Energy and Exergy Analysis of an Ammonia Fuel Cell Integrated System for Marine Vessels
Abstract
:1. Introduction
- ▪
- A novel integrated system with ammonia as the fuel in an SOFC for significantly improving the energy-conversion efficiency and green energy target for marine vessels is proposed. The SRC and EGB are integrated to serially recover the waste heat from the exhaust gas from the SOFC using an energy-cascade method.
- ▪
- A thermodynamic model of the components, sub-system, and total system is built to estimate the working performance of the proposed system.
- ▪
- A parametric study is carried out to investigate the impact of the main parameters on system performance.
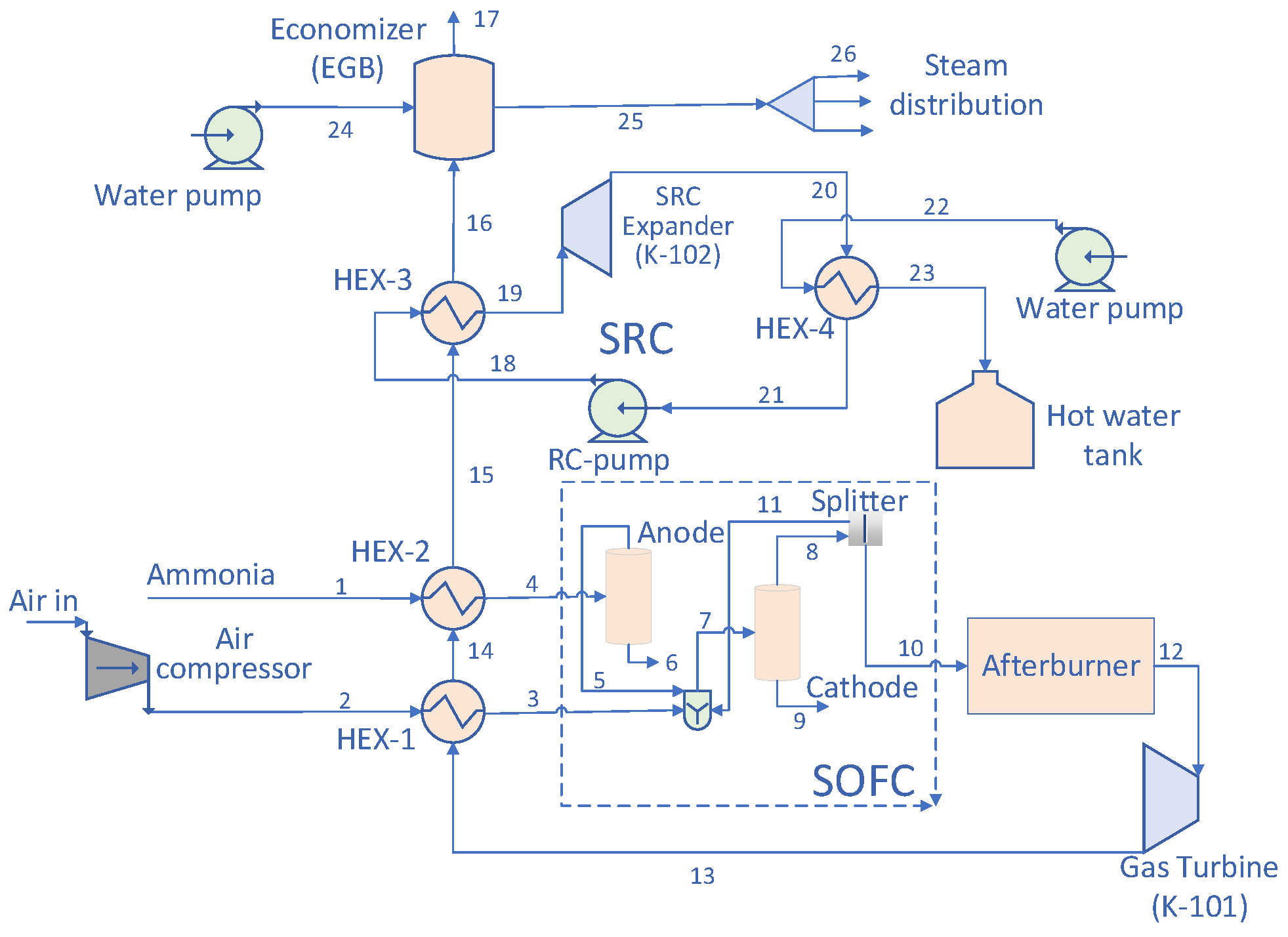
2. Process Modelling of the Power Generation Plant for the Vessel
2.1. Design
2.2. Operating Data Performances and Assumptions
- The supplied air is composed of 79% N2 and 21% O2 at 29.85 °C, 101.3 kPa;
- This simulation is accomplished in a steady state and thermodynamic equilibrium;
- The mass and pressure losses in all pipe components are negligible;
- The pressure losses on the tube side and shell side of the heat exchanger (HEX) are assumed to be 6.895 and 3.447 kPa, respectively;
- The flow temperatures of the air and fuel at the inlet and outlet of the SOFC are constant and equal to the working temperature of the SOFC.
3. Material and Methodology
3.1. Model of the SOFC
3.1.1. Fuel and Oxidant Utilization
3.1.2. Afterburner
3.2. Model of the GT
GT
3.3. Model of the SRC
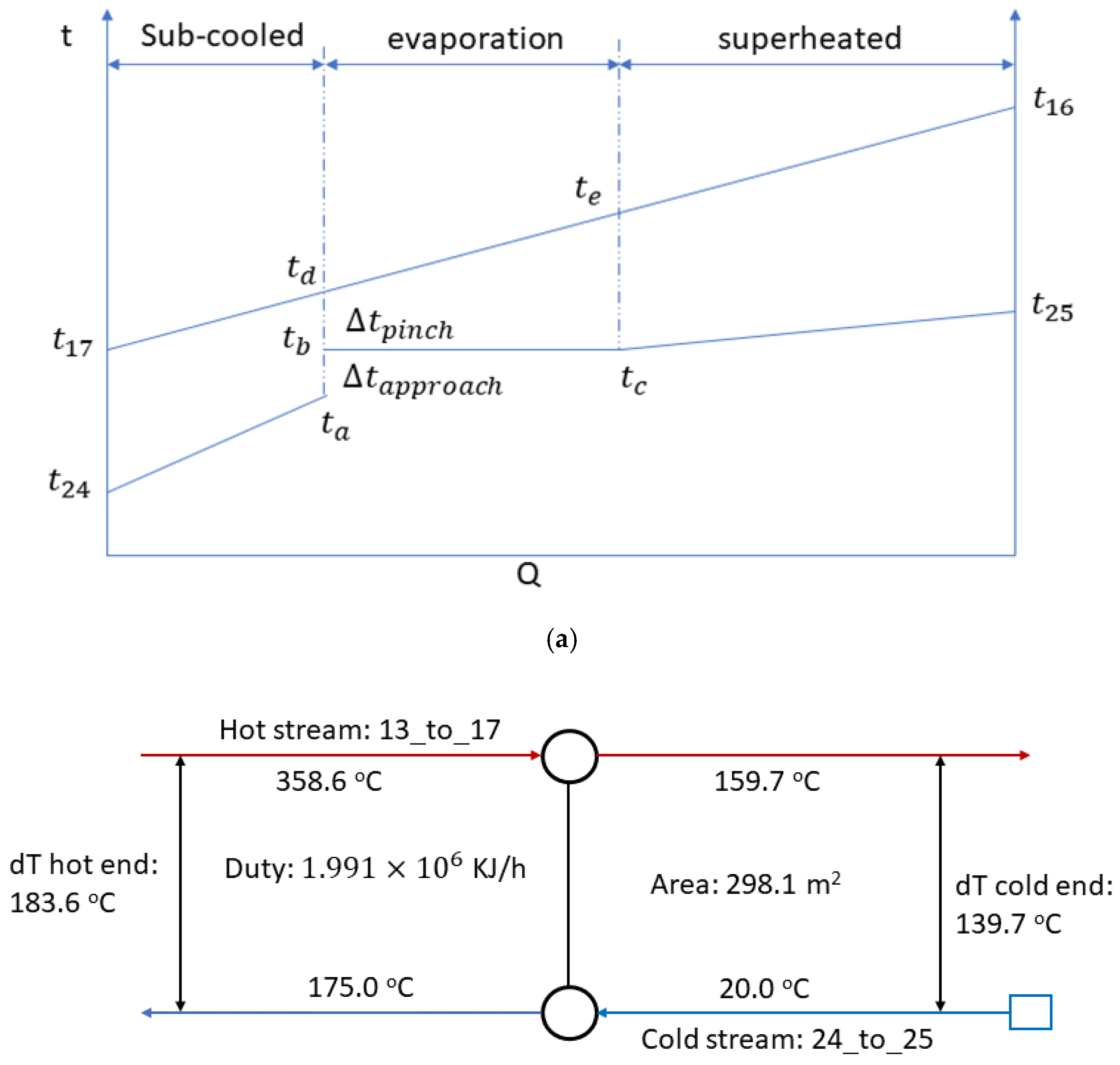
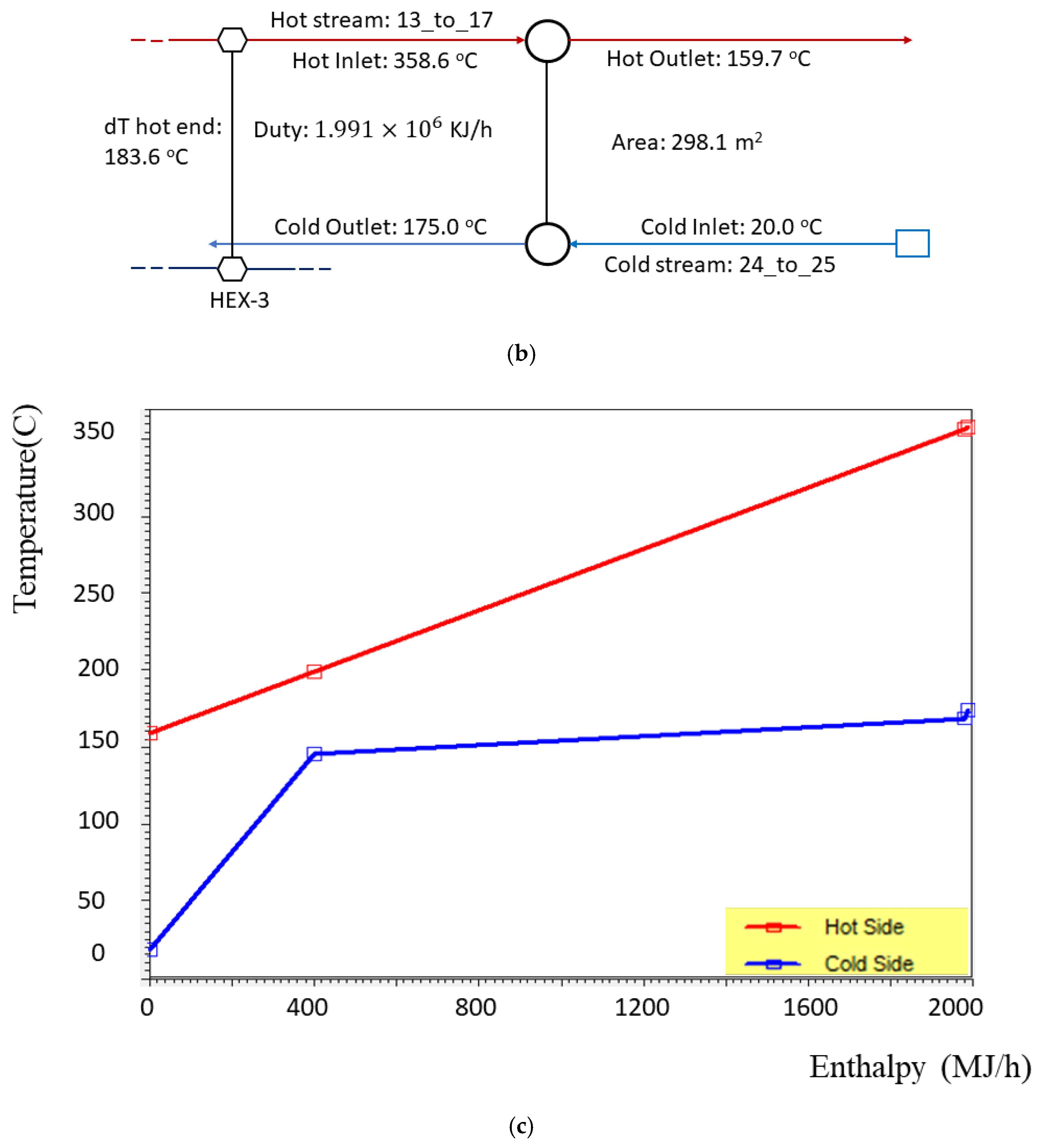
3.4. Model of the EGB
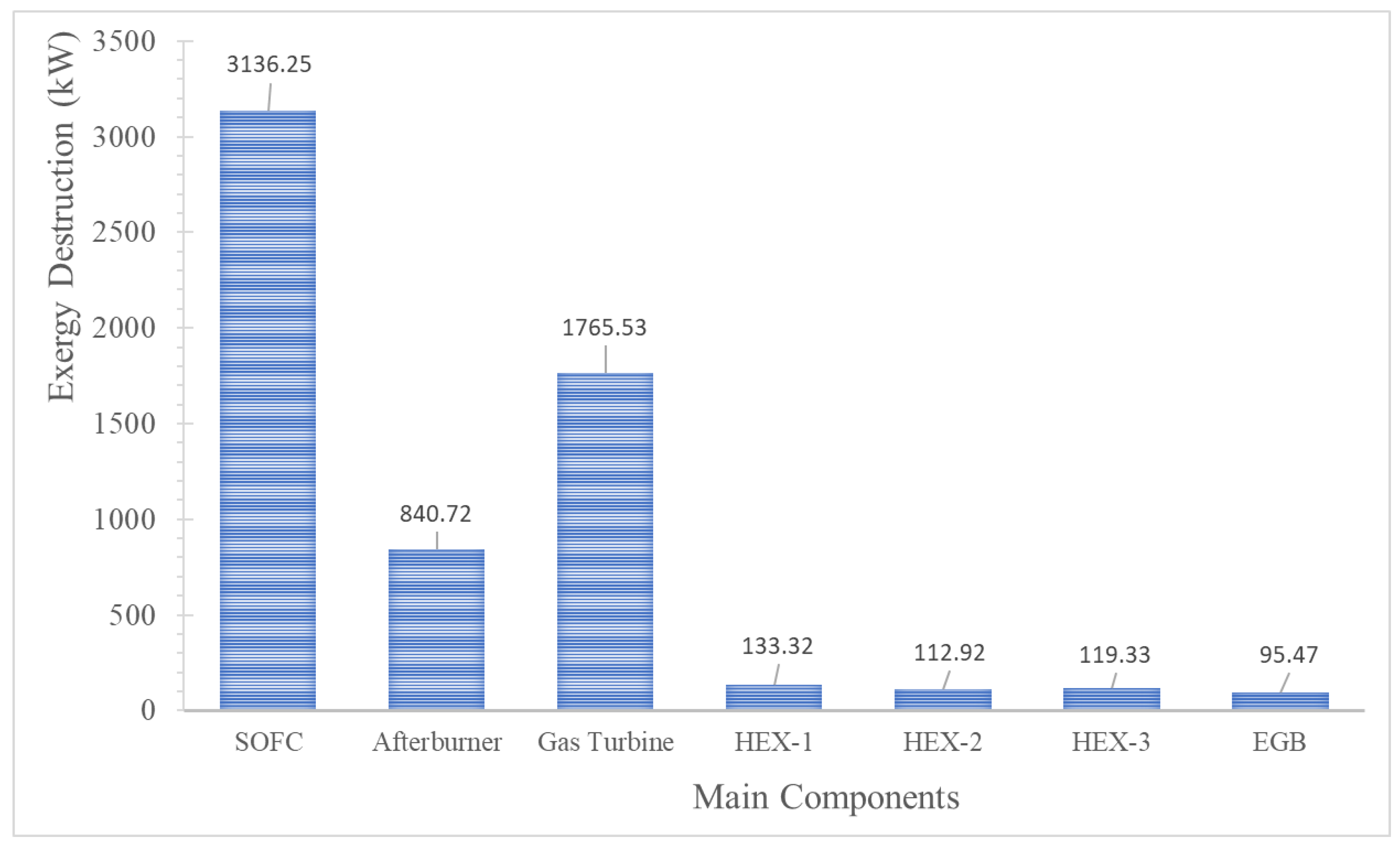
3.5. Exergy Efficiency of the Main Components
4. Model Verification
5. Results and Discussion
5.1. Energy and Exergy Efficiency of System
5.2. Influence of the Key Parameters on the System’s Performance
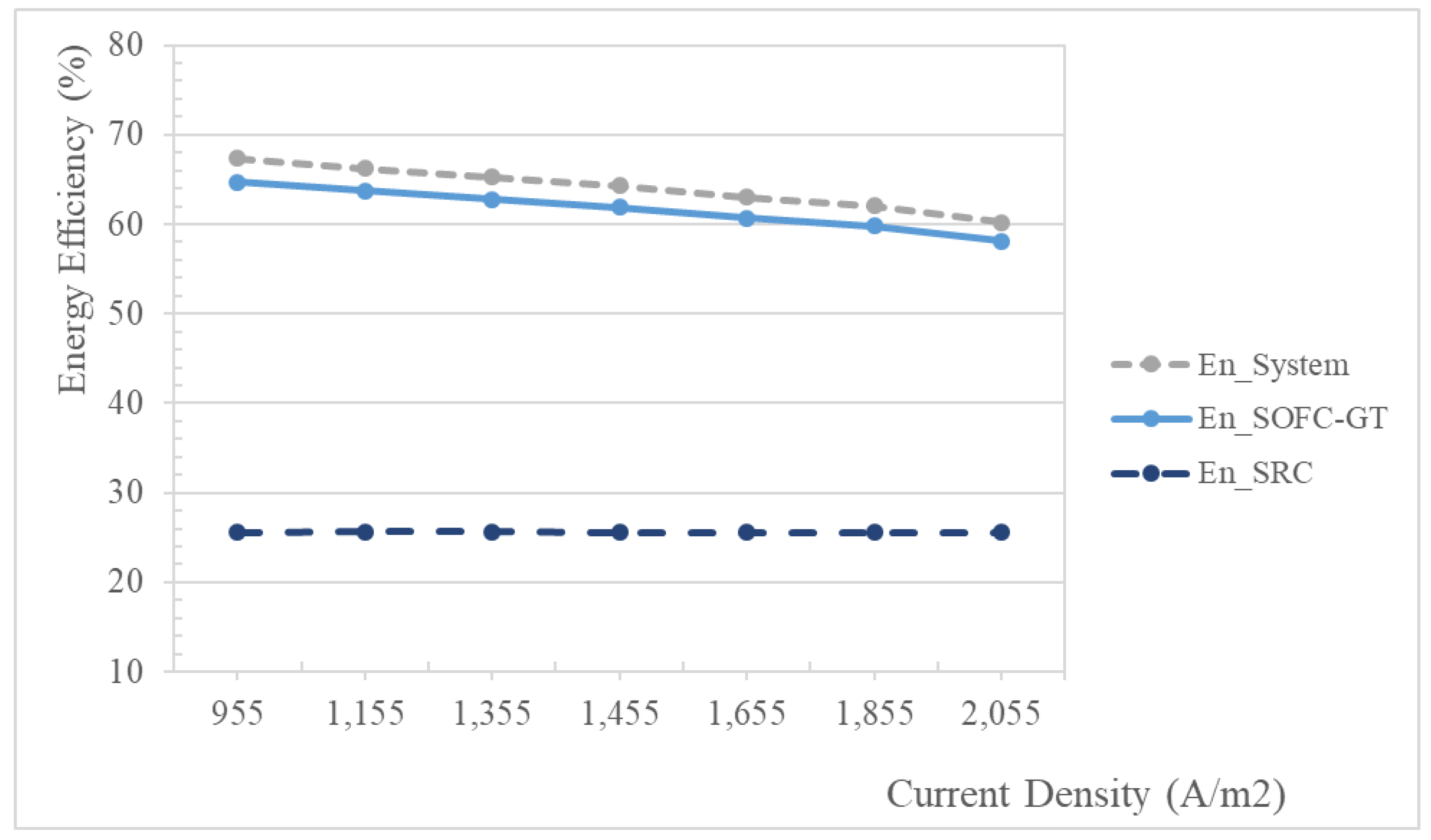
5.2.1. Effect of Current Density
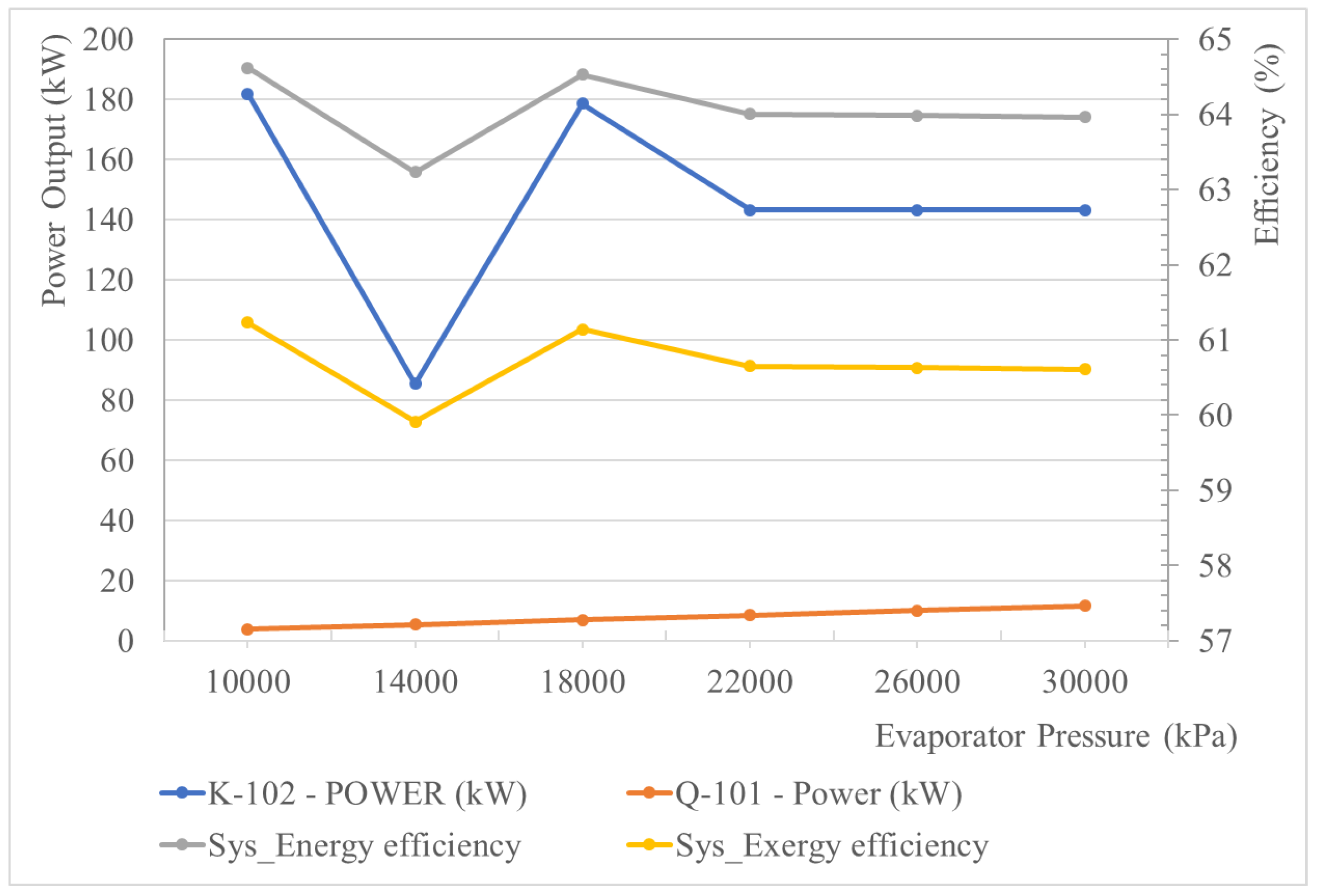
5.2.2. Effect of the Turbine Inlet Pressure of the SRC
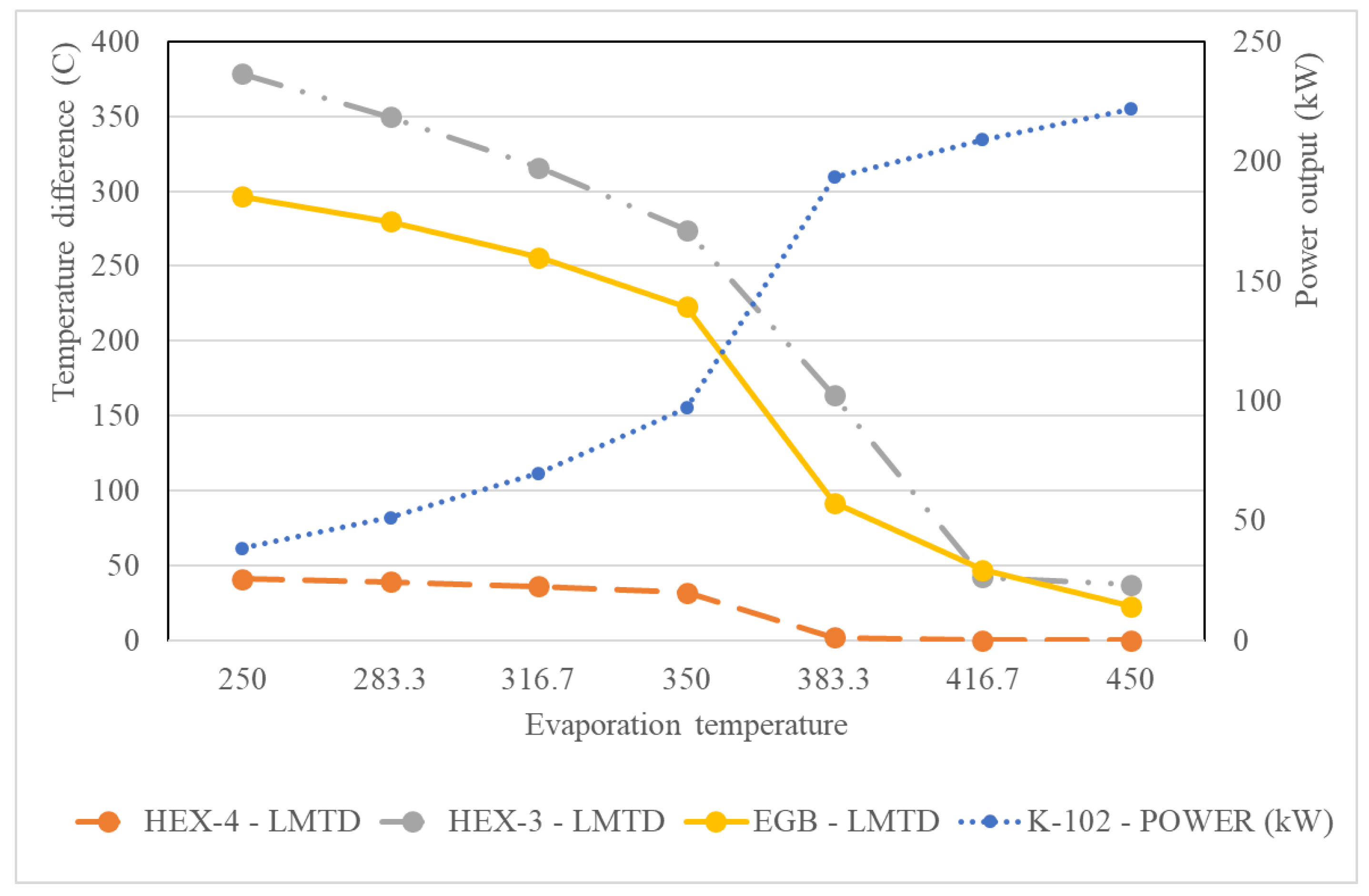
5.2.3. Effect of the Evaporation Temperature of the SRC
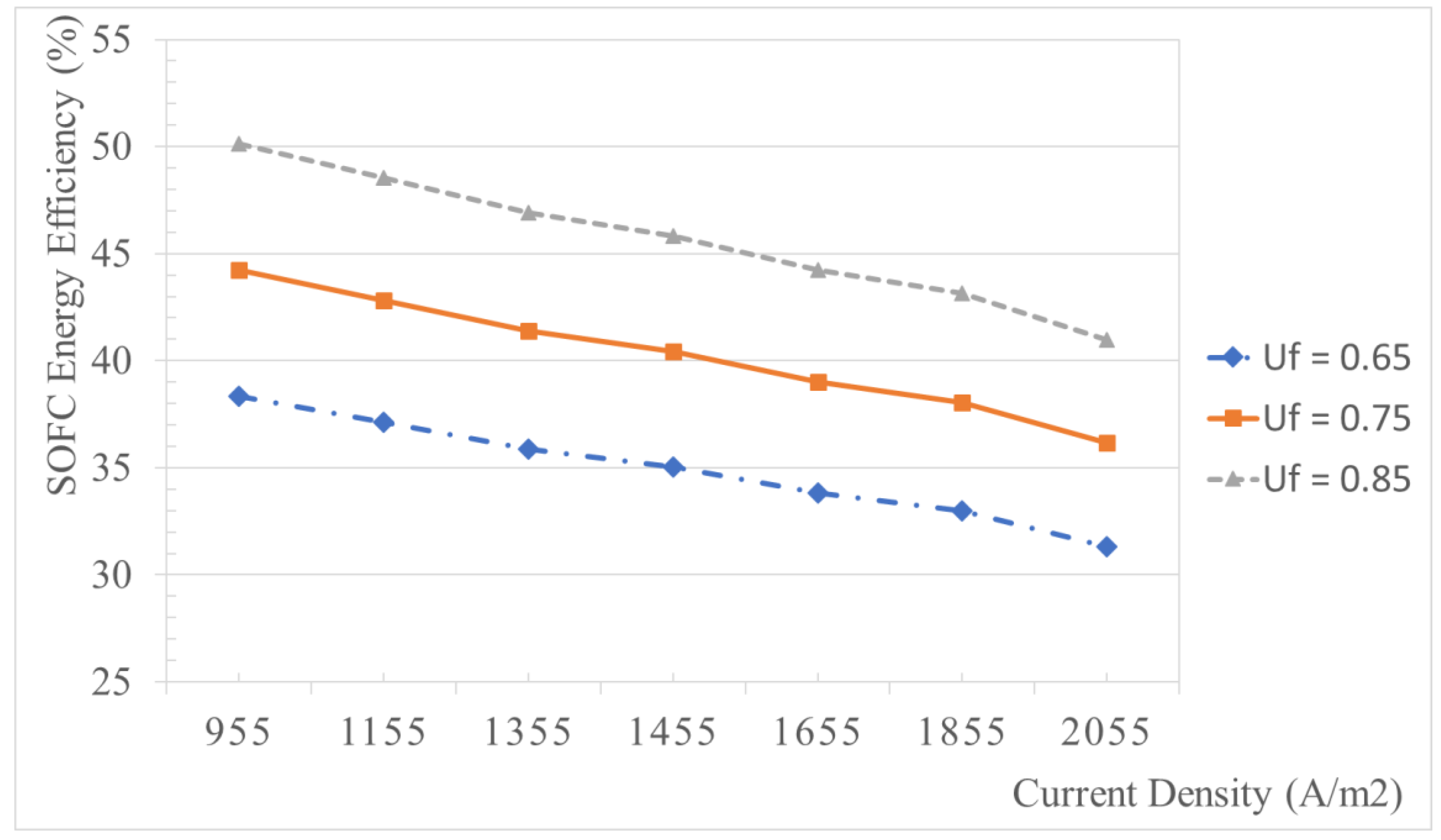
5.2.4. Effect of the Fuel Utilization Factor
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Minutillo, M.; Perna, A.; Di Trolio, P.; Di Micco, S.; Jannelli, E. Techno-economics of novel refueling stations based on ammonia-to-hydrogen route and SOFC technology. Int. J. Hydrogen Energy 2021, 46, 10059–10071. [Google Scholar] [CrossRef]
- IMO. MEPC Fourth IMO Greenhouse Gas Study. Int. Marit. Organ. 2021, 11, 951–952. [Google Scholar]
- Hu, Q.; Zhou, W.; Diao, F. Interpretation of Initial IMO Strategy on Reduction of GHG Emissions from Ships. Sh. Build. China 2019, 60, 195–201. [Google Scholar]
- Zhu, M.; Yuen, K.F.; Ge, J.W.; Li, K.X. Impact of maritime emissions trading system on fleet deployment and mitigation of CO2 emission. Transp. Res. Part D Transp. Environ. 2018, 62, 474–488. [Google Scholar] [CrossRef]
- Rathore, S.S.; Biswas, S.; Fini, D.; Kulkarni, A.P.; Giddey, S. Direct ammonia solid-oxide fuel cells: A review of progress and prospects. Int. J. Hydrogen Energy 2021, 46, 35365–35384. [Google Scholar] [CrossRef]
- Wan, Z.; Tao, Y.; Shao, J.; Zhang, Y.; You, H. Ammonia as an effective hydrogen carrier and a clean fuel for solid oxide fuel cells. Energy Convers. Manag. 2021, 228, 113729. [Google Scholar] [CrossRef]
- Baniasadi, E.; Dincer, I. Energy and exergy analyses of a combined ammonia-fed solid oxide fuel cell system for vehicular applications. Int. J. Hydrogen Energy 2011, 36, 11128–11136. [Google Scholar] [CrossRef]
- van Biert, L.; Godjevac, M.; Visser, K.; Aravind, P.V. A review of fuel cell systems for maritime applications. J. Power Sources 2016, 327, 345–364. [Google Scholar] [CrossRef] [Green Version]
- Jeerh, G.; Zhang, M.; Tao, S. Recent progress in ammonia fuel cells and their potential applications. J. Mater. Chem. A 2020, 9, 727–752. [Google Scholar] [CrossRef]
- Chiong, M.C.; Chong, C.T.; Ng, J.H.; Mashruk, S.; Chong, W.W.F.; Samiran, N.A.; Mong, G.R.; Valera-Medina, A. Advancements of combustion technologies in the ammonia-fuelled engines. Energy Convers. Manag. 2021, 244, 114460. [Google Scholar] [CrossRef]
- Miyaoka, H.; Miyaoka, H.; Ichikawa, T.; Ichikawa, T.; Kojima, Y. Highly purified hydrogen production from ammonia for PEM fuel cell. Int. J. Hydrogen Energy 2018, 43, 14486–14492. [Google Scholar] [CrossRef]
- Laval, A.; Haldor, T.; Siemens, G. Ammonfuel-an industrial view of ammonia as a marine fuel. Hafnia 2020, 7, 32–59. [Google Scholar]
- Cinti, G.; Barelli, L.; Bidini, G. The use of ammonia as a fuel for transport: Integration with solid oxide fuel cells. AIP Conf. Proc. 2019, 2191, 020048. [Google Scholar] [CrossRef]
- Parikhani, T.; Azariyan, H.; Behrad, R.; Ghaebi, H.; Jannatkhah, J. Thermodynamic and thermoeconomic analysis of a novel ammonia-water mixture combined cooling, heating, and power (CCHP) cycle. Renew. Energy 2020, 145, 1158–1175. [Google Scholar] [CrossRef]
- Wojcik, A.; Middleton, H.; Damopoulos, I.; Van Herle, J. Ammonia as a fuel in solid oxide fuel cells. J. Power Sources 2003, 118, 342–348. [Google Scholar] [CrossRef]
- Ma, Q.; Peng, R.; Lin, Y.; Gao, J.; Meng, G. A high-performance ammonia-fueled solid oxide fuel cell. J. Power Sources 2006, 161, 95–98. [Google Scholar] [CrossRef]
- Al-Hamed, K.H.M.; Dincer, I. A novel ammonia solid oxide fuel cell-based powering system with on-board hydrogen production for clean locomotives. Energy 2021, 220, 119771. [Google Scholar] [CrossRef]
- Ishak, F.; Dincer, I.; Zamfirescu, C. Thermodynamic analysis of ammonia-fed solid oxide fuel cells. J. Power Sources 2012, 202, 157–165. [Google Scholar] [CrossRef]
- Ishak, F.; Dincer, I.; Zamfirescu, C. Energy and exergy analyses of direct ammonia solid oxide fuel cell integrated with gas turbine power cycle. J. Power Sources 2012, 212, 73–85. [Google Scholar] [CrossRef]
- Siddiqui, O.; Dincer, I. Analysis and performance assessment of a new solar-based multigeneration system integrated with ammonia fuel cell and solid oxide fuel cell-gas turbine combined cycle. J. Power Sources 2017, 370, 138–154. [Google Scholar] [CrossRef]
- Lee, H.; Jung, I.; Roh, G.; Na, Y.; Kang, H. Comparative analysis of on-board methane and methanol reforming systems combined with HT-PEM fuel cell and CO2 capture/liquefaction system for hydrogen fueled ship application. Energies 2020, 13, 224. [Google Scholar] [CrossRef] [Green Version]
- Nafees, A.; Rasid, R.A. Study of natural gas powered solid oxide fuel cell simulation and modeling. IOP Conf. Ser. Mater. Sci. Eng. 2019, 702, 012017. [Google Scholar] [CrossRef]
- Yoon-Ho, L. Thermo-economic analysis of a novel regasification system with liquefied-natural-gas cold-energy. Int. J. Refrig. 2019, 101, 218–229. [Google Scholar] [CrossRef]
- Song, J.; Gu, C. wei Performance analysis of a dual-loop organic Rankine cycle (ORC) system with wet steam expansion for engine waste heat recovery. Appl. Energy 2015, 156, 280–289. [Google Scholar] [CrossRef]
- Razmi, A.R.; Janbaz, M. Exergoeconomic assessment with reliability consideration of a green cogeneration system based on compressed air energy storage (CAES). Energy Convers. Manag. 2020, 204, 112320. [Google Scholar] [CrossRef]
- Ezzat, M.F.; Dincer, I. Energy and exergy analyses of a novel ammonia combined power plant operating with gas turbine and solid oxide fuel cell systems. Energy 2020, 194, 116750. [Google Scholar] [CrossRef]
- Lan, R.; Tao, S. Ammonia as a suitable fuel for fuel cells. Front. Energy Res. 2014, 2, 3–6. [Google Scholar] [CrossRef] [Green Version]
- Larminie, J.; Dicks, A.; McDonald, M.S. Fuel Cell Systems Explained, 2nd ed.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2003; ISBN 047084857X. [Google Scholar]
- Ma, Q.; Peng, R.R.; Tian, L.; Meng, G. Direct utilization of ammonia in intermediate-temperature solid oxide fuel cells. Electrochem. Commun. 2006, 8, 1791–1795. [Google Scholar] [CrossRef]
- Cui, Z.; Wang, J.; Lior, N. Thermodynamic Analysis of a Solid Oxide Fuel Cell Based Combined Cooling, Heating, and Power System Integrated with Biomass Gasification. Entropy 2021, 23, 1029. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, O.; Dincer, I. A review and comparative assessment of direct ammonia fuel cells. Therm. Sci. Eng. Prog. 2018, 5, 568–578. [Google Scholar] [CrossRef]
- Perna, A.; Minutillo, M.; Jannelli, E.; Cigolotti, V.; Nam, S.W.; Han, J. Design and performance assessment of a combined heat, hydrogen and power (CHHP) system based on ammonia-fueled SOFC. Appl. Energy 2018, 231, 1216–1229. [Google Scholar] [CrossRef]
- Eveloy, V.; Karunkeyoon, W.; Rodgers, P.; Al Alili, A. Energy, exergy and economic analysis of an integrated solid oxide fuel cell—gas turbine—organic Rankine power generation system. Int. J. Hydrogen Energy 2016, 41, 13843–13858. [Google Scholar] [CrossRef]
- Song, M.; Zhuang, Y.; Zhang, L.; Li, W.; Du, J.; Shen, S. Thermodynamic performance assessment of SOFC-RC-KC system for multiple waste heat recovery. Energy Convers. Manag. 2021, 245, 114579. [Google Scholar] [CrossRef]
- Liu, Y.; Han, J.; You, H. Performance analysis of a CCHP system based on SOFC/GT/CO2 cycle and ORC with LNG cold energy utilization. Int. J. Hydrogen Energy 2019, 44, 29700–29710. [Google Scholar] [CrossRef]
- Chitgar, N.; Moghimi, M. Design and evaluation of a novel multi-generation system based on SOFC-GT for electricity, fresh water and hydrogen production. Energy 2020, 197, 117162. [Google Scholar] [CrossRef]
- Ghorbani, S.; Khoshgoftar-Manesh, M.H.; Nourpour, M.; Blanco-Marigorta, A.M. Exergoeconomic and exergoenvironmental analyses of an integrated SOFC-GT-ORC hybrid system. Energy 2020, 206, 118151. [Google Scholar] [CrossRef]
- Zhao, H.; Hou, X.; Yang, Q. Thermodynamic Study and Exergetic Analysis of the Integrated SOFC-GT-Kalina Power Cycle. Energy Power Eng. 2018, 10, 43–64. [Google Scholar] [CrossRef] [Green Version]
- Fuerte, A.; Valenzuela, R.X.; Escudero, M.J.; Daza, L. Ammonia as efficient fuel for SOFC. J. Power Sources 2009, 192, 170–174. [Google Scholar] [CrossRef]
- Amiri, T.; Singh, K.; Sandhu, N.K.; Hanifi, A.R.; Etsell, T.H.; Luo, J.-L.; Thangadurai, V.; Sarkar, P. High Performance Tubular Solid Oxide Fuel Cell Based on Ba 0.5 Sr 0.5 Ce 0.6 Zr 0.2 Gd 0.1 Y 0.1 O 3-δ Proton Conducting Electrolyte. J. Electrochem. Soc. 2018, 165, F764–F769. [Google Scholar] [CrossRef] [Green Version]
- Milewski, J.; Szczęśniak, A.; Szabłowski, Ł. A proton conducting solid oxide fuel cell—implementation of the reduced order model in available software and verification based on experimental data. J. Power Sources 2021, 502, 229948. [Google Scholar] [CrossRef]
- Mehrpooya, M.; Dehghani, H.; Ali Moosavian, S.M. Optimal design of solid oxide fuel cell, ammonia-water single effect absorption cycle and Rankine steam cycle hybrid system. J. Power Sources 2016, 306, 107–123. [Google Scholar] [CrossRef]
- Peters, R.; Deja, R.; Engelbracht, M.; Frank, M.; Nguyen, V.N.; Blum, L.; Stolten, D. Efficiency analysis of a hydrogen-fueled solid oxide fuel cell system with anode off-gas recirculation. J. Power Sources 2016, 328, 105–113. [Google Scholar] [CrossRef]
- Ebrahimi, M.; Moradpoor, I. Combined solid oxide fuel cell, micro-gas turbine and organic Rankine cycle for power generation (SOFC-MGT-ORC). Energy Convers. Manag. 2016, 116, 120–133. [Google Scholar] [CrossRef]
- Al-Hamed, K.H.M.; Dincer, I. A new direct ammonia solid oxide fuel cell and gas turbine based integrated system for electric rail transportation. eTransportation 2019, 2, 100027. [Google Scholar] [CrossRef]
- Barelli, L.; Bidini, G.; Cinti, G. Operation of a solid oxide fuel cell based power system with ammonia as a fuel: Experimental test and system design. Energies 2020, 13, 6173. [Google Scholar] [CrossRef]
- Aminyavari, M.; Mamaghani, A.H.; Shirazi, A.; Najafi, B.; Rinaldi, F. Exergetic, economic, and environmental evaluations and multi-objective optimization of an internal-reforming SOFC-gas turbine cycle coupled with a Rankine cycle. Appl. Therm. Eng. 2016, 108, 833–846. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, M.; Liso, V.; Samsatli, S.; Samsatli, N.J.; Jing, R.; Chen, J.; Li, N.; Zhao, Y. Parametric analysis and optimization for exergoeconomic performance of a combined system based on solid oxide fuel cell-gas turbine and supercritical carbon dioxide Brayton cycle. Energy Convers. Manag. 2019, 186, 66–81. [Google Scholar] [CrossRef]
- Venkitesh, K.; Daniel, J.; Sreekanth, M. Thermodynamic Assessment of a Gas Turbine System Combined with A Solid Oxide Fuel Cell Powered by Synthetic Gas from Municipal Solid Waste. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1128, 012036. [Google Scholar] [CrossRef]
- Rangel-Hernández, V.H.; Niño-Avendaño, A.M.; Ramírez-Minguela, J.J.; Belman-Flores, J.M.; Elizalde-Blancas, F. Advanced Exergy Analysis of an Integrated SOFC-Adsorption Refrigeration Power System. Appl. Energy 2018. [Google Scholar] [CrossRef] [Green Version]
- Gholamian, E.; Zare, V. A comparative thermodynamic investigation with environmental analysis of SOFC waste heat to power conversion employing Kalina and Organic Rankine Cycles. Energy Convers. Manag. 2016, 117, 150–161. [Google Scholar] [CrossRef]
- Saadabadi, S.A.; Patel, H.; Woudstra, T.; Aravind, P.V. Thermodynamic Analysis of Solid Oxide Fuel Cell Integrated System Fuelled by Ammonia from Struvite Precipitation Process. Fuel Cells 2020, 20, 143–157. [Google Scholar] [CrossRef]
- Ranjbar, F.; Chitsaz, A.; Mahmoudi, S.M.S.; Khalilarya, S.; Rosen, M.A. Energy and exergy assessments of a novel trigeneration system based on a solid oxide fuel cell. Energy Convers. Manag. 2014, 87, 318–327. [Google Scholar] [CrossRef]
- Ni, M.; Leung, D.Y.C.; Leung, M.K.H. Thermodynamic analysis of ammonia fed solid oxide fuel cells: Comparison between proton-conducting electrolyte and oxygen ion-conducting electrolyte. J. Power Sources 2008, 183, 682–686. [Google Scholar] [CrossRef]


| Component | Parameter | Value |
|---|---|---|
| SOFC | Operating pressure (bar) | 4 |
| Operating temperature (°C) | 814.1 | |
| Number of single cells | 14,202 | |
| Fuel cell current density (A/m2) | 1455 | |
| Active surface area (m2) | 0.22 | |
| Anode thickness (cm) | 0.002 | |
| Cathode thickness (cm) | 0.002 | |
| Electrolyte thickness (cm) | 0.004 | |
| Stoichiometric rate of hydrogen | 1.2 | |
| Stoichiometric rate of oxygen | 2 | |
| Fuel utilization factor in SOFC (%) | 85 | |
| Compressor | Isentropic efficiency (%) | 82 |
| Pumps | Isentropic efficiency (%) | 82 |
| Expanders | Isentropic efficiency (%) | 80 |
| Heat exchangers | Minimum temperature approach (°C) | 7 |
| Converter | DC-AC converter efficiency (%) | 98 |
| Components | Exergy Destruction Rate | |
|---|---|---|
| SOFC | (38) | |
| Afterburner | (39) | |
| Gas Turbine | (40) | |
| Air heat exchanger (HEX-1) | (41) | |
| Fuel heat exchanger (HEX-2) | (42) | |
| SRC heat exchanger (HEX-3) | (43) | |
| Exhaust gas boiler | (44) |
| Parameter | Model Results | Reported [52] | Difference (%) |
|---|---|---|---|
| SOFC temperature (°C) | 808.8 | 800 | 1 |
| Cell voltage (V) | 0.85 | 0.67 | 21 |
| Fuel utilization factor (%) | 85 | 80 | 5 |
| Current Density (A/m2) | 1455 | 1450 | 0.3 |
| SOFC efficiency | 43.8 | 39 | 4.8 |
| Node | Vapor Fraction | Temperature | Pressure | Molar Flow | Mass Enthalpy |
|---|---|---|---|---|---|
| Unit | C | kPa | kgmole/h | kJ/kg | |
| Air in | 1.00 | 29.85 | 101.30 | 187.62 | 4.63 |
| 1 | 1.00 | 29.85 | 400.00 | 80.10 | −2687.22 |
| 2 | 1.00 | 203.36 | 400.00 | 187.62 | 183.31 |
| 3 | 1.00 | 492.60 | 396.55 | 187.62 | 495.48 |
| 4 | 1.00 | 492.60 | 396.55 | 80.10 | −1518.26 |
| 5 | 1.00 | 152.64 | 396.55 | 105.22 | −1518.21 |
| 6 | 0.00 | 152.64 | 396.55 | 0.00 | −1518.21 |
| 7 | 1.00 | 383.66 | 396.55 | 309.58 | 90.18 |
| 8 | 1.00 | 814.11 | 396.55 | 334.66 | 90.18 |
| 9 | 0.00 | 814.11 | 396.55 | 0.00 | 90.18 |
| 10 | 1.00 | 814.11 | 396.55 | 317.93 | 90.18 |
| 11 | 1.00 | 814.11 | 396.55 | 16.73 | 90.18 |
| 12 | 1.00 | 1158.61 | 396.55 | 320.99 | 90.18 |
| 13 | 1.00 | 890.01 | 117.00 | 320.99 | −358.61 |
| 14 | 1.00 | 736.20 | 110.11 | 320.99 | −607.95 |
| 15 | 1.00 | 587.58 | 103.21 | 320.99 | −843.23 |
| 16 | 1.00 | 359.39 | 96.32 | 320.99 | −1192.75 |
| 17 | 1.00 | 159.71 | 89.42 | 320.99 | −1486.54 |
| 18 | 0.00 | 72.12 | 19,000.00 | 56.62 | −15,667.37 |
| 19 | 1.00 | 360.90 | 18,996.55 | 56.62 | −13,345.07 |
| 20 | 0.73 | 74.69 | 38.00 | 56.62 | −13,965.52 |
| 21 | 0.00 | 70.00 | 31.11 | 56.62 | −15,693.37 |
| 22 | 0.00 | 20.00 | 100.00 | 444.07 | −15,909.39 |
| 23 | 0.00 | 70.98 | 96.55 | 444.07 | −15,689.09 |
| 24 | 0.00 | 20.00 | 784.53 | 40.24 | −15,908.74 |
| 25 | 1.00 | 175.00 | 781.08 | 40.24 | −13,162.57 |
| 26 | 1.00 | 814.10 | 396.55 | 16.73 | 90.21 |
| Term | Value |
|---|---|
| SOFC power output (kW) | 3800 |
| Gas Turbine power (kW) | 844.8 |
| SRC Turbine power (kW) | 175.8 |
| SRC pump power (kW) | 7.369 |
| Air compressor power (kW) | 268.7 |
| SOFC electrical efficiency (%) | 53.92 |
| Electrical efficiency of entire system (%) | 64.49 |
| EGB mass flow rate (kg/h) | 725 |
| EGB superheated temperature (°C) | 175 |
| Subsystem | Energy Efficiency | Exergy Efficiency |
|---|---|---|
| SOFC-GT | 62.10 | 58.84 |
| SRC | 25.58 | 41.21 |
| Total System | 64.49 | 61.10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duong, P.A.; Ryu, B.; Kim, C.; Lee, J.; Kang, H. Energy and Exergy Analysis of an Ammonia Fuel Cell Integrated System for Marine Vessels. Energies 2022, 15, 3331. https://doi.org/10.3390/en15093331
Duong PA, Ryu B, Kim C, Lee J, Kang H. Energy and Exergy Analysis of an Ammonia Fuel Cell Integrated System for Marine Vessels. Energies. 2022; 15(9):3331. https://doi.org/10.3390/en15093331
Chicago/Turabian StyleDuong, Phan Anh, Borim Ryu, Chongmin Kim, Jinuk Lee, and Hokeun Kang. 2022. "Energy and Exergy Analysis of an Ammonia Fuel Cell Integrated System for Marine Vessels" Energies 15, no. 9: 3331. https://doi.org/10.3390/en15093331
APA StyleDuong, P. A., Ryu, B., Kim, C., Lee, J., & Kang, H. (2022). Energy and Exergy Analysis of an Ammonia Fuel Cell Integrated System for Marine Vessels. Energies, 15(9), 3331. https://doi.org/10.3390/en15093331







