Changes in Soil Bacterial Community Structure in Bermudagrass Turf under Short-Term Traffic Stress
Abstract
:1. Introduction
2. Materials and Methods
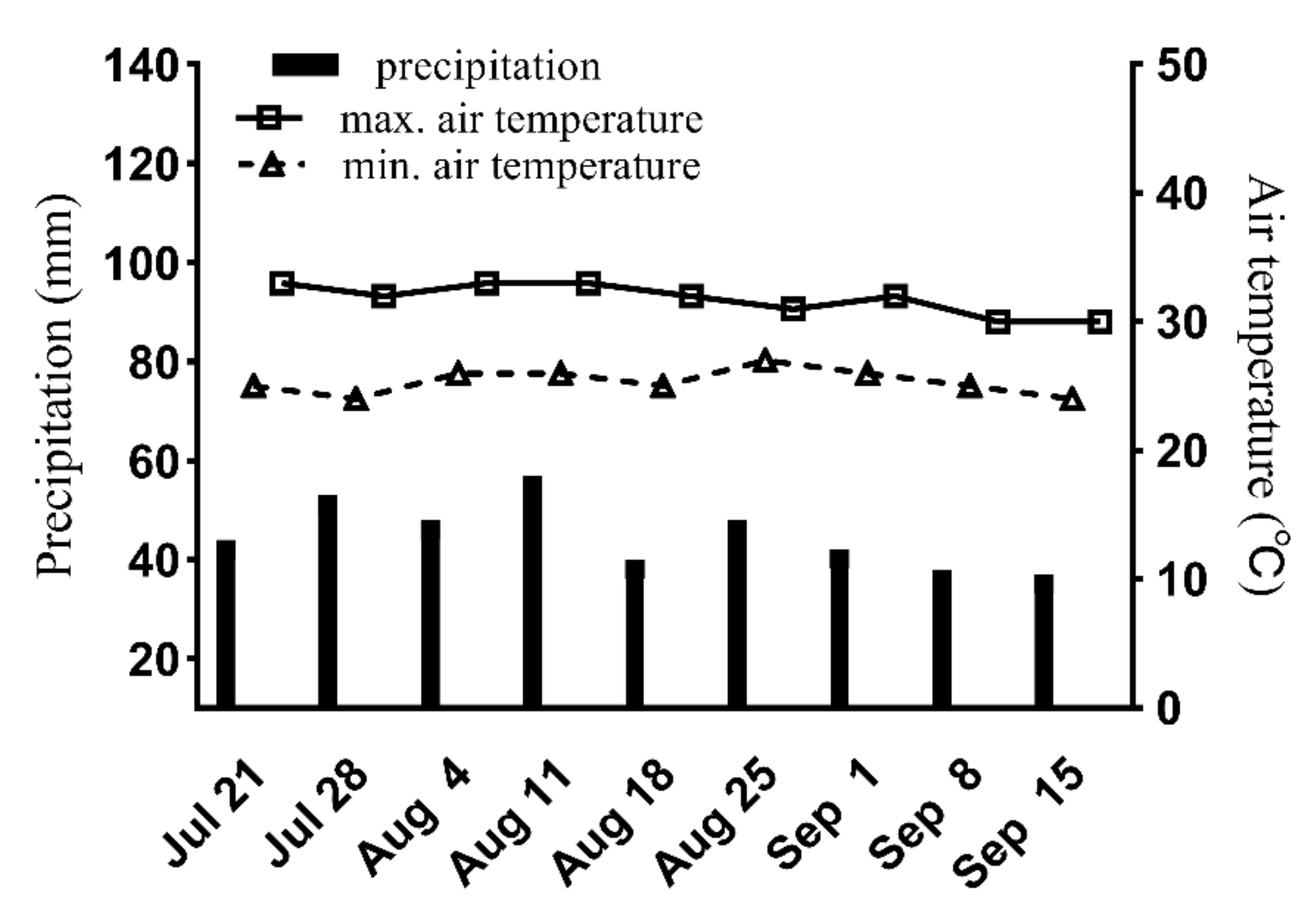
2.1. Site of Experiments
2.2. Experimental Design
2.3. Measurement of Turf Quality Indicators, Soli Physical Properties and Soil Respiration Rate
2.4. Soil Sample Collection and Analysis
2.5. gDNA Collection and PCR Amplification
2.6. Bioinformatics and Statistical Analysis
3. Results
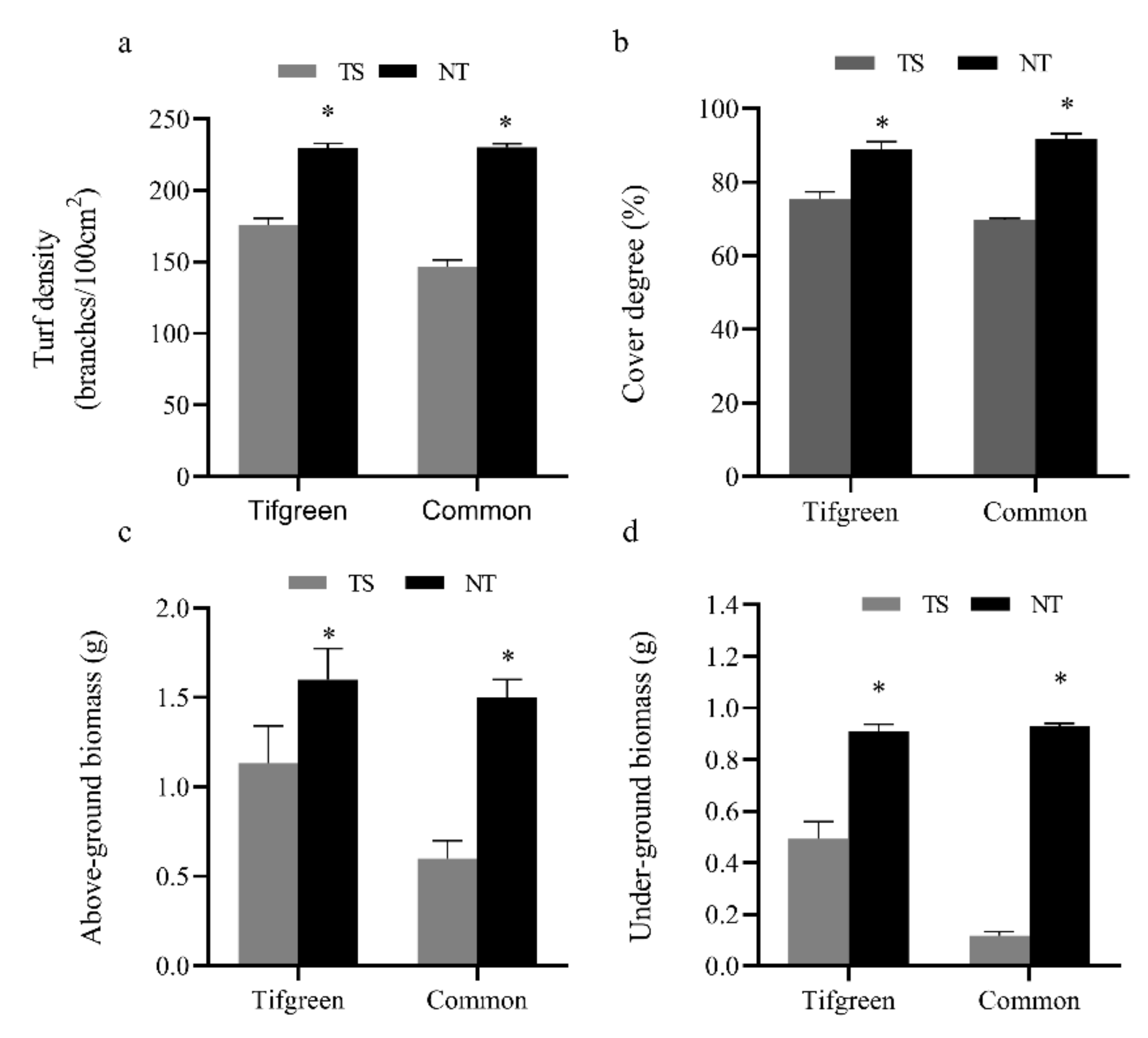
3.1. Effects of Traffic Stress on Turf Quality Indicators
3.2. Effects of Traffic Stress on Soil Physicochemical Properties
3.3. The Composition of Bacterial Communities
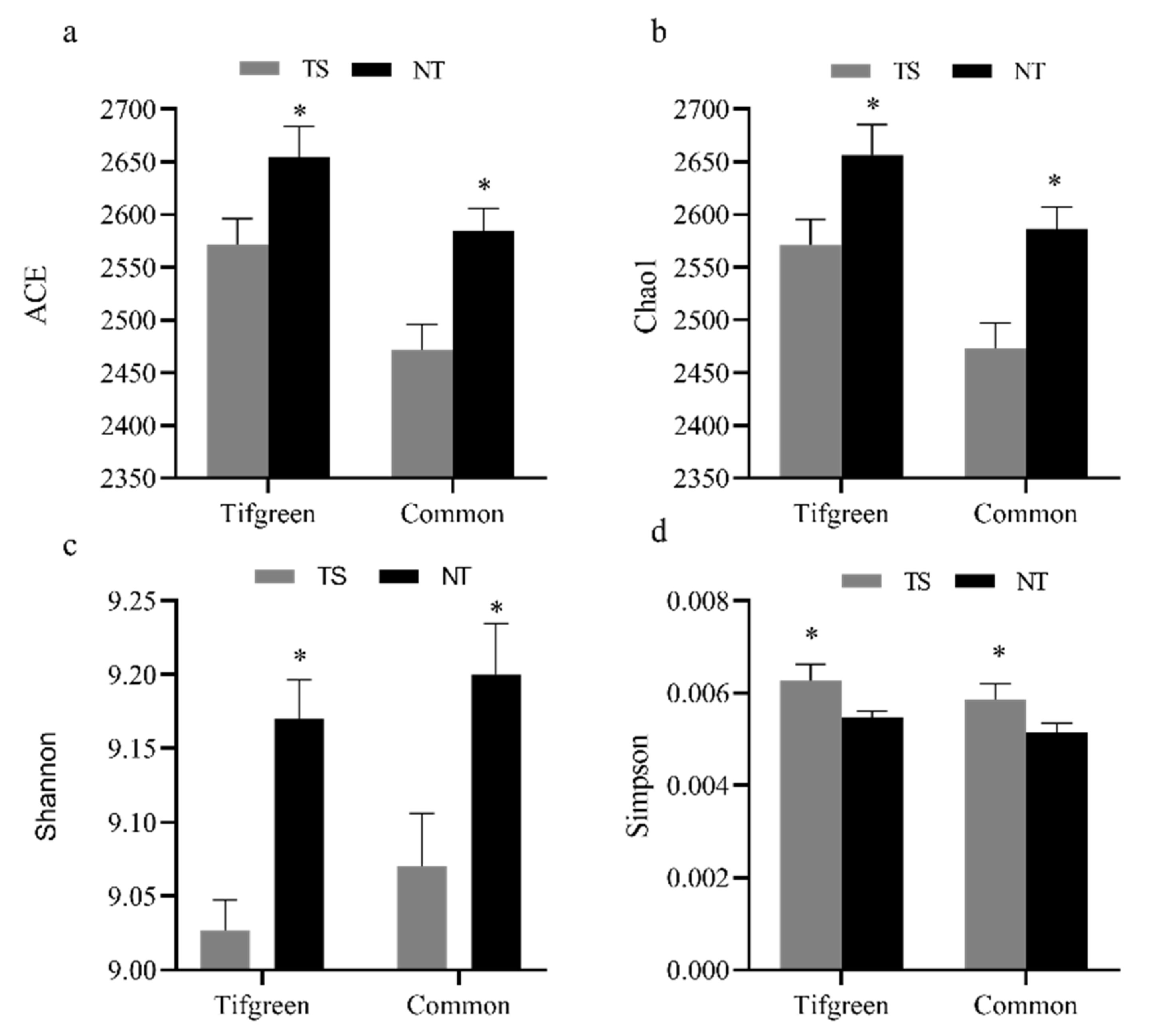
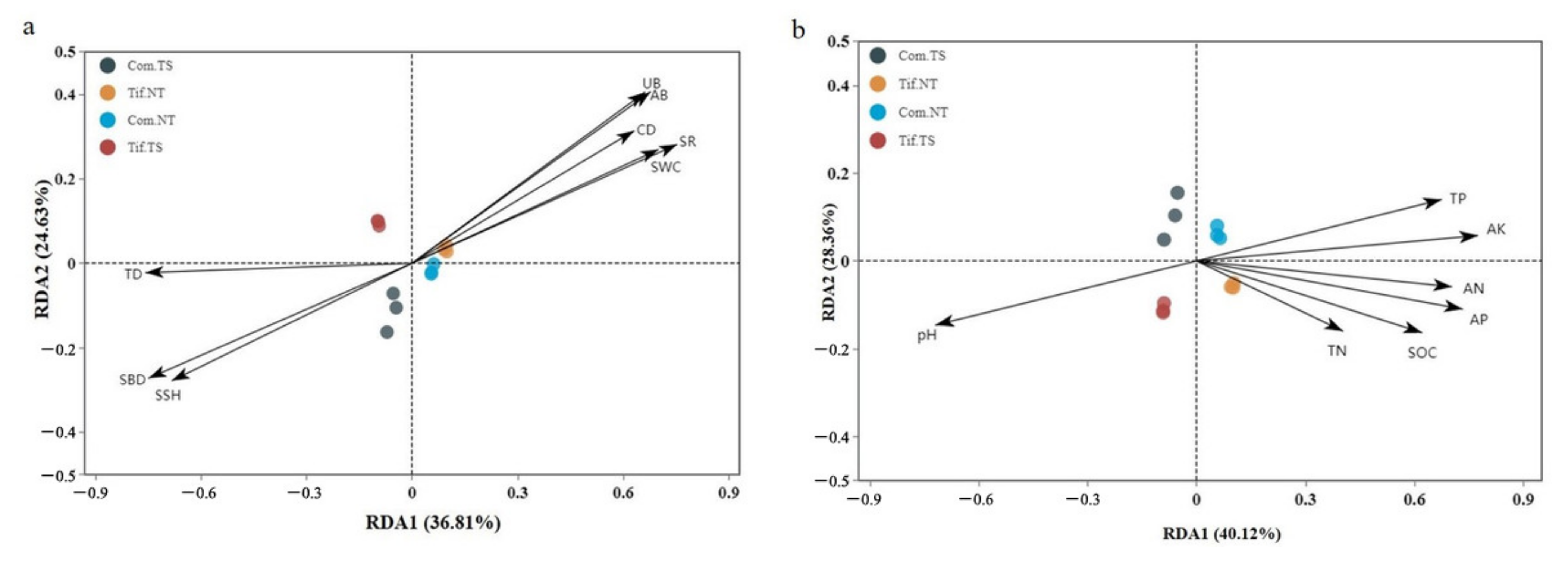
3.4. Analysis of Bacterial Alpha- and Beta-Diversities and Their Relations with Environmental Factors
3.5. LefSe
3.6. Spearman Correlation Analysis
4. Discussion
4.1. Changes in Turf Quality, Bacterial Community Diversity, and Soil Physicochemical Characters under Traffic Stress
4.2. Bacterial Community Structure Correlation with Soil Properties under Traffic Stress
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gląb, T.; Szewczyk, W.; Dubas, E.; Kowalik, K.; Jezierski, T. Anatomical and Morphological Factors Affecting Wear Tolerance of Turfgrass. Sci. Horticul. 2015, 185, 1–13. [Google Scholar] [CrossRef]
- Martiniello, P. Effect of Traffic Stress on Cool-Season Turfgrass under A Mediterranean Climate. Agron. Sust. Dev. 2007, 27, 293–301. [Google Scholar] [CrossRef]
- Wei, H.; Yang, W.; Wang, Y.; Ding, J.; Ge, L.; Richardson, M.; Liu, T.; Zhang, J. Correlations among Soil, Leaf Morphology, and Physiological Factors with Wear Tolerance of Four Warm-season Turfgrass Species. HortScience 2022, 57, 571–580. [Google Scholar] [CrossRef]
- Głąb, T.; Szewczyk, W. The Effect of Traffic on Turfgrass Root Morphological Features. Sci. Horticul. 2015, 197, 542–554. [Google Scholar] [CrossRef]
- Antille, D.L.; Peets, S.; Galambošová, J.; Botta, G.F.; Rataj, V.; Macák, M.; Tullberg, J.N.; Chamen, W.C.; White, D.R.; Misiewicz, P.A.; et al. Soil Compaction and Controlled Traffic Farming in Arable and Grass Cropping Systems. Agron. Res. 2019, 17, 653–682. [Google Scholar] [CrossRef]
- Hiernaux, P.; Bielders, C.L.; Valentin, C.; Bationo, A.; Fernandez-Rivera, S. Effects of Livestock Grazing on Physical and Chemical Properties of Sandy Soils in Sahelian Rangelands. J. Arid Environ. 1999, 41, 231–245. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.; Wang, X.; Zhang, J. Development of A Novel Traffic Simulator and Evaluation of Warm-Season Turfgrass Traffic Tolerance in Field Experiments. Acta Pratacul. Sin. 2019, 28, 41–52. [Google Scholar] [CrossRef]
- Carman, K. Compaction Characteristics of Towed Wheels on Clay Loam in a Soil Bin. Soil Till. Res. 2002, 65, 37–43. [Google Scholar] [CrossRef]
- Franzluebbers, A.; Stuedemann, J.; Schomberg, H. Spatial Distribution of Soil Carbon and Nitrogen Pools under Grazed Tall Fescue. Soil Sci. Soc. Am. J. 2000, 64, 635–639. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.L.; Deng, Y.; Ding, J.Z.; Hu, H.W.; Xu, T.L.; Li, F.; Yang, G.B.; Yang, Y.H. Distinct Microbial Communities in The Active and Permafrost Layers on The Tibetan Plateau. Mol. Ecol. 2017, 26, 6608–6620. [Google Scholar] [CrossRef]
- Singh, J.S.; Raghubanshi, A.S.; Singh, R.S.; Srivastava, S. Microbial Biomass Acts as A Source of Plant Nutrients in Dry Tropical Forest and Savanna. Nature 1989, 338, 499–500. [Google Scholar] [CrossRef]
- Bardgett, R.D.; Van Der Putten, W.H. Belowground Biodiversity and Ecosystem Functioning. Nature 2014, 515, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Baquerizo, M.; Oliverio, A.M.; Brewer, T.E.; Benavent-González, A.; Eldridge, D.J.; Bardgett, R.D.; Maestre, F.T.; Singh, B.K.; Fierer, N. A Global Atlas of the Dominant Bacteria Found in Soil. Science 2018, 359, 320–325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, S.H.; Huang, B.H.; Huang, C.L.; Li, G.; Liao, P.C. The Aboveground Vegetation Type and Underground Soil Property Mediate the Divergence of Soil Microbiomes and The Biological Interactions. Microb. Ecol. 2018, 75, 434–446. [Google Scholar] [CrossRef] [PubMed]
- Bissett, A.; Brown, M.V.; Siciliano, S.D.; Thrall, P.H. Microbial Community Responses to Anthropogenically Induced Environmental Change: Towards A Systems Approach. Ecol. Lett. 2013, 16, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Klimek, B.; Niklińska, M.; Jaźwa, M.; Tarasek, A.; Tekielak, I.; Musielok, Ł. Covariation of Soil Bacteria Functional Diversity and Vegetation Diversity Along an Altitudinal Climatic Gradient in The Western Carpathians. Pedobiologia 2015, 58, 105–112. [Google Scholar] [CrossRef]
- Bukar, M.; Sodipo, O.; Dawkins, K.; Ramirez, R.; Kaldapa, J.T.; Tarfa, M.; Esiobu, M. Microbiomes of Top and Sub-Layers of Semi-Arid Soils in North-Eastern Nigeria Are Rich in Firmicutes and Proteobacteria with Surprisingly High Diversity of Rare Species. Adv. Microbiol. 2019, 9, 102–118. [Google Scholar] [CrossRef] [Green Version]
- Brockett, B.F.; Prescott, C.E.; Grayston, S.J. Soil Moisture is the Major Factor Influencing Microbial Community Structure and Enzyme Activities across Seven Biogeoclimatic Zones in Western Canada. Soil Biol. Biochem. 2012, 44, 9–20. [Google Scholar] [CrossRef]
- Cederlund, H.; Wessén, E.; Enwall, K.; Jones, C.M.; Juhanson, J.; Pell, M.; Philippot, L.; Hallin, S. Soil Carbon Quality and Nitrogen Fertilization Structure Bacterial Communities with Predictable Responses of Major Bacterial Phyla. Appl. Soil Ecol. 2014, 84, 62–68. [Google Scholar] [CrossRef]
- He, W.; Zhang, M.; Jin, G.; Sui, X.; Zhang, T.; Song, F. Effects of Nitrogen Deposition on Nitrogen-Mineralizing Enzyme Activity and Soil Microbial Community Structure in A Korean Pine Plantation. Microb. Ecol. 2021, 81, 410–424. [Google Scholar] [CrossRef]
- Li, J.; Shen, Z.; Li, C.; Kou, Y.; Wang, Y.; Tu, B.; Zhang, S.; Li, X. Stair-Step Pattern of Soil Bacterial Diversity Mainly Driven by pH and Vegetation Types Along the Elevational Gradients of Gongga Mountain, China. Front. Microbiol. 2018, 9, 569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martınez, L.; Zinck, J. Temporal Variation of Soil Compaction and Deterioration of Soil Quality in Pasture Areas of Colombian Amazonia. Soil Till. Res. 2004, 75, 3–18. [Google Scholar] [CrossRef]
- Patkowska, E. Biostimulants Managed Fungal Phytopathogens and Enhanced Activity of Beneficial Microorganisms in Rhizosphere of Scorzonera (Scorzonera hispanica L.). Agriculture 2021, 11, 347. [Google Scholar] [CrossRef]
- Van Wyk, D.A.; Adeleke, R.; Rhode, O.H.; Bezuidenhout, C.C.; Mienie, C. Ecological Guild and Enzyme Activities of Rhizosphere Soil Microbial Communities Associated with Bt-Maize Cultivation Under Field Conditions in North West Province of South Africa. J. Basic Microbiol. 2017, 57, 781–792. [Google Scholar] [CrossRef]
- Cheng, H.; Wu, B.; Wei, M.; Wang, S.; Rong, X.; Du, D.; Wang, C. Changes in Community Structure and Metabolic Function of Soil Bacteria Depending on The Type Restoration Processing in The Degraded Alpine Grassland Ecosystems in Northern Tibet. Sci. Total Environ. 2020, 755, 142619. [Google Scholar] [CrossRef]
- Zhang, J.; Richardson, M.; Karcher, D.; McCalla, J.; Mai, J.; Luo, H. Dormant Sprigging of Bermudagrass and Zoysiagrass. HortTechnology 2021, 31, 395–404. [Google Scholar] [CrossRef]
- Magni, S.; Sportelli, M.; Grossi, N.; Volterrani, M.; Minelli, A.; Pirchio, M.; Fontanelli, M.; Frasconi, C.; Gaetani, M.; Martelloni, L.; et al. Autonomous Mowing and Turf-Type Bermudagrass as Innovations for an Environment-Friendly Floor Management of a Vineyard in Coastal Tuscany. Agriculture 2020, 10, 189. [Google Scholar] [CrossRef]
- Wei, H.; Ding, J.; Zhang, J.; Yang, W.; Wang, Y.; Liu, T. Changes in Soil Fungal Community Structure Under Bermudagrass Turf in Response to Traffic Stress. Acta Pratacul. Sin. 2022, 31, 102–112. [Google Scholar] [CrossRef]
- Olsen, S.R. Estimation of Available Phosphorus in Soils by Extraction with Sodium Bicarbonate; US Department of Agriculture: Washington, DC, USA, 1954.
- Wu, W.; Tang, X.P.; Yang, C.; Liu, H.B.; Guo, N.J. Investigation of Ecological Factors Controlling Quality of Flue-Cured Tobacco (Nicotiana tabacum L.) Using Classification Methods. Ecol. Inform. 2013, 16, 53–61. [Google Scholar] [CrossRef]
- Walkley, A. An Examination of Methods for Determining Organic Carbon and Nitrogen in Soils. (With One Text-figure.). J. Agric. Sci. 1935, 25, 598–609. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME Allows Analysis of High-Throughput Community Sequencing Data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yilmaz, P.; Parfrey, L.W.; Yarza, P.; Gerken, J.; Pruesse, E.; Quast, C.; Schweer, T.; Peplies, J.; Ludwig, W.; Glöckner, F.O. The SILVA and “All-Species Living Tree Project (LTP)” Taxonomic Frameworks. Nucl. Acids Res. 2014, 42, D643–D648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heruth, D.; Xiong, M.; Jiang, X. Microbiome-seq data analysis. In Big Data Analysis for Bioinformatics and Biomedical Discoveries; CRC Press: Boca Raton, FL, USA, 2016; Volume 97, p. 114. [Google Scholar]
- Oksanen, J.; Kindt, R.; Legendre, P.; O’Hara, B.; Stevens, M.H.H.; Oksanen, M.J.; Simpson, G.L.; Solymos, P.; Wagner, H. The vegan package. Comm. Ecol. Pack. 2007, 10, 719. Available online: http://www.R-project.org (accessed on 24 February 2021).
- Love, M.I.; Huber, W.; Anders, S. Moderated Estimation of Fold Change and Dispersion for RNA-seq Data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [Green Version]
- White, J.R.; Nagarajan, N.; Pop, M. Statistical Methods for Detecting Differentially Abundant Features in Clinical Metagenomic Samples. PLoS Comput. Biol. 2009, 5, e1000352. [Google Scholar] [CrossRef]
- Menneer, J.; Ledgard, S.; McLay, C.; Silvester, W. The Effects of Treading by Dairy Cows during Wet Soil Conditions on White Clover Productivity, Growth and Morphology in a White Clover–Perennial Ryegrass Pasture. Grass Forage Sci. 2005, 60, 46–58. [Google Scholar] [CrossRef]
- Malleshaiah, S.; Govindaswamy, V.; Murugaiah, J.; Ganga, M.; Surakshitha, N. Influence of Traffic Stress on Warm Season Turfgrass Species under Simulated Traffic. Indian J. Agric. Sci. 2017, 87, 62–68. [Google Scholar]
- Eilers, K.G.; Lauber, C.L.; Knight, R.; Fierer, N. Shifts in Bacterial Community Structure Associated with Inputs of Low Molecular Weight Carbon Compounds to Soil. Soil Biol. Biochem. 2010, 42, 896–903. [Google Scholar] [CrossRef]
- Hamza, M.; Anderson, W. Soil Compaction in Cropping Systems: A Review of The Nature, Causes and Possible Solutions. Soil Till. Res. 2005, 82, 121–145. [Google Scholar] [CrossRef]
- Jaqueth, A.L.; Turner, T.R.; Iwaniuk, M.E.; McIntosh, B.J.; Burk, A.O. Relative Traffic Tolerance of Warm-Season Grasses and Suitability for Grazing by Equine. J. Equine Vet. Sci. 2019, 103, 103244. [Google Scholar] [CrossRef]
- Zhang, Y.; Cao, C.; Peng, M.; Xu, X.; Zhang, P.; Yu, Q.; Sun, T. Diversity of Nitrogen-Fixing, Ammonia-Oxidizing, And Denitrifying Bacteria in Biological Soil Crusts of A Revegetation Area In Horqin Sandy Land, Northeast China. Ecol. Eng. 2014, 71, 71–79. [Google Scholar] [CrossRef]
- Cornwell, W.K.; Cornelissen, J.H.; Amatangelo, K.; Dorrepaal, E.; Eviner, V.T.; Godoy, O.; Hobbie, S.E.; Hoorens, B.; Kurokawa, H.; Pérez-Harguindeguy, N.; et al. Plant Species Traits Are the Predominant Control on Litter Decomposition Rates within Biomes Worldwide. Ecol. Lett. 2008, 11, 1065–1071. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.Z.; Okubo, A.; Sugiyama, S.I. Effects of Grassland Species on Decomposition of Litter and Soil Microbial Communities. Ecol. Res. 2010, 25, 255–261. [Google Scholar] [CrossRef]
- Pornaro, C.; Barolo, E.; Rimi, F.; Macolino, S.; Richardson, M. Performance of Various Cool-Season Turfgrasses as Influenced by Simulated Traffic in Northeastern Italy. Eur. J. Hortic. Sci. 2016, 81, 27–36. [Google Scholar] [CrossRef]
- Wardle, D.A.; Bardgett, R.D.; Klironomos, J.N.; Setälä, H.; Van Der Putten, W.H.; Wall, D.H. Ecological Linkages between Aboveground and Belowground Biota. Science 2004, 304, 1629–1633. [Google Scholar] [CrossRef] [PubMed]
- Trenholm, L.; Carrow, R.; Duncan, R. Mechanisms of Wear Tolerance in Seashore Paspalum and Bermudagrass. Crop Sci. 2000, 40, 1350–1357. [Google Scholar] [CrossRef]
- Frank, D.A.; McNaughton, S. The Ecology of Plants, Large Mammalian Herbivores, and Drought in Yellowstone National Park. Ecology 1992, 73, 2043–2058. [Google Scholar] [CrossRef]
- Ezeokoli, O.T.; Mashigo, S.K.; Paterson, D.G.; Bezuidenhout, C.C.; Adeleke, R.A. Microbial Community Structure and Relationship with Physicochemical Properties of Soil Stockpiles in Selected South African Opencast Coal Mines. Soil Sci. Plant Nutr. 2019, 65, 332–341. [Google Scholar] [CrossRef]
- Cao, J.; Gong, Y.; Yeh, E.T.; Holden, N.M.; Adamowski, J.F.; Deo, R.C.; Liu, M.; Zhou, J.; Zhang, J.; Zhang, W.; et al. Impact of grassland contract policy on soil organic carbon losses from alpine grassland on the Qinghai-Tibetan Plateau. Soil Use Manag. 2017, 33, 663–671. [Google Scholar] [CrossRef]
- Papp, M.; Fóti, S.; Nagy, Z.; Pintér, K.; Posta, K.; Fekete, S.; Csintalan, Z.; Balogh, J. Rhizospheric, Mycorrhizal and Heterotrophic Respiration in Dry Grasslands. Eur. J. Soil Biol. 2018, 85, 43–52. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, D.; Shi, B.; Sun, W. Differential Effects of Grazing, Water, And Nitrogen Addition on Soil Respiration and Its Components in a Meadow Steppe. Plant Soil. 2020, 447, 581–598. [Google Scholar] [CrossRef]
- Gong, J.; Ge, Z.; An, R.; Duan, Q.; You, X.; Huang, Y. Soil Respiration in Poplar Plantations in Northern China at Different Forest Ages. Plant Soil 2012, 360, 109–122. [Google Scholar] [CrossRef]
- Sogin, M.L.; Morrison, H.G.; Huber, J.A.; Welch, D.M.; Huse, S.M.; Neal, P.R.; Arrieta, J.M.; Herndl, G.J. Microbial diversity in the deep sea and the underexplored “rare biosphere”. Proc. Natl. Acad. Sci. USA 2006, 103, 12115–12120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tripathi, B.M.; Kim, M.; Singh, D.; Lee-Cruz, L.; Lai-Hoe, A.; Ainuddin, A.; Raha Abdul Rahim, R.; Husni, M.H.A.; Chun, J.; Adams, J.M. Tropical Soil Bacterial Communities in Malaysia: pH Dominates in The Equatorial Tropics Too. Microb. Ecol. 2012, 64, 474–484. [Google Scholar] [CrossRef] [PubMed]
- Bulgarelli, D.; Schlaeppi, K.; Spaepen, S.; Van, T.; Emiel, V.L.; Schulze-Lefert, P. Structure and Functions of the Bacterial Microbiota of Plants. Annu. Rev. Plant. Biol. 2013, 64, 807–838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eichorst, S.A.; Trojan, D.; Roux, S.; Herbold, C.; Rattei, T.; Woebken, D. Genomic Insights into The Acidobacteria Reveal Strategies for Their Success in Terrestrial Environments. Environ. Microbiol. 2018, 20, 1041–1063. [Google Scholar] [CrossRef] [Green Version]
- Da, W.; Rd, B.; Jn, K. Ecological linkages between above ground and below ground biota. Science 2004, 304, 1629–1633. [Google Scholar] [CrossRef]
- Neina, D. The Role of Soil pH in Plant Nutrition and Soil Remediation. Appl. Environ. Soil Sci. 2019, 2019, 1–9. [Google Scholar] [CrossRef]
- Dai, Z.; Su, W.; Chen, H.; Barberán, A.; Zhao, H.; Yu, M.; Yu, L.; Brookes, P.C.; Schadt, C.W.; Chang, X.S.; et al. Long-term Nitrogen Fertilization Decreases Bacterial Diversity and Favors the Growth of Actinobacteria and Proteobacteria in Agro-Ecosystems Across the Globe. Glob. Change Biol. 2018, 24, 3452–3461. [Google Scholar] [CrossRef]
- Jiao, S.; Lu, Y. Soil pH and Temperature Regulate Assembly Processes of Abundant and Rare Bacterial Communities in Agricultural Ecosystems. Environ. Microbiol. 2020, 22, 1052–1065. [Google Scholar] [CrossRef]
- Lauber, C.L.; Mady, M.; Knight, R.; Fierer, N. Pyrosequencing-Based Assessment of Soil pH as A Predictor of Soil Bacterial Community Structure at The Continental Scale. Appl. Environ. Microbiol. 2009, 75, 5111–5120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foesel, B.U.; Nägele, V.; Naether, A.; Wüst, P.K.; Weinert, J.; Bonkowski, M.; Lohaus, G.; Polle, A.; Alt, F.; Oelmann, Y.; et al. Determinants of Acidobacteria Activity Inferred from The Relative Abundances of 16S RRNA Transcripts in German Grassland and Forest Soils. Environ. Microbiol. 2014, 16, 658–675. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.T.; Robeson, M.S.; Lauber, C.L.; Hamady, M.; Knight, R.; Fierer, N. A Comprehensive Survey of Soil Acidobacterial Diversity Using Pyrosequencing and Clone Library Analyses. ISME J. 2009, 3, 442–453. [Google Scholar] [CrossRef]
- Fierer, N.; Bradford, M.A.; Jackson, R.B. Toward an Ecological Classification of Soil Bacteria. Ecology 2007, 88, 1354–1364. [Google Scholar] [CrossRef]
- Bledsoe, R.B.; Goodwillie, C.; Peralta, A.L. Long-term Nutrient Enrichment of an Oligotroph-Dominated Wetland Increases Bacterial Diversity in Bulk Soils and Plant Rhizospheres. Msphere 2020, 5, e00035-20. [Google Scholar] [CrossRef]
- Radajewski, S.; Duxbury, T. Motility Responses and Desiccation Survival of Zoospores from the Actinomycete Kineosporia sp. Strain SR11. Microb. Ecol. 2001, 41, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hu, N.; Ge, T.; Kuzyakov, Y.; Wang, Z.L.; Li, Z.; Tang, Z.; Chen, Y.; Wu, C.; Lou, Y. Soil Aggregation Regulates Distributions of Carbon, Microbial Community and Enzyme Activities after 23-Year Manure Amendment. Appl. Soil Ecol. 2017, 111, 65–72. [Google Scholar] [CrossRef]
- Ivanova, A.A.; Zhelezova, A.D.; Chernov, T.I.; Dedysh, S.N. Linking Ecology and Systematics of Acidobacteria: Distinct Habitat Preferences of the Acidobacteriia and Blastocatellia in Tundra Soils. PLoS ONE 2020, 15, e0230157. [Google Scholar] [CrossRef] [Green Version]
- Goldford, J.E.; Lu, N.; Bajic, D.; Estrela, S.; Tikhonov, M.; Sanchez-Gorostiaga, A.; Segrè, D.; Mehta, P.; Sanchez, A. Emergent Simplicity in Microbial Community Assembly. Science 2018, 361, 469–474. [Google Scholar] [CrossRef] [Green Version]





| Soil Properties | Tif.TS | Tif.NT | Com.TS | Com.NT |
|---|---|---|---|---|
| SSH (g) | 120.67 ± 1.45 b | 87.67 ± 1.45 c | 142.33 ± 2.33 a | 78.67 ± 2.62 d |
| SBD (g/cm3) | 1.62 ± 0.26 a | 1.28 ± 0.23 b | 1.73 ± 0.18 a | 1.35 ± 0.14 b |
| SWC (%) | 55.93 ± 1.36 b | 66.10 ± 1.11 a | 50.76 ± 1.52 c | 67.92 ± 1.18 a |
| pH | 6.83 ± 0.17 a | 6.35 ± 0.21 b | 6.79 ± 0.14 a | 6.07 ± 0.12 c |
| TN (g·kg−1) | 1.50 ± 0.06 a | 1.53 ± 0.13 a | 1.48 ± 0.03 a | 1.57 ± 0.14 a |
| TP (g·kg−1) | 0.82 ± 0.03 b | 0.87 ± 0.01 a | 0.83 ± 0.02 b | 0.88 ± 0.05 a |
| AN (mg·kg−1) | 11.28 ± 0.20 b | 12.14 ± 0.16 a | 11.12 ± 0.13 b | 12.05 ± 0.22 a |
| AP (mg·kg−1) | 45.39 ± 0.58 b | 49.08 ± 0.31 a | 44.28 ± 0.57 b | 49.15 ± 0.58 a |
| AK (mg·kg−1) | 240.33 ± 0.95 b | 250.47 ± 1.25 a | 240.48 ± 1.72 b | 249.12 ± 0.90 a |
| SOC (g·kg−1) | 15.76 ± 0.06 b | 16.21 ± 0.16 a | 15.61 ± 0.15 b | 16.23 ± 0.08 a |
| SR (μmol·m−2·s−1) | 4.58 ± 0.83 c | 6.85 ± 0.82 a | 3.78 ± 0.48 d | 6.50 ± 0.73 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, H.; Wang, Y.; Zhang, J.; Ge, L.; Liu, T. Changes in Soil Bacterial Community Structure in Bermudagrass Turf under Short-Term Traffic Stress. Agriculture 2022, 12, 668. https://doi.org/10.3390/agriculture12050668
Wei H, Wang Y, Zhang J, Ge L, Liu T. Changes in Soil Bacterial Community Structure in Bermudagrass Turf under Short-Term Traffic Stress. Agriculture. 2022; 12(5):668. https://doi.org/10.3390/agriculture12050668
Chicago/Turabian StyleWei, Hongjian, Yongqi Wang, Juming Zhang, Liangfa Ge, and Tianzeng Liu. 2022. "Changes in Soil Bacterial Community Structure in Bermudagrass Turf under Short-Term Traffic Stress" Agriculture 12, no. 5: 668. https://doi.org/10.3390/agriculture12050668
APA StyleWei, H., Wang, Y., Zhang, J., Ge, L., & Liu, T. (2022). Changes in Soil Bacterial Community Structure in Bermudagrass Turf under Short-Term Traffic Stress. Agriculture, 12(5), 668. https://doi.org/10.3390/agriculture12050668






