Application of Infrared Imaging for Early Detection of Downy Mildew (Plasmopara viticola) in Grapevine
Abstract
1. Introduction
2. Materials and Methods
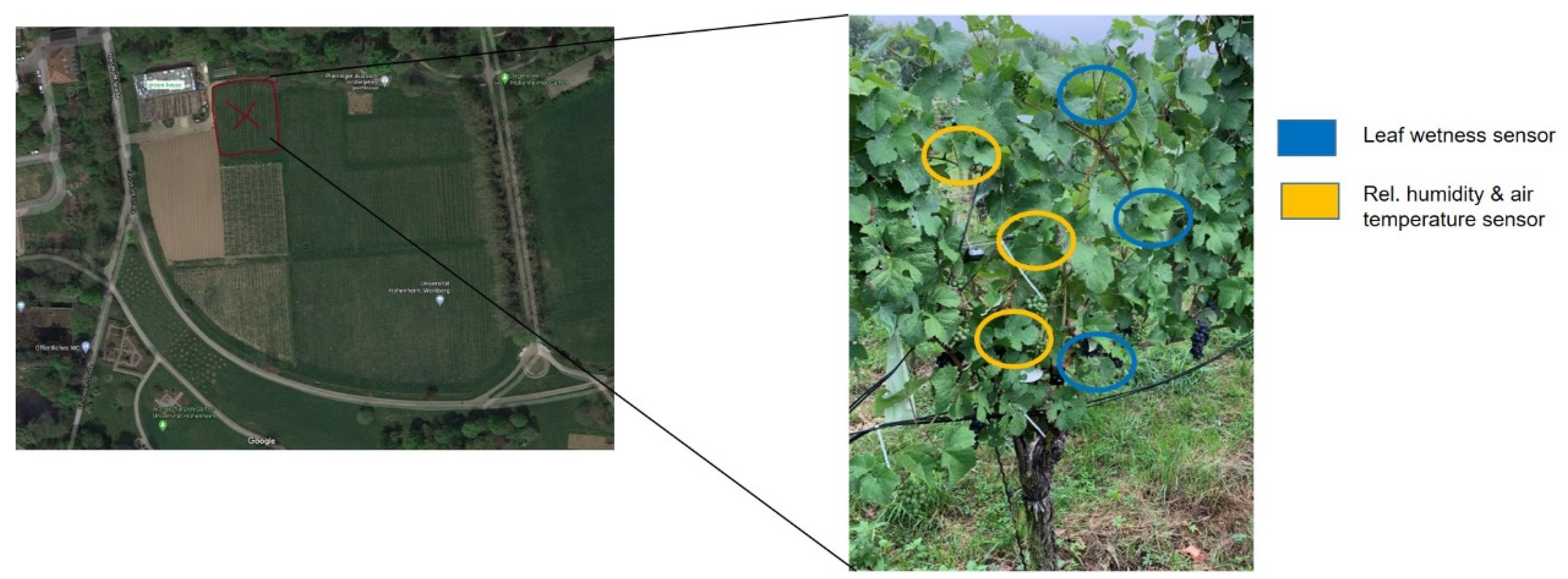
2.1. Site Description
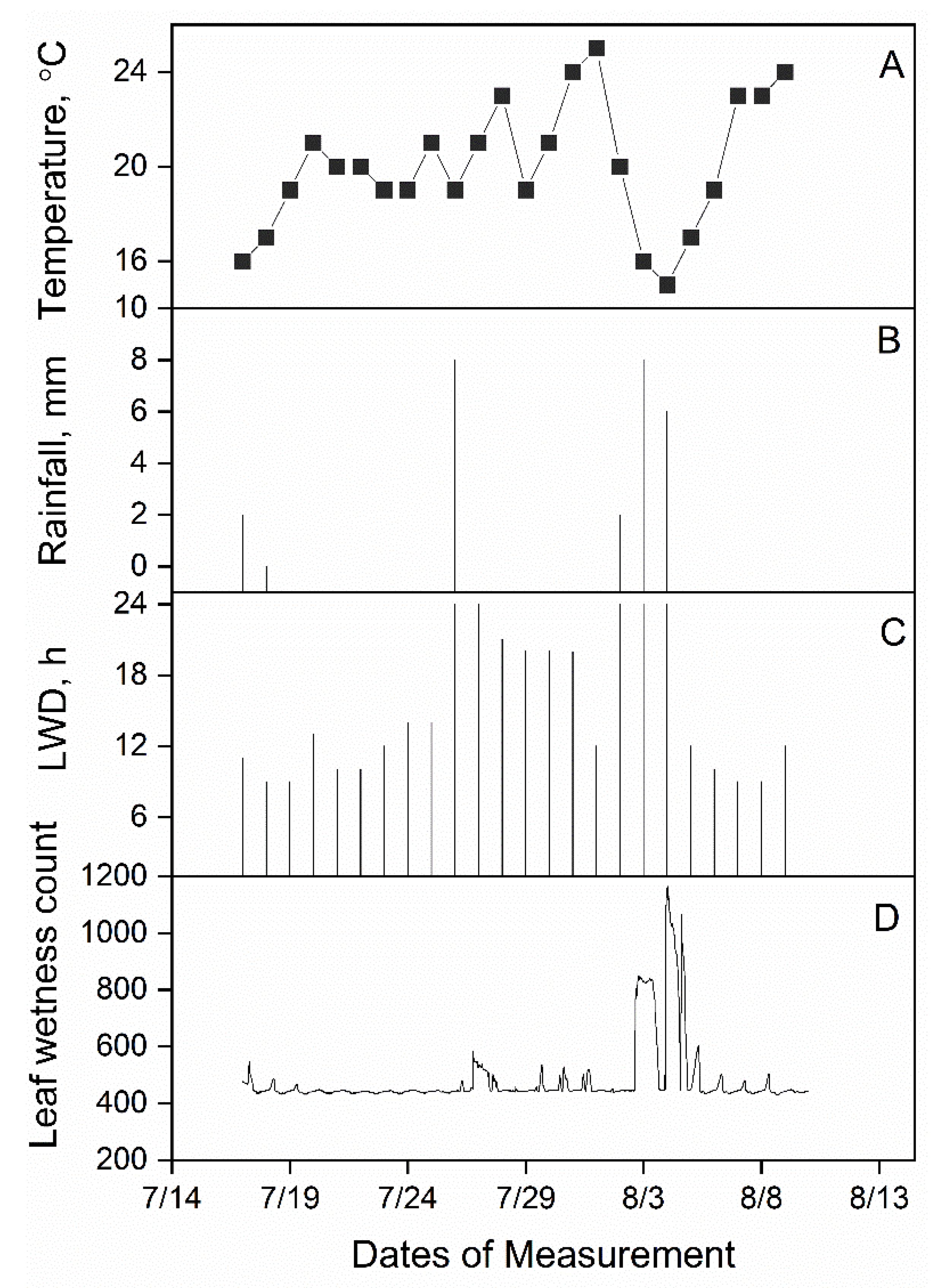
2.2. Meteorological Measurements
2.3. Visual Disease Monitoring
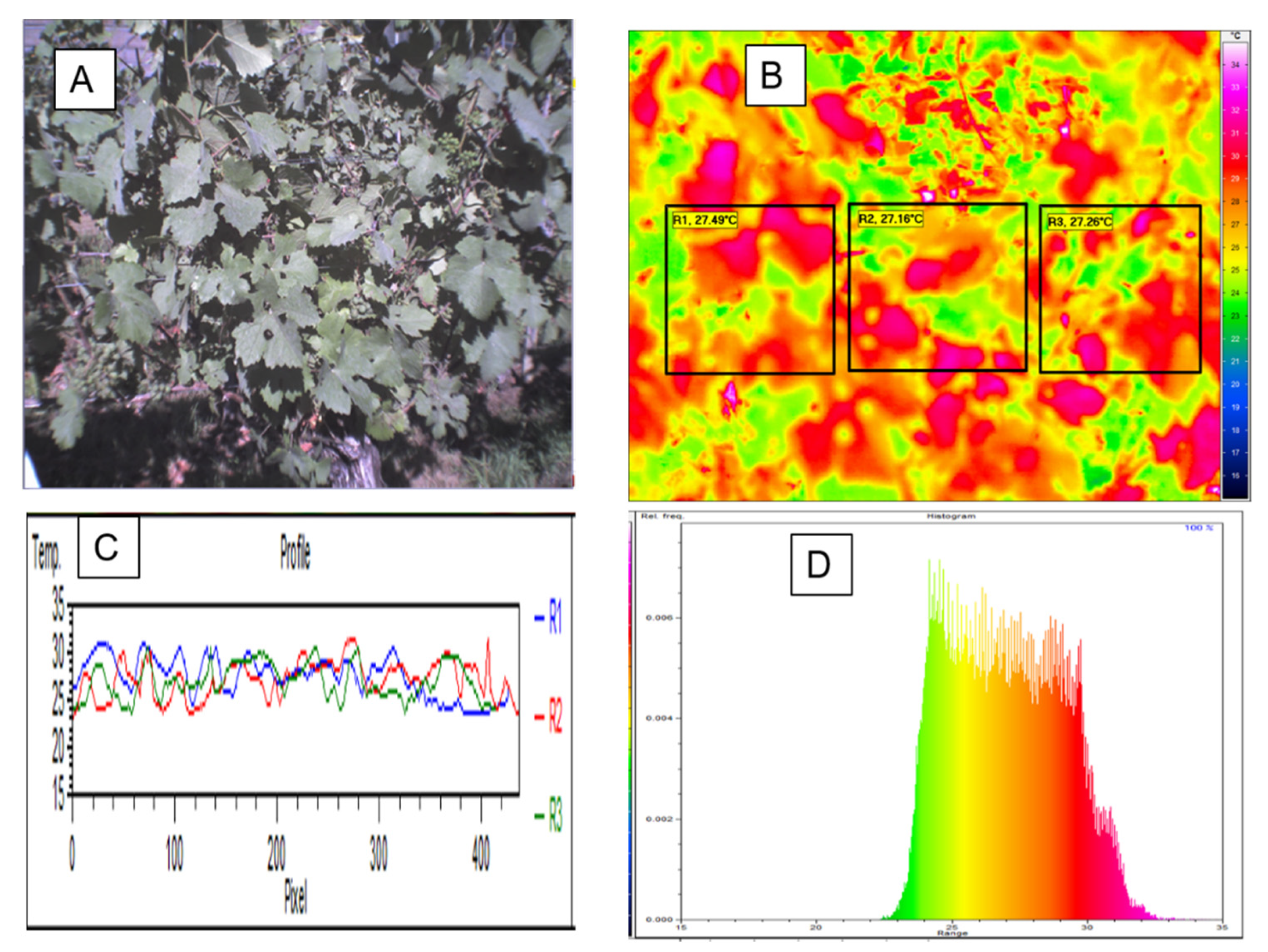
2.4. Thermographic Measurements
2.5. Data Analysis
3. Results
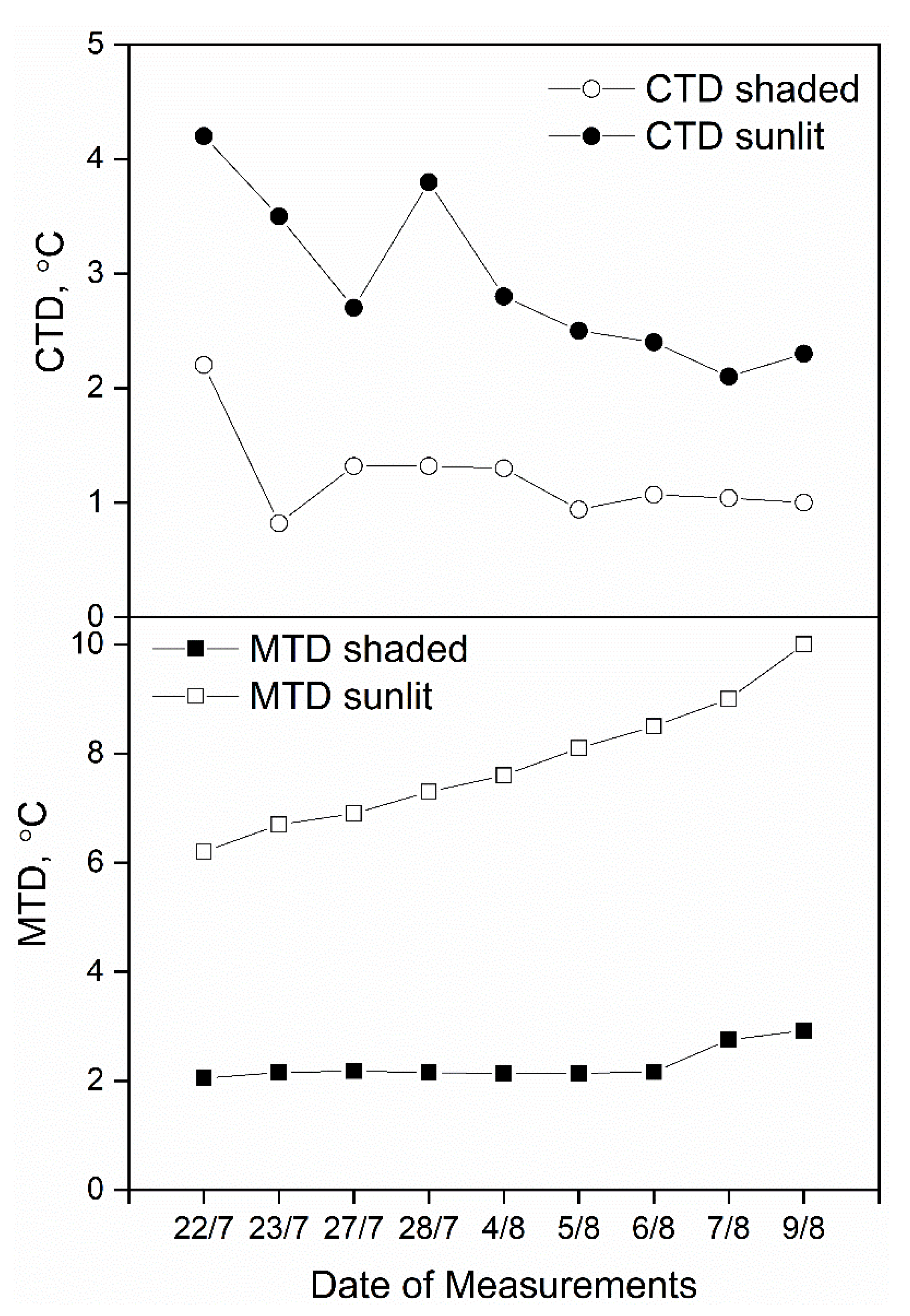
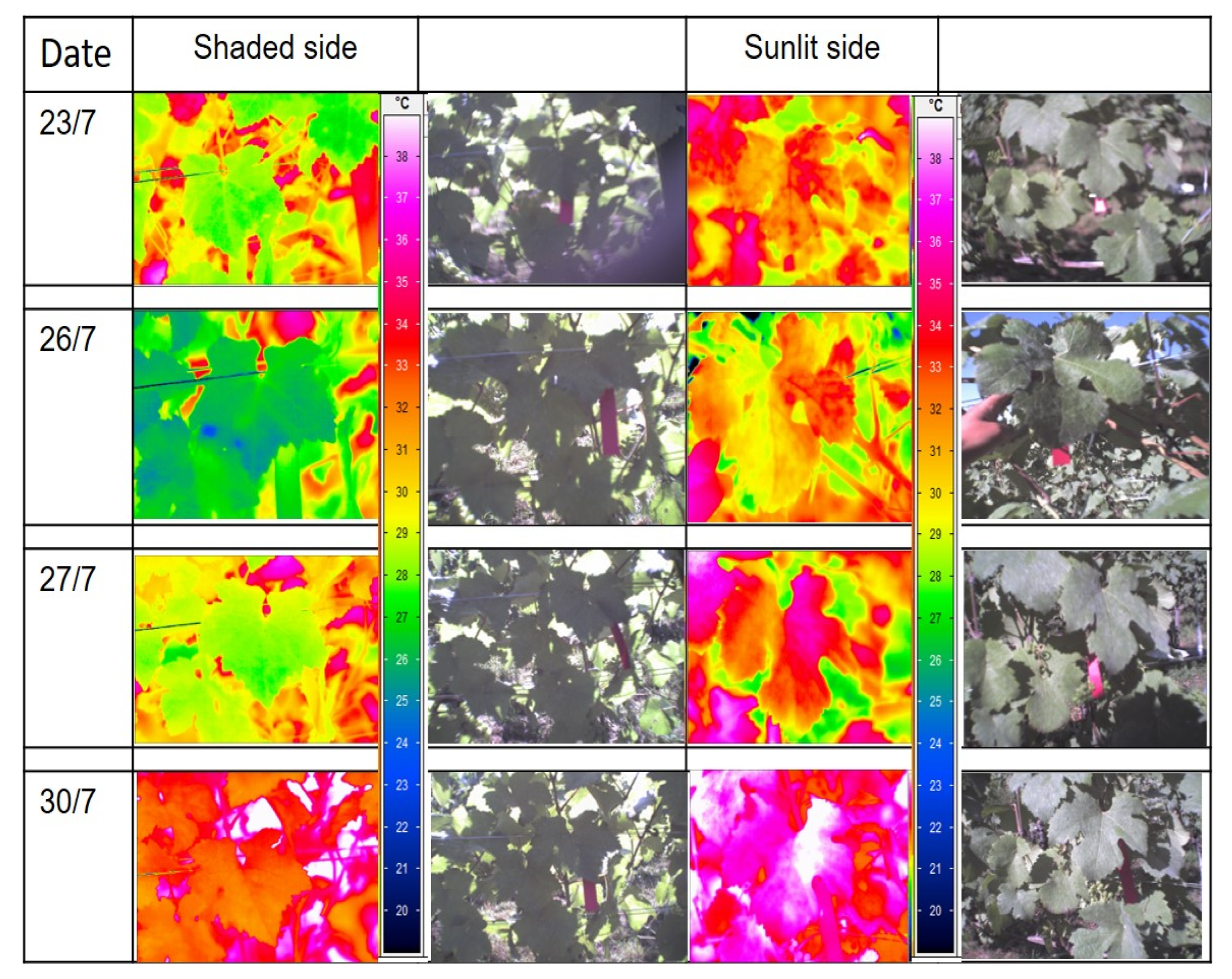
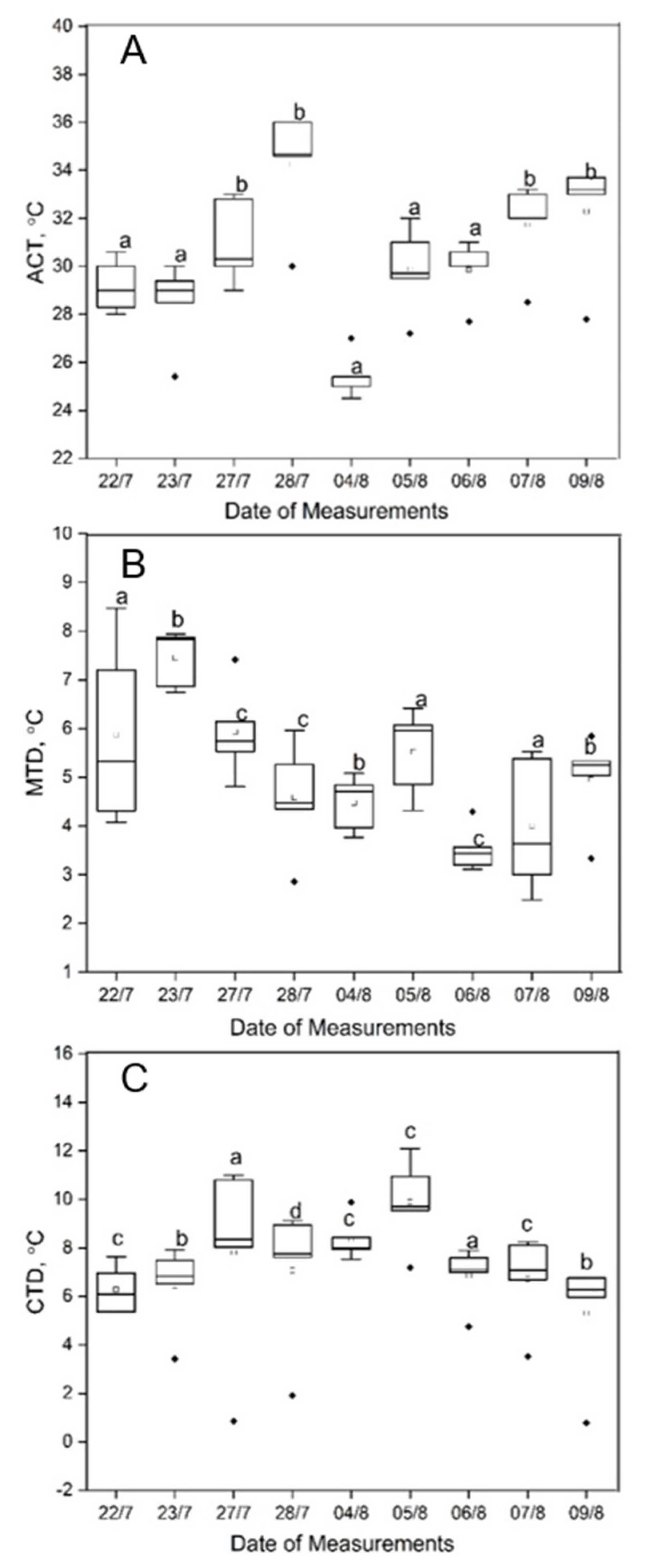
3.1. Sunlit Versus Shaded Leaves
3.2. Downy Mildew Development
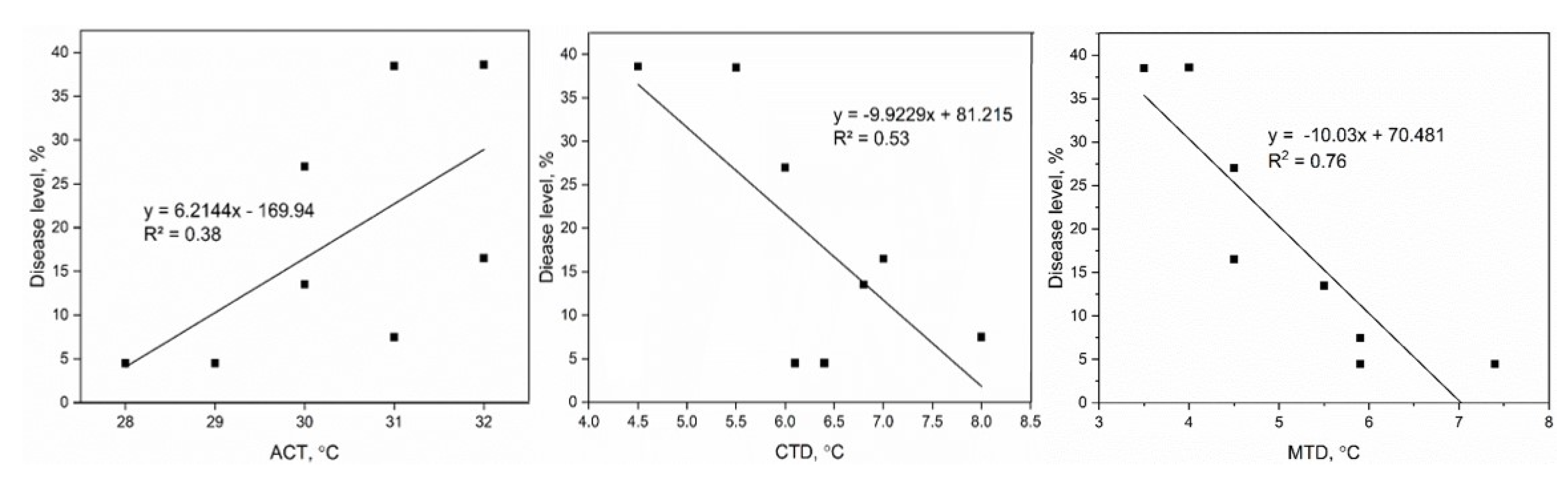
3.3. Disease Level and Temperature Effects
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Junges, A.H.; Ducati, J.R.; Lampugnani, C.S.; Kurtz, M.A.A. Detection of grapevine leaf stripe disease symptoms by hyperspectral sensor. Phytopathol. Mediterr. 2018, 57, 399–406. [Google Scholar]
- Boso, S.; KAssemeyer, H.H. Different susuceptibility of European grapevine cultivars for downy mildew. Vitis 2008, 1, 39–49. [Google Scholar]
- Allègre, M.; Daire, X.; Héloir, M.-C.; Trouvelot, S.; Mercier, L.; Adrian, M.; Pugin, A. Stomatal deregulation in Plasmopara viticola-infected grapevine leaves. New Phytol. 2006, 173, 832–840. [Google Scholar] [CrossRef] [PubMed]
- Calderón, R.; Montes-Borrego, M.; Landa, B.B.; Navas-Corté, J.A.; Zarco-Tejada, P.J. Detection of downy mildew of opium poppy using high-resolution multi-spectral and thermal imagery acquired with an unmanned aerial vehicle. Precis. Agric. 2014, 15, 639–661. [Google Scholar] [CrossRef]
- Francesca, S.; Simona, G.; Nicola, T.F.; Andrea, R.; Vittorio, R.; Federico, S.; Cynthia, R.; Lodovica, G.M. Downy mildew (Plasmopara viticola) epidemics on grapevine under climate change. Glob. Change Biol. 2006, 12, 1299–1307. [Google Scholar] [CrossRef]
- Beruski, C.G.; Gleason, L.M.; Sentelahas, C.P.; Pereira, B.A. Leaf wetness duration estimation and its influence on a soybean rust warning system. Australas. Plant Pathol. 2019, 48, 395–408. [Google Scholar] [CrossRef]
- Gleason, M.L.; Duttweiler, K.B.; Batzer, J.C.; Taylor, S.E.; Sentelhas, P.C.; Monteiro, J.E.B.A.; Gillespie, T.J. Obtaining weather data for input to crop disease-warning systems: Leaf wetness duration as a case study. Sci. Agric. (Piracicaba Braz.) 2008, 65, 76–87. [Google Scholar] [CrossRef]
- Dalla Marta, A.; Di Stefano, B.; Cerovic, Z.G.; Agati, G.; Orlandini, S. Solar radiation affects grapevine susceptibility to Plasmopara Viticola. Sci. Agric. (Piracicaba Braz.) 2008, 65, 65–70. [Google Scholar] [CrossRef][Green Version]
- Zamani, A.; Sharifi, A.; Felegari, S.; Tariq, A.; Zhao, N. AgroClimatic Zoning of Saffron Culture in Miyaneh City by Using WLC Method and Remote Sensing Data. Agriculture 2022, 12, 118. [Google Scholar] [CrossRef]
- Singh, V.; Sharma, N.; Singh, S. A review of imaging techniques for plant disease detection. Artif. Intell. Agric 2020, 4, 229–242. [Google Scholar] [CrossRef]
- Thomas, S.; Kuska, T.M.; Behnenkamp, D.; Brugger, A.; Alisaac, E.; Wahabzada, M.; Behmann, J.; Mahlein, A.-K. Benefits of hyperspectral imaging for plant disease detcetion and plant protection: Atechnical perspective. J. Plant Dis. 2018, 125, 5–20. [Google Scholar] [CrossRef]
- Ishimwe, R.; Abutaleb, K.; Ahmed, F. Application of thermal imaging in agriculture—A review. Adv. Remote Sens. 2014, 3, 128–140. [Google Scholar] [CrossRef]
- Jones, H.G.; Stoll, M.; Santos, T.; De Sousa, C.; Chaves, M.M.; Grant, O.M. Use of infrared thermography for monitoring stomatal closure in the field: Application to grapevine. J. Exp. Bot. 2002, 53, 2249–2260. [Google Scholar] [CrossRef] [PubMed]
- Lindenthal, M.; Steiner, U.; Dehne, H.W.; Oerke, E.C. Effect of downy mildew development on transpiration of cucumber leaves visualized by digital infrared thermography. Phytopathology 2005, 95, 233–240. [Google Scholar] [CrossRef]
- Yang, N.; Yuan, M.; Wang, P.; Zhang, R.; Sun, J.; Mao, H. Tea disease detection based on fast infrared thermal image processing technology. J. Sci. Food Agric. 2019, 99, 3459–3466. [Google Scholar] [CrossRef]
- Oerke, E.-C.; Herzog, K.; Toepfer, R. Hyperspectral phenotyping of the reaction of grapevine genotypes to Plasmopara viticola. J. Exp. Bot. 2016, 67, 5529–5543. [Google Scholar] [CrossRef]
- Xu, H.; Zhu, S.; Ying, Y.; Jiang, H. Early detection of plant disease using infrared thermal imaging. In Proceedings of the Optics for Natural Resources, Agriculture, and Foods, Boston, MA, USA, 23 October 2006. [Google Scholar]
- Stoll, M.; Schultz, H.R.; Berkelmann-Loehnertz, B. Exploring the sensitivity of thermal imaging for Plasmopara viticola pathogen detection in grapevines under different water status. Funct. Plant. Biol. 2008, 35, 281–288. [Google Scholar] [CrossRef]
- MacKenzie, J.S.; Peres, A.N. Use of leaf wetness and temperature to time fungicide applications to control anthracnose fruit rot of strawberry in Florida. Plant Dis. 2011, 96, 522–528. [Google Scholar] [CrossRef]
- Wang, Y.; Zia-Khan, S.; Owusu-Adu, S.; Miedaner, T.; Müller, J. Early detection of Zymoseptoria tritici in winter wheat by infrared thermography. Agriculture 2019, 9, 139. [Google Scholar] [CrossRef]
- Leinonen, I.; Jones, H.G. Combining thermal and visible imagery for estimating canopy temperature and identifying plant stress. J. Exp. Bot. 2004, 55, 1423–1431. [Google Scholar] [CrossRef]
- Fuchs, M. Infrared measurement of canopy temperature and detection of plant water stress. Theor. Appl. Climatol. 1990, 42, 253–261. [Google Scholar] [CrossRef]
- Pande, S.; Rajesh, T.R.; Rao, K.C.; Kishore, G.K. Effect of temperature and leaf wetness period on the component of resistance of late leaf spot disease in groundnut. Plant Pathol. J. 2004, 20, 67–74. [Google Scholar] [CrossRef]
- Jhorar, O.P.; Butler, D.R.; Mathauda, S.S. Effects of leaf wetness duration, relative humidity, light and dark on infection and sporulation by Didymella rabiei on chickpea. Plant Pathol. J. 1998, 47, 586–594. [Google Scholar] [CrossRef]
- Morales, G.; Moragrega, C.; Montesino, E.; Llorente, I. Effects of leaf wetness duration and temperature on infection of Prunus by Xanthomonas arboricola pv. Pruni. PLoS ONE 2018, 13, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Shtienberg, D. Effect of folia diseases on gas exchange processes: A comparative study. Phytopathology 1992, 82, 760–765. [Google Scholar] [CrossRef]
- Chaerle, L.; de Boever, F.; van Montagu, M.; van der Straeten, D. Thermographic visualization of cell death in tobacco and Arabidopsis. Plant Cell Environ. 2001, 24, 15–25. [Google Scholar] [CrossRef]
- Smith, R.C.G.; Heritage, A.D.; Stapper, M.; Barrs, H.D. Effect of stripe rust (Puccinia striiformis West.) and irrigation on the yield and foliage temperature of wheat. Field Crops Res. 1986, 14, 39–51. [Google Scholar] [CrossRef]
- Robert, C.; Bancal, M.-O.; Lannou, C.; Ney, B. Quantification of the effects of Septoria tritici blotch on wheat leaf gas exchange with respect to lesion age, leaf number, and leaf nitrogen status. J. Exp. Bot. 2006, 57, 225–234. [Google Scholar] [CrossRef]
- Rosyara, R.U.; Subedi, S.; Duveiller, E.; Sharma, R.C. The effect of spot blotch and heat stress on variation of canopy temperature depression, chlorophyll fluorescence and chlorophyll content of hexaploid wheat genotypes. Euphytica 2010, 174, 377–390. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zia-Khan, S.; Kleb, M.; Merkt, N.; Schock, S.; Müller, J. Application of Infrared Imaging for Early Detection of Downy Mildew (Plasmopara viticola) in Grapevine. Agriculture 2022, 12, 617. https://doi.org/10.3390/agriculture12050617
Zia-Khan S, Kleb M, Merkt N, Schock S, Müller J. Application of Infrared Imaging for Early Detection of Downy Mildew (Plasmopara viticola) in Grapevine. Agriculture. 2022; 12(5):617. https://doi.org/10.3390/agriculture12050617
Chicago/Turabian StyleZia-Khan, Shamaila, Melissa Kleb, Nikolaus Merkt, Steffen Schock, and Joachim Müller. 2022. "Application of Infrared Imaging for Early Detection of Downy Mildew (Plasmopara viticola) in Grapevine" Agriculture 12, no. 5: 617. https://doi.org/10.3390/agriculture12050617
APA StyleZia-Khan, S., Kleb, M., Merkt, N., Schock, S., & Müller, J. (2022). Application of Infrared Imaging for Early Detection of Downy Mildew (Plasmopara viticola) in Grapevine. Agriculture, 12(5), 617. https://doi.org/10.3390/agriculture12050617






