Abstract
Zebrafish has become a popular model to study many physiological and pathophysiological processes in humans. In recent years, it has rapidly emerged in the study of metabolic disorders, namely, obesity and diabetes, as the regulatory mechanisms and metabolic pathways of glucose and lipid homeostasis are highly conserved between fish and mammals. Zebrafish is also widely used in the field of neurosciences to study brain plasticity and regenerative mechanisms due to the high maintenance and activity of neural stem cells during adulthood. Recently, a large body of evidence has established that metabolic disorders can alter brain homeostasis, leading to neuro-inflammation and oxidative stress and causing decreased neurogenesis. To date, these pathological metabolic conditions are also risk factors for the development of cognitive dysfunctions and neurodegenerative diseases. In this review, we first aim to describe the main metabolic models established in zebrafish to demonstrate their similarities with their respective mammalian/human counterparts. Then, in the second part, we report the impact of metabolic disorders (obesity and diabetes) on brain homeostasis with a particular focus on the blood–brain barrier, neuro-inflammation, oxidative stress, cognitive functions and brain plasticity. Finally, we propose interesting signaling pathways and regulatory mechanisms to be explored in order to better understand how metabolic disorders can negatively impact neural stem cell activity.
1. Introduction
Metabolic syndrome is a combination of at least three of the following metabolic disorders: abdominal obesity, hypertriglyceridemia, low serum high-density lipoprotein (HDL) levels, hyperglycemia (associated with insulin resistance) and hypertension [1]. These biochemical and physiological dysfunctions significantly increase the risk of chronic kidney disease, hepatic steatosis and cardiovascular diseases, including myocardial infarction and stroke, among others [2,3,4]. Recently, metabolic syndrome has also been suggested to be associated with cognitive and behavioral impairments [5].
Diabetes and obesity are two metabolic diseases related to metabolic syndrome. Both diseases are closely associated: approximately 84% of people with type 2 diabetes are also obese and/or overweight [6]. In addition, diabetes and obesity cause many deleterious effects on the body. For example, they strongly affect the renal glomerular filtration rate and increase the risk of developing chronic kidney disease [7]. They are also risk factors for the development of cardiovascular diseases affecting blood pressure and endothelial cell and cardiomyocyte functions [8,9]. In mice, obesity promotes adipose tissue inflammation, leading to liver inflammation and promoting glucose intolerance and insulin resistance [10]. Similarly, insulin resistance was correlated with an increased risk of hepatic steatosis in a Korean cohort, even before the onset of a diabetic state [11]. Overall, diabetes and obesity impair the cardiovascular, renal, visual, intestinal and metabolic systems.
Obesity and diabetes have been more recently documented for their deleterious consequences on the central nervous system, especially on cognitive processes [12,13]. Both diseases disrupt the blood-brain barrier (BBB) [14,15] and promote neuro-inflammation and cerebral oxidative stress [16]. Obesity and diabetes have also been suggested to be involved in neuronal degeneration, being risk factors for the development of neurodegenerative diseases, including Alzheimer’s disease [17]. This is likely related to increased oxidative stress through mitochondrial dysfunction and neuro-inflammation [18,19,20]. Strikingly, recent data have demonstrated that diabetes and obesity have a deleterious impact on brain plasticity and, among others, on neurogenesis.
Neurogenesis is an evolutionarily conserved process that involves the division of neural stem cells, the genesis of other committed progenitors and, finally, the birth of new neurons that can migrate and differentiate within the nervous tissue [21,22,23,24]. This interesting and intriguing process occurs primarily during development but has also been documented in the adult brain of all species studied to date, including humans [25,26]. In mammals, adult neurogenesis occurs mainly in two regions: (1) the subventricular zone (SVZ) of the lateral ventricle and (2) the subgranular zone (SGZ) of the dentate gyrus of the hippocampus [27,28]. Neurogenesis is tightly regulated by many different factors, including hormones and oxygen supply, as well as trophic, immune and epigenetic factors [29,30]. The microenvironment of the neurogenic niche can consequently disrupt neural stem cell activity, namely, under chronic and/or acute inflammation, as well as under pro-oxidant conditions [31,32,33,34,35,36].
To better understand the mechanisms underlying the deleterious effects of metabolic disorders in the brain of obese and/or diabetic individuals, several animal models have been used, such as non-human primate models (i.e., chimpanzee), large animal models (i.e., dog and pig), rodent models (i.e., rat and mouse) and non-mammalian models, such as nematode (C. elegans) and zebrafish (Danio rerio) [37]. Of course, working with large mammalian models has many drawbacks, including ethical concerns and difficult manipulations, as well as high costs. For these reasons, the main models used to study neurogenesis remain rodents. However, in recent years, zebrafish have emerged as an interesting model to study the impact of metabolic disorder on the brain [38,39,40,41,42,43].
Zebrafish is indeed an attractive organism to study metabolic disorders. This small teleost fish has metabolic organs conserved with humans, including the liver, adipose tissue, pancreas and kidney [44]. As in mammals, many methods are available to measure insulin, blood glucose, lipid and cholesterol levels in this small and easily manipulated model. Finally, over 70% of the human genome has orthologs in zebrafish, and many physiological processes are conserved between fish and mammals, including humans [45]. Thus, several groups have developed models of metabolic disorders to successfully mimic the human pathologies of diabetes and obesity. These include diet-induced obesity (DIO), high-fat diets (HFDs) and hyperglycemic/diabetic models, as well as genetic models of metabolic disturbances. Interestingly, the pathological disorders induced by hyperglycemia and/or obesity are parallel to those in humans [46,47,48]. In addition, zebrafish have almost unique characteristics when considering the central nervous system. The adult zebrafish brain retains a broad distribution of active neurogenic niches throughout the encephalon [22,49,50,51]. This is in striking contrast to mammals, in which neurogenic niches are restricted to two main regions, the SVZ and SGZ [21,27,28]. Furthermore, unlike mammals, the brain of adult zebrafish is able to regenerate efficiently after large lesions, without generating persistent glial scarring and without striking residual disabilities [21,52,53,54,55].
Today, more and more efforts are being made to better understand how metabolic disorders can alter brain regeneration in order to find new therapeutic approaches to combat their impacts on the CNS under constitutive and regenerative conditions. In this general context, zebrafish is a promising model that offers new hope to understand the disrupted mechanisms occurring during metabolic disorders. It also offers the possibility to discover new effective drugs to combat the deleterious effects induced by metabolic disruptions. In this review, we discuss the main zebrafish models developed to study the effects of obesity and diabetes on CNS functions. We then highlight, in a comparative approach, the deleterious effects of these models on brain homeostasis, focusing on the BBB and constitutive and regenerative neurogenesis, as well as on cognitive functions. Finally, we discuss potential mechanisms that could explain the deleterious effect of obesity and/or diabetes on the CNS.
2. Different Models of Metabolic Disorders in Adult Zebrafish
A growing number of studies has used zebrafish to investigate its relevance to hyperglycemia/diabetes as well as overweight/obesity. In this first section, we document the main models of metabolic disorders developed in zebrafish, focusing mainly on the adult stages.
2.1. Models of Acute and Chronic Hyperglycemia
Acute hyperglycemia: An interesting model aimed to develop acute hyperglycemia in fish by intraperitoneal injection of D-glucose (2.5 g/kg body weight) [40,56]. Such an injection resulted in a rapid and transient increase in blood glucose compared to zebrafish injected with vehicle. Thus, 1.5 h after D-glucose injection, blood glucose levels reached high values compared with control-injected fish (350–500 mg/dL glucose versus 100–150 mg/dL, respectively) [40,56]. In mice, a similar injection can be performed to study the impact of acute hyperglycemia in stroke models [57,58], or in cerebral blood flow and tissue oxygen saturation [59].
Chronic hyperglycemia: Other hyperglycemic models aimed to induce chronic and persistent hyperglycemia. In mammals, chronic hyperglycemia is mainly due to an alteration in insulin production, secretion and signaling. The zebrafish pancreas is quite similar to that of mammals with the presence of endocrine islets and, in particular, insulin-producing pancreatic β-cells [60]. Two main methods have been used to establish a larger and stable diabetic state: (1) the induction of hyperglycemia by the destruction of pancreatic β-cells (relative to type 1 diabetes) and (2) the induction of chronic hyperglycemia by dissolving D-glucose in fish water (relative to type 2 diabetes).
Type 1 diabetes can be induced in fish by pancreatectomy [61,62] or chemical-dependent ablation [63,64]. The former method is difficult to perform and not really used in the zebrafish community. In contrast, chemical ablation by intraperitoneal injection of drugs such as streptozotocin (STZ) and alloxan are widely developed [63,64]. These drugs, which have been widely used in mammals [65], lead to the death of pancreatic β-cells through the generation of oxidative stress. This results in impaired insulin production and high fasting blood glucose levels [66]. In zebrafish, several studies have demonstrated that injection of STZ and/or alloxan leads to hyperglycemia in larvae and adults [61,64,67,68,69]. For example, Olsen and colleagues showed that a serial injection of STZ in adult zebrafish leads to an increase in fasting blood glucose compared to control fish (~300 mg/dL vs. 60 mg/dL) from week 1 to 3 [67]. This treatment also induces higher levels of serum glycated protein (over 300%) and a ~80% reduction in insulin levels [67]. Interestingly, after 3 weeks, these hyperglycemic fish develop renal and retinal defects, as revealed by increased glomerular basement membrane thickness and decreased retinal layer thickness [67]. This is similar to the human situation, in which type 1 diabetic patients suffer from increased serum protein glycation levels [70,71], increased glomerular basement membrane thickness [72] and retinal complications [73]. Interestingly, hyperglycemic zebrafish also exhibit a reduced ability to regenerate their caudal fin after transection [67]. This interesting feature parallels the wound-healing defects observed in diabetic patients [74]. In other experiments using STZ, hyperglycemia has a deleterious impact on the cardiovascular system, leading to the misexpression of important cardiac proteins (P53, Ampk and Klf2a) and to the loss of cardiac myofibrils and their apoptosis, as well as to cardiac dysfunction [75]. In these hyperglycemic fish, stroke volume, cardiac output and ejection fraction (end-diastolic volume minus end-systolic volume) are lower than in control fish [75]. In addition, the expression of glucose transporters (GLUTs) is decreased in the heart of zebrafish, indicating a decreased ability of the zebrafish myocardium to utilize glucose [75].
Overall, these type 1 diabetes models exhibit many features of diabetic pathology in mammals (including humans), such as increased fasting blood glucose, increased plasma protein glycation, retinal and renal dysfunctions and cardiovascular complications, as well as impaired regenerative processes [67,76,77]. However, these diabetic models using STZ and alloxan have limitations, given the ability of fish to regenerate pancreatic β-cells over time [77,78,79].
Models of type 2 diabetes have been developed by supplementing fish water with D-glucose using different concentrations of D-glucose (55, 111 and 133 mM) [42,56,80]. However, young zebrafish (4–11 months) can acclimate better to glucose immersion than old fish (1–3 years) [81]. Although the different glucose concentrations lead to significant hyperglycemia between day 2 and day 3 of treatment, the 111 mM concentration remains the frequently used one in the literature [42,56]. Immersion of zebrafish in 111 mM D-glucose solution significantly increases blood glucose levels from nearly 3 mM (54 mg/dL) to 12 mM (216 mg/dL) in control and hyperglycemic fish, respectively [42]. Accordingly, our own experiments also demonstrated the significant increase in blood glucose levels after 14 days of treatment, from 60 mg/dL to 280 mg/mL [56,82]. Interestingly, this diabetic state is dependent on D-glucose dissolved in water, as a 7-day washout after 2 weeks of D-glucose treatment is sufficient to return to normal blood glucose levels [42]. This model also leads to the induction of hyperinsulinemia and impaired glucose metabolism, as well as higher glycation of ocular proteins, altered expression levels of insulin receptors in skeletal muscle and decreased blood glucose levels after treatment with antidiabetic drugs [42].
These general characteristics are commonly observed in diabetic mammalian models and human patients [83,84,85]. Type 2 diabetic rodents correspond mainly to (1) NOD mice (non-obese diabetic mice), (2) ob/ob and db/db mice with a mutation on leptin signaling components (leptin and its receptor, respectively) and (3) HFD or DIO models. In all these models, insulinemia, hyperglycemia and increased levels of serum protein glycation are observed. Overall, this work reflects the reliability of using hyperglycemic zebrafish to mimic human pathology.
Genetic and transgenic models of hyperglycemia: The use of genetic tools such as morpholinos, CrispR-Cas9 targeted gene ablation and transgenic and/or mutant lines has also allowed to generate hyperglycemic larvae and adult fish. For example, the overexpression of foxn3, a gene associated with fasting blood glucose regulation, leads to increased hepatic gluconeogenesis and fasting blood glucose in adulthood [86,87]. In adult zebrafish, knocking out the pdx1 gene (a gene implicated in the development of type 2 diabetes) results in a reduced number of pancreatic β-cells, in decreased insulin levels and, consequently, hyperglycemia. It also delays body growth in fish [88]. Another transgenic model was achieved by overfeeding insulin-resistant skeletal muscle (zMIR) fish mutated on the IGF1 receptor [89]. Adult zMIR fish have normal glucose levels similar to control fish, but glucose levels increase significantly after the fish are overfed and describe the transitional state between insulin resistance and the development of type 2 diabetes [89]. Many other transgenic fish have been used, such as deiodinase 2 KO and aldh3a1 KO [90]. In addition to all these models, many diabetic and hyperglycemic protocols have been developed in the larva [90,91].
2.2. Models of Overweight and Obesity in Zebrafish Leading to Hyperglycemia
Obesity and overweight are characterized by hypertrophy and hyperplasia of adipocyte cells, resulting in increased body weight and body mass index (BMI). Zebrafish share with mammals the major metabolic organs regulating energy homeostasis (intestine, liver, pancreas, adipose tissue and muscle). Their respective functions are also evolutionarily conserved among taxa, including the regulation of feeding behavior, lipid storage and insulin secretion, among others [91]. Interestingly, the conserved metabolic pathways in adipocytogenesis and cholesterol metabolism between zebrafish and humans have made zebrafish an appropriate and alternative model in the field of metabolic disruptions [92]. Many zebrafish models of obesity have been developed in larvae and adults using overfeeding (diet-induced obesity—DIO) and/or high-fat diets (HFDs) [38,39,41,90,93,94,95]. Similarly, some genetic models have also been established [90,96,97,98].
Overfeeding models: Numerous obesity-inducing diet protocols have been conducted, with the difference among these models being either the nature of the food provided and/or the duration of the feeding. In 2010, Oka and colleagues overfed fish with artemia (a small shrimp used as food source in aquaculture) [99]. In their overfeeding protocol, they provided 60 mg of artemia/fish/day for 8 weeks versus 5 mg for the control [99]. At the end of their experimental procedure, the DIO fish had increased body weight, BMI and triglyceride levels and had developed hepatic steatosis.
Similar studies by Husmura and Hiramitsu showed increased visceral and subcutaneous adipose tissue volume in overfed fish and hepatic mitochondrial dysfunction [100,101]. In addition, several models of overfeeding have noted increased lipid deposition in the liver of overfed fish by Oil Red O staining, which allows the labeling of neutral lipids and cholesterol esters [38,39]. Overfed fish have also increased phosphorylation of hepatic Akt protein, a pathway involved in the development of insulin resistance [39]. The investigations of other groups have also documented that overfeeding induces higher body weight and BMI linked to the expansion of visceral and subcutaneous adipose tissue [41,95].
A different overfeeding protocol, applied for 8 months (DIO fish being fed twice as much as controls), resulted in similar disturbances: increased weight gain and steatosis of the liver (cell vacuolization), as well as an altered inflammatory response of the liver [102]. Indeed, DIO fish were unable to modulate the expression of genes involved in the inflammatory/immune system response after LPS stimulation (i.e., Toll-like receptor signaling pathway, ubiquitin-mediated proteolysis, MAPK and Jak-STAT signaling pathway, cell cycle and apoptotic genes) [102].
More recently, we established rapid and reliable models of overweight/obesity by feeding them in an “ad-libitum”-like way with conventional dry food or with a mix of artemia/conventional food for a period of 4 weeks. These models also induced higher body weight, BMI, hyperglycemia and heterogeneous liver steatosis [21,38]. Similarly, zebrafish overfed with 120 mg of commercial dry food versus 20 mg for controls for a period of 8 weeks exhibited higher body weight and BMI, hyperglycemia, glucose intolerance and increased insulin production [47].
Other experimentations have been proposed using an HFD protocol. These diets contain high amounts of fat and can be achieved using, for example, heavy whipping cream, chicken egg yolk, corn oil and lard, and ancient vegetables added (or not) to the conventional diet [39,93,103,104]. These HFD fish suffer from increased body weight including increased fat mass and hypertrophy, cardiovascular disorders, hepatic steatosis and hyperglycemia.
These different metabolic disturbances described in HFD/DIO zebrafish are also found in DIO and HFD mouse models [105,106,107,108]. Overall, these overfeeding protocols performed in zebrafish share features with human pathology: increased body weight and BMI, expansion of adipose tissue, hypertriglyceridemia, hepatic steatosis and altered expression of genes involved in lipid metabolism and inflammatory response. Hyperinsulinemia and hyperglycemia could also be observed, as well as altered expression levels of adipokines (i.e., adiponectin and leptin) and advanced glycation end products [109].
In conclusion, numerous models of diabetes and overweight/obesity have been established in zebrafish and have demonstrated that many mammalian (and human) features of these pathologies are shared with zebrafish (Figure 1). Interesting reviews document the different protocols of transgenic models to establish these different states of diabetes and/or obesity in zebrafish larvae and adults [90]. In this review, Salehpour and colleagues established a scoring system for type 2 diabetes in zebrafish compared to humans. Among the non-genetic models, glucose immersion as performed by [42,47] and the hyperglycemia obesity model have the highest score [90].
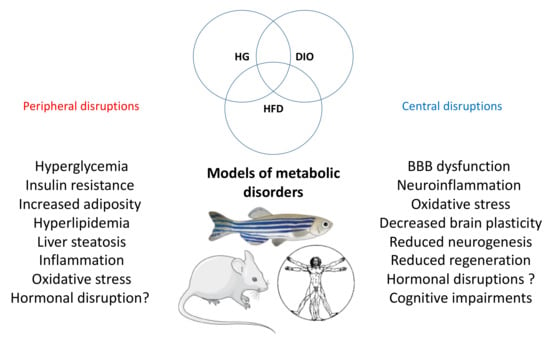
Figure 1.
General overview of the peripheral and central disruptions induced by metabolic disorder. The main peripheral and central disruptions are observed in the different models of metabolic disorders (hyperglycemia (HG), DIO and HFD) in fish and rodents. These pathological processes are also found in humans.
3. The Effects of Metabolic Disorders on Brain Plasticity and Function: Focus on Zebrafish and Comparative Aspects
As previously mentioned, the brain of adult zebrafish exhibits numerous neurogenic niches due to the persistence of many neural stem cells during adulthood. Zebrafish has also a strong capacity for nervous tissue regeneration [50,52,110,111]. The brain of zebrafish is also protected by a blood-brain barrier (BBB) that helps in maintaining brain homeostasis as in mammals [112]. Taken together, these intrinsic characteristics highlight the use of zebrafish to explore the deleterious effects of metabolic disorders on the central nervous system.
3.1. Hyperglycemia and Brain Homeostasis in Adult Zebrafish
Only a few studies have examined the impact of hyperglycemia on brain homeostasis and plasticity in adult zebrafish. While acute hyperglycemia induced by intraperitoneal injection of D-glucose (2.5 g/kg) resolves after 24 h, it nonetheless results in the upregulation of pro-inflammatory cytokines, including il1b, il6, il8 and tnfα [40]. In contrast, acute hyperglycemia has no effects on the expression of genes involved in BBB establishment (i.e., claudin5a, zonula occludens 1a and 1b). Furthermore, it does not impact brain cell proliferation in the main neurogenic niches studied (OB/TEL, ventral and dorsal telencephalic domains, pretectum and hypothalamus) [40]. Further studies are needed to understand the impact of acute hyperglycemia on neuro-inflammation, with a focus on microglia reactivity and BBB leakage by performing extravasation assays.
Chronic hyperglycemia (111 mM D-glucose for 14 days) results in more severe detrimental effects on the brain. Although it does not alter the cerebral expression of pro-inflammatory genes, probably due to compensatory mechanisms, it leads to the significant upregulation of those related to BBB integrity [40]. These results obtained in adults are to be linked with studies performed in zebrafish larvae, for which chronic glucose exposure leads to defects in tectal blood-vessel patterning and neurovascular coupling, altering both vascular NO production and the number of cells in the vascular wall. It also induces a change in the neuronal calcium concentration and leads to the upregulation of GFAP, a marker of reactive gliosis expressed in NSCs in fish [113,114].
The cerebral redox balance is also altered in diabetic fish, as evidenced by increased levels of lipid peroxidation (TBARS analysis) and carbonylated brain proteins [115]. Similarly, the activity of the antioxidant enzyme superoxide dismutase (SOD) is reduced, as well as the expression of some redox-sensitive genes (sod1 and -2, gpx3a, nrf2) [115]. These data were also partially corroborated in the retina of hyperglycemic fish [116] and in another hyperglycemic zebrafish model displaying modification in brain catalase activity [117].
Interestingly, chronic hyperglycemia alters neurogenesis within the major neurogenic areas of adult zebrafish (OB/TEL; ventral and dorsal telencephalic domains, pretectum and hypothalamus) [40]. It furthermore impairs the injury-induced neurogenesis process observed after a telencephalic injury [40]. This blunted regenerative capacity was also reported after caudal fin amputation in hyperglycemic fish, as previously mentioned [67].
From a behavioral perspective, hyperglycemic fish exhibit anxiety-like behavior and memory impairment, as shown by the inhibitory avoidance test [43,118]. These cognitive defects could originate from an alteration in the purinergic system [43]. Indeed, a significant decrease in brain ATP, ADP and AMP hydrolysis levels is observed in hyperglycemic fish, linked to the down-regulation of ectonucleoside triphosphate diphosphohydrolases (entpd2a.1, -2a.2, -3 and entpd8) and adenosine receptors (adora1, adora2aa, adora2ab and adora2b). Interestingly, acetylcholinesterase gene expression and activity are also altered [43].
Overall, these data demonstrate that hyperglycemia in zebrafish promotes BBB alterations, neuroinflammation and oxidative stress in the central nervous system. It also leads to reactive gliosis and to impaired neurogenesis, as well as the development of cognitive defects and depressive-like behavior. All these disrupted processes reported in zebrafish are also observed in mammals during diabetes. For example, claudin-5 and occludin expressions are downregulated, reflecting the increased BBB permeability observed in the brain of hyperglycemic mice [119]. Similarly, the number of microglia and reactive astrocytes in the hippocampus of hyperglycemic animals is increased, demonstrating a neuro-inflammatory state [120]. Other studies have shown the upregulation of pro-inflammatory genes (i.e., tnfα) associated with microglia activation in type 1 and type 2 diabetic mice [119]. The levels of antioxidant defenses and enzymes (i.e., glutathione -GSH- and glutathione peroxidase -GPX-) are decreased in the brain of diabetic rodents [121]. To date, numerous studies have found reduced hippocampal neurogenesis in diabetic rodents associated with cognitive defects and depressive behaviors [122,123,124,125,126,127,128,129].
Therefore, in both zebrafish and mammals, diabetes impacts the BBB, inflammatory and redox status, brain plasticity and cognitive functions (Figure 2). However, data should be reinforced in zebrafish to better understand the global impact of hyperglycemia on brain homeostasis, looking at, for instance, microglia reactivity and BBB physiology, as well as cell death under constitutive and brain injury conditions in hyperglycemic zebrafish.
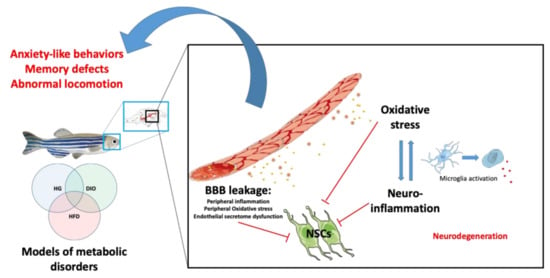
Figure 2.
Peripheral and central mechanisms impacting brain homeostasis in metabolic diseases. Under hyperglycemic (HG), DIO and HFD conditions, BBB breakdown occurs and leads to central oxidative stress and neuroinflammation through the activation of microglia (switch from ramified to ameboid state). It can result in neurodegeneration. In addition, metabolic disorders could lead to impaired secretion of endothelial factors that could, in synergy with the disrupted peripheral and central factors, impair neural stem cell (NSC) activity. The ultimate consequence of such disruptions is the development of cognitive impairments (locomotion, anxiety, memory) and neurodegenerative diseases.
3.2. Obesity and Brain Homeostasis in Zebrafish
Similar to diabetes, overweight and obesity are associated with a range of physiological disorders affecting the central nervous system. Models of overfeeding developed in zebrafish share many pathophysiological disturbances with their human counterparts, as described previously.
In zebrafish, 4-week overfeeding with a mixture of artemia and dry food induces BBB disruption, as shown by Evans blue leakage [38]. In comparison, overfeeding with only dry food results in lower BBB leakage [130], suggesting that “diet quality” may impair differentially BBB dysfunction. Similarly, an HFD protocol provided for 11 weeks with a mixture of standard food and lard (80% + 20%, respectively) results in the downregulation of genes involved in blood–brain barrier functions [103]. BBB disruption has also been associated with an increase in the number of activated microglia (amoeboid) in the ventral telencephalon and hypothalamus and a general increase in the brain expression of pro-inflammatory cytokines (il1b, il6 and tnfa) and of the inflammatory transcription factor nfkb [38]. These data were corroborated in another overfed zebrafish model showing increased amoeboid and dystrophic microglia in the hypothalamus [131]. In a hybrid obesity model (high glucose/high cholesterol experimental protocol), the treated fish upregulated the cerebral expression of pro-inflammatory cytokine and apoptotic genes [132].
Interestingly, obese fish also have a disturbed brain redox balance. They exhibit higher levels of 4-HNE (4-Hydroxynonenal), a lipid peroxidation product known as a marker of oxidative stress [38]. The activities of antioxidant enzymes peroxidase and catalase in the brain are increased, suggesting the activation of the antioxidant response [38]. Meguro and colleagues also showed that HFD zebrafish exhibit disrupted expression of genes involved in antioxidant stress [103].
In general, obese fish (HFD and/or DIO) exhibit a decreased brain plasticity, as revealed by the mis-regulation of bdnf (brain derived neurotrophic factor) and psd95 (postsynaptic density protein 95) genes [95,103]. The ptn growth factor is also significantly reduced in obese fish, while caspase 9 gene expression is upregulated, suggesting increased cell death. Interestingly, several genes involved in β-amyloid metabolism are also modulated, suggesting links between diet and neurodegeneration [103]. This decrease in the expression of genes involved in brain plasticity parallels the consistent reduction in cell proliferation along the neurogenic niches of obese fish; this was found through PCNA immunohistochemistry and qPCR analysis (decreased expression of pcna and the progenitor marker sox2) [38,130,133,134]. Very interestingly, Stankiewicz and colleagues showed that obese fish display a decrease in the daily amplitude of central clock gene expression associated with the misalignment or decreased amplitude of daily patterns of key cell-cycle regulators (e.g., cyclins A and B, and p20) [134]. Clock genes are known to be involved in the regulation of stem cell activity and are expressed in the neurogenic niches in zebrafish [135,136], raising the question of the links between circadian clock perturbations and the decreased neurogenesis observed in obese fish. Considering neurological functions, the active avoidance test and locomotion are impaired in obese fish compared with controls [38,103,130].
Taken together, these central disruptions are also described in obese mammals. For example, HFD rodents exhibit BBB leakage associated with decreased expression of tight junctions (claudin-5 and occludins) [137,138]. Similarly, HFD induces hippocampal and/or hypothalamic neuroinflammation with microglia activation and increased oxidative stress and leads to decreases in synaptic density and expression of genes involved in synaptogenesis [139,140,141,142]. Numerous studies have also highlighted the effect of a HFD on neurogenesis. For example, obese mice show decreased cell proliferation in neurogenic niches, namely, in the hypothalamus and hippocampus, associated with decreased memory and mood-related disruptive behavior [143]. Such brain alterations are strongly associated with cognitive impairments and increased anxiety [139,140,141,144,145].
Overall, these studies have demonstrated similar effects of obesity on the mammalian and fish brain, with impairment of the BBB leading to increased oxidative stress and neuro-inflammation, decreased brain plasticity including neurogenesis and altered cognitive behaviors (Figure 2). This also raises the question of the mechanisms sustaining such deleterious effects. Indeed, most zebrafish and mammalian models of obesity are hyperglycemic, suggesting that this condition could be already sufficient to impair BBB function and brain homeostasis.
3.3. Effects of Hypercholesterolemia on the CNS
Hypercholesterolemia is defined by high levels of cholesterol in the blood circulation [146]. Under hypercholersterolemic conditions, total cholesterol levels exceed 240 mg/dL, with LDL (low-density lipoproteins) higher than 160–190 mg/dL and HDL (high-density lipoproteins) lower than 40 mg/dL [147]. Although some gene mutations are responsible for familial hypercholesterolemia, dietary cholesterol consumption is associated with higher blood cholesterol levels [148,149]. Atherosclerosis is a subsequent result of the long-lasting elevated levels of cholesterol in the blood [150]. The blockage of a coronary artery may result in a heart attack. Similarly, at the level of the brain, vessel obstruction results in stroke.
In rodents, hypercholesterolemia has been shown to impact BBB functions [151,152]. It also results in increased neuroinflammation [153], increased cerebral oxidative stress biomarkers and decreased antioxidant activities [152]. Dietary cholesterol and hypercholesterolemia impact brain plasticity and neurogenesis [154,155]. In addition, early exposure to elevated cholesterol may be a risk factor for mild cognitive impairment, and hypercholesterolemia is an early risk factor for the development of Alzheimer’s disease [156]. Indeed, hypercholesterolemia has been shown to accelerate Aβ accumulation and tau pathology, which subsequently leads to cognitive impairment [157]. The link among hypercholesterolemia, cognitive dysfunction and Alzheimer’s disease is potentially mediated by increased neuroinflammation and oxidative stress [158].
Among the treatments that can be prescribed to decrease cholesterol are the statins. This drug family competitively inhibits the enzyme HMG-CoA reductase in the hepatic pathway that plays a central role in the production of cholesterol [159]. Several studies have documented the beneficial role of such cholesterol-lowering drugs in decreasing the risk of developing dementia and cognitive decline [158]. On the contrary, another body of evidence of high-cholesterol diets and controlled randomized trials shows no effects of statin treatment in terms of improving cognitive performance [160]. More strikingly, the food and drug administration indicated a potential effect of statins of inducing reversible cognitive impairments [158].
Therefore, despite the abundance of the available literature, the effects of statins on cognitive functions remain controversial [161,162,163]. While epidemiological evidence suggests a role for statins under neurodegenerative conditions, including vascular dementia, Alzheimer’s disease (AD) and Parkinson’s disease (PD), several large studies, as well as a number of case reports, contradict these findings [158]. One possible explanation for the contribution of statins to cognitive impairment is the inhibition of the protein geranylgeranyltransferase-1 (GGT) by statins. GGT is important for synapse formation and remodeling. Impaired synaptic plasticity in the hippocampus and reduced dendritic spine density in cortical neurons were observed in GGT-haplodeficient mice [164]. In addition, cholesterol depletion induced by prolonged statin exposure enhances neuroserpin protein aggregation [165]. Neuroserpin protein aggregates have been shown to be more numerous in patients with Alzheimer’s disease, and there is an association between neuroserpin and Aβ plaques in the brain of AD patients [166]. In addition, mitochondria are key organelles involved in the development of neurodegenerative diseases. Some data document the effects of statins on mitochondria acting on oxidative phosphorylation, generation of oxidative stress, uncoupling protein 3 concentration and interference in amyloid-β metabolism [167]. Interestingly, in vitro experiments have shown that rosuvastatin restores neurite outgrowth in hypoxic neurons by preserving mitochondrial functions and improving mitochondrial biogenesis. Interestingly, atorvastatin but not pravastatin impairs mitochondrial function in human pancreatic islets and rat β-cells [168], suggesting different effects of lipid-lowering drugs on mitochondria. Thus, it is becoming important to further investigate the contribution of the different statins to brain health.
Zebrafish is also emerging as a model for studying cholesterol metabolism and the effect of hypercholesterolemia and cholesterol-lowering drugs on the brain. Indeed, zebrafish have VLDL (very-low-density lipoprotein), LDL and HDL and their metabolism is quite similar to that of humans [169,170]. It consequently allows the study of new mechanisms and molecular pathways resulting from disorders associated with dyslipidemia [171]. There are also similarities between zebrafish and humans in intestinal cholesterol absorption [172]. Moreover, cholesterol-lowering drugs have been successfully used in zebrafish model and have shown a decrease in cholesterol levels within the tested fish. For example, the administration of ezetimibe and simvastatin reduces intestinal cholesterol levels in zebrafish [173,174]. Therefore, zebrafish could be a powerful model to uncover the implications of hypercholesterolemia and cholesterol-lowering drugs in brain homeostasis, health and cognition. However, to date, there are virtually no studies on the impact of hypercholesterolemia on brain functions in zebrafish.
4. Brain Dis-Plasticity and Metabolic Disorders: Molecular Mechanisms to Investigate
Neurogenesis is a tightly orchestrated process regulated by a combination of extrinsic and intrinsic factors [175,176,177]. Among them, inflammation and oxidative stress (induced during diabetes and obesity) are known to modulate the proliferation of neural stem/progenitor cells and the differentiation, migration and survival of new born cells. Zebrafish share many neurogenic signaling pathways and transcriptional regulations with mammals under both healthy and regenerative conditions [21,22,49,53,178,179,180]. However, there are not so many data highlighting the mechanisms regulating key neurogenic signaling pathways in these pathologies (diabetes and obesity) in neither fish nor mammals.
4.1. Notch Signaling in Mammal and Zebrafish Neurogenesis
One of the important signaling pathways that directly affects neural stem/progenitor proliferation and differentiation is the Notch 1 pathway [22,181,182,183,184,185,186,187,188,189]. This conserved signaling from drosophila to human plays a critical role in neural stem cell maintenance and neurogenesis during embryonic development and adult stages. The deregulation of Notch signaling has been implicated in many neurodegenerative diseases [185]. In mice, the conditional knock-out of this gene increases the proliferation of neural stem cells, while the constitutive expression of Notch-1 increases the number of progenitor cells [181,190]. Similarly, the pharmacological inhibition of Notch signaling in zebrafish increases the proliferation of neural stem cells [180,183,186,191].
Studies support the role of Notch signaling as a possible candidate for decreasing neurogenesis in case of metabolic disorders [192,193]. The offspring from HFD mice suffer from decreased neuronal progenitor proliferation, differentiation and synaptic plasticity, correlated with increased expression levels of Notch-1 signaling and its effector genes, namely, Hes5 [192,193]. Furthermore, in another study, HFD mice displayed increased Notch signaling and exhibited defects in hypothalamic neurogenesis (differentiation) [194]. In these mice, the inhibition of Notch signaling or of the inflammatory transcription factor NF-kB improved hypothalamic NSC differentiation. Therefore, in HFD mice, the resulting inflammation could activate Notch signaling in neural stem cells and lead to neurogenic defects [194]
To our knowledge, in diabetic and/or obese fish, there are no clear data showing the disruption of Notch signaling. The DIO and hyperglycemic zebrafish models have increased brain inflammation associated with defective neurogenesis [38,40,130,134]. Similar to mammals, Notch signaling is an important regulator of adult zebrafish neurogenesis [182,183]. The different notch receptors are expressed in several neurogenic niches [183,187]. However, the links between Notch signaling and impaired neurogenesis in obese and/or hyperglycemic zebrafish has not yet been investigated. The preliminary results of our study on the expression of the target gene of Notch signaling in zebrafish, her4.1, did not show striking gene expression differences in the main neurogenic niches of control and obese fish (Figure 3). Such investigations can widely contribute to the understanding of Notch involvement in neurogenic disruption in the case of metabolic diseases.
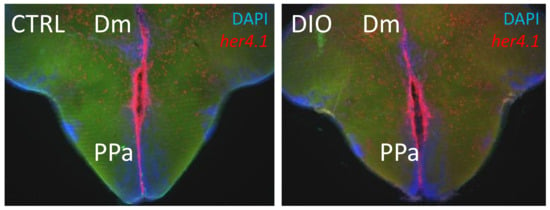
Figure 3.
her4.1 in situ hybridization on brain section of CTRL and DIO fish. Preliminary data on DIO fish (4 weeks) did not show striking differences in the expression of the Notch target gene, her4.1 (red), in CTRL and DIO fish. Cell nuclei were counterstained with DAPI (blue). Dm: dorsomedian telencephalon. PPa: anterior part of the preoptic area.
In addition, it has been shown that NSCs from the embryo of pregnant diabetic mice exhibit altered expression of genes implied in the proliferation and cell fate specification, such as delta-like 1 (a Notch ligand), Hes1 and Hes5 (key factors for Notch/Delta signaling) [195]. Furthermore, methylglyoxal, a highly reactive glycolytic intermediate metabolite, has been shown to regulate Notch signaling and, subsequently, neural progenitor fate [196].
Therefore, the disruption of Notch signaling in NSCs during obesity and/or diabetes may occur in zebrafish and modulate NSC proliferation and cell fate. However, these hypotheses definitively require further investigations.
4.2. BMP/Id1 Signaling and Cross-Talk with Notch Signaling in NSCs
Bone morphogenetic proteins (BMPs) are members of the transforming growth factor b (TGF-β) family [197], binding to transmembrane type I and type II receptors and leading to the activation of Smad proteins (Smad1/5/8). Smad1/5/8 bind to Smad4 [22,198]. This complex translocates to the nucleus and activates many target genes, including id1, the inhibitor of differentiation/DNA binding 1 [199]. In zebrafish, there are five helix–loop–helix transcriptional regulators in the Id family and four members in mice [200,201]. In both mammals and zebrafish, Id1 has overlapping and distinct functions during development and body homeostasis, controlling many cellular events, such as cell quiescence, differentiation and migration of different cell types [200].
In mammals, Id1 is an important factor regulating NSC quiescence in the mouse SVZ (a neurogenic niche), with quiescent adult NSCs strongly expressing Id1 [202]. In zebrafish, Id1 is only expressed in radial glial cells (neural stem cells) and mainly in the quiescent ones [201,203,204,205]. Interestingly, Id1 gain- and loss-of-function studies have shown that Id1 promotes the quiescence of neural stem cells, while its down-regulation allows the entry of the neural stem cell into the cell cycle [205]. The Id1 promoter regulation appears evolutionarily conserved, and BMP signaling is important for the correct expression of Id1 in zebrafish NSCs and in the regulation of their quiescent/proliferative state [204]. Id1 could promote NSC quiescence in both mouse and fish through Id1 interaction with members of the Hes/Her protein family (Notch target genes) [22,205].
Together, these data show that BMPs and Id1 are important regulators of neurogenic processes promoting neural stem cell quiescence in both mammals and fish [206]. With Notch, BMP/Id1 are also important actors of brain plasticity that should be studied in zebrafish during metabolic disorders. Indeed, in rodents, recent data suggest a role of BMPs in the development of metabolic disorders. Indeed, reduced BMP4 signaling may promote the development of obesity, insulin resistance and associated disruptions [207]. Other data show a role of BMP signaling in the regulation of feeding behavior in different mice models [208,209]. So far, BMPs play key roles in the regulation of energy balance in the brain and in adult neural plasticity [206]. It would be interesting to better understand the modulation and role of BMP/id signaling in the neurogenic niche of diabetic and/or obese models of mice and zebrafish, namely, in the hypothalamus, a key neurogenic region controlling the genesis of anorexigenic and orexigenic neurons.
4.3. The Role of Stress Hormone Signaling in Neurogenesis
Glucocorticoids are steroid hormones secreted mainly by the adrenal cortex. They are involved in several processes, such as metabolism [210], immune response [211], cardiovascular functions [212] and development [213]. De novo glucocorticoid synthesis also occurs in the brain, suggesting key roles of local synthesis in neurological functions [214,215]. Under normal physiological conditions, glucocorticoids are involved in adaptive responses. However, chronic exposure to glucocorticoids has been shown to be associated with a chronic stress state and several metabolic disorders, such as obesity, insulin resistance, glucose intolerance dyslipidemia and hypertension [216]. For instance, glucocorticoid levels have been shown to be upregulated in the adrenal glands in the case of obesity [217,218,219]. Indeed, the 11β-HSD1 enzyme that transforms cortisone into cortisol, the main active form of glucocorticoid, is increased in the adipose tissue of obese humans [220,221]. Similarly, diabetic individuals are also subjected to higher levels of glucocorticoids associated with chronic stress and depression [222,223]. The glucocorticoid responsive gene, fkbp5, has also been shown to be upregulated in the subcutaneous adipose tissue of type 2 diabetic patients, linked to the increase in glucose levels [224]. In addition, glucocorticoid levels are associated with non-alcoholic fatty liver, a hepatic disorder found in both obesity and diabetes [225].
In the central nervous system, chronic exposure to glucocorticoids increases neuro-inflammation and hippocampal neuronal damage [226]. Several studies have shown that high glucocorticoid levels are associated with decreased brain plasticity. Indeed, in vitro studies performed on embryonic neural stem/progenitor cells have confirmed that chronic exposure to glucocorticoids suppresses the differentiation and survival of NSCs affecting several signaling pathways and decreases the expression of neuronal and synaptic markers [227]. In parallel, long-term exposure leads, in vivo, to blunted hippocampal neurogenesis [228,229], which is restored through the use of glucocorticoid receptor antagonists [230]. Together, glucocorticoids increase the loss of hippocampal neurons, reduce adult neurogenesis and compromise cognitive functions, leading to an increased risk for developing Alzheimer’s disease [229,231]. This has been further corroborated by the study of diabetic mice that display higher levels of corticosteroids and have increased tau protein phosphorylation correlated with memory impairments [232]. Overall, these data support the fact that chronic exposure to glucocorticoids negatively affects brain homeostasis and plasticity, promoting behavioral change and increasing the risk factors for cognitive defects and neurodegenerative disease.
In zebrafish, the inter-renal gland ensures the function of the adrenal gland found in mammals and secretes glucocorticoids [233]. In addition, the brain is able to perform de novo steroidogenesis and glucocorticoid synthesis [234,235,236,237]. In a zebrafish model of obesity associated with hyperglycemia, the expression of the glucocorticoid responsive gene fkbp5 was up-regulated but did not reach a significant level [130]. Unpublished data from our laboratory using a stronger model of overfeeding also demonstrated an increase in the cerebral expression of fkbp5. Together, these data suggest that the peripheral and/or locally produced glucocorticoids may be increased under overfeeding conditions. Our preliminary data for measuring glucocorticoid levels in the brain of obese/hyperglycemic fish tend to show an increase in glucocorticoid levels. Further investigations are consequently required to understand whether glucocorticoids are indeed significantly increased during zebrafish obesity and diabetes or not and what are their roles in brain plasticity in these pathologies in zebrafish.
Many other factors well known to modulate and regulate neurogenesis should also be investigated, such as Wnt signaling, SHH, endothelial factors, inflammation, oxidative stress and steroids other than glucocorticoids. Figure 4 provides a schematic overview of some pathways to be investigated in the brain and NSCs under metabolic disease conditions.
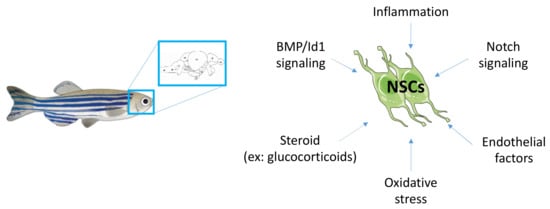
Figure 4.
Mechanisms to be investigated regarding the misregulated neurogenic signaling pathways under conditions of metabolic diseases.
5. Conclusions
Under diabetic and obese conditions, the central nervous system is greatly affected through the disruption of the BBB and subsequent neuro-inflammation and oxidative stress. In both mammalian and zebrafish models, metabolic disorders induce decreased brain plasticity, impaired cognitive functions and abnormal behaviors. However, the precise cellular and molecular mechanisms sustaining such impairments are not well understood. In this review, we aim to demonstrate that zebrafish is an alternative model to study the impact of metabolic disorders on brain homeostasis and NSC activity. Several groups have developed successful metabolic models of zebrafish, mimicking overweight, obese and hyperglycemic/diabetic states. Indeed, zebrafish under appropriate experimental procedures can show many features of human metabolic disruption: higher body weight and BMI, expansion of visceral and subcutaneous adipose tissues, liver steatosis, disturbed lipidic profiles (LDL, HDL, body cholesterol and triglycerides), insulin resistance and hyperglycemia. Furthermore, zebrafish is well-recognized as an interesting model for studying brain plasticity, including homeostatic and regenerative neurogenesis [21,22,49,55,180,187,205,238,239]. Consequently, this model is appropriate to further investigate the role of metabolic disorders in adult neurogenesis. Now, further explorations of the molecular mechanisms and signaling pathways disrupted in NSCs should be performed under metabolic disorder conditions.
Author Contributions
Writing and review: B.G. and N.D. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the European Regional Development Funds (RE0022527) ZEBRATOX (EU-Région Réunion—French State national counterpart).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
We thank Olivier Meilhac and Jean-Loup Bascands for their help in developing the obese zebrafish models.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Saklayen, M.G. The Global Epidemic of the Metabolic Syndrome. Curr. Hypertens Rep. 2018, 20, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gurgel, M.H.C.; Montenegro Junior, R.M.; Melo Ponte, C.M.; Sousa, T.C.S.; Silva, P.G.B.; de Sousa Belem, L.; Furtado, F.L.B.; de Araujo Batista, L.A.; Pereira, A.C.; Santos, R.D. Metabolic syndrome, diabetes and inadequate lifestyle in first-degree relatives of acute myocardial infarction survivors younger than 45 years old. Lipids Health Dis. 2017, 16, 224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DeBoer, M.D.; Filipp, S.L.; Sims, M.; Musani, S.K.; Gurka, M.J. Risk of Ischemic Stroke Increases Over the Spectrum of Metabolic Syndrome Severity. Stroke 2020, 51, 2548–2552. [Google Scholar] [CrossRef] [PubMed]
- Katsiki, N.; Anagnostis, P.; Kotsa, K.; Goulis, D.G.; Mikhailidis, D.P. Obesity, Metabolic Syndrome and the Risk of Microvascular Complications in Patients with Diabetes mellitus. Curr. Pharm. Des. 2019, 25, 2051–2059. [Google Scholar] [CrossRef] [PubMed]
- Bangen, K.J.; Armstrong, N.M.; Au, R.; Gross, A.L. Metabolic Syndrome and Cognitive Trajectories in the Framingham Offspring Study. J. Alzheimers Dis. 2019, 71, 931–943. [Google Scholar] [CrossRef]
- Blumentals, W.A.; Hwu, P.; Kobayashi, N.; Ogura, E. Obesity in hospitalized type 2 diabetes patients: A descriptive study. Med. Sci. Monit. 2013, 19, 359–365. [Google Scholar]
- Ritz, E. Metabolic syndrome and kidney disease. Blood Purif. 2008, 26, 59–62. [Google Scholar] [CrossRef]
- Tune, J.D.; Goodwill, A.G.; Sassoon, D.J.; Mather, K.J. Cardiovascular consequences of metabolic syndrome. Transl. Res. 2017, 183, 57–70. [Google Scholar] [CrossRef] [Green Version]
- Artunc, F.; Schleicher, E.; Weigert, C.; Fritsche, A.; Stefan, N.; Haring, H.U. The impact of insulin resistance on the kidney and vasculature. Nat. Rev. Nephrol. 2016, 12, 721–737. [Google Scholar] [CrossRef] [Green Version]
- Van der Heijden, R.A.; Sheedfar, F.; Morrison, M.C.; Hommelberg, P.P.; Kor, D.; Kloosterhuis, N.J.; Gruben, N.; Youssef, S.A.; de Bruin, A.; Hofker, M.H.; et al. High-fat diet induced obesity primes inflammation in adipose tissue prior to liver in C57BL/6j mice. Aging 2015, 7, 256–268. [Google Scholar] [CrossRef] [Green Version]
- Hazlehurst, J.M.; Woods, C.; Marjot, T.; Cobbold, J.F.; Tomlinson, J.W. Non-alcoholic fatty liver disease and diabetes. Metabolism 2016, 65, 1096–1108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andre, C.; Dinel, A.L.; Ferreira, G.; Laye, S.; Castanon, N. Diet-induced obesity progressively alters cognition, anxiety-like behavior and lipopolysaccharide-induced depressive-like behavior: Focus on brain indoleamine 2,3-dioxygenase activation. Brain Behav Immun 2014, 41, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Vieira, M.N.N.; Lima-Filho, R.A.S.; De Felice, F.G. Connecting Alzheimer’s disease to diabetes: Underlying mechanisms and potential therapeutic targets. Neuropharmacology 2018, 136, 160–171. [Google Scholar] [CrossRef] [PubMed]
- Roh, H.T.; Cho, S.Y.; So, W.Y. Obesity promotes oxidative stress and exacerbates blood-brain barrier disruption after high-intensity exercise. J. Sport Health Sci. 2017, 6, 225–230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoo, D.Y.; Yim, H.S.; Jung, H.Y.; Nam, S.M.; Kim, J.W.; Choi, J.H.; Seong, J.K.; Yoon, Y.S.; Kim, D.W.; Hwang, I.K. Chronic type 2 diabetes reduces the integrity of the blood-brain barrier by reducing tight junction proteins in the hippocampus. J. Vet. Med. Sci. 2016, 78, 957–962. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pugazhenthi, S.; Qin, L.; Reddy, P.H. Common neurodegenerative pathways in obesity, diabetes, and Alzheimer’s disease. Biochim. Biophys Acta Mol. Basis Dis. 2017, 1863, 1037–1045. [Google Scholar] [CrossRef]
- Miller, A.A.; Spencer, S.J. Obesity and neuroinflammation: A pathway to cognitive impairment. Brain Behav Immun 2014, 42, 10–21. [Google Scholar] [CrossRef]
- Milanski, M.; Degasperi, G.; Coope, A.; Morari, J.; Denis, R.; Cintra, D.E.; Tsukumo, D.M.; Anhe, G.; Amaral, M.E.; Takahashi, H.K.; et al. Saturated fatty acids produce an inflammatory response predominantly through the activation of TLR4 signaling in hypothalamus: Implications for the pathogenesis of obesity. J. Neurosci. 2009, 29, 359–370. [Google Scholar] [CrossRef]
- Sarparanta, J.; Garcia-Macia, M.; Singh, R. Autophagy and Mitochondria in Obesity and Type 2 Diabetes. Curr Diabetes Rev. 2017, 13, 352–369. [Google Scholar] [CrossRef]
- Bondia-Pons, I.; Ryan, L.; Martinez, J.A. Oxidative stress and inflammation interactions in human obesity. J. Physiol Biochem 2012, 68, 701–711. [Google Scholar] [CrossRef]
- Ghaddar, B.; Lubke, L.; Couret, D.; Rastegar, S.; Diotel, N. Cellular Mechanisms Participating in Brain Repair of Adult Zebrafish and Mammals after Injury. Cells 2021, 10, 391. [Google Scholar] [CrossRef] [PubMed]
- Diotel, N.; Lubke, L.; Strahle, U.; Rastegar, S. Common and Distinct Features of Adult Neurogenesis and Regeneration in the Telencephalon of Zebrafish and Mammals. Front. Neurosci 2020, 14, 568930. [Google Scholar] [CrossRef] [PubMed]
- Fares, J.; Bou Diab, Z.; Nabha, S.; Fares, Y. Neurogenesis in the adult hippocampus: History, regulation, and prospective roles. Int. J. Neurosci. 2019, 129, 598–611. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.J.; Kitamura, T.; Saitoh, Y.; Ohkawa, N.; Kondo, T.; Inokuchi, K. Adult Neurogenesis Conserves Hippocampal Memory Capacity. J. Neurosci 2018, 38, 6854–6863. [Google Scholar] [CrossRef] [Green Version]
- Kempermann, G.; Gage, F.H.; Aigner, L.; Song, H.; Curtis, M.A.; Thuret, S.; Kuhn, H.G.; Jessberger, S.; Frankland, P.W.; Cameron, H.A.; et al. Human Adult Neurogenesis: Evidence and Remaining Questions. Cell Stem Cell 2018, 23, 25–30. [Google Scholar] [CrossRef] [Green Version]
- Boldrini, M.; Fulmore, C.A.; Tartt, A.N.; Simeon, L.R.; Pavlova, I.; Poposka, V.; Rosoklija, G.B.; Stankov, A.; Arango, V.; Dwork, A.J.; et al. Human Hippocampal Neurogenesis Persists throughout Aging. Cell Stem Cell 2018, 22, 589–599.e5. [Google Scholar] [CrossRef] [Green Version]
- Ernst, A.; Frisen, J. Adult neurogenesis in humans- common and unique traits in mammals. PLoS Biol. 2015, 13, e1002045. [Google Scholar] [CrossRef]
- Lindsey, B.W.; Tropepe, V. A comparative framework for understanding the biological principles of adult neurogenesis. Prog. Neurobiol. 2006, 80, 281–307. [Google Scholar] [CrossRef]
- Kohman, R.A.; Rhodes, J.S. Neurogenesis, inflammation and behavior. Brain Behav. Immun. 2013, 27, 22–32. [Google Scholar] [CrossRef] [Green Version]
- Yirmiya, R.; Goshen, I. Immune modulation of learning, memory, neural plasticity and neurogenesis. Brain Behav. Immun. 2011, 25, 181–213. [Google Scholar] [CrossRef]
- Ryan, S.M.; Nolan, Y.M. Neuroinflammation negatively affects adult hippocampal neurogenesis and cognition: Can exercise compensate? Neurosci. Biobehav. Rev. 2015, 61, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Covacu, R.; Brundin, L. Effects of Neuroinflammation on Neural Stem Cells. Neuroscientist 2017, 23, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Mishra, A.; Tiwari, V.; Shukla, S. Enhanced neuroinflammation and oxidative stress are associated with altered hippocampal neurogenesis in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine treated mice. Behav. Pharmacol 2019, 30, 689–699. [Google Scholar] [CrossRef] [PubMed]
- Borsini, A.; Zunszain, P.A.; Thuret, S.; Pariante, C.M. The role of inflammatory cytokines as key modulators of neurogenesis. Trends Neurosci. 2015, 38, 145–157. [Google Scholar] [CrossRef] [Green Version]
- Zonis, S.; Ljubimov, V.A.; Mahgerefteh, M.; Pechnick, R.N.; Wawrowsky, K.; Chesnokova, V. p21Cip restrains hippocampal neurogenesis and protects neuronal progenitors from apoptosis during acute systemic inflammation. Hippocampus 2013, 23, 1383–1394. [Google Scholar] [CrossRef] [Green Version]
- Zonis, S.; Pechnick, R.N.; Ljubimov, V.A.; Mahgerefteh, M.; Wawrowsky, K.; Michelsen, K.S.; Chesnokova, V. Chronic intestinal inflammation alters hippocampal neurogenesis. J. Neuroinflamm. 2015, 12, 65. [Google Scholar] [CrossRef] [Green Version]
- Kleinert, M.; Clemmensen, C.; Hofmann, S.M.; Moore, M.C.; Renner, S.; Woods, S.C.; Huypens, P.; Beckers, J.; de Angelis, M.H.; Schurmann, A.; et al. Animal models of obesity and diabetes mellitus. Nat. Rev. Endocrinol. 2018, 14, 140–162. [Google Scholar] [CrossRef] [Green Version]
- Ghaddar, B.; Veeren, B.; Rondeau, P.; Bringart, M.; Lefebvre d’Hellencourt, C.; Meilhac, O.; Bascands, J.L.; Diotel, N. Impaired brain homeostasis and neurogenesis in diet-induced overweight zebrafish: A preventive role from A. borbonica extract. Sci. Rep. 2020, 10, 14496. [Google Scholar] [CrossRef]
- Landgraf, K.; Schuster, S.; Meusel, A.; Garten, A.; Riemer, T.; Schleinitz, D.; Kiess, W.; Korner, A. Short-term overfeeding of zebrafish with normal or high-fat diet as a model for the development of metabolically healthy versus unhealthy obesity. BMC Physiol. 2017, 17, 4. [Google Scholar] [CrossRef] [Green Version]
- Dorsemans, A.C.; Soule, S.; Weger, M.; Bourdon, E.; Lefebvre d’Hellencourt, C.; Meilhac, O.; Diotel, N. Impaired constitutive and regenerative neurogenesis in adult hyperglycemic zebrafish. J. Comp. Neurol. 2017, 525, 442–458. [Google Scholar] [CrossRef]
- Montalbano, G.; Mania, M.; Guerrera, M.C.; Laura, R.; Abbate, F.; Levanti, M.; Maugeri, A.; Germana, A.; Navarra, M. Effects of a Flavonoid-Rich Extract from Citrus sinensis Juice on a Diet-Induced Obese Zebrafish. Int. J. Mol. Sci. 2019, 20, 5116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Capiotti, K.M.; Antonioli, R., Jr.; Kist, L.W.; Bogo, M.R.; Bonan, C.D.; Da Silva, R.S. Persistent impaired glucose metabolism in a zebrafish hyperglycemia model. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2014, 171, 58–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Capiotti, K.M.; De Moraes, D.A.; Menezes, F.P.; Kist, L.W.; Bogo, M.R.; Da Silva, R.S. Hyperglycemia induces memory impairment linked to increased acetylcholinesterase activity in zebrafish (Danio rerio). Behav. Brain Res. 2014, 274, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Menke, A.L.; Spitsbergen, J.M.; Wolterbeek, A.P.; Woutersen, R.A. Normal anatomy and histology of the adult zebrafish. Toxicol. Pathol. 2011, 39, 759–775. [Google Scholar] [CrossRef]
- Howe, K.; Clark, M.D.; Torroja, C.F.; Torrance, J.; Berthelot, C.; Muffato, M.; Collins, J.E.; Humphray, S.; McLaren, K.; Matthews, L.; et al. The zebrafish reference genome sequence and its relationship to the human genome. Nature 2013, 496, 498–503. [Google Scholar] [CrossRef] [Green Version]
- Heckler, K.; Kroll, J. Zebrafish as a Model for the Study of Microvascular Complications of Diabetes and Their Mechanisms. Int. J. Mol. Sci. 2017, 18, 2002. [Google Scholar] [CrossRef] [Green Version]
- Zang, L.; Shimada, Y.; Nishimura, N. Development of a Novel Zebrafish Model for Type 2 Diabetes Mellitus. Sci. Rep. 2017, 7, 1461. [Google Scholar] [CrossRef] [Green Version]
- Wiggenhauser, L.M.; Kroll, J. Vascular Damage in Obesity and Diabetes: Highlighting Links Between Endothelial Dysfunction and Metabolic Disease in Zebrafish and Man. Curr. Vasc. Pharmacol. 2019, 17, 476–490. [Google Scholar] [CrossRef]
- Grandel, H.; Brand, M. Comparative aspects of adult neural stem cell activity in vertebrates. Dev. Genes Evol. 2013, 223, 131–147. [Google Scholar] [CrossRef]
- Grandel, H.; Kaslin, J.; Ganz, J.; Wenzel, I.; Brand, M. Neural stem cells and neurogenesis in the adult zebrafish brain: Origin, proliferation dynamics, migration and cell fate. Dev. Biol. 2006, 295, 263–277. [Google Scholar] [CrossRef] [Green Version]
- Pellegrini, E.; Mouriec, K.; Anglade, I.; Menuet, A.; Le Page, Y.; Gueguen, M.M.; Marmignon, M.H.; Brion, F.; Pakdel, F.; Kah, O. Identification of aromatase-positive radial glial cells as progenitor cells in the ventricular layer of the forebrain in zebrafish. J. Comp. Neurol. 2007, 501, 150–167. [Google Scholar] [CrossRef] [PubMed]
- Lucini, C.; D’Angelo, L.; Cacialli, P.; Palladino, A.; de Girolamo, P. BDNF, Brain, and Regeneration: Insights from Zebrafish. Int. J. Mol. Sci. 2018, 19, 3155. [Google Scholar] [CrossRef] [Green Version]
- Zambusi, A.; Ninkovic, J. Regeneration of the central nervous system-principles from brain regeneration in adult zebrafish. World J. Stem Cells 2020, 12, 8–24. [Google Scholar] [CrossRef] [PubMed]
- Lupperger, V.; Buggenthin, F.; Chapouton, P.; Marr, C. Image analysis of neural stem cell division patterns in the zebrafish brain. Cytometry A 2018, 93, 314–322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmidt, R.; Beil, T.; Strähle, U.; Rastegar, S. Stab wound injury of the zebrafish adult telencephalon: A method to investigate vertebrate brain neurogenesis and regeneration. J. Vis. Exp. 2014, 90, e51753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dorsemans, A.C.; Lefebvre d’Hellencourt, C.; Ait-Arsa, I.; Jestin, E.; Meilhac, O.; Diotel, N. Acute and Chronic Models of Hyperglycemia in Zebrafish: A Method to Assess the Impact of Hyperglycemia on Neurogenesis and the Biodistribution of Radiolabeled Molecules. J. Vis. Exp. 2017, 124, e55203. [Google Scholar] [CrossRef] [PubMed]
- Couret, D.; Bourane, S.; Catan, A.; Nativel, B.; Planesse, C.; Dorsemans, A.C.; Ait-Arsa, I.; Cournot, M.; Rondeau, P.; Patche, J.; et al. A hemorrhagic transformation model of mechanical stroke therapy with acute hyperglycemia in mice. J. Comp. Neurol. 2018, 526, 1006–1016. [Google Scholar] [CrossRef] [PubMed]
- Couret, D.; Planesse, C.; Patche, J.; Diotel, N.; Nativel, B.; Bourane, S.; Meilhac, O. Lack of Neuroprotective Effects of High-Density Lipoprotein Therapy in Stroke under Acute Hyperglycemic Conditions. Molecules 2021, 26, 6365. [Google Scholar] [CrossRef] [PubMed]
- Abookasis, D.; Shemesh, D.; Bokobza, N.; Bloygrund, H.; Franjy-Tal, Y.; Rozenberg, K.; Rosenzweig, T. Evaluation the effect of acute hyperglycemia on cerebral tissue properties with diffuse optical imaging systems. In Proceedings of the Neurophotonics. International Society for Optics and Photonics, Online, 1 April 2020; p. 11360. [Google Scholar] [CrossRef]
- Kinkel, M.D.; Eames, S.C.; Philipson, L.H.; Prince, V.E. Intraperitoneal injection into adult zebrafish. J. Vis. Exp. 2010, 30, 2126. [Google Scholar] [CrossRef] [PubMed]
- Moss, J.B.; Koustubhan, P.; Greenman, M.; Parsons, M.J.; Walter, I.; Moss, L.G. Regeneration of the pancreas in adult zebrafish. Diabetes 2009, 58, 1844–1851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delaspre, F.; Beer, R.L.; Rovira, M.; Huang, W.; Wang, G.; Gee, S.; Vitery Mdel, C.; Wheelan, S.J.; Parsons, M.J. Centroacinar Cells Are Progenitors That Contribute to Endocrine Pancreas Regeneration. Diabetes 2015, 64, 3499–3509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Intine, R.V.; Olsen, A.S.; Sarras, M.P., Jr. A zebrafish model of diabetes mellitus and metabolic memory. J. Vis. Exp. 2013, 28, e50232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benchoula, K.; Khatib, A.; Quzwain, F.M.C.; Che Mohamad, C.A.; Wan Sulaiman, W.M.A.; Abdul Wahab, R.; Ahmed, Q.U.; Abdul Ghaffar, M.; Saiman, M.Z.; Alajmi, M.F.; et al. Optimization of Hyperglycemic Induction in Zebrafish and Evaluation of Its Blood Glucose Level and Metabolite Fingerprint Treated with Psychotria malayana Jack Leaf Extract. Molecules 2019, 24, 1506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Islam, M.S.; Loots du, T. Experimental rodent models of type 2 diabetes: A review. Methods Find. Exp. Clin. Pharmacol. 2009, 31, 249–261. [Google Scholar] [CrossRef] [PubMed]
- Szkudelski, T. The mechanism of alloxan and streptozotocin action in B cells of the rat pancreas. Physiol. Res. 2001, 50, 537–546. [Google Scholar] [PubMed]
- Olsen, A.S.; Sarras, M.P., Jr.; Intine, R.V. Limb regeneration is impaired in an adult zebrafish model of diabetes mellitus. Wound Repair Regen. 2010, 18, 532–542. [Google Scholar] [CrossRef] [Green Version]
- Nam, Y.H.; Hong, B.N.; Rodriguez, I.; Ji, M.G.; Kim, K.; Kim, U.J.; Kang, T.H. Synergistic Potentials of Coffee on Injured Pancreatic Islets and Insulin Action via KATP Channel Blocking in Zebrafish. J. Agric. Food Chem. 2015, 63, 5612–5621. [Google Scholar] [CrossRef]
- Shin, E.; Na Hong, B.; Ho Kang, T. An optimal establishment of an acute hyperglycemia zebrafish model. Afr. J. Pharm. Pharmacol. 2012, 6, 2922–2928. [Google Scholar] [CrossRef] [Green Version]
- Neelofar, K.; Arif, Z.; Alam, K.; Ahmad, J. Hyperglycemia induced structural and functional changes in human serum albumin of diabetic patients: A physico-chemical study. Mol. Biosyst. 2016, 12, 2481–2489. [Google Scholar] [CrossRef]
- Steele, C.; Hagopian, W.A.; Gitelman, S.; Masharani, U.; Cavaghan, M.; Rother, K.I.; Donaldson, D.; Harlan, D.M.; Bluestone, J.; Herold, K.C. Insulin secretion in type 1 diabetes. Diabetes 2004, 53, 426–433. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Wang, Y.; Gurung, P.; Wang, T.; Li, L.; Zhang, R.; Li, H.; Guo, R.; Han, Q.; Zhang, J.; et al. The relationship between the thickness of glomerular basement membrane and renal outcomes in patients with diabetic nephropathy. Acta. Diabetol. 2018, 55, 669–679. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Cao, D.; Yu, H.; Yang, D.; Zhuang, X.; Hu, Y.; Li, J.; Yang, J.; Wu, Q.; Liu, B.; et al. Early retinal neurovascular impairment in patients with diabetes without clinically detectable retinopathy. Br. J. Ophthalmol. 2019, 103, 1747–1752. [Google Scholar] [PubMed]
- Patel, S.; Srivastava, S.; Singh, M.R.; Singh, D. Mechanistic insight into diabetic wounds: Pathogenesis, molecular targets and treatment strategies to pace wound healing. Biomed. Pharmacother. 2019, 112, 108615. [Google Scholar] [CrossRef]
- Wang, Q.; Luo, C.; Lu, G.; Chen, Z. Effect of adenosine monophosphate-activated protein kinase-p53-Kruppel-like factor 2a pathway in hyperglycemia-induced cardiac remodeling in adult zebrafish. J. Diabetes Investig. 2021, 12, 320–333. [Google Scholar] [CrossRef] [PubMed]
- Olsen, A.S.; Sarras, M.P., Jr.; Leontovich, A.; Intine, R.V. Heritable transmission of diabetic metabolic memory in zebrafish correlates with DNA hypomethylation and aberrant gene expression. Diabetes 2012, 61, 485–491. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Yang, X.L.; Liu, K.C.; Sheng, W.L.; Xia, Q.; Wang, R.C.; Chen, X.Q.; Zhang, Y. Effects of streptozotocin on pancreatic islet beta-cell apoptosis and glucose metabolism in zebrafish larvae. Fish. Physiol. Biochem. 2020, 46, 1025–1038. [Google Scholar] [CrossRef]
- Prince, V.E.; Anderson, R.M.; Dalgin, G. Zebrafish Pancreas Development and Regeneration: Fishing for Diabetes Therapies. Curr. Top. Dev. Biol. 2017, 124, 235–276. [Google Scholar]
- Matsuda, H. Zebrafish as a model for studying functional pancreatic beta cells development and regeneration. Dev. Growth Differ. 2018, 60, 393–399. [Google Scholar] [CrossRef] [Green Version]
- Benchoula, K.; Khatib, A.; Jaffar, A.; Ahmed, Q.U.; Sulaiman, W.; Wahab, R.A.; El-Seedi, H.R. The promise of zebrafish as a model of metabolic syndrome. Exp. Anim. 2019, 68, 407–416. [Google Scholar] [CrossRef] [Green Version]
- Connaughton, V.P.; Baker, C.; Fonde, L.; Gerardi, E.; Slack, C. Alternate Immersion in an External Glucose Solution Differentially Affects Blood Sugar Values in Older Versus Younger Zebrafish Adults. Zebrafish 2016, 13, 87–94. [Google Scholar] [CrossRef]
- Gence, L.; Fernezelian, D.; Bringart, M.; Veeren, B.; Christophe, A.; Brion, F.; Meilhac, O.; Bascands, J.L.; Diotel, N. Hypericum lanceolatum Lam. Medicinal Plant: Potential Toxicity and Therapeutic Effects Based on a Q2 Zebrafish Model. Front. Pharmacol. 2022, 13, 832928. [Google Scholar] [CrossRef] [PubMed]
- Alsahli, M.; Gerich, J.E. Renal glucose metabolism in normal physiological conditions and in diabetes. Diabetes Res. Clin. Pract. 2017, 133, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Schwartsburd, P. Glucose-lowering Strategies in Diabetes: Pharmacological Development of New Antidiabetic Drugs. Curr. Pharm. Des. 2018, 24, 1007–1011. [Google Scholar] [CrossRef] [PubMed]
- Block, N.E.; Buse, M.G. Effects of hypercortisolemia and diabetes on skeletal muscle insulin receptor function in vitro and in vivo. Am. J. Physiol. 1989, 256, E39–E48. [Google Scholar] [CrossRef] [PubMed]
- Karanth, S.; Chaurasia, B.; Bowman, F.M.; Tippetts, T.S.; Holland, W.L.; Summers, S.A.; Schlegel, A. FOXN3 controls liver glucose metabolism by regulating gluconeogenic substrate selection. Physiol. Rep. 2019, 7, e14238. [Google Scholar] [CrossRef] [PubMed]
- Karanth, S.; Zinkhan, E.K.; Hill, J.T.; Yost, H.J.; Schlegel, A. FOXN3 Regulates Hepatic Glucose Utilization. Cell Rep. 2016, 15, 2745–2755. [Google Scholar] [CrossRef] [Green Version]
- Kimmel, R.A.; Dobler, S.; Schmitner, N.; Walsen, T.; Freudenblum, J.; Meyer, D. Diabetic pdx1-mutant zebrafish show conserved responses to nutrient overload and anti-glycemic treatment. Sci. Rep. 2015, 5, 14241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maddison, L.A.; Joest, K.E.; Kammeyer, R.M.; Chen, W. Skeletal muscle insulin resistance in zebrafish induces alterations in beta-cell number and glucose tolerance in an age- and diet-dependent manner. Am. J. Physiol. Endocrinol. Metab. 2015, 308, E662–E669. [Google Scholar] [CrossRef] [Green Version]
- Salehpour, A.; Rezaei, M.; Khoradmehr, A.; Tahamtani, Y.; Tamadon, A. Which Hyperglycemic Model of Zebrafish (Danio rerio) Suites My Type 2 Diabetes Mellitus Research? A Scoring System for Available Methods. Front. Cell Dev. Biol. 2021, 9, 652061. [Google Scholar] [CrossRef]
- Zang, L.; Maddison, L.A.; Chen, W. Zebrafish as a Model for Obesity and Diabetes. Front. Cell Dev. Biol. 2018, 6, 91. [Google Scholar] [CrossRef] [Green Version]
- Anderson, J.L.; Carten, J.D.; Farber, S.A. Zebrafish lipid metabolism: From mediating early patterning to the metabolism of dietary fat and cholesterol. Methods Cell Biol. 2011, 101, 111–141. [Google Scholar] [PubMed] [Green Version]
- Meguro, S.; Hasumura, T.; Hase, T. Body fat accumulation in zebrafish is induced by a diet rich in fat and reduced by supplementation with green tea extract. PLoS ONE 2015, 10, e0120142. [Google Scholar] [CrossRef] [PubMed]
- Montalbano, G.; Mania, M.; Abbate, F.; Navarra, M.; Guerrera, M.C.; Laura, R.; Vega, J.A.; Levanti, M.; Germana, A. Melatonin treatment suppresses appetite genes and improves adipose tissue plasticity in diet-induced obese zebrafish. Endocrine 2018, 62, 381–393. [Google Scholar] [CrossRef] [PubMed]
- Montalbano, G.; Mania, M.; Guerrera, M.C.; Abbate, F.; Laura, R.; Navarra, M.; Vega, J.A.; Ciriaco, E.; Germana, A. Morphological differences in adipose tissue and changes in BDNF/Trkb expression in brain and gut of a diet induced obese zebrafish model. Ann. Anat. 2016, 204, 36–44. [Google Scholar] [CrossRef]
- Ahima, R.S.; Lazar, M.A. Physiology. The health risk of obesity–better metrics imperative. Science 2013, 341, 856–858. [Google Scholar] [CrossRef]
- Chu, C.Y.; Chen, C.F.; Rajendran, R.S.; Shen, C.N.; Chen, T.H.; Yen, C.C.; Chuang, C.K.; Lin, D.S.; Hsiao, C.D. Overexpression of Akt1 enhances adipogenesis and leads to lipoma formation in zebrafish. PLoS ONE 2012, 7, e36474. [Google Scholar]
- Hsu, C.C.; Lai, C.Y.; Lin, C.Y.; Yeh, K.Y.; Her, G.M. MicroRNA-27b Depletion Enhances Endotrophic and Intravascular Lipid Accumulation and Induces Adipocyte Hyperplasia in Zebrafish. Int. J. Mol. Sci. 2017, 19, 93. [Google Scholar] [CrossRef] [Green Version]
- Oka, T.; Nishimura, Y.; Zang, L.; Hirano, M.; Shimada, Y.; Wang, Z.; Umemoto, N.; Kuroyanagi, J.; Nishimura, N.; Tanaka, T. Diet-induced obesity in zebrafish shares common pathophysiological pathways with mammalian obesity. BMC Physiol. 2010, 10, 21. [Google Scholar] [CrossRef] [Green Version]
- Hasumura, T.; Shimada, Y.; Kuroyanagi, J.; Nishimura, Y.; Meguro, S.; Takema, Y.; Tanaka, T. Green tea extract suppresses adiposity and affects the expression of lipid metabolism genes in diet-induced obese zebrafish. Nutr. Metab. 2012, 9, 73. [Google Scholar] [CrossRef] [Green Version]
- Hiramitsu, M.; Shimada, Y.; Kuroyanagi, J.; Inoue, T.; Katagiri, T.; Zang, L.; Nishimura, Y.; Nishimura, N.; Tanaka, T. Eriocitrin ameliorates diet-induced hepatic steatosis with activation of mitochondrial biogenesis. Sci. Rep. 2014, 4, 3708. [Google Scholar] [CrossRef] [Green Version]
- Forn-Cuni, G.; Varela, M.; Fernandez-Rodriguez, C.M.; Figueras, A.; Novoa, B. Liver immune responses to inflammatory stimuli in a diet-induced obesity model of zebrafish. J. Endocrinol. 2015, 224, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Meguro, S.; Hosoi, S.; Hasumura, T. High-fat diet impairs cognitive function of zebrafish. Sci. Rep. 2019, 9, 17063. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Mueller, O.; Bagwell, J.; Bagnat, M.; Liddle, R.A.; Rawls, J.F. High fat diet induces microbiota-dependent silencing of enteroendocrine cells. Elife 2019, 8, e48479. [Google Scholar] [CrossRef] [PubMed]
- Grisotto, C.; Taile, J.; Planesse, C.; Diotel, N.; Gonthier, M.P.; Meilhac, O.; Couret, D. High-Fat Diet Aggravates Cerebral Infarct, Hemorrhagic Transformation and Neuroinflammation in a Mouse Stroke Model. Int. J. Mol. Sci. 2021, 22, 4571. [Google Scholar] [CrossRef]
- Kothari, V.; Luo, Y.; Tornabene, T.; O’Neill, A.M.; Greene, M.W.; Geetha, T.; Babu, J.R. High fat diet induces brain insulin resistance and cognitive impairment in mice. Biochim. Biophys. Acta. Mol. Basis. Dis. 2017, 1863, 499–508. [Google Scholar] [CrossRef]
- Della Vedova, M.C.; Munoz, M.D.; Santillan, L.D.; Plateo-Pignatari, M.G.; Germano, M.J.; Rinaldi Tosi, M.E.; Garcia, S.; Gomez, N.N.; Fornes, M.W.; Gomez Mejiba, S.E.; et al. A Mouse Model of Diet-Induced Obesity Resembling Most Features of Human Metabolic Syndrome. Nutr. Metab. Insights 2016, 9, 93–102. [Google Scholar] [CrossRef] [Green Version]
- Cao, M.; Pan, Q.; Dong, H.; Yuan, X.; Li, Y.; Sun, Z.; Dong, X.; Wang, H. Adipose-derived mesenchymal stem cells improve glucose homeostasis in high-fat diet-induced obese mice. Stem. Cell Res. Ther. 2015, 6, 208. [Google Scholar] [CrossRef] [Green Version]
- Carnovali, M.; Luzi, L.; Terruzzi, I.; Banfi, G.; Mariotti, M. Metabolic and bone effects of high-fat diet in adult zebrafish. Endocrine 2018, 61, 317–326. [Google Scholar] [CrossRef]
- Diotel, N.; Rodriguez Viales, R.; Armant, O.; Marz, M.; Ferg, M.; Rastegar, S.; Strahle, U. Comprehensive expression map of transcription regulators in the adult zebrafish telencephalon reveals distinct neurogenic niches. J. Comp. Neurol. 2015, 523, 1202–1221. [Google Scholar] [CrossRef] [Green Version]
- Kizil, C.; Kaslin, J.; Kroehne, V.; Brand, M. Adult neurogenesis and brain regeneration in zebrafish. Dev. Neurobiol. 2012, 72, 429–461. [Google Scholar] [CrossRef]
- Eliceiri, B.P.; Gonzalez, A.M.; Baird, A. Zebrafish model of the blood-brain barrier: Morphological and permeability studies. Methods Mol. Biol. 2011, 686, 371–378. [Google Scholar] [PubMed] [Green Version]
- Chhabria, K.; Vouros, A.; Gray, C.; MacDonald, R.B.; Jiang, Z.; Wilkinson, R.N.; Plant, K.; Vasilaki, E.; Howarth, C.; Chico, T.J.A. Sodium nitroprusside prevents the detrimental effects of glucose on the neurovascular unit and behaviour in zebrafish. Dis. Model. Mech. 2019, 12, dmm039867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chhabria, K.; Plant, K.; Bandmann, O.; Wilkinson, R.N.; Martin, C.; Kugler, E.; Armitage, P.A.; Santoscoy, P.L.; Cunliffe, V.T.; Huisken, J.; et al. The effect of hyperglycemia on neurovascular coupling and cerebrovascular patterning in zebrafish. J. Cereb. Blood Flow Metab. 2020, 40, 298–313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dos Santos, M.M.; de Macedo, G.T.; Prestes, A.S.; Ecker, A.; Muller, T.E.; Leitemperger, J.; Fontana, B.D.; Ardisson-Araujo, D.M.P.; Rosemberg, D.B.; Barbosa, N.V. Modulation of redox and insulin signaling underlie the anti-hyperglycemic and antioxidant effects of diphenyl diselenide in zebrafish. Free. Radic. Biol. Med. 2020, 158, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Nellore, J.; Pauline, P.C.; Mohanan, S.P.; Ravikumar, R.; Pillai, R.C. Vinca rosea Normalizes Oxidative Stress and Inhibits Hyperglycemia Induced Increase in VEGF in Zebrafish Retina. Res. J. Pharm. Biol. Chem. Sci. 2013, 4, 927–936. [Google Scholar]
- Oyelaja-Akinsipo, O.B.; Dare, E.O.; Katare, D.P. Protective role of diosgenin against hyperglycaemia-mediated cerebral ischemic brain injury in zebrafish model of type II diabetes mellitus. Heliyon 2020, 6, e03296. [Google Scholar] [CrossRef] [Green Version]
- Dos Santos, M.M.; de Macedo, G.T.; Prestes, A.S.; Loro, V.L.; Heidrich, G.M.; Picoloto, R.S.; Rosemberg, D.B.; Barbosa, N.V. Hyperglycemia elicits anxiety-like behaviors in zebrafish: Protective role of dietary diphenyl diselenide. Prog. Neuropsychopharmacol. Biol. Psychiatry 2018, 85, 128–135. [Google Scholar] [CrossRef]
- Rom, S.; Zuluaga-Ramirez, V.; Gajghate, S.; Seliga, A.; Winfield, M.; Heldt, N.A.; Kolpakov, M.A.; Bashkirova, Y.V.; Sabri, A.K.; Persidsky, Y. Hyperglycemia-Driven Neuroinflammation Compromises BBB Leading to Memory Loss in Both Diabetes Mellitus (DM) Type 1 and Type 2 Mouse Models. Mol. Neurobiol. 2019, 56, 1883–1896. [Google Scholar] [CrossRef]
- Wanrooy, B.J.; Kumar, K.P.; Wen, S.W.; Qin, C.X.; Ritchie, R.H.; Wong, C.H.Y. Distinct contributions of hyperglycemia and high-fat feeding in metabolic syndrome-induced neuroinflammation. J. Neuroinflamm. 2018, 15, 293. [Google Scholar] [CrossRef]
- Alvarez-Nolting, R.; Arnal, E.; Barcia, J.M.; Miranda, M.; Romero, F.J. Protection by DHA of early hippocampal changes in diabetes: Possible role of CREB and NF-kappaB. Neurochem. Res. 2012, 37, 105–115. [Google Scholar] [CrossRef]
- Piazza, F.V.; Pinto, G.V.; Trott, G.; Marcuzzo, S.; Gomez, R.; Fernandes Mda, C. Enriched environment prevents memory deficits in type 1 diabetic rats. Behav. Brain Res. 2011, 217, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Balu, D.T.; Hodes, G.E.; Hill, T.E.; Ho, N.; Rahman, Z.; Bender, C.N.; Ring, R.H.; Dwyer, J.M.; Rosenzweig-Lipson, S.; Hughes, Z.A.; et al. Flow cytometric analysis of BrdU incorporation as a high-throughput method for measuring adult neurogenesis in the mouse. J. Pharmacol. Toxicol. Methods 2009, 59, 100–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beauquis, J.; Roig, P.; Homo-Delarche, F.; De Nicola, A.; Saravia, F. Reduced hippocampal neurogenesis and number of hilar neurones in streptozotocin-induced diabetic mice: Reversion by antidepressant treatment. Eur. J. Neurosci. 2006, 23, 1539–1546. [Google Scholar] [CrossRef] [PubMed]
- Ho, N.; Sommers, M.S.; Lucki, I. Effects of diabetes on hippocampal neurogenesis: Links to cognition and depression. Neurosci. Biobehav. Rev. 2013, 37, 1346–1362. [Google Scholar] [CrossRef] [Green Version]
- Corem, N.; Anzi, S.; Gelb, S.; Ben-Zvi, A. Leptin receptor deficiency induces early, transient and hyperglycaemia-independent blood-brain barrier dysfunction. Sci. Rep. 2019, 9, 2884. [Google Scholar] [CrossRef] [Green Version]
- Fujihara, R.; Chiba, Y.; Nakagawa, T.; Nishi, N.; Murakami, R.; Matsumoto, K.; Kawauchi, M.; Yamamoto, T.; Ueno, M. Albumin microvascular leakage in brains with diabetes mellitus. Microsc. Res. Tech. 2016, 79, 833–837. [Google Scholar] [CrossRef]
- Nerurkar, P.V.; Johns, L.M.; Buesa, L.M.; Kipyakwai, G.; Volper, E.; Sato, R.; Shah, P.; Feher, D.; Williams, P.G.; Nerurkar, V.R. Momordica charantia (bitter melon) attenuates high-fat diet-associated oxidative stress and neuroinflammation. J. Neuroinflamm. 2011, 8, 64. [Google Scholar] [CrossRef]
- Muriach, M.; Flores-Bellver, M.; Romero, F.J.; Barcia, J.M. Diabetes and the brain: Oxidative stress, inflammation, and autophagy. Oxid. Med. Cell. Longev. 2014, 2014, 102158. [Google Scholar] [CrossRef] [Green Version]
- Ghaddar, B.; Bringart, M.; Lefebvre d’Hellencourt, C.; Meilhac, O.; Diotel, N. Deleterious Effects of Overfeeding on Brain Homeostasis and Plasticity in Adult Zebrafish. Zebrafish 2021, 18, 190–206. [Google Scholar] [CrossRef]
- Imperatore, R.; Tunisi, L.; Mavaro, I.; D’Angelo, L.; Attanasio, C.; Safari, O.; Motlagh, H.A.; De Girolamo, P.; Cristino, L.; Varricchio, E.; et al. Immunohistochemical Analysis of Intestinal and Central Nervous System Morphology in an Obese Animal Model (Danio rerio) Treated with 3,5-T2: A Possible Farm Management Practice? Animals 2020, 10, 1131. [Google Scholar] [CrossRef]
- Wang, J.; Li, Y.; Lai, K.; Zhong, Q.; Demin, K.A.; Kalueff, A.V.; Song, C. High-glucose/high-cholesterol diet in zebrafish evokes diabetic and affective pathogenesis: The role of peripheral and central inflammation, microglia and apoptosis. Prog. Neuropsychopharmacol. Biol. Psychiatry 2020, 96, 109752. [Google Scholar] [CrossRef] [PubMed]
- Luzio, A.; Figueiredo, M.; Matos, M.M.; Coimbra, A.M.; Alvaro, A.R.; Monteiro, S.M. Effects of short-term exposure to genistein and overfeeding diet on the neural and retinal progenitor competence of adult zebrafish (Danio rerio). Neurotoxicol. Teratol. 2021, 88, 107030. [Google Scholar] [CrossRef] [PubMed]
- Stankiewicz, A.J.; McGowan, E.M.; Yu, L.; Zhdanova, I.V. Impaired Sleep, Circadian Rhythms and Neurogenesis in Diet-Induced Premature Aging. Int. J. Mol. Sci. 2017, 18, 2243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weger, M.; Diotel, N.; Dorsemans, A.C.; Dickmeis, T.; Weger, B.D. Stem cells and the circadian clock. Dev. Biol. 2017, 431, 111–123. [Google Scholar] [CrossRef] [PubMed]
- Weger, M.; Weger, B.D.; Diotel, N.; Rastegar, S.; Hirota, T.; Kay, S.A.; Strahle, U.; Dickmeis, T. Real-time in vivo monitoring of circadian E-box enhancer activity: A robust and sensitive zebrafish reporter line for developmental, chemical and neural biology of the circadian clock. Dev. Biol. 2013, 380, 259–273. [Google Scholar] [CrossRef] [Green Version]
- Ogata, S.; Ito, S.; Masuda, T.; Ohtsuki, S. Changes of Blood-Brain Barrier and Brain Parenchymal Protein Expression Levels of Mice under Different Insulin-Resistance Conditions Induced by High-Fat Diet. Pharm. Res. 2019, 36, 141. [Google Scholar] [CrossRef]
- Salameh, T.S.; Mortell, W.G.; Logsdon, A.F.; Butterfield, D.A.; Banks, W.A. Disruption of the hippocampal and hypothalamic blood-brain barrier in a diet-induced obese model of type II diabetes: Prevention and treatment by the mitochondrial carbonic anhydrase inhibitor, topiramate. Fluids Barriers CNS 2019, 16, 1. [Google Scholar] [CrossRef] [Green Version]
- Valcarcel-Ares, M.N.; Tucsek, Z.; Kiss, T.; Giles, C.B.; Tarantini, S.; Yabluchanskiy, A.; Balasubramanian, P.; Gautam, T.; Galvan, V.; Ballabh, P.; et al. Obesity in Aging Exacerbates Neuroinflammation, Dysregulating Synaptic Function-Related Genes and Altering Eicosanoid Synthesis in the Mouse Hippocampus: Potential Role in Impaired Synaptic Plasticity and Cognitive Decline. J. Gerontol A Biol. Sci Med. Sci. 2019, 74, 290–298. [Google Scholar] [CrossRef]
- Hersey, M.; Woodruff, J.L.; Maxwell, N.; Sadek, A.T.; Bykalo, M.K.; Bain, I.; Grillo, C.A.; Piroli, G.G.; Hashemi, P.; Reagan, L.P. High-fat diet induces neuroinflammation and reduces the serotonergic response to escitalopram in the hippocampus of obese rats. Brain Behav. Immun. 2021, 96, 63–72. [Google Scholar] [CrossRef]
- Ly, M.; Raji, C.A.; Yu, G.Z.; Wang, Q.; Wang, Y.; Schindler, S.E.; An, H.; Samara, A.; Eisenstein, S.A.; Hershey, T.; et al. Obesity and White Matter Neuroinflammation Related Edema in Alzheimer’s Disease Dementia Biomarker Negative Cognitively Normal Individuals. J. Alzheimers Dis. 2021, 79, 1801–1811. [Google Scholar] [CrossRef]
- Nam, S.M.; Kim, J.W.; Kwon, H.J.; Yoo, D.Y.; Jung, H.Y.; Kim, D.W.; Hwang, I.K.; Seong, J.K.; Yoon, Y.S. Differential Effects of Low- and High-dose Zinc Supplementation on Synaptic Plasticity and Neurogenesis in the Hippocampus of Control and High-fat Diet-fed Mice. Neurochem. Res. 2017, 42, 3149–3159. [Google Scholar] [CrossRef] [PubMed]
- Suarez, J.; Rivera, P.; Aparisi Rey, A.; Perez-Martin, M.; Arrabal, S.; Rodriguez de Fonseca, F.; Ruiz de Azua, I.; Lutz, B. Adipocyte cannabinoid CB1 receptor deficiency alleviates high fat diet-induced memory deficit, depressive-like behavior, neuroinflammation and impairment in adult neurogenesis. Psychoneuroendocrinology 2019, 110, 104418. [Google Scholar] [CrossRef] [PubMed]
- Wongchitrat, P.; Lansubsakul, N.; Kamsrijai, U.; Sae-Ung, K.; Mukda, S.; Govitrapong, P. Melatonin attenuates the high-fat diet and streptozotocin-induced reduction in rat hippocampal neurogenesis. Neurochem. Int. 2016, 100, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Saiyasit, N.; Chunchai, T.; Prus, D.; Suparan, K.; Pittayapong, P.; Apaijai, N.; Pratchayasakul, W.; Sripetchwandee, J.; Chattipakorn, M.D.P.D.N.; Chattipakorn, S.C. Gut dysbiosis develops before metabolic disturbance and cognitive decline in high-fat diet-induced obese condition. Nutrition 2020, 69, 110576. [Google Scholar] [CrossRef]
- Durrington, P. Dyslipidaemia. Lancet 2003, 362, 717–731. [Google Scholar] [CrossRef]
- Grundy, S.M.; Stone, N.J.; Bailey, A.L.; Beam, C.; Birtcher, K.K.; Blumenthal, R.S.; Braun, L.T.; de Ferranti, S.; Faiella-Tommasino, J.; Forman, D.E.; et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019, 139, e1082–e1143. [Google Scholar]
- Grundy, S.M. Does Dietary Cholesterol Matter? Curr. Atheroscler. Rep. 2016, 18, 68. [Google Scholar] [CrossRef]
- Vincent, M.J.; Allen, B.; Palacios, O.M.; Haber, L.T.; Maki, K.C. Meta-regression analysis of the effects of dietary cholesterol intake on LDL and HDL cholesterol. Am. J. Clin. Nutr. 2019, 109, 7–16. [Google Scholar] [CrossRef]
- Bhatnagar, D.; Soran, H.; Durrington, P.N. Hypercholesterolaemia and its management. BMJ 2008, 337, a993. [Google Scholar] [CrossRef] [Green Version]
- De Oliveira, J.; Engel, D.F.; de Paula, G.C.; Dos Santos, D.B.; Lopes, J.B.; Farina, M.; Moreira, E.L.G.; de Bem, A.F. High Cholesterol Diet Exacerbates Blood-Brain Barrier Disruption in LDLr-/- Mice: Impact on Cognitive Function. J. Alzheimers Dis. 2020, 78, 97–115. [Google Scholar] [CrossRef]
- Czuba, E.; Steliga, A.; Lietzau, G.; Kowianski, P. Cholesterol as a modifying agent of the neurovascular unit structure and function under physiological and pathological conditions. Metab. Brain Dis. 2017, 32, 935–948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thirumangalakudi, L.; Prakasam, A.; Zhang, R.; Bimonte-Nelson, H.; Sambamurti, K.; Kindy, M.S.; Bhat, N.R. High cholesterol-induced neuroinflammation and amyloid precursor protein processing correlate with loss of working memory in mice. J. Neurochem. 2008, 106, 475–485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Engel, D.F.; Grzyb, A.N.; de Oliveira, J.; Potzsch, A.; Walker, T.L.; Brocardo, P.S.; Kempermann, G.; de Bem, A.F. Impaired adult hippocampal neurogenesis in a mouse model of familial hypercholesterolemia: A role for the LDL receptor and cholesterol metabolism in adult neural precursor cells. Mol. Metab. 2019, 30, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Singh, C.P. Histopathological changes in different tissues of T. fasciatus under the acute impact of BHC. Toxicol. Lett. 1982, 14, 151–156. [Google Scholar] [CrossRef]
- Zambon, D.; Quintana, M.; Mata, P.; Alonso, R.; Benavent, J.; Cruz-Sanchez, F.; Gich, J.; Pocovi, M.; Civeira, F.; Capurro, S.; et al. Higher incidence of mild cognitive impairment in familial hypercholesterolemia. Am. J. Med. 2010, 123, 267–274. [Google Scholar] [CrossRef] [Green Version]
- Park, S.H.; Kim, J.H.; Choi, K.H.; Jang, Y.J.; Bae, S.S.; Choi, B.T.; Shin, H.K. Hypercholesterolemia accelerates amyloid beta-induced cognitive deficits. Int J. Mol. Med. 2013, 31, 577–582. [Google Scholar] [CrossRef] [Green Version]
- Wu, M.; Zhai, Y.; Liang, X.; Chen, W.; Lin, R.; Ma, L.; Huang, Y.; Zhao, D.; Liang, Y.; Zhao, W.; et al. Connecting the Dots Between Hypercholesterolemia and Alzheimer’s Disease: A Potential Mechanism Based on 27-Hydroxycholesterol. Front. Neurosci. 2022, 16, 842814. [Google Scholar] [CrossRef]
- Maron, D.J.; Fazio, S.; Linton, M.F. Current perspectives on statins. Circulation 2000, 101, 207–213. [Google Scholar] [CrossRef] [Green Version]
- Van Vliet, P. Cholesterol and late-life cognitive decline. J. Alzheimers Dis. 2012, 30 (Suppl. S2), S147–S162. [Google Scholar] [CrossRef]
- Swiger, K.J.; Manalac, R.J.; Blumenthal, R.S.; Blaha, M.J.; Martin, S.S. Statins and cognition: A systematic review and meta-analysis of short- and long-term cognitive effects. Mayo Clin. Proc. 2013, 88, 1213–1221. [Google Scholar] [CrossRef]
- Wagstaff, L.R.; Mitton, M.W.; Arvik, B.M.; Doraiswamy, P.M. Statin-associated memory loss: Analysis of 60 case reports and review of the literature. Pharmacotherapy 2003, 23, 871–880. [Google Scholar] [CrossRef] [PubMed]
- Mandas, A.; Congiu, M.G.; Abete, C.; Dessi, S.; Manconi, P.E.; Musio, M.; Columbu, S.; Racugno, W. Cognitive decline and depressive symptoms in late-life are associated with statin use: Evidence from a population-based study of Sardinian old people living in their own home. Neurol Res. 2014, 36, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Hottman, D.; Cheng, S.; Gram, A.; LeBlanc, K.; Yuan, L.L.; Li, L. Systemic or Forebrain Neuron-Specific Deficiency of Geranylgeranyltransferase-1 Impairs Synaptic Plasticity and Reduces Dendritic Spine Density. Neuroscience 2018, 373, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Giampietro, C.; Lionetti, M.C.; Costantini, G.; Mutti, F.; Zapperi, S.; La Porta, C.A. Cholesterol impairment contributes to neuroserpin aggregation. Sci. Rep. 2017, 7, 43669. [Google Scholar] [CrossRef] [Green Version]
- Fabbro, S.; Seeds, N.W. Plasminogen activator activity is inhibited while neuroserpin is up-regulated in the Alzheimer disease brain. J. Neurochem. 2009, 109, 303–315. [Google Scholar] [CrossRef]
- Mollazadeh, H.; Tavana, E.; Fanni, G.; Bo, S.; Banach, M.; Pirro, M.; von Haehling, S.; Jamialahmadi, T.; Sahebkar, A. Effects of statins on mitochondrial pathways. J. Cachexia Sarcopenia Muscle 2021, 12, 237–251. [Google Scholar] [CrossRef]
- Urbano, F.; Bugliani, M.; Filippello, A.; Scamporrino, A.; Di Mauro, S.; Di Pino, A.; Scicali, R.; Noto, D.; Rabuazzo, A.M.; Averna, M.; et al. Atorvastatin but Not Pravastatin Impairs Mitochondrial Function in Human Pancreatic Islets and Rat beta-Cells. Direct Effect of Oxidative Stress. Sci. Rep. 2017, 7, 11863. [Google Scholar] [CrossRef] [Green Version]
- Stoletov, K.; Fang, L.; Choi, S.H.; Hartvigsen, K.; Hansen, L.F.; Hall, C.; Pattison, J.; Juliano, J.; Miller, E.R.; Almazan, F.; et al. Vascular lipid accumulation, lipoprotein oxidation, and macrophage lipid uptake in hypercholesterolemic zebrafish. Circ. Res. 2009, 104, 952–960. [Google Scholar] [CrossRef]
- Babin, P.J.; Vernier, J.M. Plasma lipoproteins in fish. J. Lipid Res. 1989, 30, 467–489. [Google Scholar] [CrossRef]
- Fang, L.; Liu, C.; Miller, Y.I. Zebrafish models of dyslipidemia: Relevance to atherosclerosis and angiogenesis. Transl. Res. 2014, 163, 99–108. [Google Scholar] [CrossRef] [Green Version]
- Holtta-Vuori, M.; Salo, V.T.; Nyberg, L.; Brackmann, C.; Enejder, A.; Panula, P.; Ikonen, E. Zebrafish: Gaining popularity in lipid research. Biochem. J. 2010, 429, 235–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clifton, J.D.; Lucumi, E.; Myers, M.C.; Napper, A.; Hama, K.; Farber, S.A.; Smith, A.B., III; Huryn, D.M.; Diamond, S.L.; Pack, M. Identification of novel inhibitors of dietary lipid absorption using zebrafish. PLoS ONE 2010, 5, e12386. [Google Scholar] [CrossRef] [PubMed]
- Baek, J.S.; Fang, L.; Li, A.C.; Miller, Y.I. Ezetimibe and simvastatin reduce cholesterol levels in zebrafish larvae fed a high-cholesterol diet. Cholesterol 2012, 2012, 564705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsieh, J. Orchestrating transcriptional control of adult neurogenesis. Genes Dev. 2012, 26, 1010–1021. [Google Scholar] [CrossRef] [Green Version]
- Beckervordersandforth, R.; Zhang, C.L.; Lie, D.C. Transcription-Factor-Dependent Control of Adult Hippocampal Neurogenesis. Cold Spring Harb. Perspect. Biol. 2015, 7, a018879. [Google Scholar] [CrossRef]
- Faigle, R.; Song, H. Signaling mechanisms regulating adult neural stem cells and neurogenesis. Biochim. Biophys. Acta 2013, 1830, 2435–2448. [Google Scholar] [CrossRef] [Green Version]
- Jurisch-Yaksi, N.; Yaksi, E.; Kizil, C. Radial glia in the zebrafish brain: Functional, structural, and physiological comparison with the mammalian glia. Glia 2020, 68, 2451–2470. [Google Scholar] [CrossRef]
- Chapouton, P.; Jagasia, R.; Bally-Cuif, L. Adult neurogenesis in non-mammalian vertebrates. Bioessays 2007, 29, 745–757. [Google Scholar] [CrossRef] [Green Version]
- Labusch, M.; Mancini, L.; Morizet, D.; Bally-Cuif, L. Conserved and Divergent Features of Adult Neurogenesis in Zebrafish. Front. Cell Dev. Biol. 2020, 8, 525. [Google Scholar] [CrossRef]
- Aujla, P.K.; Naratadam, G.T.; Xu, L.; Raetzman, L.T. Notch/Rbpjkappa signaling regulates progenitor maintenance and differentiation of hypothalamic arcuate neurons. Development 2013, 140, 3511–3521. [Google Scholar] [CrossRef] [Green Version]
- Chapouton, P.; Webb, K.J.; Stigloher, C.; Alunni, A.; Adolf, B.; Hesl, B.; Topp, S.; Kremmer, E.; Bally-Cuif, L. Expression of hairy/enhancer of split genes in neural progenitors and neurogenesis domains of the adult zebrafish brain. J. Comp. Neurol. 2011, 519, 1748–1769. [Google Scholar] [CrossRef] [PubMed]
- Chapouton, P.; Skupien, P.; Hesl, B.; Coolen, M.; Moore, J.C.; Madelaine, R.; Kremmer, E.; Faus-Kessler, T.; Blader, P.; Lawson, N.D.; et al. Notch activity levels control the balance between quiescence and recruitment of adult neural stem cells. J. Neurosci. 2010, 30, 7961–7974. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sueda, R.; Kageyama, R. Regulation of active and quiescent somatic stem cells by Notch signaling. Dev. Growth Differ. 2020, 62, 59–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, R.; Engler, A.; Taylor, V. Notch: An interactive player in neurogenesis and disease. Cell Tissue Res. 2018, 371, 73–89. [Google Scholar] [CrossRef]
- Alunni, A.; Bally-Cuif, L. A comparative view of regenerative neurogenesis in vertebrates. Development 2016, 143, 741–753. [Google Scholar] [CrossRef] [Green Version]
- De Oliveira-Carlos, V.; Ganz, J.; Hans, S.; Kaslin, J.; Brand, M. Notch receptor expression in neurogenic regions of the adult zebrafish brain. PLoS ONE 2013, 8, e73384. [Google Scholar] [CrossRef] [Green Version]
- Ables, J.L.; Decarolis, N.A.; Johnson, M.A.; Rivera, P.D.; Gao, Z.; Cooper, D.C.; Radtke, F.; Hsieh, J.; Eisch, A.J. Notch1 is required for maintenance of the reservoir of adult hippocampal stem cells. J. Neurosci. 2010, 30, 10484–10492. [Google Scholar] [CrossRef] [Green Version]
- Gaiano, N.; Nye, J.S.; Fishell, G. Radial glial identity is promoted by Notch1 signaling in the murine forebrain. Neuron 2000, 26, 395–404. [Google Scholar] [CrossRef] [Green Version]
- Piccin, D.; Yu, F.; Morshead, C.M. Notch signaling imparts and preserves neural stem characteristics in the adult brain. Stem Cells Dev. 2013, 22, 1541–1550. [Google Scholar] [CrossRef]
- Kishimoto, N.; Shimizu, K.; Sawamoto, K. Neuronal regeneration in a zebrafish model of adult brain injury. Dis. Models Mech. 2012, 5, 200–209. [Google Scholar] [CrossRef] [Green Version]
- Mendes-da-Silva, C.; Lemes, S.F.; Baliani Tda, S.; Versutti, M.D.; Torsoni, M.A. Increased expression of Hes5 protein in Notch signaling pathway in the hippocampus of mice offspring of dams fed a high-fat diet during pregnancy and suckling. Int. J. Dev. Neurosci. 2015, 40, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Jiang, M.; Yang, C.; Wu, Y.; Liu, Y.; Cui, Y.; Huang, G. Maternal high-fat diet affects Msi/Notch/Hes signaling in neural stem cells of offspring mice. J. Nutr. Biochem. 2014, 25, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Tang, Y.; Cai, D. IKKbeta/NF-kappaB disrupts adult hypothalamic neural stem cells to mediate a neurodegenerative mechanism of dietary obesity and pre-diabetes. Nat. Cell Biol. 2012, 14, 999–1012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, J.; Tay, S.S.; Ling, E.A.; Dheen, S.T. High glucose alters the expression of genes involved in proliferation and cell-fate specification of embryonic neural stem cells. Diabetologia 2006, 49, 1027–1038. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, D.C.; Harvey, E.M.; Suraj, R.; Erickson, S.L.; Mohammad, L.; Ren, M.; Liu, H.; He, G.; Kaplan, D.R.; Ellis, J.; et al. Methylglyoxal couples metabolic and translational control of Notch signalling in mammalian neural stem cells. Nat. Commun. 2020, 11, 2018. [Google Scholar] [CrossRef] [Green Version]
- Miyazono, K.; Kamiya, Y.; Morikawa, M. Bone morphogenetic protein receptors and signal transduction. J. Biochem 2010, 147, 35–51. [Google Scholar] [CrossRef] [Green Version]
- Shi, Y.; Massague, J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell 2003, 113, 685–700. [Google Scholar] [CrossRef] [Green Version]
- Hollnagel, A.; Oehlmann, V.; Heymer, J.; Ruther, U.; Nordheim, A. Id genes are direct targets of bone morphogenetic protein induction in embryonic stem cells. J. Biol. Chem. 1999, 274, 19838–19845. [Google Scholar] [CrossRef] [Green Version]
- Ling, F.; Kang, B.; Sun, X.H. Id proteins: Small molecules, mighty regulators. Curr. Top. Dev. Biol. 2014, 110, 189–216. [Google Scholar]
- Diotel, N.; Beil, T.; Strahle, U.; Rastegar, S. Differential expression of id genes and their potential regulator znf238 in zebrafish adult neural progenitor cells and neurons suggests distinct functions in adult neurogenesis. Gene Expr. Patterns 2015, 19, 1–13. [Google Scholar] [CrossRef]
- Nam, H.S.; Benezra, R. High levels of Id1 expression define B1 type adult neural stem cells. Cell Stem. Cell 2009, 5, 515–526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, G.; Lubke, L.; Chen, F.; Beil, T.; Takamiya, M.; Diotel, N.; Strahle, U.; Rastegar, S. Neuron-Radial Glial Cell Communication via BMP/Id1 Signaling Is Key to Long-Term Maintenance of the Regenerative Capacity of the Adult Zebrafish Telencephalon. Cells 2021, 10, 2794. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Ferg, M.; Lubke, L.; Takamiya, M.; Beil, T.; Gourain, V.; Diotel, N.; Strahle, U.; Rastegar, S. Bone morphogenetic protein signaling regulates Id1-mediated neural stem cell quiescence in the adult zebrafish brain via a phylogenetically conserved enhancer module. Stem Cells 2020, 38, 875–889. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez Viales, R.; Diotel, N.; Ferg, M.; Armant, O.; Eich, J.; Alunni, A.; Marz, M.; Bally-Cuif, L.; Rastegar, S.; Strahle, U. The helix-loop-helix protein id1 controls stem cell proliferation during regenerative neurogenesis in the adult zebrafish telencephalon. Stem Cells 2015, 33, 892–903. [Google Scholar] [CrossRef]
- Jensen, G.S.; Leon-Palmer, N.E.; Townsend, K.L. Bone morphogenetic proteins (BMPs) in the central regulation of energy balance and adult neural plasticity. Metab. Clin. Exp. 2021, 123, 154837. [Google Scholar] [CrossRef]
- Baboota, R.K.; Bluher, M.; Smith, U. Emerging Role of Bone Morphogenetic Protein 4 in Metabolic Disorders. Diabetes 2021, 70, 303–312. [Google Scholar] [CrossRef]
- Townsend, K.L.; Suzuki, R.; Huang, T.L.; Jing, E.; Schulz, T.J.; Lee, K.; Taniguchi, C.M.; Espinoza, D.O.; McDougall, L.E.; Zhang, H.; et al. Bone morphogenetic protein 7 (BMP7) reverses obesity and regulates appetite through a central mTOR pathway. FASEB J. 2012, 26, 2187–2196. [Google Scholar] [CrossRef] [Green Version]
- Townsend, K.L.; Madden, C.J.; Blaszkiewicz, M.; McDougall, L.; Tupone, D.; Lynes, M.D.; Mishina, Y.; Yu, P.; Morrison, S.F.; Tseng, Y.H. Reestablishment of Energy Balance in a Male Mouse Model With POMC Neuron Deletion of BMPR1A. Endocrinology 2017, 158, 4233–4245. [Google Scholar] [CrossRef] [Green Version]
- Vegiopoulos, A.; Herzig, S. Glucocorticoids, metabolism and metabolic diseases. Mol. Cell Endocrinol 2007, 275, 43–61. [Google Scholar] [CrossRef] [Green Version]
- Cruz-Topete, D.; Cidlowski, J.A. One hormone, two actions: Anti- and pro-inflammatory effects of glucocorticoids. Neuroimmunomodulation 2015, 22, 20–32. [Google Scholar] [CrossRef] [Green Version]
- Nussinovitch, U.; de Carvalho, J.F.; Pereira, R.M.; Shoenfeld, Y. Glucocorticoids and the cardiovascular system: State of the art. Curr. Pharm. Des. 2010, 16, 3574–3585. [Google Scholar] [CrossRef] [PubMed]
- Fowden, A.L.; Forhead, A.J. Glucocorticoids as regulatory signals during intrauterine development. Exp. Physiol. 2015, 100, 1477–1487. [Google Scholar] [CrossRef] [PubMed]
- Taves, M.D.; Gomez-Sanchez, C.E.; Soma, K.K. Extra-adrenal glucocorticoids and mineralocorticoids: Evidence for local synthesis, regulation, and function. Am. J. Physiol. Endocrinol. Metab. 2011, 301, E11–E24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Timmermans, S.; Souffriau, J.; Libert, C. A General Introduction to Glucocorticoid Biology. Front. Immunol. 2019, 10, 1545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McEwen, B.S. Physiology and neurobiology of stress and adaptation: Central role of the brain. Physiol. Rev. 2007, 87, 873–904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vierhapper, H.; Nowotny, P.; Waldhausl, W. Production rates of cortisol in obesity. Obes. Res. 2004, 12, 1421–1425. [Google Scholar] [CrossRef] [PubMed]
- Stewart, P.M.; Boulton, A.; Kumar, S.; Clark, P.M.; Shackleton, C.H. Cortisol metabolism in human obesity: Impaired cortisone-->cortisol conversion in subjects with central adiposity. J. Clin. Endocrinol. Metab. 1999, 84, 1022–1027. [Google Scholar] [CrossRef]
- Akalestou, E.; Genser, L.; Rutter, G.A. Glucocorticoid Metabolism in Obesity and Following Weight Loss. Front. Endocrinol. 2020, 11, 59. [Google Scholar] [CrossRef] [Green Version]
- Stomby, A.; Andrew, R.; Walker, B.R.; Olsson, T. Tissue-specific dysregulation of cortisol regeneration by 11betaHSD1 in obesity: Has it promised too much? Diabetologia 2014, 57, 1100–1110. [Google Scholar] [CrossRef] [Green Version]
- Rebuffe-Scrive, M.; Bronnegard, M.; Nilsson, A.; Eldh, J.; Gustafsson, J.A.; Bjorntorp, P. Steroid hormone receptors in human adipose tissues. J. Clin. Endocrinol. Metab. 1990, 71, 1215–1219. [Google Scholar] [CrossRef]
- Chiodini, I.; Adda, G.; Scillitani, A.; Coletti, F.; Morelli, V.; Di Lembo, S.; Epaminonda, P.; Masserini, B.; Beck-Peccoz, P.; Orsi, E.; et al. Cortisol secretion in patients with type 2 diabetes: Relationship with chronic complications. Diabetes Care 2007, 30, 83–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roy, M.S.; Roy, A.; Brown, S. Increased urinary-free cortisol outputs in diabetic patients. J. Diabetes Complicat. 1998, 12, 24–27. [Google Scholar] [CrossRef]
- Sidibeh, C.O.; Pereira, M.J.; Abalo, X.M.; Boersma, J.G.; Skrtic, S.; Lundkvist, P.; Katsogiannos, P.; Hausch, F.; Castillejo-Lopez, C.; Eriksson, J.W. FKBP5 expression in human adipose tissue: Potential role in glucose and lipid metabolism, adipogenesis and type 2 diabetes. Endocrine 2018, 62, 116–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woods, C.P.; Hazlehurst, J.M.; Tomlinson, J.W. Glucocorticoids and non-alcoholic fatty liver disease. J. Steroid Biochem. Mol. Biol. 2015, 154, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zhang, Y.; Wu, W.; Xu, T.; Yin, Y.; Zhang, J.; Huang, D.; Li, W. Chronic glucocorticoid exposure activates BK-NLRP1 signal involving in hippocampal neuron damage. J. Neuroinflamm. 2017, 14, 139. [Google Scholar] [CrossRef] [PubMed]
- Odaka, H.; Numakawa, T.; Yoshimura, A.; Nakajima, S.; Adachi, N.; Ooshima, Y.; Inoue, T.; Kunugi, H. Chronic glucocorticoid exposure suppressed the differentiation and survival of embryonic neural stem/progenitor cells: Possible involvement of ERK and PI3K/Akt signaling in the neuronal differentiation. Neurosci. Res. 2016, 113, 28–36. [Google Scholar] [CrossRef] [Green Version]
- Morais, M.; Santos, P.A.; Mateus-Pinheiro, A.; Patricio, P.; Pinto, L.; Sousa, N.; Pedroso, P.; Almeida, S.; Filipe, A.; Bessa, J.M. The effects of chronic stress on hippocampal adult neurogenesis and dendritic plasticity are reversed by selective MAO-A inhibition. J. Psychopharmacol. 2014, 28, 1178–1183. [Google Scholar] [CrossRef] [Green Version]
- Vyas, S.; Rodrigues, A.J.; Silva, J.M.; Tronche, F.; Almeida, O.F.; Sousa, N.; Sotiropoulos, I. Chronic Stress and Glucocorticoids: From Neuronal Plasticity to Neurodegeneration. Neural. Plast. 2016, 2016, 6391686. [Google Scholar] [CrossRef] [Green Version]
- Hu, P.; Oomen, C.; van Dam, A.M.; Wester, J.; Zhou, J.N.; Joels, M.; Lucassen, P.J. A single-day treatment with mifepristone is sufficient to normalize chronic glucocorticoid induced suppression of hippocampal cell proliferation. PLoS ONE 2012, 7, e46224. [Google Scholar] [CrossRef]
- Caruso, A.; Nicoletti, F.; Mango, D.; Saidi, A.; Orlando, R.; Scaccianoce, S. Stress as risk factor for Alzheimer’s disease. Pharmacol. Res. 2018, 132, 130–134. [Google Scholar] [CrossRef]
- Dey, A.; Hao, S.; Wosiski-Kuhn, M.; Stranahan, A.M. Glucocorticoid-mediated activation of GSK3beta promotes tau phosphorylation and impairs memory in type 2 diabetes. Neurobiol. Aging 2017, 57, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Wendelaar Bonga, S.E. The stress response in fish. Physiol. Rev. 1997, 77, 591–625. [Google Scholar] [CrossRef] [PubMed]
- Weger, M.; Diotel, N.; Weger, B.D.; Beil, T.; Zaucker, A.; Eachus, H.L.; Oakes, J.A.; do Rego, J.L.; Storbeck, K.H.; Gut, P.; et al. Expression and activity profiling of the steroidogenic enzymes of glucocorticoid biosynthesis and the fdx1 co-factors in zebrafish. J. Neuroendocrinol. 2018, 30, e12586. [Google Scholar] [CrossRef] [PubMed]
- Diotel, N.; Do Rego, J.L.; Anglade, I.; Vaillant, C.; Pellegrini, E.; Vaudry, H.; Kah, O. The brain of teleost fish, a source, and a target of sexual steroids. Front. Neurosci. 2011, 5, 137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diotel, N.; Do Rego, J.L.; Anglade, I.; Vaillant, C.; Pellegrini, E.; Gueguen, M.M.; Mironov, S.; Vaudry, H.; Kah, O. Activity and expression of steroidogenic enzymes in the brain of adult zebrafish. Eur. J. Neurosci. 2011, 34, 45–56. [Google Scholar] [CrossRef]
- Sakamoto, H.; Ukena, K.; Tsutsui, K. Activity and localization of 3beta-hydroxysteroid dehydrogenase/Delta5-Delta4-isomerase in the zebrafish central nervous system. J. Comp. Neurol. 2001, 439, 291–305. [Google Scholar] [CrossRef]
- Than-Trong, E.; Kiani, B.; Dray, N.; Ortica, S.; Simons, B.; Rulands, S.; Alunni, A.; Bally-Cuif, L. Lineage hierarchies and stochasticity ensure the long-term maintenance of adult neural stem cells. Sci. Adv. 2020, 6, eaaz5424. [Google Scholar] [CrossRef]
- Baumgart, E.V.; Barbosa, J.S.; Bally-Cuif, L.; Gotz, M.; Ninkovic, J. Stab wound injury of the zebrafish telencephalon: A model for comparative analysis of reactive gliosis. Glia 2012, 60, 343–357. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).