Pyruvate Upregulates Hepatic FGF21 Expression by Activating PDE and Inhibiting cAMP–Epac–CREB Signaling Pathway
Abstract
:1. Introduction
2. Results
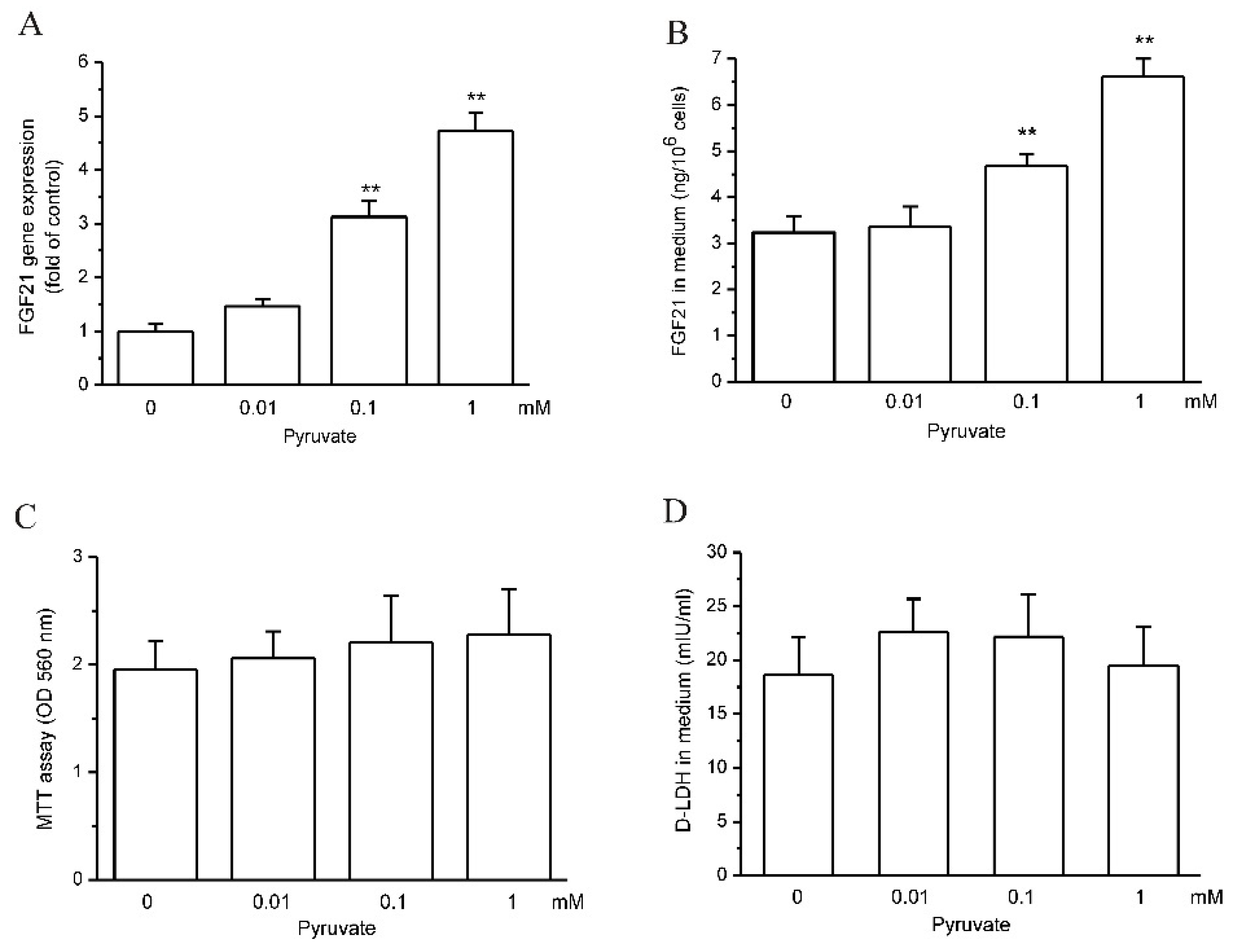
2.1. Pyruvate Upregulated FGF21 Expression and Secretion in HepG2 Cells
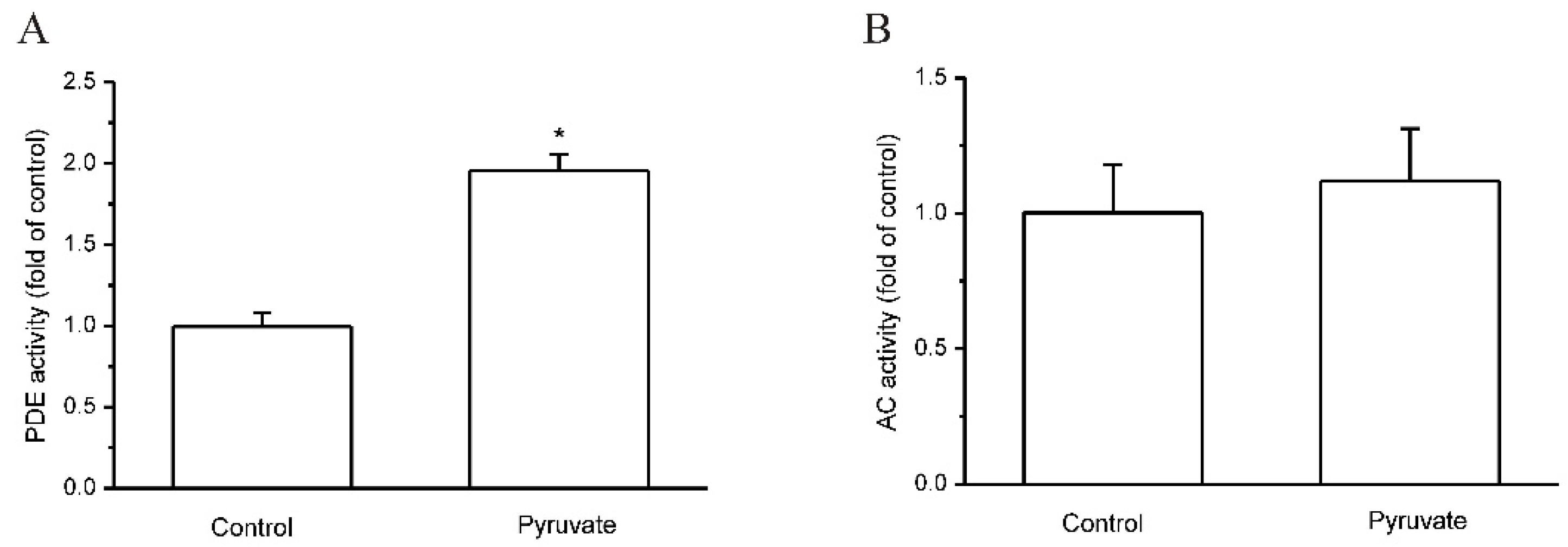
2.2. Pyruvate Activated PDEs and Reduced cAMP to Upregulate FGF21 Expression in HepG2 Cells
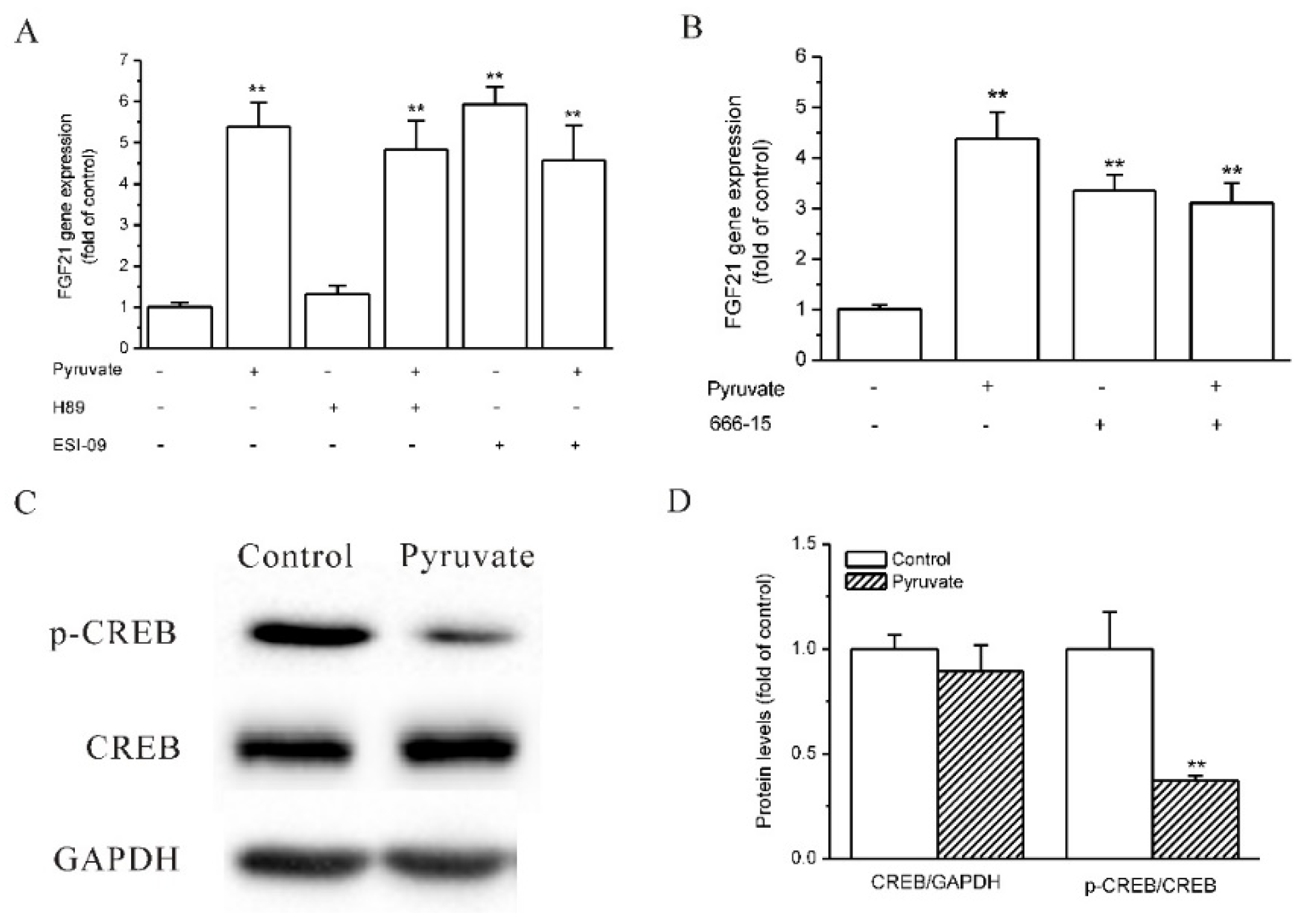
2.3. Epac and CREB Were Involved in Pyruvate-Stimulated FGF21 Expression in HepG2 Cells
2.4. Pyruvate Stimulated FGF21 Expression in Mouse AML-12 Hepatocytes and Mouse Liver In Vivo
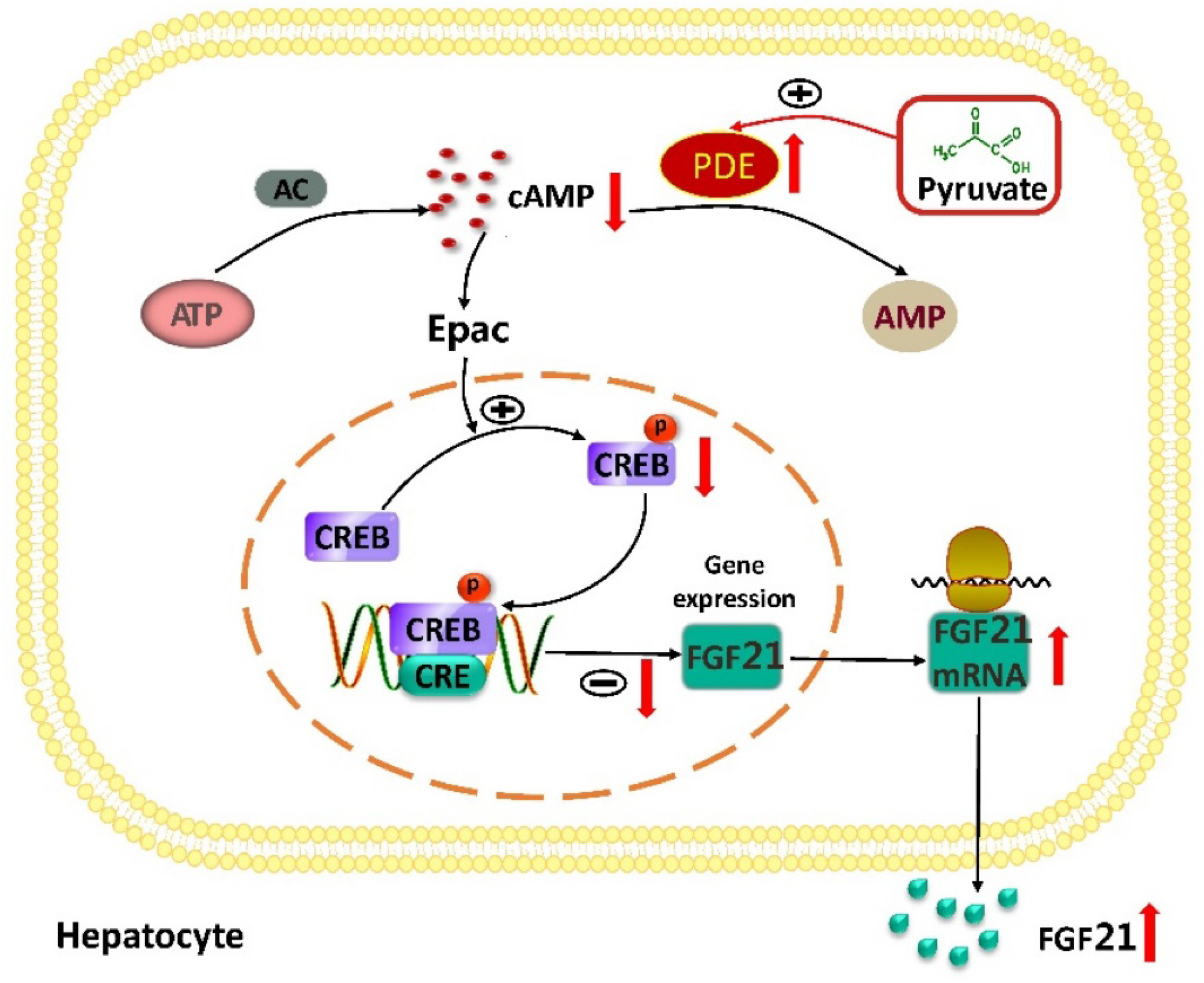
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Culture
4.3. RT-qPCR
4.4. ELISA
4.5. Cell Viability Assay
4.6. Western Blot
4.7. cAMP Measurement
4.8. Adenylyl Cyclase (AC) and Phosphodiesterases (PDEs) Activity Assay
4.9. Animal Experiment
4.10. Pyruvate Measurement
4.11. ALT and AST Activity Assay
4.12. H&E Staining
4.13. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- De Oliveira Dos Santos, A.R.; de Oliveira Zanuso, B.; Miola, V.F.B.; Barbalho, S.M.; Santos Bueno, P.C.; Flato, U.A.P.; Detregiachi, C.R.P.; Buchaim, D.V.; Buchaim, R.L.; Tofano, R.J.; et al. Adipokines, Myokines, and Hepatokines: Crosstalk and Metabolic Repercussions. Int. J. Mol. Sci. 2021, 22, 2639. [Google Scholar] [CrossRef] [PubMed]
- Jensen-Cody, S.O.; Potthoff, M.J. Hepatokines and metabolism: Deciphering communication from the liver. Mol. Metab. 2021, 44, 101138. [Google Scholar] [CrossRef] [PubMed]
- Guerra, S.; Gastaldelli, A. The role of the liver in the modulation of glucose and insulin in non alcoholic fatty liver disease and type 2 diabetes. Curr. Opin. Pharmacol. 2020, 55, 165–174. [Google Scholar] [CrossRef]
- Staiger, H.; Keuper, M.; Berti, L.; de Angelis, M.H.; Häring, H.U. Fibroblast Growth Factor 21-Metabolic Role in Mice and Men. Endocr. Rev. 2017, 38, 468–488. [Google Scholar] [CrossRef]
- Erickson, A.; Moreau, R. The regulation of FGF21 gene expression by metabolic factors and nutrients. Horm. Mol. Biol. Clin. Investig. 2016, 30, 20160016. [Google Scholar] [CrossRef] [PubMed]
- Tillman, E.J.; Rolph, T. FGF21: An Emerging Therapeutic Target for Non-Alcoholic Steatohepatitis and Related Metabolic Diseases. Front. Endocrinol. 2020, 11, 601290. [Google Scholar] [CrossRef] [PubMed]
- Keuper, M.; Häring, H.U.; Staiger, H. Circulating FGF21 Levels in Human Health and Metabolic Disease. Exp. Clin. Endocrinol. Diabetes 2020, 128, 752–770. [Google Scholar] [CrossRef] [Green Version]
- Lewis, J.E.; Ebling, F.J.P.; Samms, R.J.; Tsintzas, K. Going Back to the Biology of FGF21: New Insights. Trends Endocrinol. Metab. 2019, 30, 491–504. [Google Scholar] [CrossRef]
- Asrih, M.; Montessuit, C.; Philippe, J.; Jornayvaz, F.R. Free Fatty Acids Impair FGF21 Action in HepG2 Cells. Cell Physiol. Biochem. 2015, 37, 1767–1778. [Google Scholar] [CrossRef]
- So, W.Y.; Leung, P.S. Fibroblast Growth Factor 21 As an Emerging Therapeutic Target for Type 2 Diabetes Mellitus. Med. Res. Rev. 2016, 36, 672–704. [Google Scholar] [CrossRef]
- Prochownik, E.V.; Wang, H. The Metabolic Fates of Pyruvate in Normal and Neoplastic Cells. Cells 2021, 10, 762. [Google Scholar] [CrossRef] [PubMed]
- Cortez, M.Y.; Torgan, C.E.; Brozinick, J.T., Jr.; Miller, R.H.; Ivy, J.L. Effects of pyruvate and dihydroxyacetone consumption on the growth and metabolic state of obese Zucker rats. Am. J. Clin. Nutr. 1991, 53, 847–853. [Google Scholar] [CrossRef] [PubMed]
- Stanko, R.T.; Reynolds, H.R.; Hoyson, R.; Janosky, J.E.; Wolf, R. Pyruvate supplementation of a low-cholesterol, low-fat diet: Effects on plasma lipid concentrations and body composition in hyperlipidemic patients. Am. J. Clin. Nutr. 1994, 59, 423–427. [Google Scholar] [CrossRef] [PubMed]
- Stone, M.H.; Sanborn, K.; Smith, L.L.; O’Bryant, H.S.; Hoke, T.; Utter, A.C.; Johnson, R.L.; Boros, R.; Hruby, J.; Pierce, K.C.; et al. Effects of in-season (5 weeks) creatine and pyruvate supplementation on anaerobic performance and body composition in American football players. Int. J. Sport Nutr. 1999, 9, 146–165. [Google Scholar] [CrossRef] [PubMed]
- Sileri, P.; Brown, M.; Morini, S.; Rastellini, C.; Benedetti, E.; Cicalese, L. Pyruvate prevents intestinal functional changes following ischemia-reperfusion injury. Transplant. Proc. 2001, 33, 852. [Google Scholar] [CrossRef]
- Crestanello, J.A.; Kamelgard, J.; Whitman, G.J. The cumulative nature of pyruvate’s dual mechanism for myocardial protection. J. Surg. Res. 1995, 59, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.J.; Deng, J.T.; Shen, H.Q.; Jiang, L.L.; He, Q.W.; Zhan, J.; Zhang, Z.Z.; Wang, Y.L. Pyruvate Protects Against Intestinal Injury by Inhibiting the JAK/STAT Signaling Pathway in Rats with Hemorrhagic Shock. J. Surg. Res. 2020, 248, 98–108. [Google Scholar] [CrossRef]
- Mallet, R.T.; Olivencia-Yurvati, A.H.; Bünger, R. Pyruvate enhancement of cardiac performance: Cellular mechanisms and clinical application. Exp. Biol. Med. 2018, 243, 198–210. [Google Scholar] [CrossRef]
- Bonazzola, P.; Ragone, M.I.; Consolini, A.E. Effects of pyruvate on the energetics of rat ventricles stunned by ischemia-reperfusion. Can. J. Physiol. Pharmacol. 2014, 92, 386–398. [Google Scholar] [CrossRef]
- Gurji, H.A.; White, D.W.; Hoxha, B.; Sun, J.; Harbor, J.P.; Schulz, D.R.; Williams, A.G., Jr.; Olivencia-Yurvati, A.H.; Mallet, R.T. Pyruvate-enriched resuscitation: Metabolic support of post-ischemic hindlimb muscle in hypovolemic goats. Exp. Biol. Med. 2014, 239, 240–249. [Google Scholar] [CrossRef]
- Regitz, V.; Azumi, T.; Stephan, H.; Naujocks, S.; Schaper, W. Biochemical mechanism of infarct size reduction by pyruvate. Cardiovasc. Res. 1981, 15, 652–658. [Google Scholar] [CrossRef] [PubMed]
- Varma, S.D.; Devamanoharan, P.S.; Ali, A.H. Prevention of intracellular oxidative stress to lens by pyruvate and its ester. Free Radic. Res. 1998, 28, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Constantopoulos, G.; Barranger, J.A. Nonenzymatic decarboxylation of pyruvate. Anal. Biochem. 1984, 139, 353–358. [Google Scholar] [CrossRef]
- Guarino, V.A.; Oldham, W.M.; Loscalzo, J.; Zhang, Y.Y. Reaction rate of pyruvate and hydrogen peroxide: Assessing antioxidant capacity of pyruvate under biological conditions. Sci. Rep. 2019, 9, 19568. [Google Scholar] [CrossRef] [PubMed]
- Salminen, A.; Kaarniranta, K.; Kauppinen, A. Integrated stress response stimulates FGF21 expression: Systemic enhancer of longevity. Cell. Signal. 2017, 40, 10–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Sousa-Coelho, A.L.; Marrero, P.F.; Haro, D. Activating transcription factor 4-dependent induction of FGF21 during amino acid deprivation. Biochem. J. 2012, 443, 165–171. [Google Scholar] [CrossRef] [Green Version]
- Wan, X.S.; Lu, X.H.; Xiao, Y.C.; Lin, Y.; Zhu, H.; Ding, T.; Yang, Y.; Huang, Y.; Zhang, Y.; Liu, Y.L.; et al. ATF4- and CHOP-dependent induction of FGF21 through endoplasmic reticulum stress. Biomed. Res. Int. 2014, 2014, 807874. [Google Scholar] [CrossRef]
- Zhou, F.; Bai, M.; Zhang, Y.; Zhu, Q.; Zhang, L.; Zhang, Q.; Wang, S.; Zhu, K.; Liu, Y.; Wang, X.; et al. Berberine-induced activation of AMPK increases hepatic FGF21 expression via NUR77. Biochem. Biophys. Res. Commun. 2018, 495, 1936–1941. [Google Scholar] [CrossRef]
- Badman, M.K.; Pissios, P.; Kennedy, A.R.; Koukos, G.; Flier, J.S.; Maratos-Flier, E. Hepatic fibroblast growth factor 21 is regulated by PPARalpha and is a key mediator of hepatic lipid metabolism in ketotic states. Cell Metab. 2007, 5, 426–437. [Google Scholar] [CrossRef] [Green Version]
- Cyphert, H.A.; Alonge, K.M.; Ippagunta, S.M.; Hillgartner, F.B. Glucagon stimulates hepatic FGF21 secretion through a PKA- and EPAC-dependent posttranscriptional mechanism. PLoS ONE 2014, 9, e94996. [Google Scholar] [CrossRef] [Green Version]
- Han, H.S.; Choi, B.H.; Kim, J.S.; Kang, G.; Koo, S.H. Hepatic Crtc2 controls whole body energy metabolism via a miR-34a-Fgf21 axis. Nat. Commun. 2017, 8, 1878. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khannpnavar, B.; Mehta, V.; Qi, C.; Korkhov, V. Structure and function of adenylyl cyclases, key enzymes in cellular signaling. Curr. Opin. Struct. Biol. 2020, 63, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Wahlang, B.; McClain, C.; Barve, S.; Gobejishvili, L. Role of cAMP and phosphodiesterase signaling in liver health and disease. Cell. Signal. 2018, 49, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Gancedo, J.M. Biological roles of cAMP: Variations on a theme in the different kingdoms of life. Biol. Rev. Camb. Philos. Soc. 2013, 88, 645–668. [Google Scholar] [CrossRef]
- Robichaux, W.G., III; Cheng, X. Intracellular cAMP Sensor EPAC: Physiology, Pathophysiology, and Therapeutics Development. Physiol. Rev. 2018, 98, 919–1053. [Google Scholar] [CrossRef]
- Liu, N.; Wu, Y.G.; Lan, G.C.; Sui, H.S.; Ge, L.; Wang, J.Z.; Liu, Y.; Qiao, T.W.; Tan, J.H. Pyruvate prevents aging of mouse oocytes. Reproduction 2009, 138, 223–234. [Google Scholar] [CrossRef] [Green Version]
- Varma, S.D.; Hegde, K.R.; Kovtun, S. Oxidative damage to lens in culture: Reversibility by pyruvate and ethyl pyruvate. Ophthalmologica 2006, 220, 52–57. [Google Scholar] [CrossRef]
- Yako, H.; Niimi, N.; Kato, A.; Takaku, S.; Tatsumi, Y.; Nishito, Y.; Kato, K.; Sango, K. Role of pyruvate in maintaining cell viability and energy production under high-glucose conditions. Sci. Rep. 2021, 11, 18910. [Google Scholar] [CrossRef]
- Kamenetsky, M.; Middelhaufe, S.; Bank, E.M.; Levin, L.R.; Buck, J.; Steegborn, C. Molecular details of cAMP generation in mammalian cells: A tale of two systems. J. Mol. Biol. 2006, 362, 623–639. [Google Scholar] [CrossRef] [Green Version]
- Lugnier, C. Cyclic nucleotide phosphodiesterase (PDE) superfamily: A new target for the development of specific therapeutic agents. Pharmacol. Ther. 2006, 109, 366–398. [Google Scholar] [CrossRef]
- Neves-Zaph, S.R. Phosphodiesterase Diversity and Signal Processing Within cAMP Signaling Networks. Adv. Neurobiol. 2017, 17, 3–14. [Google Scholar]
- Azevedo, M.F.; Faucz, F.R.; Bimpaki, E.; Horvath, A.; Levy, I.; de Alexandre, R.B.; Ahmad, F.; Manganiello, V.; Stratakis, C.A. Clinical and molecular genetics of the phosphodiesterases (PDEs). Endocr. Rev. 2014, 35, 195–233. [Google Scholar] [CrossRef]
- Kreisel, W.; Schaffner, D.; Lazaro, A.; Trebicka, J.; Merfort, I.; Schmitt-Graeff, A.; Deibert, P. Phosphodiesterases in the Liver as Potential Therapeutic Targets of Cirrhotic Portal Hypertension. Int. J. Mol. Sci. 2020, 21, 6223. [Google Scholar] [CrossRef]
- Lewis, M.J.; Khaliulin, I.; Hall, K.; Suleiman, M.S. Cardioprotection of Immature Heart by Simultaneous Activation of PKA and Epac: A Role for the Mitochondrial Permeability Transition Pore. Int. J. Mol. Sci. 2022, 23, 1720. [Google Scholar] [CrossRef]
- Vitali, E.; Peverelli, E.; Giardino, E.; Locatelli, M.; Lasio, G.B.; Beck-Peccoz, P.; Spada, A.; Lania, A.G.; Mantovani, G. Cyclic adenosine 3′-5′-monophosphate (cAMP) exerts proliferative and anti-proliferative effects in pituitary cells of different types by activating both cAMP-dependent protein kinase A (PKA) and exchange proteins directly activated by cAMP (Epac). Mol. Cell. Endocrinol. 2014, 383, 193–202. [Google Scholar] [CrossRef]
- Murray, A.J.; Tucker, S.J.; Shewan, D.A. cAMP-dependent axon guidance is distinctly regulated by Epac and protein kinase A. J. Neurosci. 2009, 29, 15434–15444. [Google Scholar] [CrossRef] [Green Version]
- Hoque, K.M.; Woodward, O.M.; van Rossum, D.B.; Zachos, N.C.; Chen, L.; Leung, G.P.; Guggino, W.B.; Guggino, S.E.; Tse, C.M. Epac1 mediates protein kinase A-independent mechanism of forskolin-activated intestinal chloride secretion. J. Gen. Physiol. 2010, 135, 43–58. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Gu, Y.; Li, G.W.; Huang, L.Y. A critical role of the cAMP sensor Epac in switching protein kinase signalling in prostaglandin E2-induced potentiation of P2X3 receptor currents in inflamed rats. J. Physiol. 2007, 584, 191–203. [Google Scholar] [CrossRef]
- Itzhakov, D.; Nitzan, Y.; Breitbart, H. Protein kinase A inhibition induces EPAC-dependent acrosomal exocytosis in human sperm. Asian J. Androl. 2019, 21, 337–344. [Google Scholar]
- Benchoula, K.; Parhar, I.S.; Madhavan, P.; Hwa, W.E. CREB nuclear transcription activity as a targeting factor in the treatment of diabetes and diabetes complications. Biochem. Pharmacol. 2021, 188, 114531. [Google Scholar] [CrossRef]
- Sands, W.A.; Palmer, T.M. Regulating gene transcription in response to cyclic AMP elevation. Cell. Signal. 2008, 20, 460–466. [Google Scholar] [CrossRef]
- Grimes, M.T.; Powell, M.; Gutierrez, S.M.; Darby-King, A.; Harley, C.W.; McLean, J.H. Epac activation initiates associative odor preference memories in the rat pup. Learn Mem. 2015, 22, 74–82. [Google Scholar] [CrossRef] [Green Version]
- Ramos-Alvarez, I.; Lee, L.; Jensen, R.T. Cyclic AMP-dependent protein kinase A and EPAC mediate VIP and secretin stimulation of PAK4 and activation of Na(+),K(+)-ATPase in pancreatic acinar cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2019, 316, G263–G277. [Google Scholar] [CrossRef]
- Ogiwara, K.; Hoyagi, M.; Takahashi, T. A central role for cAMP/EPAC/RAP/PI3K/AKT/CREB signaling in LH-induced follicular Pgr expression at medaka ovulation. Biol. Reprod. 2021, 105, 413–426. [Google Scholar] [CrossRef]
- Berman, B.G.; Halperin, M.L. Effect of insulin on pyruvate metabolism in epididymal adipose tissue of the rat. Correlation of intracellular pyruvate contents and pyruvate dehydrogenase activity. Biochem. J. 1973, 134, 885–889. [Google Scholar]
- McDaniel, H.G. Nutritional and hormonal regulation of pyruvate metabolism in the liver. Am. J. Physiol. 1979, 236, E501–E507. [Google Scholar] [CrossRef]
- Exton, J.H. Mechanisms of hormonal regulation of hepatic glucose metabolism. Diabetes Metab. Rev. 1987, 3, 163–183. [Google Scholar] [CrossRef]
- Ravnskjaer, K.; Madiraju, A.; Montminy, M. Role of the cAMP Pathway in Glucose and Lipid Metabolism. Handb. Exp. Pharmacol. 2016, 233, 29–49. [Google Scholar]
- Karnib, N.; El-Ghandour, R.; El Hayek, L.; Nasrallah, P.; Khalifeh, M.; Barmo, N.; Jabre, V.; Ibrahim, P.; Bilen, M.; Stephan, J.S.; et al. Lactate is an antidepressant that mediates resilience to stress by modulating the hippocampal levels and activity of histone deacetylases. Neuropsychopharmacology 2019, 44, 1152–1162. [Google Scholar] [CrossRef]
- Moreno-Felici, J.; Hyroššová, P.; Aragó, M.; Rodríguez-Arévalo, S.; García-Rovés, P.M.; Escolano, C.; Perales, J.C. Phosphoenolpyruvate from Glycolysis and PEPCK Regulate Cancer Cell Fate by Altering Cytosolic Ca(2). Cells 2019, 9, 18. [Google Scholar] [CrossRef] [Green Version]
- Ho, P.C.; Bihuniak, J.D.; Macintyre, A.N.; Staron, M.; Liu, X.; Amezquita, R.; Tsui, Y.C.; Cui, G.; Micevic, G.; Perales, J.C.; et al. Phosphoenolpyruvate Is a Metabolic Checkpoint of Anti-tumor T Cell Responses. Cell 2015, 162, 1217–1228. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, M.T.; Yudell, B.E.; Loor, J.J. Regulation of energy metabolism by long-chain fatty acids. Prog. Lipid Res. 2014, 53, 124–144. [Google Scholar] [CrossRef]
- Gonzalez, S.V.; Nguyen, N.H.; Rise, F.; Hassel, B. Brain metabolism of exogenous pyruvate. J. Neurochem. 2005, 95, 284–293. [Google Scholar] [CrossRef]
- Sharma, A.B.; Barlow, M.A.; Yang, S.H.; Simpkins, J.W.; Mallet, R.T. Pyruvate enhances neurological recovery following cardiopulmonary arrest and resuscitation. Resuscitation 2008, 76, 108–119. [Google Scholar] [CrossRef] [Green Version]
- Soto, M.; Orliaguet, L.; Reyzer, M.L.; Manier, M.L.; Caprioli, R.M.; Kahn, C.R. Pyruvate induces torpor in obese mice. Proc. Natl. Acad. Sci. USA 2018, 115, 810–815. [Google Scholar] [CrossRef] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.-Y.; Zhang, L.-J.; Liang, X.-Y.; Zhang, X.-C.; Chang, J.-R.; Shi, M.; Liu, H.; Zhou, Y.; Sun, Z.; Zhao, Y.-F. Pyruvate Upregulates Hepatic FGF21 Expression by Activating PDE and Inhibiting cAMP–Epac–CREB Signaling Pathway. Int. J. Mol. Sci. 2022, 23, 5490. https://doi.org/10.3390/ijms23105490
Zhao Y-Y, Zhang L-J, Liang X-Y, Zhang X-C, Chang J-R, Shi M, Liu H, Zhou Y, Sun Z, Zhao Y-F. Pyruvate Upregulates Hepatic FGF21 Expression by Activating PDE and Inhibiting cAMP–Epac–CREB Signaling Pathway. International Journal of Molecular Sciences. 2022; 23(10):5490. https://doi.org/10.3390/ijms23105490
Chicago/Turabian StyleZhao, Yan-Yan, Li-Jun Zhang, Xiang-Yan Liang, Xiao-Chun Zhang, Jin-Rui Chang, Man Shi, Huan Liu, Ying Zhou, Zhuo Sun, and Yu-Feng Zhao. 2022. "Pyruvate Upregulates Hepatic FGF21 Expression by Activating PDE and Inhibiting cAMP–Epac–CREB Signaling Pathway" International Journal of Molecular Sciences 23, no. 10: 5490. https://doi.org/10.3390/ijms23105490
APA StyleZhao, Y.-Y., Zhang, L.-J., Liang, X.-Y., Zhang, X.-C., Chang, J.-R., Shi, M., Liu, H., Zhou, Y., Sun, Z., & Zhao, Y.-F. (2022). Pyruvate Upregulates Hepatic FGF21 Expression by Activating PDE and Inhibiting cAMP–Epac–CREB Signaling Pathway. International Journal of Molecular Sciences, 23(10), 5490. https://doi.org/10.3390/ijms23105490





