar] [CrossRef]
- Manotas, M.C.; González, D.M.; Céspedes, C.; Forero, C.; Moreno, A.P.R. Genetic and Epigenetic Control of Puberty. Sex. Dev. 2022, 16, 1–10. [Google Scholar] [CrossRef]
- Kanherkar, R.R.; Bhatia-Dey, N.; Csoka, A.B. Epigenetics across the human lifespan. Front. Cell Dev. Biol. 2014, 2, 49. [Google Scholar] [CrossRef] [PubMed]
- Ryan, C.P. “Epigenetic clocks”: Theory and applications in human biology. Am. J. Hum. Biol. 2021, 33, e23488. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Ye, Z.; Mather, K.A.; Nguyen, T.L.; Dite, G.S.; Armstrong, N.J.; Wong, E.M.; Thalamuthu, A.; Giles, G.G.; Craig, J.M.; et al. Early life affects late-life health through determining DNA methylation across the lifespan: A twin study. EBioMedicine 2022, 77, 103927. [Google Scholar] [CrossRef] [PubMed]
- Hart, D.A. Sex Differences in Biological Systems and the Conundrum of Menopause: Potential Commonalities in Post-Menopausal Disease Mechanisms. Int. J. Mol. Sci. 2022, 23, 4119. [Google Scholar] [CrossRef]
- Hart, D.A.; Zernicke, R.F. Optimal Human Functioning Requires Exercise Across the Lifespan: Mobility in a 1g Environment Is Intrinsic to the Integrity of Multiple Biological Systems. Front. Physiol. 2020, 11, 156. [Google Scholar] [CrossRef]
- Hart, D.A. Learning From Human Responses to Deconditioning Environments: Improved Understanding of the “Use It or Lose It” Principle. Front. Sports Act. Living 2021, 3, 685845. [Google Scholar] [CrossRef]
- James, A.W.; Péault, B. Perivascular Mesenchymal Progenitors for Bone Regeneration. J. Orthop. Res. 2019, 37, 1221–1228. [Google Scholar] [CrossRef]
- Lee, L.L.; Chintalgattu, V. Pericytes in the Heart. Adv. Exp. Med. Biol. 2019, 1122, 187–210. [Google Scholar] [CrossRef]
- Grassel, S.G. The role of peripheral nerve fibres and their neurotransmitters in cartilage and bone physiology and pathophysiology. Arthritis Res. Ther. 2014, 16, 485. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, J.; Zhang, P.; Liu, T.; Xu, J.; Fan, Z.; Shen, Y.; Li, W.; Zhang, H. Calcitonin gene-related peptide is a key factor in the homing of transplanted human MSCs to sites of spinal cord injury. Sci. Rep. 2016, 6, 27724. [Google Scholar] [CrossRef]
- Dong, P.; Gu, X.; Zhu, G.; Li, M.; Ma, B.; Zi, Y. Melatonin Induces Osteoblastic Differentiation of Mesenchymal Stem Cells and Promotes Fracture Healing in a Rat Model of Femoral Fracture via Neuropeptide Y/Neuropeptide Y Receptor Y1 Signaling. Pharmacology 2018, 102, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.-Q.; Jiang, N.; Yu, B. Mechanisms of action of neuropeptide Y on stem cells and its potential applications in orthopaedic disorders. World J. Stem Cells 2020, 12, 986–1000. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.W.; Wang, Y.-L.; Christensen, J.M.; Khalifian, S.; Schneeberger, S.; Raimondi, G.; Cooney, D.S.; Lee, W.A.; Brandacher, G. Donor age negatively affects the immunoregulatory properties of both adipose and bone marrow derived mesenchymal stem cells. Transpl. Immunol. 2014, 30, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-Q.; Shao, Y.; Ma, C.-Y.; Chen, W.; Sun, L.; Liu, W.; Zhang, D.-Y.; Fu, B.-C.; Liu, K.-Y.; Jia, Z.-B.; et al. Decreased SIRT3 in aged human mesenchymal stromal/stem cells increases cellular susceptibility to oxidative stress. J. Cell. Mol. Med. 2014, 18, 2298–2310. [Google Scholar] [CrossRef] [PubMed]
- Peffers, M.J.; Collins, J.; Loughlin, J.; Proctor, C.; Clegg, P.D. A proteomic analysis of chondrogenic, osteogenic and tenogenic constructs from aging mesenchymal stem cells. Stem Cell Res. Ther. 2016, 7, 133. [Google Scholar] [CrossRef]
- Fan, M.; Chen, W.; Liu, W.; Du, G.-Q.; Jiang, S.-L.; Tian, W.-C.; Sun, L.; Li, R.-K.; Tian, H. The Effect of Age on the Efficacy of Human Mesenchymal Stem Cell Transplantation after a Myocardial Infarction. Rejuvenation Res. 2010, 13, 429–438. [Google Scholar] [CrossRef] [PubMed]
- Tashiro, J.; Elliot, S.J.; Gerth, D.J.; Xia, X.; Pereira-Simon, S.; Choi, R.; Catanuto, P.; Shahzeidi, S.; Toonkel, R.L.; Shah, R.H.; et al. Therapeutic benefits of young, but not old, adipose-derived mesenchymal stem cells in a chronic mouse model of bleomycin-induced pulmonary fibrosis. Transl. Res. 2015, 166, 554–567. [Google Scholar] [CrossRef]
- Maacha, S.; Sidahmed, H.; Jacob, S.; Gentilcore, G.; Calzone, R.; Grivel, J.-C.; Cugno, C. Paracrine Mechanisms of Mesenchymal Stromal Cells in Angiogenesis. Stem Cells Int. 2020, 2020, 4356359. [Google Scholar] [CrossRef]
- Caplan, A.I. New MSC: MSCs as pericytes are Sentinels and gatekeepers. J. Orthop. Res. 2017, 35, 1151–1159. [Google Scholar] [CrossRef]
- Li, C.; Chai, Y.; Wang, L.; Gao, B.; Chen, H.; Gao, P.; Zhou, F.-Q.; Luo, X.; Crane, J.L.; Yu, B.; et al. Programmed cell senescence in skeleton during late puberty. Nat. Commun. 2017, 8, 1312. [Google Scholar] [CrossRef]
- Garay-Mendoza, D.; Villarreal-Martínez, L.; Garza-Bedolla, A.; Pérez-Garza, D.M.; Acosta-Olivo, C.; Vilchez-Cavazos, F.; Diaz-Hutchinson, C.; Gómez-Almaguer, D.; Jaime-Pérez, J.C.; Mancías-Guerra, C. The effect of intra-articular injection of autologous bone marrow stem cells on pain and knee function in patients with osteoarthritis. Int. J. Rheum. Dis. 2018, 21, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Bolia, I.K.; Bougioukli, S.; Hill, W.J.; Trasolini, N.A.; Petrigliano, F.A.; Lieberman, J.R.; Weber, A.E. Clinical Efficacy of Bone Marrow Aspirate Concentrate Versus Stromal Vascular Fraction Injection in Patients With Knee Osteoarthritis: A Systematic Review and Meta-analysis. Am. J. Sports Med. 2021, 3635465211014500. [Google Scholar] [CrossRef] [PubMed]
- Kunze, K.N.; Burnett, R.A.; Wright-Chisem, J.; Frank, R.M.; Chahla, J. Adipose-Derived Mesenchymal Stem Cell Treatments and Available Formulations. Curr. Rev. Musculoskelet. Med. 2020, 13, 264–280. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-I.; Kim, M.-S.; Kim, J.-H. Intra-articular Injection of Autologous Adipose-Derived Stem Cells or Stromal Vascular Fractions: Are They Effective for Patients With Knee Osteoarthritis? A Systematic Review With Meta-analysis of Randomized Controlled Trials. Am. J. Sports Med. 2022, 3635465211053893. [Google Scholar] [CrossRef]
- Zhuang, Y.; Li, D.; Fu, J.; Shi, Q.; Lu, Y.; Ju, X. Comparison of biological properties of umbilical cord-derived mesenchymal stem cells from early and late passages: Immunoreulatory ability is enhanced in aged cells. Mol. Med. Rep. 2015, 11, 166–174. [Google Scholar] [CrossRef]
- Franzen, J.; Georgomanolis, T.; Selich, A.; Kuo, C.-C.; Stöger, R.; Brant, L.; Mulabdić, M.S.; Fernandez-Rebollo, E.; Grezella, C.; Ostrowska, A.; et al. DNA methylation changes during long-term in vitro cell culture are caused by epigenetic drift. Commun. Biol. 2021, 4, 598. [Google Scholar] [CrossRef]
- Gunawardene, P.; Bermeo, S.; Vidal, C.; Al Saedi, A.; Chung, P.; Boersma, D.; Phu, S.; Pokorski, I.; Suriyaarachchi, P.; Demontiero, O.; et al. Association between circulating osteoprogenitor cells and disability and frailty in older persons: The Nepan Osteoporosis and Frailty study. J. Gerontol. Biol. Sci. 2016, 71, 1124–1130. [Google Scholar] [CrossRef]
- Krawetz, R.J.; Wu, Y.E.; Martin, L.; Rattner, J.B.; Matyas, J.R.; Hart, D.A. Synovial Fluid Progenitors Expressing CD90+ from Normal but Not osteoarthritis Joints Undergo Chondrogenic Differentiation without Micro-Mass Culture. PLoS ONE 2012, 7, e43616. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, W.; Ran, Q.; Xiang, Y.; Zhong, J.F.; Li, S.C.; Li, Z. The Differentiation Balance of Bone Marrow Mesenchymal Stem Cells Is critical to Hematopoiesis. Stem Cells Int. 2018, 2018, 1540148. [Google Scholar] [CrossRef]
- Zorina, T.D. New Insights on the Role of the Mesenchymal–Hematopoietic Stem Cell Axis in Autologous and Allogeneic Hematopoiesis. Stem Cells Dev. 2021, 30, 2–16. [Google Scholar] [CrossRef]
- Zhao, Q.; Liu, X.; Yu, C.; Xiao, Y. Macrophages and Bone Marrow-Derived Mesenchymal Stem Cells Work in Concert to Promote Fracture Healing: A Brief Review. DNA Cell Biol. 2022, 41, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Wagner, D.R.; Karnik, S.; Gunderson, Z.J.; Nielson, J.J.; Fennimore, A.; Promer, H.J.; Lowerey, J.W.; Loghmani, M.T.; Low, P.S.; McKinley, T.O.; et al. Dysfunctional stem and progenitor cells impair fracture healing with age. World J. Stem Cells 2019, 11, 281–296. [Google Scholar] [CrossRef] [PubMed]
- Bragdon, B.C.; Bahney, C.S. Origin of Reparative Stem Cells in Fracture Healing. Curr. Osteoporos. Rep. 2018, 16, 490–503. [Google Scholar] [CrossRef] [PubMed]
- de Sousa, E.B.; Casado, P.L.; Neto, V.M.; Duarte, M.E.L.; Aguiar, D.P. Synovial fluid and synovial membrane mesenchymal stem cells: Latest discoveries and therapeutic perspectives. Stem Cell Res. Ther. 2014, 5, 112. [Google Scholar] [CrossRef]
- Murata, D.; Miyakoshi, D.; Hatazoe, T.; Miura, N.; Tokunaga, S.; Fujiki, M.; Nakayama, K.; Misumi, K. Multipotency of equine mesenchymal stem cells derived from synovial fluid. Vet.-J. 2014, 202, 53–61. [Google Scholar] [CrossRef]
- Huang, Y.-Z.; Xie, H.-Q.; Silini, A.; Parolini, O.; Zhang, Y.; Deng, L.; Huang, Y.-C. Mesenchymal Stem/Progenitor Cells Derived from Articular Cartilage, Synovial Membrane and Synovial Fluid for Cartilage Regeneration: Current Status and Future Perspectives. Stem Cell Rev. Rep. 2017, 5, 575–586. [Google Scholar] [CrossRef]
- Jiang, Z.; Yu, S.; Lin, H.; Bi, R. Expression and function of cartilage-derived pluripotent cells in joint development and repair. Stem Cell Res. Ther. 2020, 11, 111. [Google Scholar] [CrossRef]
- Wilson, A.S.; Legg, P.G.; McNeur, J.C. Studies on the innervation of the medial meniscus in the human knee joint. Anat. Rec. 1969, 165, 485–491. [Google Scholar] [CrossRef]
- Arnoczky, S.P.; Warren, R.F. Microvasculature of the human meniscus. Am. J. Sports Med. 1982, 10, 90–95. [Google Scholar] [CrossRef]
- Gray, J.C. Neural and Vascular Anatomy of the Menisci of the Human Knee. J. Orthop. Sports Phys. Ther. 1999, 29, 23–30. [Google Scholar] [CrossRef]
- Radin, E.L.; Burr, D.B.; Caterson, B.; Fyhrie, D.; Brown, T.D.; Boyd, R.D. Mechanical determinants of osteoarthrosis. Semin Rheum. 1991, 21 (Suppl. 2), 12–21. [Google Scholar] [CrossRef]
- Frank, C.B.; Shrive, N.G.; Boorman, R.S.; Lo, I.K.Y.; Hart, D.A. New Perspectives on Bioengineering of Joint Tissues: Joint Adaptation Creates a Moving Target for Engineering Replacement Tissues. Ann. Biomed. Eng. 2004, 32, 458–463. [Google Scholar] [CrossRef] [PubMed]
- Harris, Q.; Seto, J.; O’Brien, K.; Lee, P.S.; Kondo, C.; Heard, B.J.; Hart, D.A.; Krawetz, R.J. Monocyte chemotactic protein-1 inhibits chondrogenesis of synovial mesenchymal progenitor cells: An in vitro study. Stem Cells 2013, 31, 2253–2265. [Google Scholar] [CrossRef] [PubMed]
- Gunawardene, P.; Al Saedi, A.; Singh, L.; Bermeo, S.; Vogrin, S.; Phu, S.; Suriyaarachchi, P.; Pignolo, R.J.; Duque, G. Age, gender, and percentage of circulating osteoprogenitor (COP) cells: The COP Study. Exp. Gerontol. 2017, 96, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Zupan, J.; Strazar, K.; Kocijan, R.; Nau, T.; Grillari, J.; Presen, D.M. Age-related alterations and senescence of mesenchymal stromal cells: Implications for regenerative treatments of bones and joints. Mech. Aging Dev. 2021, 198, 111539. [Google Scholar] [CrossRef]
- Franzen, J.; Zirkel, A.; Blake, J.; Rath, B.; Benes, V.; Papantonis, A.; Wagner, W. Senescence-associated DNA methylation is stochastically acquired in subpopulations of mesenchymal stem cells. Aging Cell 2017, 16, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Cakouros, D.; Gronthos, S. The changing epigenetic landscape of Mesenchymal Stem/Stromal Cells during aging. Bone 2020, 137, 115440. [Google Scholar] [CrossRef]
- Yasui, Y.; Hart, D.A.; Sugita, N.; Chijimatsu, R.; Koizumi, K.; Ando, W.; Moriguchi, Y.; Shimomura, K.; Myoui, A.; Yoshikawa, H.; et al. Time-Dependent Recovery of Human Synovial Membrane Mesenchymal Stem Cell Function After High-Dose Steroid Therapy: Case Report and Laboratory Study. Am. J. Sports Med. 2018, 46, 695–701. [Google Scholar] [CrossRef]
- Kruk, J.S.; Bermeo, S.; Skarratt, K.K.; Fuller, S.J.; Duque, G. The Effect of Antidepressants on Mesenchymal Stem Cell Differentiation. J. Bone Metab. 2018, 25, 43–51. [Google Scholar] [CrossRef]
- Hart, D.A.; Nakamura, N.; Shrive, N.G. Perspective: Challenges Presented for Regeneration of Heterogeneous Musculoskeletal Tissues that Normally Develop in Unique Biomechanical Environments. Front. Bioeng. Biotechnol. 2021, 9, 760273. [Google Scholar] [CrossRef]
- Bullough, W.S. The chalones: A review. Natl. Cancer Inst. Monogr. 1973, 38, 5–16. [Google Scholar] [PubMed]
- Rytomaa, T. The chalone concept. Int. Rev. Exp. Pathol. 1976, 16, 135–206. [Google Scholar] [PubMed]
- Allen, J.C.; Smith, C.J. Chalones: A Reappraisal. Biochem. Soc. Trans. 1979, 7, 584–592. [Google Scholar] [CrossRef] [PubMed]
- Reichlin, S. Somatostatin: Historical Aspects. Scand. J. Gastroenterol. 1986, 119, 1–10. [Google Scholar] [CrossRef]
- Gamer, L.W.; Nove, J.; Rosen, V. Return of the Chalones. Dev. Cell 2003, 4, 143–144. [Google Scholar] [CrossRef]
- Elgio, K.; Reichelt, K.L. Chalones: From aqueous extracts to oliopeptides. Cell Cycle 2004, 3, 1208–1211. [Google Scholar]
- Conboy, I.M.; Conboy, M.J.; Wagers, A.J.; Girma, E.R.; Weissman, I.L.; Rando, T.A. Rejuvenation of aged progenitor cells by exposure to young systemic environment. Nature 2005, 433, 760–764. [Google Scholar] [CrossRef]
- Hu, Y.; Xu, R.; Chen, C.-Y.; Rao, S.-S.; Xia, K.; Huang, J.; Yin, H.; Wang, Z.-X.; Cao, J.; Liu, Z.Z.; et al. Extracellular vesicles from human umbilical cord blood ameliorate bone loss in senile osteoporotic mice. Metabolism 2019, 95, 93–101. [Google Scholar] [CrossRef]
- Kiss, T.; Nyul-Toth, A.; Gulej, R.; Tarantini, S.; Csipo, T.; Mukli, P.; Ungvari, A.; Balasubramanian, P.; Yabluchansky, A.; Benya, Z.; et al. Old blodd from heterochronic parabionts accelerates vascular aging in young mice: Transcriptomics signature of pathologic smooth muscle remodeling. Geroscience 2022, 1–29. [Google Scholar] [CrossRef]
- Latifi, N.; Lecce, M.; Simmons, C.A. Porcine Umbilical Cord Perivascular Cells for Preclinical Testing of Tissue-Engineered Heart Valves. Tissue Eng. Part C Methods 2021, 27, 35–46. [Google Scholar] [CrossRef]
- Raposa, L.; Lourenço, A.P.; Nascimento, D.S.; Cerqueira, R.; Cardim, N.; Leite-Moreira, A. Human umbilical cord tissue-derived mesenchymal stromal cells as adjuvant therapy for myocardial infarction: A review of current evidence focusing on pre-clinical large animal models and early human trials. Cytotherapy 2021, 23, 974–979. [Google Scholar] [CrossRef] [PubMed]
- Spehar, K.; Pan, A.; Beerman, I. Restoring stem cell functionality: Current progress and future directions. Stem Cells 2020, 8, 1060–1077. [Google Scholar] [CrossRef] [PubMed]
- Mendelsohn, A.R.; Larrick, J.W. Stem cell rejuvenation by restoration of youthful metabolic compartmentalization. Rejuvenation Res. 2021, 24, 470–474. [Google Scholar] [CrossRef] [PubMed]
- Ando, W.; Tateishi, K.; Hart, D.A.; Katakai, D.; Tanaka, Y.; Nakata, K.; Hashimoto, J.; Fujie, H.; Shino, K.; Yoshikawa, H.; et al. Cartilage repair using an in vitro generated scaffold-free tissue-engineered construct derived from porcine synovial mesenchymal stem cells. Biomaterials 2007, 28, 5462–5470. [Google Scholar] [CrossRef] [PubMed]
- Shimomura, K.; Ando, W.; Tateishi, K.; Nansai, N.; Fujie, H.; Hart, D.A.; Kohda, H.; Kita, K.; Kanamoto, T.; Mae, T.; et al. The influence of skeletal maturity on allogeneic synovial mesenchymal stem cell-based repair of cartilage in a large animal model. Biomaterials 2010, 31, 8004–8011. [Google Scholar] [CrossRef]
- Caplan, A.I.; Dennis, J.E. Mesenchymal stem cells as trophic mediators. J. Cell. Biochem. 2006, 98, 1076–1084. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hart, D.A. One of the Primary Functions of Tissue-Resident Pluripotent Pericytes Cells May Be to Regulate Normal Organ Growth and Maturation: Implications for Attempts to Repair Tissues Later in Life. Int. J. Mol. Sci. 2022, 23, 5496. https://doi.org/10.3390/ijms23105496
Hart DA. One of the Primary Functions of Tissue-Resident Pluripotent Pericytes Cells May Be to Regulate Normal Organ Growth and Maturation: Implications for Attempts to Repair Tissues Later in Life. International Journal of Molecular Sciences. 2022; 23(10):5496. https://doi.org/10.3390/ijms23105496
Chicago/Turabian StyleHart, David A. 2022. "One of the Primary Functions of Tissue-Resident Pluripotent Pericytes Cells May Be to Regulate Normal Organ Growth and Maturation: Implications for Attempts to Repair Tissues Later in Life" International Journal of Molecular Sciences 23, no. 10: 5496. https://doi.org/10.3390/ijms23105496
APA StyleHart, D. A. (2022). One of the Primary Functions of Tissue-Resident Pluripotent Pericytes Cells May Be to Regulate Normal Organ Growth and Maturation: Implications for Attempts to Repair Tissues Later in Life. International Journal of Molecular Sciences, 23(10), 5496. https://doi.org/10.3390/ijms23105496




