Antimalarial Drug Strategies to Target Plasmodium Gametocytes
Abstract
1. Introduction
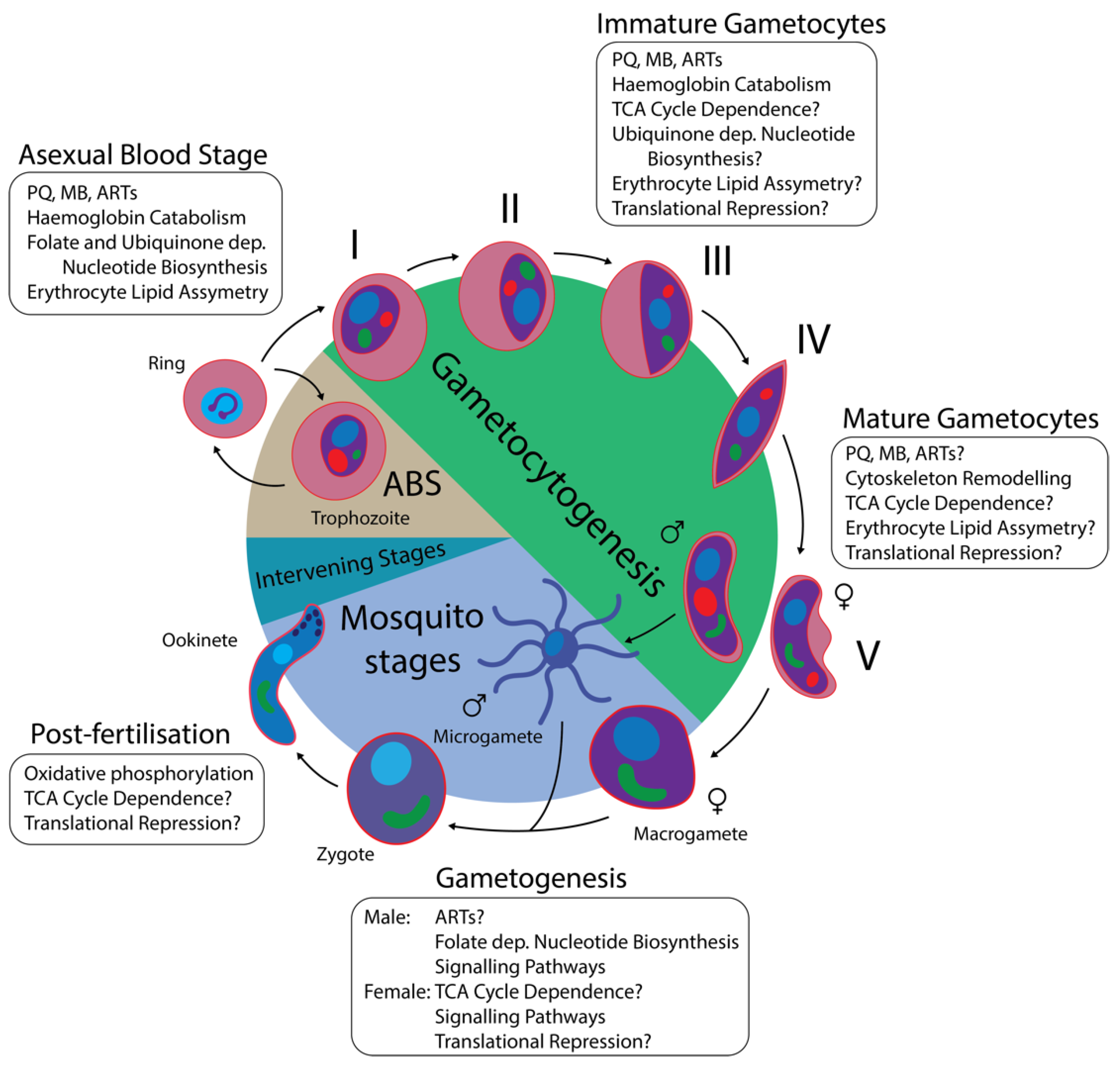
1.1. The P. falciparum Lifecycle
1.2. Gametocyte Biology
1.3. Gametocytocidal Compounds
2. Antimalarials with Variable Gametocytocidal Activity
Haemoglobin Catabolism
3. Antimalarials with Known Gametocytocidal Activity
3.1. Primaquine
3.2. Methylene Blue
3.3. Artemisinin Derivatives
4. Potential Directions Based on Gametocyte Biology
4.1. Cytoskeleton Remodelling
4.2. TCA Cycle Dependence
4.3. Mitochondrial Activity
4.4. Lipid Metabolism
5. Targeting Gametogenesis and the Post-Fertilisation Stages
5.1. Folate Dependent Pyrimidine Biosynthesis
5.2. Gametogenesis Signalling Pathways
5.3. Female Translational Repression
6. Future Perspectives
7. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABS | Asexual blood stage |
| AMP | Antimicrobial peptide |
| ARTs | Artemisinin derivatives |
| ATP | Adenosine triphosphate |
| cAMP | Cyclic adenosine monophosphate |
| CDPK | Calcium-dependent protein kinase |
| cGMP | Cyclic guanosine monophosphate |
| CITH | CAR-I/Trailer Hitch |
| DHA | Dihydroartemisinin |
| DHFR | Dihydrofolate reductase |
| DHODH | Dihydroorotate dehydrogenase |
| DOZI | Development of zygote inhibited |
| DV | Digestive vacuole |
| G6PD | Glucose 6 phosphate dehydrogenase |
| Hb | Haemoglobin |
| HTS | High throughput screen |
| IMC | Inner membrane complex |
| MB | Methylene blue |
| MDA | Mass drug administration |
| MMV | Medicines for Malaria Venture |
| MOA | Mechanism of action |
| mRNA | Messenger ribonucleic acid |
| mRNP | Messenger ribonucleoprotein |
| mtETC | Mitochondrial electron transport chain |
| NADPH | Nicotinamide adenine dinucleotide phosphate |
| PDE | Phosphodiesterase |
| PF4 | Platelet factor 4 |
| PI(4)K | phosphatidylinositol-4-OH kinase |
| PKA | Protein kinase A |
| PKG | Protein kinase G |
| PQ | Primaquine |
| PUF2 | Pumilio/FBF 2 |
| SMFA | Standard membrane feeding assay |
| STEVOR | Subtelomeric variable open reading frame |
| TCA cycle | Tricarboxylic acid cycle |
| WHO | World Health Organisation |
References
- World Health Organization. World Malaria Report 2021; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Nkumama, I.N.; O’Meara, W.P.; Osier, F.H.A. Changes in Malaria Epidemiology in Africa and New Challenges for Elimination. Trends Parasitol. 2017, 33, 128–140. [Google Scholar] [CrossRef] [PubMed]
- Leroy, D.; Campo, B.; Ding, X.C.; Burrows, J.N.; Cherbuin, S. Defining the biology component of the drug discovery strategy for malaria eradication. Trends Parasitol. 2014, 30, 478–490. [Google Scholar] [CrossRef]
- Smith, R.C.; Vega-Rodríguez, J.; Jacobs-Lorena, M. The Plasmodium bottleneck: Malaria parasite losses in the mosquito vector. Mem. Inst. Oswaldo Cruz 2014, 109, 644–661. [Google Scholar] [CrossRef]
- Phillips, R.S. Current status of malaria and potential for control. Clin. Microbiol. Rev. 2001, 14, 208–226. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine Committee on the Economics of Antimalarial Drugs. Saving Lives, Buying Time: Economics of Malaria Drugs in an Age of Resistance; Arrow, K.J., Panosian, C., Gelband, H., Eds.; National Academies Press (US): Washington, DC, USA, 2004. [Google Scholar]
- Delves, M.; Lafuente-Monasterio, M.J.; Upton, L.; Ruecker, A.; Leroy, D.; Gamo, F.-J.; Sinden, R. Fueling Open Innovation for Malaria Transmission-Blocking Drugs: Hundreds of Molecules Targeting Early Parasite Mosquito Stages. Front. Microbiol. 2019, 10, 2134. [Google Scholar] [CrossRef] [PubMed]
- Hyde, J.E. Drug-resistant malaria—An insight. FEBS J. 2007, 274, 4688–4698. [Google Scholar] [CrossRef] [PubMed]
- Ashley, E.A.; Dhorda, M.; Fairhurst, R.M.; Amaratunga, C.; Lim, P.; Suon, S.; Sreng, S.; Anderson, J.M.; Mao, S.; Sam, B.; et al. Spread of Artemisinin Resistance in Plasmodium falciparum Malaria. N. Engl. J. Med. 2014, 371, 411–423. [Google Scholar] [CrossRef]
- malERA Refresh Consultative Panel on Characterising the Reservoir and Measuring Transmission. malERA: An updated research agenda for characterising the reservoir and measuring transmission in malaria elimination and eradication. PLoS Med. 2017, 14, e1002452. [Google Scholar] [CrossRef]
- Burrows, J.N.; Duparc, S.; Gutteridge, W.E.; Hooft van Huijsduijnen, R.; Kaszubska, W.; Macintyre, F.; Mazzuri, S.; Mohrle, J.J.; Wells, T.N.C. New developments in anti-malarial target candidate and product profiles. Malar. J. 2017, 16, 26. [Google Scholar] [CrossRef]
- Sinden, R.E. Mitosis and meiosis in malarial parasites. Acta Leiden 1991, 60, 19–27. [Google Scholar]
- Prudencio, M.; Rodriguez, A.; Mota, M.M. The silent path to thousands of merozoites: The Plasmodium liver stage. Nat. Rev. Microbiol. 2006, 4, 849–856. [Google Scholar] [CrossRef] [PubMed]
- Laishram, D.D.; Sutton, P.L.; Nanda, N.; Sharma, V.L.; Sobti, R.C.; Carlton, J.M.; Joshi, H. The complexities of malaria disease manifestations with a focus on asymptomatic malaria. Malar. J. 2012, 11, 29. [Google Scholar] [CrossRef] [PubMed]
- Phillips, M.A.; Burrows, J.N.; Manyando, C.; van Huijsduijnen, R.H.; Van Voorhis, W.C.; Wells, T.N.C. Malaria. Nat. Rev. Dis. Primers 2017, 3, 17050. [Google Scholar] [CrossRef] [PubMed]
- Carter, L.M.; Kafsack, B.F.C.; Llinás, M.; Mideo, N.; Pollitt, L.C.; Reece, S.E. Stress and sex in malaria parasites: Why does commitment vary? Evol. Med. Public Health 2013, 2013, 135–147. [Google Scholar] [CrossRef]
- Rono, M.K.; Nyonda, M.A.; Simam, J.J.; Ngoi, J.M.; Mok, S.; Kortok, M.M.; Abdullah, A.S.; Elfaki, M.M.; Waitumbi, J.N.; El-Hassan, I.M.; et al. Adaptation of Plasmodium falciparum to its transmission environment. Nat. Ecol. Evol. 2018, 2, 377–387. [Google Scholar] [CrossRef]
- Kafsack, B.F.C.; Rovira-Graells, N.; Clark, T.G.; Bancells, C.; Crowley, V.M.; Campino, S.G.; Williams, A.E.; Drought, L.G.; Kwiatkowski, D.P.; Baker, D.A.; et al. A transcriptional switch underlies commitment to sexual development in malaria parasites. Nature 2014, 507, 248–252. [Google Scholar] [CrossRef]
- Josling, G.A.; Williamson, K.C.; Llinás, M. Regulation of Sexual Commitment and Gametocytogenesis in Malaria Parasites. Annu. Rev. Microbiol. 2018, 72, 501–519. [Google Scholar] [CrossRef]
- Bruce, M.C.; Alano, P.; Duthie, S.; Carter, R. Commitment of the malaria parasite Plasmodium falciparum to sexual and asexual development. Parasitology 1990, 100, 191–200. [Google Scholar] [CrossRef]
- Bancells, C.; Llora-Batlle, O.; Poran, A.; Notzel, C.; Rovira-Graells, N.; Elemento, O.; Kafsack, B.F.C.; Cortes, A. Revisiting the initial steps of sexual development in the malaria parasite Plasmodium falciparum. Nat. Microbiol. 2019, 4, 144–154. [Google Scholar] [CrossRef]
- Paul, R.E.; Coulson, T.N.; Raibaud, A.; Brey, P.T. Sex determination in malaria parasites. Science 2000, 287, 128–131. [Google Scholar] [CrossRef]
- Silvestrini, F.; Alano, P.; Williams, J.L. Commitment to the production of male and female gametocytes in the human malaria parasite Plasmodium falciparum. Parasitology 2000, 121, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Carreno, R.A.; Kissinger, J.C.; McCutchan, T.F.; Barta, J.R. Phylogenetic analysis of haemosporinid parasites (apicomplexa: Haemosporina) and their coevolution with vectors and intermediate hosts. Arch. Protistenkd. 1997, 148, 245–252. [Google Scholar] [CrossRef]
- Smith, T.G.; Walliker, D.; Ranford-Cartwright, L.C. Sexual differentiation and sex determination in the Apicomplexa. Trends Parasitol. 2002, 18, 315–323. [Google Scholar] [CrossRef]
- Sinden, R.E. Gametocytogenesis of Plasmodium falciparum in vitro: An electron microscopic study. Parasitology 1982, 84, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Joice, R.; Nilsson, S.K.; Montgomery, J.; Dankwa, S.; Egan, E.; Morahan, B.; Seydel, K.B.; Bertuccini, L.; Alano, P.; Williamson, K.C.; et al. Plasmodium falciparum transmission stages accumulate in the human bone marrow. Sci. Transl. Med. 2014, 6, 244re5. [Google Scholar] [CrossRef]
- Venugopal, K.; Hentzschel, F.; Valkiūnas, G.; Marti, M. Plasmodium asexual growth and sexual development in the haematopoietic niche of the host. Nat. Rev. Microbiol. 2020, 18, 177–189. [Google Scholar] [CrossRef]
- Bousema, T.; Okell, L.; Shekalaghe, S.; Griffin, J.T.; Omar, S.; Sawa, P.; Sutherland, C.; Sauerwein, R.; Ghani, A.C.; Drakeley, C. Revisiting the circulation time of Plasmodium falciparum gametocytes: Molecular detection methods to estimate the duration of gametocyte carriage and the effect of gametocytocidal drugs. Malar. J. 2010, 9, 136. [Google Scholar] [CrossRef]
- Miao, J.; Chen, Z.; Wang, Z.; Shrestha, S.; Li, X.; Li, R.; Cui, L. Sex-Specific Biology of the Human Malaria Parasite Revealed from the Proteomes of Mature Male and Female Gametocytes. Mol. Cell Proteom. 2017, 16, 537–551. [Google Scholar] [CrossRef]
- Garcia, G.E.; Wirtz, R.A.; Barr, J.R.; Woolfitt, A.; Rosenberg, R. Xanthurenic Acid Induces Gametogenesis in Plasmodium, the Malaria Parasite. J. Biol. Chem. 1998, 273, 12003–12005. [Google Scholar] [CrossRef]
- Billker, O.; Lindo, V.; Panico, M.; Etienne, A.E.; Paxton, T.; Dell, A.; Rogers, M.; Sinden, R.E.; Morris, H.R. Identification of xanthurenic acid as the putative inducer of malaria development in the mosquito. Nature 1998, 392, 289–292. [Google Scholar] [CrossRef]
- Sinden, R.E.; Canning, E.U.; Spain, B. Gametogenesis and Fertilization in Plasmodium yoelii nigeriensis: A Transmission Electron Microscope Study. Proc. R. Soc. Lond. Ser. B Biol. Sci. 1976, 193, 55–76. [Google Scholar]
- McRobert, L.; Taylor, C.J.; Deng, W.; Fivelman, Q.L.; Cummings, R.M.; Polley, S.D.; Billker, O.; Baker, D.A. Gametogenesis in Malaria Parasites Is Mediated by the cGMP-Dependent Protein Kinase. PLoS Biol. 2008, 6, e139. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Tewari, R.; Ning, J.; Blagborough, A.M.; Garbom, S.; Pei, J.; Grishin, N.V.; Steele, R.E.; Sinden, R.E.; Snell, W.J.; et al. The conserved plant sterility gene HAP2 functions after attachment of fusogenic membranes in Chlamydomonas and Plasmodium gametes. Genes Dev. 2008, 22, 1051–1068. [Google Scholar] [CrossRef] [PubMed]
- Sinden, R.E. Plasmodium differentiation in the mosquito. Parassitologia 1999, 41, 139–148. [Google Scholar] [PubMed]
- Ukegbu, C.V.; Christophides, G.K.; Vlachou, D. Identification of Three Novel Plasmodium Factors Involved in Ookinete to Oocyst Developmental Transition. Front. Cell. Infect. Microbiol. 2021, 11, 634273. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, R.; Rungsiwongse, J. The number of sporozoites produced by individual malaria oocysts. Am. J. Trop. Med. Hyg. 1991, 45, 574–577. [Google Scholar] [CrossRef]
- Hillyer, J.F.; Barreau, C.; Vernick, K.D. Efficiency of salivary gland invasion by malaria sporozoites is controlled by rapid sporozoite destruction in the mosquito haemocoel. Int. J. Parasitol. 2007, 37, 673–681. [Google Scholar] [CrossRef]
- Sinden, R.E.; Billingsley, P.F. Plasmodium invasion of mosquito cells: Hawk or dove? Trends Parasitol. 2001, 17, 209–212. [Google Scholar] [CrossRef]
- Gouagna, L.C.; Yao, F.; Yameogo, B.; Dabiré, R.K.; Ouédraogo, J.-B. Comparison of field-based xenodiagnosis and direct membrane feeding assays for evaluating host infectiousness to malaria vector Anopheles gambiae. Acta Trop. 2014, 130, 131–139. [Google Scholar] [CrossRef]
- Gardner, M.J.; Hall, N.; Fung, E.; White, O.; Berriman, M.; Hyman, R.W.; Carlton, J.M.; Pain, A.; Nelson, K.E.; Bowman, S.; et al. Genome sequence of the human malaria parasite Plasmodium falciparum. Nature 2002, 419, 498–511. [Google Scholar] [CrossRef]
- Trevino, S.G.; Nkhoma, S.C.; Nair, S.; Daniel, B.J.; Moncada, K.; Khoswe, S.; Banda, R.L.; Nosten, F.; Cheeseman, I.H. High-Resolution Single-Cell Sequencing of Malaria Parasites. Genome Biol. Evol. 2017, 9, 3373–3383. [Google Scholar] [CrossRef] [PubMed]
- Reid, A.J.; Talman, A.M.; Bennett, H.M.; Gomes, A.R.; Sanders, M.J.; Illingworth, C.J.R.; Billker, O.; Berriman, M.; Lawniczak, M.K. Single-cell RNA-seq reveals hidden transcriptional variation in malaria parasites. eLife 2018, 7, e33105. [Google Scholar] [CrossRef] [PubMed]
- Howick Virginia, M.; Russell Andrew, J.C.; Andrews, T.; Heaton, H.; Reid Adam, J.; Natarajan, K.; Butungi, H.; Metcalf, T.; Verzier Lisa, H.; Rayner Julian, C.; et al. The Malaria Cell Atlas: Single parasite transcriptomes across the complete Plasmodium life cycle. Science 2019, 365, eaaw2619. [Google Scholar] [CrossRef]
- Lasonder, E.; Rijpma, S.R.; van Schaijk, B.C.; Hoeijmakers, W.A.; Kensche, P.R.; Gresnigt, M.S.; Italiaander, A.; Vos, M.W.; Woestenenk, R.; Bousema, T.; et al. Integrated transcriptomic and proteomic analyses of P. falciparum gametocytes: Molecular insight into sex-specific processes and translational repression. Nucleic Acids Res. 2016, 44, 6087–6101. [Google Scholar] [CrossRef]
- Florens, L.; Washburn, M.P.; Raine, J.D.; Anthony, R.M.; Grainger, M.; Haynes, J.D.; Moch, J.K.; Muster, N.; Sacci, J.B.; Tabb, D.L.; et al. A proteomic view of the Plasmodium falciparum life cycle. Nature 2002, 419, 520–526. [Google Scholar] [CrossRef] [PubMed]
- Lasonder, E.; Ishihama, Y.; Andersen, J.S.; Vermunt, A.M.W.; Pain, A.; Sauerwein, R.W.; Eling, W.M.C.; Hall, N.; Waters, A.P.; Stunnenberg, H.G.; et al. Analysis of the Plasmodium falciparum proteome by high-accuracy mass spectrometry. Nature 2002, 419, 537–542. [Google Scholar] [CrossRef] [PubMed]
- Lamour, S.D.; Straschil, U.; Saric, J.; Delves, M.J. Changes in metabolic phenotypes of Plasmodium falciparum in vitro cultures during gametocyte development. Malar. J. 2014, 13, 468. [Google Scholar] [CrossRef]
- Sexton, A.E.; Doerig, C.; Creek, D.J.; Carvalho, T.G. Post-Genomic Approaches to Understanding Malaria Parasite Biology: Linking Genes to Biological Functions. ACS Infect. Dis. 2019, 5, 1269–1278. [Google Scholar] [CrossRef]
- Counihan, N.A.; Modak, J.K.; de Koning-Ward, T.F. How Malaria Parasites Acquire Nutrients From Their Host. Front. Cell Dev. Biol. 2021, 9, 649184. [Google Scholar] [CrossRef]
- Srivastava, A.; Philip, N.; Hughes, K.R.; Georgiou, K.; Macrae, J.I.; Barrett, M.P.; Creek, D.J.; McConville, M.J.; Waters, A.P. Stage-Specific Changes in Plasmodium Metabolism Required for Differentiation and Adaptation to Different Host and Vector Environments. PLoS Pathog. 2016, 12, e1006094. [Google Scholar] [CrossRef]
- Delves, M.; Plouffe, D.; Scheurer, C.; Meister, S.; Wittlin, S.; Winzeler, E.; Sinden, R.E.; Leroy, D. The Activities of Current Antimalarial Drugs on the Life Cycle Stages of Plasmodium: A Comparative Study with Human and Rodent Parasites. PLoS Med. 2012, 9, e1001169. [Google Scholar] [CrossRef] [PubMed]
- Sinden, R.E. The cell biology of malaria infection of mosquito: Advances and opportunities. Cell Microbiol. 2015, 17, 451–466. [Google Scholar] [CrossRef] [PubMed]
- Birkholtz, L.M.; Coetzer, T.L.; Mancama, D.; Leroy, D.; Alano, P. Discovering New Transmission-Blocking Antimalarial Compounds: Challenges and Opportunities. Trends Parasitol. 2016, 32, 669–681. [Google Scholar] [CrossRef] [PubMed][Green Version]
- World Health Organization. WHO Guidelines for Malaria; WHO: Geneva, Switzerland, 2021. [Google Scholar]
- Peatey, C.L.; Skinner-Adams, T.S.; Dixon, M.W.A.; McCarthy, J.S.; Gardiner, D.L.; Trenholme, K.R. Effect of Antimalarial Drugs on Plasmodium falciparum Gametocytes. J. Infect. Dis. 2009, 200, 1518–1521. [Google Scholar] [CrossRef] [PubMed]
- Gupta, Y.; Gupta, N.; Singh, S.; Wu, L.; Chhikara, B.S.; Rawat, M.; Rathi, B. Multistage inhibitors of the malaria parasite: Emerging hope for chemoprotection and malaria eradication. Med. Res. Rev. 2018, 38, 1511–1535. [Google Scholar] [CrossRef]
- Barnes, K.I.; Little, F.; Mabuza, A.; Mngomezulu, N.; Govere, J.; Durrheim, D.; Roper, C.; Watkins, B.; White, N.J. Increased Gametocytemia after Treatment: An Early Parasitological Indicator of Emerging Sulfadoxine-Pyrimethamine Resistance in Falciparum Malaria. J. Infect. Dis. 2008, 197, 1605–1613. [Google Scholar] [CrossRef]
- Thommen, B.T.; Passecker, A.; Buser, T.; Hitz, E.; Voss, T.S.; Brancucci, N.M.B. Revisiting the Effect of Pharmaceuticals on Transmission Stage Formation in the Malaria Parasite Plasmodium falciparum. Front. Cell. Infect. Microbiol. 2022, 12, 802341. [Google Scholar] [CrossRef]
- Van Voorhis, W.C.; Adams, J.H.; Adelfio, R.; Ahyong, V.; Akabas, M.H.; Alano, P.; Alday, A.; Alemán Resto, Y.; Alsibaee, A.; Alzualde, A.; et al. Open Source Drug Discovery with the Malaria Box Compound Collection for Neglected Diseases and Beyond. PLoS Pathog. 2016, 12, e1005763. [Google Scholar] [CrossRef]
- Reader, J.; van der Watt, M.E.; Taylor, D.; Le Manach, C.; Mittal, N.; Ottilie, S.; Theron, A.; Moyo, P.; Erlank, E.; Nardini, L.; et al. Multistage and transmission-blocking targeted antimalarials discovered from the open-source MMV Pandemic Response Box. Nat. Commun. 2021, 12, 269. [Google Scholar] [CrossRef]
- Malebo, H.M.; D’Alessandro, S.; Ebstie, Y.A.; Sorè, H.; Tenoh Guedoung, A.R.; Katani, S.J.; Parapini, S.; Taramelli, D.; Habluetzel, A. In vitro Multistage Malaria Transmission Blocking Activity of Selected Malaria Box Compounds. Drug Des. Dev. Ther. 2020, 14, 1593–1607. [Google Scholar] [CrossRef]
- Plouffe, D.M.; Wree, M.; Du, A.Y.; Meister, S.; Li, F.; Patra, K.; Lubar, A.; Okitsu, S.L.; Flannery, E.L.; Kato, N.; et al. High-Throughput Assay and Discovery of Small Molecules that Interrupt Malaria Transmission. Cell Host Microbe 2016, 19, 114–126. [Google Scholar] [CrossRef] [PubMed]
- Sanders, N.G.; Sullivan, D.J.; Mlambo, G.; Dimopoulos, G.; Tripathi, A.K. Gametocytocidal Screen Identifies Novel Chemical Classes with Plasmodium falciparum Transmission Blocking Activity. PLoS ONE 2014, 9, e105817. [Google Scholar] [CrossRef] [PubMed]
- Almela, M.J.; Lozano, S.; Lelièvre, J.; Colmenarejo, G.; Coterón, J.M.; Rodrigues, J.; Gonzalez, C.; Herreros, E. A New Set of Chemical Starting Points with Plasmodium falciparum Transmission-Blocking Potential for Antimalarial Drug Discovery. PLoS ONE 2015, 10, e0135139. [Google Scholar] [CrossRef] [PubMed]
- Lucantoni, L.; Silvestrini, F.; Signore, M.; Siciliano, G.; Eldering, M.; Dechering, K.J.; Avery, V.M.; Alano, P. A simple and predictive phenotypic High Content Imaging assay for Plasmodium falciparum mature gametocytes to identify malaria transmission blocking compounds. Sci. Rep. 2015, 5, 16414. [Google Scholar] [CrossRef]
- D’Alessandro, S.; Camarda, G.; Corbett, Y.; Siciliano, G.; Parapini, S.; Cevenini, L.; Michelini, E.; Roda, A.; Leroy, D.; Taramelli, D.; et al. A chemical susceptibility profile of the Plasmodium falciparum transmission stages by complementary cell-based gametocyte assays. J. Antimicrob. Chemother. 2016, 71, 1148–1158. [Google Scholar] [CrossRef]
- Wadi, I.; Anvikar, A.R.; Nath, M.; Pillai, C.R.; Sinha, A.; Valecha, N. Critical examination of approaches exploited to assess the effectiveness of transmission-blocking drugs for malaria. Future Med. Chem. 2018, 10, 2619–2639. [Google Scholar] [CrossRef]
- Lucantoni, L.; Fidock, D.A.; Avery, V.M. Luciferase-Based, High-Throughput Assay for Screening and Profiling Transmission-Blocking Compounds against Plasmodium falciparum Gametocytes. Antimicrob. Agents Chemother. 2016, 60, 2097–2107. [Google Scholar] [CrossRef]
- Delves, M.J.; Ruecker, A.; Straschil, U.; Lelievre, J.; Marques, S.; Lopez-Barragan, M.J.; Herreros, E.; Sinden, R.E. Male and female Plasmodium falciparum mature gametocytes show different responses to antimalarial drugs. Antimicrob. Agents Chemother. 2013, 57, 3268–3274. [Google Scholar] [CrossRef]
- Delves, M.J.; Miguel-Blanco, C.; Matthews, H.; Molina, I.; Ruecker, A.; Yahiya, S.; Straschil, U.; Abraham, M.; Leon, M.L.; Fischer, O.J.; et al. A high throughput screen for next-generation leads targeting malaria parasite transmission. Nat. Commun. 2018, 9, 3805. [Google Scholar] [CrossRef]
- Ruecker, A.; Mathias, D.K.; Straschil, U.; Churcher, T.S.; Dinglasan, R.R.; Leroy, D.; Sinden, R.E.; Delves, M.J. A Male and Female Gametocyte Functional Viability Assay To Identify Biologically Relevant Malaria Transmission-Blocking Drugs. Antimicrob. Agents Chemother. 2014, 58, 7292–7302. [Google Scholar] [CrossRef]
- Vos, M.W.; Stone, W.J.R.; Koolen, K.M.; van Gemert, G.-J.; van Schaijk, B.; Leroy, D.; Sauerwein, R.W.; Bousema, T.; Dechering, K.J. A semi-automated luminescence based standard membrane feeding assay identifies novel small molecules that inhibit transmission of malaria parasites by mosquitoes. Sci. Rep. 2015, 5, 18704. [Google Scholar] [CrossRef] [PubMed]
- Miura, K.; Stone, W.J.R.; Koolen, K.M.; Deng, B.; Zhou, L.; van Gemert, G.-J.; Locke, E.; Morin, M.; Bousema, T.; Sauerwein, R.W.; et al. An inter-laboratory comparison of standard membrane-feeding assays for evaluation of malaria transmission-blocking vaccines. Malar. J. 2016, 15, 463. [Google Scholar] [CrossRef] [PubMed]
- Miura, K.; Swihart, B.J.; Deng, B.; Zhou, L.; Pham, T.P.; Diouf, A.; Fay, M.P.; Long, C.A. Strong concordance between percent inhibition in oocyst and sporozoite intensities in a Plasmodium falciparum standard membrane-feeding assay. Parasites Vectors 2019, 12, 206. [Google Scholar] [CrossRef] [PubMed]
- Hovlid, M.L.; Winzeler, E.A. Phenotypic Screens in Antimalarial Drug Discovery. Trends Parasitol. 2016, 32, 697–707. [Google Scholar] [CrossRef] [PubMed]
- Ross, L.S.; Fidock, D.A. Elucidating Mechanisms of Drug-Resistant Plasmodium falciparum. Cell Host Microbe 2019, 26, 35–47. [Google Scholar] [CrossRef]
- Pagola, S.; Stephens, P.W.; Bohle, D.S.; Kosar, A.D.; Madsen, S.K. The structure of malaria pigment β-haematin. Nature 2000, 404, 307–310. [Google Scholar] [CrossRef]
- Egan, T.J.; Combrinck, J.M.; Egan, J.; Hearne, G.R.; Marques, H.M.; Ntenteni, S.; Sewell, B.T.; Smith, P.J.; Taylor, D.; van Schalkwyk, D.A.; et al. Fate of haem iron in the malaria parasite Plasmodium falciparum. Biochem. J. 2002, 365, 343–347. [Google Scholar] [CrossRef]
- Egan, T.J.; Ross, D.C.; Adams, P.A. Quinoline anti-malarial drugs inhibit spontaneous formation of β-haematin (malaria pigment). FEBS Lett. 1994, 352, 54–57. [Google Scholar] [CrossRef]
- Olafson, K.N.; Ketchum, M.A.; Rimer, J.D.; Vekilov, P.G. Mechanisms of hematin crystallization and inhibition by the antimalarial drug chloroquine. Proc. Natl. Acad. Sci. USA 2015, 112, 4946–4951. [Google Scholar] [CrossRef]
- Francis, S.E.; Sullivan, D.J., Jr.; Goldberg, A.D.E. Hemoglobin metabolism in the malaria parasite Plasmodium falciparum. Annu. Rev. Microbiol. 1997, 51, 97–123. [Google Scholar] [CrossRef]
- Hanssen, E.; Knoechel, C.; Dearnley, M.; Dixon, M.W.A.; Le Gros, M.; Larabell, C.; Tilley, L. Soft X-ray microscopy analysis of cell volume and hemoglobin content in erythrocytes infected with asexual and sexual stages of Plasmodium falciparum. J. Struct. Biol. 2012, 177, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Duffy, S.; Avery, V.M. Identification of inhibitors of Plasmodium falciparum gametocyte development. Malar. J. 2013, 12, 408. [Google Scholar] [CrossRef] [PubMed]
- Sinden, R.E. Gametocytogenesis of Plasmodium falciparum in vitro:ultrastructural observations on the lethal action of chloroquine. Ann. Trop. Med. Parasitol. 1982, 76, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Adjalley, S.H.; Johnston, G.L.; Li, T.; Eastman, R.T.; Ekland, E.H.; Eappen, A.G.; Richman, A.; Sim, B.K.L.; Lee, M.C.S.; Hoffman, S.L.; et al. Quantitative assessment of Plasmodium falciparum sexual development reveals potent transmission-blocking activity by methylene blue. Proc. Natl. Acad. Sci. USA 2011, 108, E1214–E1223. [Google Scholar] [CrossRef]
- Pasay, C.J.; Rockett, R.; Sekuloski, S.; Griffin, P.; Marquart, L.; Peatey, C.; Wang, C.Y.T.; O’Rourke, P.; Elliott, S.; Baker, M.; et al. Piperaquine Monotherapy of Drug-Susceptible Plasmodium falciparum Infection Results in Rapid Clearance of Parasitemia but is Followed by the Appearance of Gametocytemia. J. Infect. Dis. 2016, 214, 105–113. [Google Scholar] [CrossRef]
- Assefa, D.G.; Zeleke, E.D.; Bekele, D.; Tesfahunei, H.A.; Getachew, E.; Joseph, M.; Manyazewal, T. Efficacy and safety of dihydroartemisinin–piperaquine versus artemether–lumefantrine for treatment of uncomplicated Plasmodium falciparum malaria in Ugandan children: A systematic review and meta-analysis of randomized control trials. Malar. J. 2021, 20, 174. [Google Scholar] [CrossRef]
- Assefa, D.G.; Yismaw, G.; Makonnen, E. Comparative effect of dihydroartemisinin-piperaquine and artemether-lumefantrine on gametocyte clearance and haemoglobin recovery in children with uncomplicated Plasmodium falciparum malaria in Africa: A systematic review and meta-analysis of randomized control trials. Int. J. Infect. Dis. 2021, 113, 136–147. [Google Scholar]
- Ahmad, A.; Prom, A.; Bradley, J.; Ndiath, M.; Etoketim, B.; Bah, M.; Van Geertruyden, J.-P.; Drakeley, C.; Bousema, T.; Achan, J.; et al. Gametocyte carriage after seasonal malaria chemoprevention in Plasmodium falciparum infected asymptomatic children. Malar. J. 2021, 20, 169. [Google Scholar] [CrossRef]
- Ouologuem, D.T.; Kone, C.O.; Fofana, B.; Sidibe, B.; Togo, A.H.; Dembele, D.; Toure, S.; Koumare, S.; Toure, O.; Sagara, I.; et al. Differential infectivity of gametocytes after artemisinin-based combination therapy of uncomplicated falciparum malaria. Afr. J. Lab. Med. 2018, 7, 784. [Google Scholar] [CrossRef]
- Dicko, A.; Roh, M.E.; Diawara, H.; Mahamar, A.; Soumare, H.M.; Lanke, K.; Bradley, J.; Sanogo, K.; Kone, D.T.; Diarra, K.; et al. Efficacy and safety of primaquine and methylene blue for prevention of Plasmodium falciparum transmission in Mali: A phase 2, single-blind, randomised controlled trial. Lancet Infect. Dis. 2018, 18, 627–639. [Google Scholar] [CrossRef]
- Howes, R.E.; Piel, F.B.; Patil, A.P.; Nyangiri, O.A.; Gething, P.W.; Dewi, M.; Hogg, M.M.; Battle, K.E.; Padilla, C.D.; Baird, J.K.; et al. G6PD Deficiency Prevalence and Estimates of Affected Populations in Malaria Endemic Countries: A Geostatistical Model-Based Map. PLoS Med. 2012, 9, e1001339. [Google Scholar] [CrossRef]
- Avula, B.; Tekwani, B.L.; Chaurasiya, N.D.; Fasinu, P.; Dhammika Nanayakkara, N.P.; Bhandara Herath, H.M.T.; Wang, Y.H.; Bae, J.Y.; Khan, S.I.; Elsohly, M.A.; et al. Metabolism of primaquine in normal human volunteers: Investigation of phase I and phase II metabolites from plasma and urine using ultra-high performance liquid chromatography-quadrupole time-of-flight mass spectrometry. Malar. J. 2018, 17, 294. [Google Scholar] [CrossRef] [PubMed]
- Camarda, G.; Jirawatcharadech, P.; Priestley, R.S.; Saif, A.; March, S.; Wong, M.H.L.; Leung, S.; Miller, A.B.; Baker, D.A.; Alano, P.; et al. Antimalarial activity of primaquine operates via a two-step biochemical relay. Nat. Commun. 2019, 10, 3226. [Google Scholar] [CrossRef] [PubMed]
- Frampton, J.E. Tafenoquine: First Global Approval. Drugs 2018, 78, 1517–1523. [Google Scholar] [CrossRef] [PubMed]
- Stone, W.; Mahamar, A.; Smit, M.J.; Sanogo, K.; Sinaba, Y.; Niambele, S.M.; Sacko, A.; Keita, S.; Dicko, O.M.; Diallo, M.; et al. Single low-dose tafenoquine combined with dihydroartemisinin–piperaquine to reduce Plasmodium falciparum transmission in Ouelessebougou, Mali: A phase 2, single-blind, randomised clinical trial. Lancet Microbe 2022, 3, E336–E347. [Google Scholar] [CrossRef]
- Schirmer, R.H.; Adler, H.; Pickhardt, M.; Mandelkow, E. Lest we forget you—Methylene blue …. Neurobiol. Aging 2011, 32, 2325.e7–2325.e16. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.; Nagbanshi, M.; Goldau, N.; Mendes Jorge, M.; Meissner, P.; Jahn, A.; Mockenhaupt, F.P.; Müller, O. Efficacy and safety of methylene blue in the treatment of malaria: A systematic review. BMC Med. 2018, 16, 59. [Google Scholar] [CrossRef]
- Schirmer, R.H.; Coulibaly, B.; Stich, A.; Scheiwein, M.; Merkle, H.; Eubel, J.; Becker, K.; Becher, H.; Müller, O.; Zich, T.; et al. Methylene blue as an antimalarial agent. Redox Rep. 2003, 8, 272–275. [Google Scholar] [CrossRef]
- Jorge, M.M.; Ouermi, L.; Meissner, P.; Compaoré, G.; Coulibaly, B.; Nebie, E.; Krisam, J.; Klose, C.; Kieser, M.; Jahn, A.; et al. Safety and efficacy of artesunate-amodiaquine combined with either methylene blue or primaquine in children with falciparum malaria in Burkina Faso: A randomized controlled trial. PLoS ONE 2019, 14, e0222993. [Google Scholar] [CrossRef]
- McDonagh, E.M.; Bautista, J.M.; Youngster, I.; Altman, R.B.; Klein, T.E. PharmGKB summary: Methylene blue pathway. Pharmacogenet. Genom. 2013, 23, 498–508. [Google Scholar] [CrossRef]
- Buchholz, K.; Schirmer, R.H.; Eubel, J.K.; Akoachere, M.B.; Dandekar, T.; Becker, K.; Gromer, S. Interactions of methylene blue with human disulfide reductases and their orthologues from Plasmodium falciparum. Antimicrob. Agents Chemother. 2008, 52, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Haynes, R.K.; Cheu, K.-W.; Li, K.-Y.; Tang, M.M.-K.; Wong, H.-N.; Chen, M.-J.; Guo, Z.-F.; Guo, Z.-H.; Coghi, P.; Monti, D. A Partial Convergence in Action of Methylene Blue and Artemisinins: Antagonism with Chloroquine, a Reversal with Verapamil, and an Insight into the Antimalarial Activity of Chloroquine. ChemMedChem 2011, 6, 1603–1615. [Google Scholar] [CrossRef] [PubMed]
- Haynes, R.K.; Cheu, K.-W.; Chan, H.-W.; Wong, H.-N.; Li, K.-Y.; Tang, M.M.-K.; Chen, M.-J.; Guo, Z.-F.; Guo, Z.-H.; Sinniah, K.; et al. Interactions between Artemisinins and other Antimalarial Drugs in Relation to the Cofactor Model—A Unifying Proposal for Drug Action. ChemMedChem 2012, 7, 2204–2226. [Google Scholar] [CrossRef] [PubMed]
- Meshnick, S.R. The mode of action of antimalarial endoperoxides. Trans. R. Soc. Trop. Med. Hyg. 1994, 88 (Suppl. S1), S31–S32. [Google Scholar] [CrossRef]
- Bridgford, J.L.; Xie, S.C.; Cobbold, S.A.; Pasaje, C.F.A.; Herrmann, S.; Yang, T.; Gillett, D.L.; Dick, L.R.; Ralph, S.A.; Dogovski, C.; et al. Artemisinin kills malaria parasites by damaging proteins and inhibiting the proteasome. Nat. Commun. 2018, 9, 3801. [Google Scholar] [CrossRef]
- Peatey, C.L.; Leroy, D.; Gardiner, D.L.; Trenholme, K.R. Anti-malarial drugs: How effective are they against Plasmodium falciparum gametocytes? Malar. J. 2012, 11, 34. [Google Scholar] [CrossRef]
- Bolscher, J.M.; Koolen, K.M.; van Gemert, G.J.; van de Vegte-Bolmer, M.G.; Bousema, T.; Leroy, D.; Sauerwein, R.W.; Dechering, K.J. A combination of new screening assays for prioritization of transmission-blocking antimalarials reveals distinct dynamics of marketed and experimental drugs. J. Antimicrob. Chemother. 2015, 70, 1357–1366. [Google Scholar] [CrossRef]
- Lelièvre, J.; Almela, M.J.; Lozano, S.; Miguel, C.; Franco, V.; Leroy, D.; Herreros, E. Activity of clinically relevant antimalarial drugs on Plasmodium falciparum mature gametocytes in an ATP bioluminescence “transmission blocking” assay. PLoS ONE 2012, 7, e35019. [Google Scholar] [CrossRef]
- Makanga, M. A review of the effects of artemether-lumefantrine on gametocyte carriage and disease transmission. Malar. J. 2014, 13, 291. [Google Scholar] [CrossRef]
- Bousema, J.T.; Schneider, P.; Gouagna, L.C.; Drakeley, C.J.; Tostmann, A.; Houben, R.; Githure, J.I.; Ord, R.; Sutherland, C.J.; Omar, S.A.; et al. Moderate Effect of Artemisinin-Based Combination Therapy on Transmission of Plasmodium falciparum. J. Infect. Dis. 2006, 193, 1151–1159. [Google Scholar] [CrossRef]
- Dearnley, M.K.; Yeoman, J.A.; Hanssen, E.; Kenny, S.; Turnbull, L.; Whitchurch, C.B.; Tilley, L.; Dixon, M.W. Origin, composition, organization and function of the inner membrane complex of Plasmodium falciparum gametocytes. J. Cell Sci. 2012, 125, 2053–2063. [Google Scholar] [CrossRef] [PubMed]
- Tiburcio, M.; Niang, M.; Deplaine, G.; Perrot, S.; Bischoff, E.; Ndour, P.A.; Silvestrini, F.; Khattab, A.; Milon, G.; David, P.H.; et al. A switch in infected erythrocyte deformability at the maturation and blood circulation of Plasmodium falciparum transmission stages. Blood 2012, 119, e172–e180. [Google Scholar] [CrossRef] [PubMed]
- Warncke Jan, D.; Beck, H.-P. Host Cytoskeleton Remodeling throughout the Blood Stages of Plasmodium falciparum. Microbiol. Mol. Biol. Rev. 2019, 83, e00013-19. [Google Scholar] [CrossRef] [PubMed]
- Aingaran, M.; Zhang, R.; Law, S.K.; Peng, Z.; Undisz, A.; Meyer, E.; Diez-Silva, M.; Burke, T.A.; Spielmann, T.; Lim, C.T.; et al. Host cell deformability is linked to transmission in the human malaria parasite Plasmodium falciparum. Cell. Microbiol. 2012, 14, 983–993. [Google Scholar] [CrossRef] [PubMed]
- Hliscs, M.; Millet, C.; Dixon, M.W.; Siden-Kiamos, I.; McMillan, P.; Tilley, L. Organization and function of an actin cytoskeleton in Plasmodium falciparum gametocytes. Cell. Microbiol. 2015, 17, 207–225. [Google Scholar] [CrossRef] [PubMed]
- Parkyn Schneider, M.; Liu, B.; Glock, P.; Suttie, A.; McHugh, E.; Andrew, D.; Batinovic, S.; Williamson, N.; Hanssen, E.; McMillan, P.; et al. Disrupting assembly of the inner membrane complex blocks Plasmodium falciparum sexual stage development. PLoS Pathog. 2017, 13, e1006659. [Google Scholar] [CrossRef]
- Naissant, B.; Dupuy, F.; Duffier, Y.; Lorthiois, A.; Duez, J.; Scholz, J.; Buffet, P.; Merckx, A.; Bachmann, A.; Lavazec, C. Plasmodium falciparum STEVOR phosphorylation regulates host erythrocyte deformability enabling malaria parasite transmission. Blood 2016, 127, e42–e53. [Google Scholar] [CrossRef]
- Ramdani, G.; Naissant, B.; Thompson, E.; Breil, F.; Lorthiois, A.; Dupuy, F.; Cummings, R.; Duffier, Y.; Corbett, Y.; Mercereau-Puijalon, O.; et al. cAMP-Signalling Regulates Gametocyte-Infected Erythrocyte Deformability Required for Malaria Parasite Transmission. PLoS Pathog. 2015, 11, e1004815. [Google Scholar] [CrossRef]
- De Niz, M.; Meibalan, E.; Mejia, P.; Ma, S.; Brancucci, N.M.B.; Agop-Nersesian, C.; Mandt, R.; Ngotho, P.; Hughes, K.R.; Waters, A.P.; et al. Plasmodium gametocytes display homing and vascular transmigration in the host bone marrow. Sci. Adv. 2018, 4, eaat3775. [Google Scholar] [CrossRef]
- Duffier, Y.; Lorthiois, A.; Cisteró, P.; Dupuy, F.; Jouvion, G.; Fiette, L.; Mazier, D.; Mayor, A.; Lavazec, C.; Moreno Sabater, A. A humanized mouse model for sequestration of Plasmodium falciparum sexual stages and in vivo evaluation of gametocytidal drugs. Sci. Rep. 2016, 6, 35025. [Google Scholar] [CrossRef]
- Duez, J.; Carucci, M.; Garcia-Barbazan, I.; Corral, M.; Perez, O.; Presa, J.L.; Henry, B.; Roussel, C.; Ndour, P.A.; Rosa, N.B.; et al. High-throughput microsphiltration to assess red blood cell deformability and screen for malaria transmission-blocking drugs. Nat. Protoc. 2018, 13, 1362–1376. [Google Scholar] [CrossRef] [PubMed]
- Duez, J.; Holleran, J.P.; Ndour, P.A.; Loganathan, S.; Amireault, P.; Français, O.; Nemer, W.E.; Pioufle, B.L.; Amado, I.F.; Garcia, S.; et al. Splenic Retention of Plasmodium falciparum Gametocytes To Block the Transmission of Malaria. Antimicrob. Agents Chemother. 2015, 59, 4206–4214. [Google Scholar] [CrossRef] [PubMed]
- Lawniczak, M.K.N.; Eckhoff, P.A. A computational lens for sexual-stage transmission, reproduction, fitness and kinetics in Plasmodium falciparum. Malar. J. 2016, 15, 487. [Google Scholar] [CrossRef] [PubMed]
- Nixon, C.P. Plasmodium falciparum gametocyte transit through the cutaneous microvasculature: A new target for malaria transmission blocking vaccines? Hum. Vaccin Immunother. 2016, 12, 3189–3195. [Google Scholar] [CrossRef] [PubMed]
- Nacher, M. Does the shape of Plasmodium falciparum gametocytes have a function? Med. Hypotheses 2004, 62, 618–619. [Google Scholar] [CrossRef] [PubMed]
- MacRae, J.I.; Dixon, M.W.; Dearnley, M.K.; Chua, H.H.; Chambers, J.M.; Kenny, S.; Bottova, I.; Tilley, L.; McConville, M.J. Mitochondrial metabolism of sexual and asexual blood stages of the malaria parasite Plasmodium falciparum. BMC Biol. 2013, 11, 67. [Google Scholar] [CrossRef]
- Olszewski, K.L.; Llinás, M. Central carbon metabolism of Plasmodium parasites. Mol. Biochem. Parasitol. 2011, 175, 95–103. [Google Scholar] [CrossRef]
- Sinden, R.E.; Carter, R.; Drakeley, C.; Leroy, D. The biology of sexual development of Plasmodium: The design and implementation of transmission-blocking strategies. Malar. J. 2012, 11, 70. [Google Scholar] [CrossRef]
- Ke, H.; Lewis, I.A.; Morrisey, J.M.; McLean, K.J.; Ganesan, S.M.; Painter, H.J.; Mather, M.W.; Jacobs-Lorena, M.; Llinás, M.; Vaidya, A.B. Genetic investigation of tricarboxylic acid metabolism during the Plasmodium falciparum life cycle. Cell Rep. 2015, 11, 164–174. [Google Scholar] [CrossRef]
- Young, J.A.; Fivelman, Q.L.; Blair, P.L.; de la Vega, P.; Le Roch, K.G.; Zhou, Y.; Carucci, D.J.; Baker, D.A.; Winzeler, E.A. The Plasmodium falciparum sexual development transcriptome: A microarray analysis using ontology-based pattern identification. Mol. Biochem. Parasitol. 2005, 143, 67–79. [Google Scholar] [CrossRef]
- Jayaraman, V.; Suryavanshi, A.; Kalale, P.; Kunala, J.; Balaram, H. Biochemical characterization and essentiality of Plasmodium fumarate hydratase. J. Biol. Chem. 2018, 293, 5878–5894. [Google Scholar] [CrossRef] [PubMed]
- Cobbold, S.A.; Tutor, M.V.; Frasse, P.; McHugh, E.; Karnthaler, M.; Creek, D.J.; John, A.O.; Tilley, L.; Ralph, S.A.; McConville, M.J. Non-canonical metabolic pathways in the malaria parasite detected by isotope-tracing metabolomics. Mol. Syst. Biol. 2021, 17, e10023. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, N.; Spurck, T.P.; Goodman, C.D.; McFadden, G.I. Apicoplast and mitochondrion in gametocytogenesis of Plasmodium falciparum. Eukaryot. Cell 2009, 8, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Evers, F.; Cabrera-Orefice, A.; Elurbe, D.M.; Kea-Te Lindert, M.; Boltryk, S.D.; Voss, T.S.; Huynen, M.A.; Brandt, U.; Kooij, T.W.A. Composition and stage dynamics of mitochondrial complexes in Plasmodium falciparum. Nat. Commun. 2021, 12, 3820. [Google Scholar] [CrossRef] [PubMed]
- Hino, A.; Hirai, M.; Tanaka, T.Q.; Watanabe, Y.; Matsuoka, H.; Kita, K. Critical roles of the mitochondrial complex II in oocyst formation of rodent malaria parasite Plasmodium berghei. J. Biochem. 2012, 152, 259–268. [Google Scholar] [CrossRef]
- Matz, J.M.; Goosmann, C.; Matuschewski, K.; Kooij, T.W.A. An Unusual Prohibitin Regulates Malaria Parasite Mitochondrial Membrane Potential. Cell Rep. 2018, 23, 756–767. [Google Scholar] [CrossRef]
- Goodman Christopher, D.; Siregar Josephine, E.; Mollard, V.; Vega-Rodríguez, J.; Syafruddin, D.; Matsuoka, H.; Matsuzaki, M.; Toyama, T.; Sturm, A.; Cozijnsen, A.; et al. Parasites resistant to the antimalarial atovaquone fail to transmit by mosquitoes. Science 2016, 352, 349–353. [Google Scholar] [CrossRef]
- Painter, H.J.; Morrisey, J.M.; Mather, M.W.; Vaidya, A.B. Specific role of mitochondrial electron transport in blood-stage Plasmodium falciparum. Nature 2007, 446, 88–91. [Google Scholar] [CrossRef]
- Krungkrai, J. Purification, characterization and localization of mitochondrial dihydroorotate dehydrogenase in Plasmodium falciparum, human malaria parasite. Biochim. Biophys. Acta 1995, 1243, 351–360. [Google Scholar] [CrossRef]
- Barton, V.; Fisher, N.; Biagini, G.A.; Ward, S.A.; O’Neill, P.M. Inhibiting Plasmodium cytochrome bc1: A complex issue. Curr. Opin. Chem. Biol. 2010, 14, 440–446. [Google Scholar] [CrossRef]
- Nixon, G.L.; Moss, D.M.; Shone, A.E.; Lalloo, D.G.; Fisher, N.; O’Neill, P.M.; Ward, S.A.; Biagini, G.A. Antimalarial pharmacology and therapeutics of atovaquone. J. Antimicrob. Chemother. 2013, 68, 977–985. [Google Scholar] [CrossRef] [PubMed]
- Fleck, S.L.; Pudney, M.; Sinden, R.E. The effect of atovaquone (566C80) on the maturation and viability of Plasmodium falciparum gametocytes in vitro. Trans. R. Soc. Trop. Med. Hyg. 1996, 90, 309–312. [Google Scholar] [CrossRef]
- Azevedo, R.; Markovic, M.; Machado, M.; Franke-Fayard, B.; Mendes, A.M.; Prudêncio, M. Bioluminescence Method for In Vitro Screening of Plasmodium Transmission-Blocking Compounds. Antimicrob. Agents Chemother. 2017, 61, e02699-16. [Google Scholar] [CrossRef] [PubMed]
- Paton, D.G.; Childs, L.M.; Itoe, M.A.; Holmdahl, I.E.; Buckee, C.O.; Catteruccia, F. Exposing Anopheles mosquitoes to antimalarials blocks Plasmodium parasite transmission. Nature 2019, 567, 239–243. [Google Scholar] [CrossRef]
- Creasey, A.; Mendis, K.; Carlton, J.; Williamson, D.; Wilson, I.; Carter, R. Maternal inheritance of extrachromosomal DNA in malaria parasites. Mol. Biochem. Parasitol. 1994, 65, 95–98. [Google Scholar] [CrossRef]
- Beerahee, M. Clinical pharmacology of atovaquone and proguanil hydrochloride. J. Travel. Med. 1999, 6 (Suppl. S1), S13–S17. [Google Scholar]
- Butcher, G.A.; Sinden, R.E. Persistence of atovaquone in human sera following treatment: Inhibition of Plasmodium falciparum development in vivo and in vitro. Am. J. Trop. Med. Hyg. 2003, 68, 111–114. [Google Scholar] [CrossRef]
- Coteron, J.M.; Marco, M.; Esquivias, J.; Deng, X.; White, K.L.; White, J.; Koltun, M.; El Mazouni, F.; Kokkonda, S.; Katneni, K.; et al. Structure-Guided Lead Optimization of Triazolopyrimidine-Ring Substituents Identifies Potent Plasmodium falciparum Dihydroorotate Dehydrogenase Inhibitors with Clinical Candidate Potential. J. Med. Chem. 2011, 54, 5540–5561. [Google Scholar] [CrossRef]
- Dini, S.; Zaloumis, S.G.; Price, D.J.; Gobeau, N.; Kümmel, A.; Cherkaoui, M.; Moehrle, J.J.; McCarthy, J.S.; Simpson, J.A. Seeking an optimal dosing regimen for OZ439/DSM265 combination therapy for treating uncomplicated falciparum malaria. J. Antimicrob. Chemother. 2021, 76, 2325–2334. [Google Scholar] [CrossRef]
- Llanos-Cuentas, A.; Casapia, M.; Chuquiyauri, R.; Hinojosa, J.-C.; Kerr, N.; Rosario, M.; Toovey, S.; Arch, R.H.; Phillips, M.A.; Rozenberg, F.D.; et al. Antimalarial activity of single-dose DSM265, a novel plasmodium dihydroorotate dehydrogenase inhibitor, in patients with uncomplicated Plasmodium falciparum or Plasmodium vivax malaria infection: A proof-of-concept, open-label, phase 2a study. Lancet Infect. Dis. 2018, 18, 874–883. [Google Scholar] [CrossRef]
- Phillips, M.A.; Lotharius, J.; Marsh, K.; White, J.; Dayan, A.; White, K.L.; Njoroge, J.W.; El Mazouni, F.; Lao, Y.; Kokkonda, S.; et al. A long-duration dihydroorotate dehydrogenase inhibitor (DSM265) for prevention and treatment of malaria. Sci. Transl. Med. 2015, 7, 296ra111. [Google Scholar] [CrossRef] [PubMed]
- Collins, K.A.; Rückle, T.; Elliott, S.; Marquart, L.; Ballard, E.; Chalon, S.; Griffin, P.; Möhrle, J.J.; McCarthy, J.S. DSM265 at 400 Milligrams Clears Asexual Stage Parasites but Not Mature Gametocytes from the Blood of Healthy Subjects Experimentally Infected with Plasmodium falciparum. Antimicrob. Agents Chemother. 2019, 63, e01837-18. [Google Scholar] [CrossRef] [PubMed]
- McCarthy James, S.; Rückle, T.; Elliott Suzanne, L.; Ballard, E.; Collins Katharine, A.; Marquart, L.; Griffin, P.; Chalon, S.; Möhrle Jörg, J. A Single-Dose Combination Study with the Experimental Antimalarials Artefenomel and DSM265 To Determine Safety and Antimalarial Activity against Blood-Stage Plasmodium falciparum in Healthy Volunteers. Antimicrob. Agents Chemother. 2019, 64, e01371-19. [Google Scholar] [CrossRef] [PubMed]
- Tran, P.N.; Brown, S.H.; Rug, M.; Ridgway, M.C.; Mitchell, T.W.; Maier, A.G. Changes in lipid composition during sexual development of the malaria parasite Plasmodium falciparum. Malar. J. 2016, 15, 73. [Google Scholar] [CrossRef]
- Ridgway, M.C.; Cihalova, D.; Brown, S.H.J.; Tran, P.; Mitchell, T.W.; Maier, A.G. Analysis of sex-specific lipid metabolism of Plasmodium falciparum points to the importance of sphingomyelin for gametocytogenesis. J. Cell Sci. 2022, 135, jcs259592. [Google Scholar] [CrossRef]
- Ben Mamoun, C.; Prigge, S.T.; Vial, H. Targeting the Lipid Metabolic Pathways for the Treatment of Malaria. Drug Dev. Res. 2010, 71, 44–55. [Google Scholar] [CrossRef]
- Gulati, S.; Ekland, E.H.; Ruggles, K.V.; Chan, R.B.; Jayabalasingham, B.; Zhou, B.; Mantel, P.-Y.; Lee, M.C.S.; Spottiswoode, N.; Coburn-Flynn, O.; et al. Profiling the Essential Nature of Lipid Metabolism in Asexual Blood and Gametocyte Stages of Plasmodium falciparum. Cell Host Microbe 2015, 18, 371–381. [Google Scholar] [CrossRef]
- McNamara, C.W.; Lee, M.C.S.; Lim, C.S.; Lim, S.H.; Roland, J.; Nagle, A.; Simon, O.; Yeung, B.K.S.; Chatterjee, A.K.; McCormack, S.L.; et al. Targeting Plasmodium PI(4)K to eliminate malaria. Nature 2013, 504, 248–253. [Google Scholar] [CrossRef]
- Fraser, M.; Jing, W.; Bröer, S.; Kurth, F.; Sander, L.E.; Matuschewski, K.; Maier, A.G. Breakdown in membrane asymmetry regulation leads to monocyte recognition of P. falciparum-infected red blood cells. PLoS Pathog. 2021, 17, e1009259. [Google Scholar] [CrossRef]
- Fraser, M.; Matuschewski, K.; Maier, A.G. Of membranes and malaria: Phospholipid asymmetry in Plasmodium falciparum-infected red blood cells. Cell. Mol. Life Sci. 2021, 78, 4545–4561. [Google Scholar] [CrossRef]
- McMorran, B.J.; Wieczorski, L.; Drysdale, K.E.; Chan, J.A.; Huang, H.M.; Smith, C.; Mitiku, C.; Beeson, J.G.; Burgio, G.; Foote, S.J. Platelet factor 4 and Duffy antigen required for platelet killing of Plasmodium falciparum. Science 2012, 338, 1348–1351. [Google Scholar] [CrossRef]
- Kho, S.; Barber, B.E.; Johar, E.; Andries, B.; Poespoprodjo, J.R.; Kenangalem, E.; Piera, K.A.; Ehmann, A.; Price, R.N.; William, T.; et al. Platelets kill circulating parasites of all major Plasmodium species in human malaria. Blood 2018, 132, 1332–1344. [Google Scholar] [CrossRef]
- Love, M.S.; Millholland, M.G.; Mishra, S.; Kulkarni, S.; Freeman, K.B.; Pan, W.; Kavash, R.W.; Costanzo, M.J.; Jo, H.; Daly, T.M.; et al. Platelet factor 4 activity against P. falciparum and its translation to nonpeptidic mimics as antimalarials. Cell Host Microbe 2012, 12, 815–823. [Google Scholar] [CrossRef]
- Lawrence, N.; Dennis, A.S.M.; Lehane, A.M.; Ehmann, A.; Harvey, P.J.; Benfield, A.H.; Cheneval, O.; Henriques, S.T.; Craik, D.J.; McMorran, B.J. Defense Peptides Engineered from Human Platelet Factor 4 Kill Plasmodium by Selective Membrane Disruption. Cell Chem. Biol. 2018, 25, 1140–1150.e5. [Google Scholar] [CrossRef]
- Slavic, K.; Delves, M.J.; Prudêncio, M.; Talman, A.M.; Straschil, U.; Derbyshire, E.T.; Xu, Z.; Sinden, R.E.; Mota, M.M.; Morin, C.; et al. Use of a selective inhibitor to define the chemotherapeutic potential of the plasmodial hexose transporter in different stages of the parasite’s life cycle. Antimicrob. Agents Chemother. 2011, 55, 2824–2830. [Google Scholar] [CrossRef]
- Bennink, S.; Kiesow, M.J.; Pradel, G. The development of malaria parasites in the mosquito midgut. Cell Microbiol. 2016, 18, 905–918. [Google Scholar] [CrossRef]
- Mair, G.R.; Braks, J.A.; Garver, L.S.; Wiegant, J.C.; Hall, N.; Dirks, R.W.; Khan, S.M.; Dimopoulos, G.; Janse, C.J.; Waters, A.P. Regulation of sexual development of Plasmodium by translational repression. Science 2006, 313, 667–669. [Google Scholar] [CrossRef]
- Siciliano, G.; Costa, G.; Suarez-Cortes, P.; Valleriani, A.; Alano, P.; Levashina, E.A. Critical Steps of Plasmodium falciparum Ookinete Maturation. Front. Microbiol. 2020, 11, 269. [Google Scholar] [CrossRef]
- Yuda, M.; Kaneko, I.; Iwanaga, S.; Murata, Y.; Kato, T. Female-specific gene regulation in malaria parasites by an AP2-family transcription factor. Mol. Microbiol. 2020, 113, 40–51. [Google Scholar] [CrossRef]
- Nzila, A. Inhibitors of de novo folate enzymes in Plasmodium falciparum. Drug Discov. Today 2006, 11, 939–944. [Google Scholar] [CrossRef]
- Yuthavong, Y.; Tarnchompoo, B.; Vilaivan, T.; Chitnumsub, P.; Kamchonwongpaisan, S.; Charman, S.A.; McLennan, D.N.; White, K.L.; Vivas, L.; Bongard, E.; et al. Malarial dihydrofolate reductase as a paradigm for drug development against a resistance-compromised target. Proc. Natl. Acad. Sci. USA 2012, 109, 16823–16828. [Google Scholar] [CrossRef]
- Gregson, A.; Plowe, C.V. Mechanisms of Resistance of Malaria Parasites to Antifolates. Pharmacol. Rev. 2005, 57, 117–145. [Google Scholar] [CrossRef]
- Peterson, D.S.; Milhous, W.K.; Wellems, T.E. Molecular basis of differential resistance to cycloguanil and pyrimethamine in Plasmodium falciparum malaria. Proc. Natl. Acad. Sci. USA 1990, 87, 3018–3022. [Google Scholar] [CrossRef]
- Teklehaimanot, A.; Nguyen-Dinh, P.; Collins, W.E.; Barber, A.M.; Campbell, C.C. Evaluation of sporontocidal compounds using Plasmodium falciparum gametocytes produced in vitro. Am. J. Trop. Med. Hyg. 1985, 34, 429–434. [Google Scholar] [CrossRef]
- Brochet, M.; Billker, O. Calcium signalling in malaria parasites. Mol. Microbiol. 2016, 100, 397–408. [Google Scholar] [CrossRef]
- Garg, S.; Agarwal, S.; Kumar, S.; Shams Yazdani, S.; Chitnis, C.E.; Singh, S. Calcium-dependent permeabilization of erythrocytes by a perforin-like protein during egress of malaria parasites. Nat. Commun. 2013, 4, 1736. [Google Scholar] [CrossRef]
- Weiss, G.E.; Gilson, P.R.; Taechalertpaisarn, T.; Tham, W.-H.; de Jong, N.W.M.; Harvey, K.L.; Fowkes, F.J.I.; Barlow, P.N.; Rayner, J.C.; Wright, G.J.; et al. Revealing the Sequence and Resulting Cellular Morphology of Receptor-Ligand Interactions during Plasmodium falciparum Invasion of Erythrocytes. PLoS Pathog. 2015, 11, e1004670. [Google Scholar] [CrossRef]
- Carey, A.F.; Singer, M.; Bargieri, D.; Thiberge, S.; Frischknecht, F.; Ménard, R.; Amino, R. Calcium dynamics of Plasmodium berghei sporozoite motility. Cell. Microbiol. 2014, 16, 768–783. [Google Scholar] [CrossRef]
- Baker, D.A.; Kelly, J.M. Purine nucleotide cyclases in the malaria parasite. Trends Parasitol. 2004, 20, 227–232. [Google Scholar] [CrossRef]
- Baker, D.A.; Drought, L.G.; Flueck, C.; Nofal, S.D.; Patel, A.; Penzo, M.; Walker, E.M. Cyclic nucleotide signalling in malaria parasites. Open Biol. 2017, 7, 170213. [Google Scholar] [CrossRef]
- Brochet, M.; Balestra, A.C.; Brusini, L. cGMP homeostasis in malaria parasites-The key to perceiving and integrating environmental changes during transmission to the mosquito. Mol. Microbiol. 2021, 115, 829–838. [Google Scholar] [CrossRef]
- Billker, O.; Dechamps, S.; Tewari, R.; Wenig, G.; Franke-Fayard, B.; Brinkmann, V. Calcium and a Calcium-Dependent Protein Kinase Regulate Gamete Formation and Mosquito Transmission in a Malaria Parasite. Cell 2004, 117, 503–514. [Google Scholar] [CrossRef]
- Bansal, A.; Molina-Cruz, A.; Brzostowski, J.; Liu, P.; Luo, Y.; Gunalan, K.; Li, Y.; Ribeiro, J.M.C.; Miller, L.H. CDPK1 is critical for malaria parasite gametogenesis and mosquito infection. Proc. Natl. Acad. Sci. USA 2018, 115, 774–779. [Google Scholar] [CrossRef]
- Kato, N.; Sakata, T.; Breton, G.; Le Roch, K.G.; Nagle, A.; Andersen, C.; Bursulaya, B.; Henson, K.; Johnson, J.; Kumar, K.A.; et al. Gene expression signatures and small-molecule compounds link a protein kinase to Plasmodium falciparum motility. Nat. Chem. Biol. 2008, 4, 347–356. [Google Scholar] [CrossRef]
- Bansal, A.; Singh, S.; More, K.R.; Hans, D.; Nangalia, K.; Yogavel, M.; Sharma, A.; Chitnis, C.E. Characterization of Plasmodium falciparum calcium-dependent protein kinase 1 (PfCDPK1) and its role in microneme secretion during erythrocyte invasion. J. Biol. Chem. 2013, 288, 1590–1602. [Google Scholar] [CrossRef]
- Taylor, C.J.; McRobert, L.; Baker, D.A. Disruption of a Plasmodium falciparum cyclic nucleotide phosphodiesterase gene causes aberrant gametogenesis. Mol. Microbiol. 2008, 69, 110–118. [Google Scholar] [CrossRef]
- Collins, C.R.; Hackett, F.; Strath, M.; Penzo, M.; Withers-Martinez, C.; Baker, D.A.; Blackman, M.J. Malaria Parasite cGMP-dependent Protein Kinase Regulates Blood Stage Merozoite Secretory Organelle Discharge and Egress. PLoS Pathog. 2013, 9, e1003344. [Google Scholar] [CrossRef]
- Brochet, M.; Collins, M.O.; Smith, T.K.; Thompson, E.; Sebastian, S.; Volkmann, K.; Schwach, F.; Chappell, L.; Gomes, A.R.; Berriman, M.; et al. Phosphoinositide Metabolism Links cGMP-Dependent Protein Kinase G to Essential Ca2+ Signals at Key Decision Points in the Life Cycle of Malaria Parasites. PLoS Biol. 2014, 12, e1001806. [Google Scholar] [CrossRef]
- Rotella, D.; Siekierka, J.; Bhanot, P. Plasmodium falciparum cGMP-Dependent Protein Kinase—A Novel Chemotherapeutic Target. Front. Microbiol. 2021, 11, 610408. [Google Scholar] [CrossRef]
- Vanaerschot, M.; Murithi, J.M.; Pasaje, C.F.A.; Ghidelli-Disse, S.; Dwomoh, L.; Bird, M.; Spottiswoode, N.; Mittal, N.; Arendse, L.B.; Owen, E.S.; et al. Inhibition of Resistance-Refractory P. falciparum Kinase PKG Delivers Prophylactic, Blood Stage, and Transmission-Blocking Antiplasmodial Activity. Cell Chem. Biol. 2020, 27, 806–816.e8. [Google Scholar] [CrossRef]
- Reddy, B.P.N.; Shrestha, S.; Hart, K.J.; Liang, X.; Kemirembe, K.; Cui, L.; Lindner, S.E. A bioinformatic survey of RNA-binding proteins in Plasmodium. BMC Genom. 2015, 16, 890. [Google Scholar] [CrossRef]
- Bunnik, E.M.; Batugedara, G.; Saraf, A.; Prudhomme, J.; Florens, L.; Le Roch, K.G. The mRNA-bound proteome of the human malaria parasite Plasmodium falciparum. Genome Biol. 2016, 17, 147. [Google Scholar] [CrossRef]
- Caro, F.; Ahyong, V.; Betegon, M.; DeRisi, J.L. Genome-wide regulatory dynamics of translation in the Plasmodium falciparum asexual blood stages. eLife 2014, 3, e04106. [Google Scholar] [CrossRef]
- Lindner, S.E.; Swearingen, K.E.; Shears, M.J.; Walker, M.P.; Vrana, E.N.; Hart, K.J.; Minns, A.M.; Sinnis, P.; Moritz, R.L.; Kappe, S.H.I. Transcriptomics and proteomics reveal two waves of translational repression during the maturation of malaria parasite sporozoites. Nat. Commun. 2019, 10, 4964. [Google Scholar] [CrossRef]
- Bennink, S.; Pradel, G. The molecular machinery of translational control in malaria parasites. Mol. Microbiol. 2019, 112, 1658–1673. [Google Scholar] [CrossRef]
- Walzer Katelyn, A.; Kubicki Danielle, M.; Tang, X.; Chi Jen-Tsan, A.; Dzikowski, R. Single-Cell Analysis Reveals Distinct Gene Expression and Heterogeneity in Male and Female Plasmodium falciparum Gametocytes. mSphere 2018, 3, e00130-18. [Google Scholar] [CrossRef]
- Rios, K.T.; Lindner, S.E. Protein–RNA interactions important for Plasmodium transmission. PLoS Pathog. 2019, 15, e1008095. [Google Scholar] [CrossRef]
- Sebastian, S.; Brochet, M.; Collins, M.O.; Schwach, F.; Jones, M.L.; Goulding, D.; Rayner, J.C.; Choudhary, J.S.; Billker, O. A Plasmodium Calcium-Dependent Protein Kinase Controls Zygote Development and Transmission by Translationally Activating Repressed mRNAs. Cell Host Microbe 2012, 12, 9–19. [Google Scholar] [CrossRef]
- Bennink, S.; von Bohl, A.; Ngwa, C.J.; Henschel, L.; Kuehn, A.; Pilch, N.; Weißbach, T.; Rosinski, A.N.; Scheuermayer, M.; Repnik, U.; et al. A seven-helix protein constitutes stress granules crucial for regulating translation during human-to-mosquito transmission of Plasmodium falciparum. PLoS Pathog. 2018, 14, e1007249. [Google Scholar] [CrossRef]
- Müller, K.; Silvie, O.; Mollenkopf, H.-J.; Matuschewski, K. Pleiotropic Roles for the Plasmodium berghei RNA Binding Protein UIS12 in Transmission and Oocyst Maturation. Front. Cell. Infect. Microbiol. 2021, 11, 624945. [Google Scholar] [CrossRef] [PubMed]
- Hirai, M.; Maeta, A.; Mori, T.; Mita, T. Pb103 Regulates Zygote/Ookinete Development in Plasmodium berghei via Double Zinc Finger Domains. Pathogens 2021, 10, 1536. [Google Scholar] [CrossRef] [PubMed]
- Mair, G.R.; Lasonder, E.; Garver, L.S.; Franke-Fayard, B.M.D.; Carret, C.K.; Wiegant, J.C.A.G.; Dirks, R.W.; Dimopoulos, G.; Janse, C.J.; Waters, A.P. Universal Features of Post-Transcriptional Gene Regulation Are Critical for Plasmodium Zygote Development. PLoS Pathog. 2010, 6, e1000767. [Google Scholar] [CrossRef] [PubMed]
- Yuda, M.; Iwanaga, S.; Shigenobu, S.; Mair, G.R.; Janse, C.J.; Waters, A.P.; Kato, T.; Kaneko, I. Identification of a transcription factor in the mosquito-invasive stage of malaria parasites. Mol. Microbiol. 2009, 71, 1402–1414. [Google Scholar] [CrossRef] [PubMed]
- Guerreiro, A.; Deligianni, E.; Santos, J.M.; Silva, P.A.; Louis, C.; Pain, A.; Janse, C.J.; Franke-Fayard, B.; Carret, C.K.; Siden-Kiamos, I.; et al. Genome-wide RIP-Chip analysis of translational repressor-bound mRNAs in the Plasmodium gametocyte. Genome Biol. 2014, 15, 493. [Google Scholar] [CrossRef] [PubMed]
- Birkholtz, L.M.; Alano, P.; Leroy, D. Transmission-blocking drugs for malaria elimination. Trends Parasitol. 2022, 38, 390–403. [Google Scholar] [CrossRef] [PubMed]
- Chawla, J.; Oberstaller, J.; Adams, J.H. Targeting Gametocytes of the Malaria Parasite Plasmodium falciparum in a Functional Genomics Era: Next Steps. Pathogens 2021, 10, 346. [Google Scholar] [CrossRef]
- Duffey, M.; Blasco, B.; Burrows, J.N.; Wells, T.N.C.; Fidock, D.A.; Leroy, D. Assessing risks of Plasmodium falciparum resistance to select next-generation antimalarials. Trends Parasitol. 2021, 37, 709–721. [Google Scholar] [CrossRef]
- Ecker, A.; Lakshmanan, V.; Sinnis, P.; Coppens, I.; Fidock, D.A. Evidence that mutant PfCRT facilitates the transmission to mosquitoes of chloroquine-treated Plasmodium gametocytes. J. Infect. Dis. 2011, 203, 228–236. [Google Scholar] [CrossRef]
- Witmer, K.; Dahalan Farah, A.; Delves Michael, J.; Yahiya, S.; Watson Oliver, J.; Straschil, U.; Chiwcharoen, D.; Sornboon, B.; Pukrittayakamee, S.; Pearson Richard, D.; et al. Transmission of Artemisinin-Resistant Malaria Parasites to Mosquitoes under Antimalarial Drug Pressure. Antimicrob. Agents Chemother. 2020, 65, e00898-20. [Google Scholar] [CrossRef]
- Delves, M.J.; Angrisano, F.; Blagborough, A.M. Antimalarial Transmission-Blocking Interventions: Past, Present, and Future. Trends Parasitol. 2018, 34, 735–746. [Google Scholar] [CrossRef]
- Clyde, D.F. Mass administration of an antimalarial drug combining 4-aminoquinoline and 8-aminoquinoline in Tanganyika. Bull. World Health Organ. 1962, 27, 203–212. [Google Scholar] [PubMed]
- Yu, S.; Wang, J.; Luo, X.; Zheng, H.; Wang, L.; Yang, X.; Wang, Y. Transmission-Blocking Strategies Against Malaria Parasites During Their Mosquito Stages. Front. Cell. Infect. Microbiol. 2022, 12, 820650. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Policy Brief on Single-Dose Primaquine as a Gametocytocide in Plasmodium Falciparum Malaria; WHO: Geneva, Switzerland, 2015. [Google Scholar]
- Dondorp, A.M.; Smithuis, F.M.; Woodrow, C.; Seidlein, L.v. How to Contain Artemisinin- and Multidrug-Resistant Falciparum Malaria. Trends Parasitol. 2017, 33, 353–363. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. A Framework for Malaria Elimination; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Koepfli, C.; Nguitragool, W.; de Almeida, A.C.G.; Kuehn, A.; Waltmann, A.; Kattenberg, E.; Ome-Kaius, M.; Rarau, P.; Obadia, T.; Kazura, J.; et al. Identification of the asymptomatic Plasmodium falciparum and Plasmodium vivax gametocyte reservoir under different transmission intensities. PLoS Negl. Trop. Dis. 2021, 15, e0009672. [Google Scholar] [CrossRef]
- Koepfli, C.; Yan, G. Plasmodium Gametocytes in Field Studies: Do We Measure Commitment to Transmission or Detectability? Trends Parasitol. 2018, 34, 378–387. [Google Scholar] [CrossRef]
- von Seidlein, L.; Dondorp, A. Fighting fire with fire: Mass antimalarial drug administrations in an era of antimalarial resistance. Expert Rev. Anti-Infect. Ther. 2015, 13, 715–730. [Google Scholar] [CrossRef]
- Lubell, Y.; White, L.; Varadan, S.; Drake, T.; Yeung, S.; Cheah, P.Y.; Maude, R.J.; Dondorp, A.; Day, N.P.J.; White, N.J.; et al. Ethics, Economics, and the Use of Primaquine to Reduce Falciparum Malaria Transmission in Asymptomatic Populations. PLoS Med. 2014, 11, e1001704. [Google Scholar] [CrossRef]
- Adhikari, B.; James, N.; Newby, G.; von Seidlein, L.; White, N.J.; Day, N.P.J.; Dondorp, A.M.; Pell, C.; Cheah, P.Y. Community engagement and population coverage in mass anti-malarial administrations: A systematic literature review. Malar. J. 2016, 15, 523. [Google Scholar] [CrossRef]
- Peto, T.J.; Tripura, R.; Davoeung, C.; Nguon, C.; Nou, S.; Heng, C.; Kunthea, P.; Adhikari, B.; Lim, R.; James, N.; et al. Reflections on a Community Engagement Strategy for Mass Antimalarial Drug Administration in Cambodia. Am. J. Trop. Med. Hyg. 2018, 98, 100–104. [Google Scholar] [CrossRef]
- Baird, J.K.; Surjadjaja, C. Consideration of ethics in primaquine therapy against malaria transmission. Trends Parasitol. 2011, 27, 11–16. [Google Scholar] [CrossRef]
- Nasir, S.M.I.; Amarasekara, S.; Wickremasinghe, R.; Fernando, D.; Udagama, P. Prevention of re-establishment of malaria: Historical perspective and future prospects. Malar. J. 2020, 19, 452. [Google Scholar] [CrossRef] [PubMed]
- White, N.J. Does antimalarial mass drug administration increase or decrease the risk of resistance? Lancet Infect. Dis. 2017, 17, e15–e20. [Google Scholar] [CrossRef]
- Cheah, P.Y.; Parker, M.; Day, N.P.J. Ethics and Antimalarial Drug Resistance. Ethics Drug Resist. Collect. Responsib. Glob. Public Health 2020, 5, 55–73. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Munro, B.A.; McMorran, B.J. Antimalarial Drug Strategies to Target Plasmodium Gametocytes. Parasitologia 2022, 2, 101-124. https://doi.org/10.3390/parasitologia2020011
Munro BA, McMorran BJ. Antimalarial Drug Strategies to Target Plasmodium Gametocytes. Parasitologia. 2022; 2(2):101-124. https://doi.org/10.3390/parasitologia2020011
Chicago/Turabian StyleMunro, Bruce A., and Brendan J. McMorran. 2022. "Antimalarial Drug Strategies to Target Plasmodium Gametocytes" Parasitologia 2, no. 2: 101-124. https://doi.org/10.3390/parasitologia2020011
APA StyleMunro, B. A., & McMorran, B. J. (2022). Antimalarial Drug Strategies to Target Plasmodium Gametocytes. Parasitologia, 2(2), 101-124. https://doi.org/10.3390/parasitologia2020011





