Abstract
Owing to environmental pollution and increasingly strict regulations, heavy metals have attracted the attention of many researchers in various disciplines. Alginate and chitosan derivatives have gained popularity as biosorbents for water treatment. An increase in the number of publications on modified biosorbents for the biosorption of toxic compounds reveals widespread interest in examining the requirements and positive contribution of each modification type. This paper reviews the advantages and disadvantages of using alginate and chitosan for adsorption. Well-known modifications based on chitosan and alginate, namely, grafting, functionalization, copolymerization and cross-linking, as well as applications in the field of adsorption processes, especially amino acid functionalization, are reviewed. The selection criteria for the best biosorbents and their effectiveness and proposed mechanism of adsorption are discussed critically. In the conclusion, the question of why these adsorbents need modification before use is addressed.
1. Introduction
Hazardous pollutants in water are classified into two categories: organic and inorganic. The treatment of contaminated water is necessary for recycling/reuse, and includes the recovery of metals. Treatment processes, the type of pollutant and adsorbent affect the quality of recycled water, as these factors are dependent on the requirements of the purification process and the toxicity of some metals. Water used in agricultural, industrial, household, recreational and environmental activities may become contaminated. However, wastewater from metallurgical processes, chemical industries, industrial effluent, the textile industry, agricultural runoff, sewage treatment plants, and mines includes a high percentage of metal ions, rare metals, radioactive metals or/and dyes [1,2,3,4]. Often, the concentration of heavy metals present in wastewater is higher than guideline values and therefore, must be eliminated [5,6,7].
Consequently, several toxic metal ions have been successfully removed from concentrated and diluted aqueous solutions using polysaccharide-based adsorptive materials. The literature is rich in published works in the field of metal contaminant treatment by means of these biosorbents [8,9,10,11,12,13,14,15,16]. Several biosorbents have been used to purify aqueous solutions of various pollutants, which leads us to ask: what is the best adsorbent and can we compare adsorbents for the same treatment?
The increasing number of publications on alginate and chitosan with and without modification for the treatment of effluent containing metal ions raises the questions: Are modifications required, and if so, what kind of physicochemical modification is necessary? Additionally, the base material (in natural form) might be effective and only need a few modifications in terms of its sorption parameters, like temperature, dosage, stirring time and pH. Selectivity toward some metals in a complex solution might be promoted by a specific modification. The best adsorbents from a large list of adsorbents and related changes in the adsorption mechanisms should be applied. These considerations are addressed in this review.
2. Well-Known Materials Used in Sorption Processes
Adsorption involves one or more interactions, including covalent, electrostatic, or in terms of the physic-chemical bonds between the adsorbate and the adsorbent [17]. The core functional groups that may be engaged in adsorption reactions, according to Schindler and Stumm [18], are –COOH, –CO–, –NH2, –OH, and –SH. In theory, all solids are adsorbents, and some interesting adsorbents have been examined in the decontamination/recovery of metals from aqueous solutions, either as real treatments on a pilot scale (in laboratories) or on an industrial scale.
Researchers have focused on the synthesis and application of various types of adsorbents. Among the best commonly applied adsorbents to remove heavy metals are activated carbon [19,20,21,22], iron oxides [23,24,25,26], zeolites [27,28,29,30,31], and sawdust [32]. The high adsorption capacities of these biomaterials are partly linked to their highly developed porous structures and their highly specific surface areas. Related technology using silica ceramics is an efficient and widely used process for the treatment of a As(III) due to its simplicity, moderate operational conditions and economic profitability [33,34].
Algae [35,36,37,38,39], cellulose [40], and chitin [41,42,43,44] have also been used in adsorption processes. Several research teams have concentrated on using the most abundant biopolymers for the sorption of hazardous heavy metal ions and other toxic pollutants. According to the research, the best polymers for adsorption are not cellulose or starch, but chitin, algae, and their main derivatives, chitosan and alginate. Thus, we review the well-known chitosan and alginate adsorbents and their efficacy in terms of removing hazardous metal ions.
3. Metal Ion Removal by Alginate, Chitosan, and Related Adsorbent Materials
Adsorption into the surface of natural polymers and their modified derivatives has been shown to be able to eliminate hazardous and poisonous pollutants in wastewater [45]. In this regard, the polymers chitosan [46,47,48,49,50], alginate [8,10,51,52,53,54,55], and their derivatives chitosan [50,56,57] and alginate deserve special attention.
A number of papers have revealed the efficiency of chitosan for the elimination of a variety of heavy metals like As(V) and As(III) [58,59], Hg(II) [60,61], U(VI) [15,61,62,63,64,65], Cd(II) and Hg(II) [15], These articles were dedicated to investigating the effect of pH and ionic strength on the adsorption of metal ions [66,67,68,69,70]. Yang et al. [71] studied the adsorption of various hazardous metal ions such as Cu(II), Zn(II), Cd(II), Cr(VI), and Pb(II) using chitosan [39]. These studies confirmed that chitosan has an exceptional adsorption capacity, i.e., more than 1 mmol of pollutant for every 1 g of adsorbent, except for Cr and some precious and radioactive metals.
Alginate and derived adsorbents are frequently used in the treatment of water and wastewater contaminated by toxic heavy metals. For example, alginate beads have been widely studied for Pb(II) adsorption [8,10,52,55,72], in the treatment of Cu(II) and Cd(II) ions [11,52,53,73], alginate modified for efficient sorption of Cd(II), Cu(II), Pb(II), Zn(II), Ni(II) and Hg(II) [73,74,75], in the trapping of Hg(II), Cd(II), Pb(II) and Zn(II) [12], for U(VI) [76], As(V) [77,78], and Cr(VI) [79]. Composites based on chitosan and alginate play a central role in increasing the adsorption capacity. Several researchers have taken an interest in the manufacture of new adsorbents based on chitosan–alginate, and the effectiveness of these composites has been tested in the treatment of several pollutants like As(III) [80], Cr(VI) [81], Cu(II), and U(VI) [82], as well as Cu(II) and Cd(II) [83].
There are benefits and drawbacks with using adsorbents like chitosan, alginate, and related materials for the removal of metal ions. Recent research has shown the potential of biosorbents produced from natural and agricultural resources for the sorption of various pollutants. In this context, commercial applications of chitosan and alginate have advanced, because they originate from natural, renewable sources and are economically affordable compared to traditional synthetic resins. Both biomaterials and synthetic materials trap metal ions with identical functional groups [84]. The emphasis on environmentally friendly technology has spurred interest in biopolymers, which are versatile, biodegradable, and less toxic than their synthetic equivalents. The choice of these biomaterials is based on their versatility and high adsorption capacity for metal ions with modification (grafted or crosslinked (ionic, chemical, or covalent)) and without (natural), as well as in different physical forms.
Several properties occur naturally in chitosan and alginate biosorbents or can be easily included in their surface. Various ways to improve the sorption performance of alginate are proposed, including increasing the percentage of Ca(II) in the matrix, which gives rise to the involvement of several sorption mechanisms. The cross-linking of alginate by calcium ions (Ca(II)) can be carried out by diffusion or internal regulation, which can easily promote ion exchange as the initial sorption process. The diffusion method produces gels with a difference in Ca(II) ion concentration, while internal regulation yields gels with uniform ion concentrations throughout [85]. One of the benefits of crosslinking is that it offers other possibilities regarding sorption mechanisms. However, by comparing the two polysaccharides, chitosan has more efficient fixing capacities due to its richness in amine functional groups, which makes it possible to make complexes with most toxic metal ions. The nitrogen in amino sites constitutes the most important reactive group toward metal ions; additionally, this is responsible for the occurrence of bonds by chelation mechanisms. It is for this reason that many researchers are attempting to functionalize adsorbents with the addition of new amines or –SH.
Alginate has proven its usability and potential as an extremely efficient adsorptive material for the treatment of solutions containing toxic metal ions and pollutants. Like chitosan, alginate is a naturally anionic polymer which is typically obtained from brown seaweed by extraction. Alginate plays an instrumental role in wastewater treatment to eliminate heavy metal ions, because it is biodegradable, biocompatible, economical, environmentally friendly, and nontoxic to microorganisms and the environment [86]. Thus, alginate and chitosan deserve special attention because of their structural characteristics [8,87,88,89,90].
In many cases, the adsorption properties of these materials have been linked directly to their proteins, polysaccharides, and phenolic compounds. These biopolymers have active groups, such as carboxylic, sulphate, phosphate, amine, and hydroxyl groups, which have the capacity to fix metal ions. Research has suggested that the binding mechanisms are influenced by both the type of ion and the functional groupings of the active sites [87]. The existence of chemical reactive functions (hydroxyl, carboxyl, acetamide or amine) in the chains of biopolymers justifies their use in the field of biosorption and the treatment of industrial wastewater.
The advantages of using alginate and chitosan as bio-adsorbents include the possibility of chemical modifications, ease of use at laboratory scale, varied formatting, biodegradability, biocompatibility, and use at several pH intervals. Chitosan and alginate are versatile and can be tailored to different forms and sizes such as powder, threads, membranes, films, nanoparticles, beads, fibers, hollow fibers, grains, resins, flakes, and sponges.
Chitosan and alginate have a high capacity for chelating metal ions compared to some other natural polymers; this property depends on their physical and chemical states. Many approaches and related modifications have been studied to adapt alginate and chitosan adsorbents for use in the chemical industry [7,9,50,56,57,91,92]. Chemical modifications, which influence the composition and depend on the type of modification (quantitative and qualitative composition of –C, –N, –O, –H, –S) and presence of Na+ and Ca2+, also play an important role in the choice of the sorption mechanism.
Not only chitosan but also alginate have numerous unique advantages and characteristics, such as their abundance, non-toxicity, biocompatibility, reactivity, biodegradability, and effectiveness in treating metallic contaminants. Chitosan is a polymer that possesses various functional amino groups [88]. Bailey et al. (1999) noted that chitosan is the most widely used biosorbent owing to the presence of free amino groups which enhance its adsorption capacity [44]. In addition, it presents a higher adsorption potential than commercial activated carbon [89].
Alginate and chitosan offer many advantages over other adsorbents which are frequently used for pollution control, such as commercial activated carbon, polysaccharide materials, etc. However, alginate and chitosan adsorbents also have several disadvantages. Table 1 summarizes the advantages and disadvantages of chitosan and alginate adsorbents.

Table 1.
Some advantages and disadvantages of applying bio-adsorbents based on chitosan and alginate to eliminate hazardous pollutants from aqueous solutions.
4. Alginate and Chitosan Modifications
4.1. Well-Known Modifications for Alginate and Chitosan
It is widely accepted that polysaccharides, which are abundant, renewable, and biodegradable resources, are able to associate with a great number of molecules through physical and chemical interactions (either by physical modification of the structure or chemical modifications). Chitosan and alginate possess a variety of free groups such as –NH2, –OH, and –COOH, that are distributed along the backbone and have excellent potential for chemical adjustment.
The goal of the modifications appears to be to alter some properties like solubility, water absorption capacity, adsorption capacity and temperature resistance. However, chemical modification does not change the basic structure of these materials; rather, it offers a variety of derivatives with desirable properties for particular uses and applications in different fields. Derivatization by incorporating chemical groups into chitosan generally include –NH2 group (specific reactions) or –OH groups (non-specific reactions), and for alginate, –OH (non-specific reactions) and –COOH (specific reactions). The amino functionality is capable of causing chemical reactions such as acetylation, quaternization, reaction with aldehydes and ketones (to give a Schiff base), alkylation, grafting, and chelation of metals. The hydroxyl groups also lead to reactions such as o-acetylation, H-bonding with polar atoms, and grafting. Researchers have confirmed that both alginate and chitosan can undergo many types of reactions such as acylation, alkylation, arylidation, carboxymethylation, quaternization, etherification, esterification, cross-linking, and graft copolymerization [84,90]. The –OH and –COOH groups of alginate participate in several chemical reactions. The –OH can participate in oxidation, reductive-amination of oxidized alginate, sulfation, and copolymerization, and cyclodextrin can be linked with alginate. The –COOH can participate in esterification, ugi reactions, and amidation.
According to Abegunde et al. (2020), the modification of the surface is the act of tailoring the surface of a material by adjusting the physical, chemical, or biological characteristics to achieve a desired purpose [91]. In adsorption, modifications make more functional groups available so that adsorbates can be selectively adsorbed by adsorption sites, which enhances adsorption capacity and selectivity [92,93].
Thus, adsorption/biosorption on materials based on polysaccharide can be an inexpensive method for water decontamination, compound extraction and separation [94]. These molecules become even more efficient when combined with other carefully chosen compounds, and their structural properties can be tailored for the desired applications. In addition, modifications can be carried out by several methods, including mechanical and thermal processes to create pores, and chemical processes to improve the surface [91].
There are several terms (methods/reactions) used for chemical modifications of biomaterials, such as grafting, cross-linking (ionic or covalent), combining with other adsorbents, polymerization, and copolymerization. There are also terms for physically modified materials, such as magnetic adsorbents (beads and powders), nano- and micro-particles, as well as hydrogel and aerogel adsorbents. After the modification has been made, different chitosan-based and alginate-based biosorbents can be produced for metal sorption, including: (1) graft copolymers in chitosan using –NH2, –COOH, –OH, –S or –P containing compounds, and hybrid compounds; (2) various types of cross-linked magnetic, ionic [(triphosphate (STPP)] or chemical [glutaraldehyde (GLA), ethylene glycol diglycidyl ether (EDGE), polyethyleneimine (PEI)] and imprinted chitosan with target metal; and (3) chitosan and alginate derivatives.
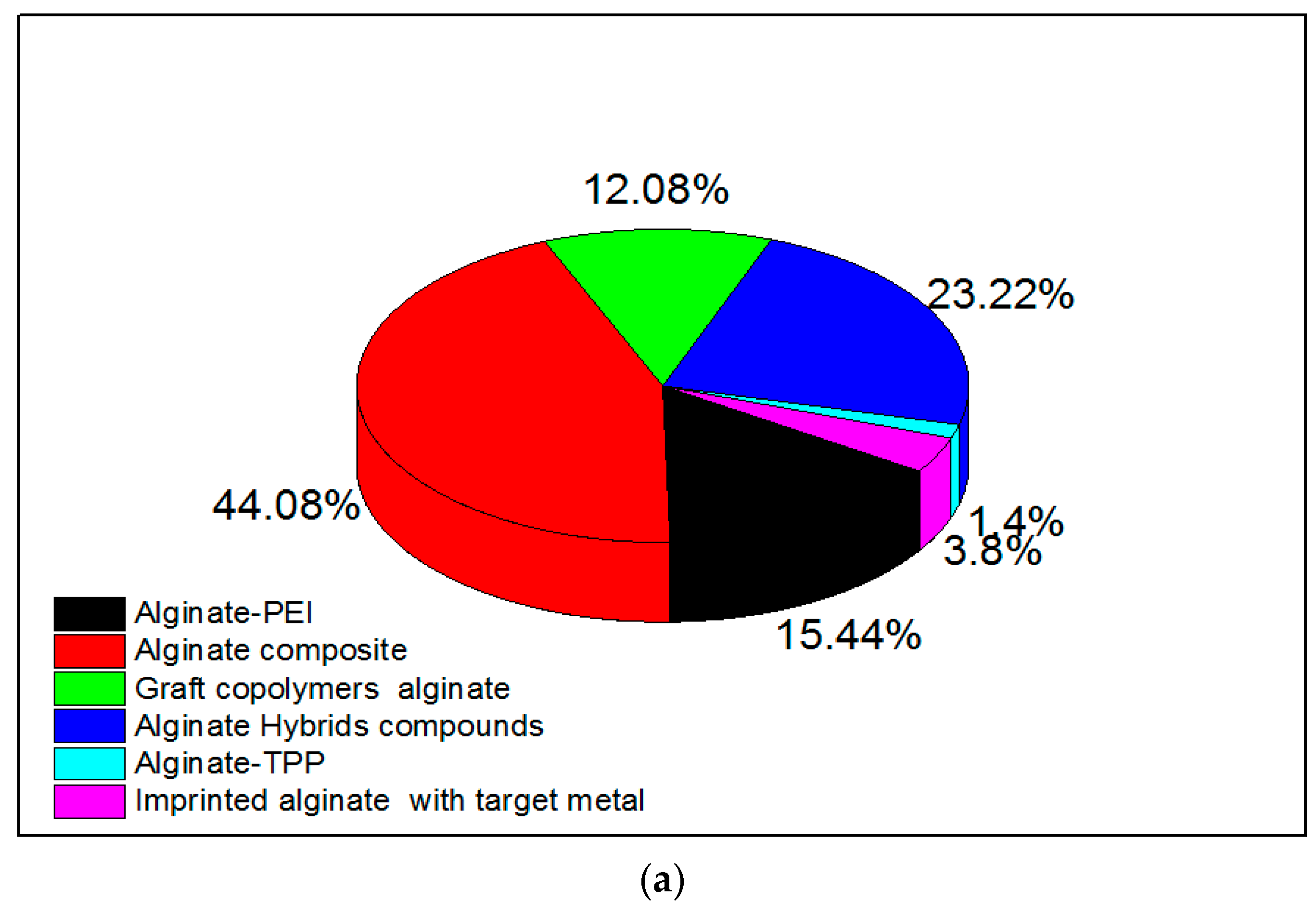
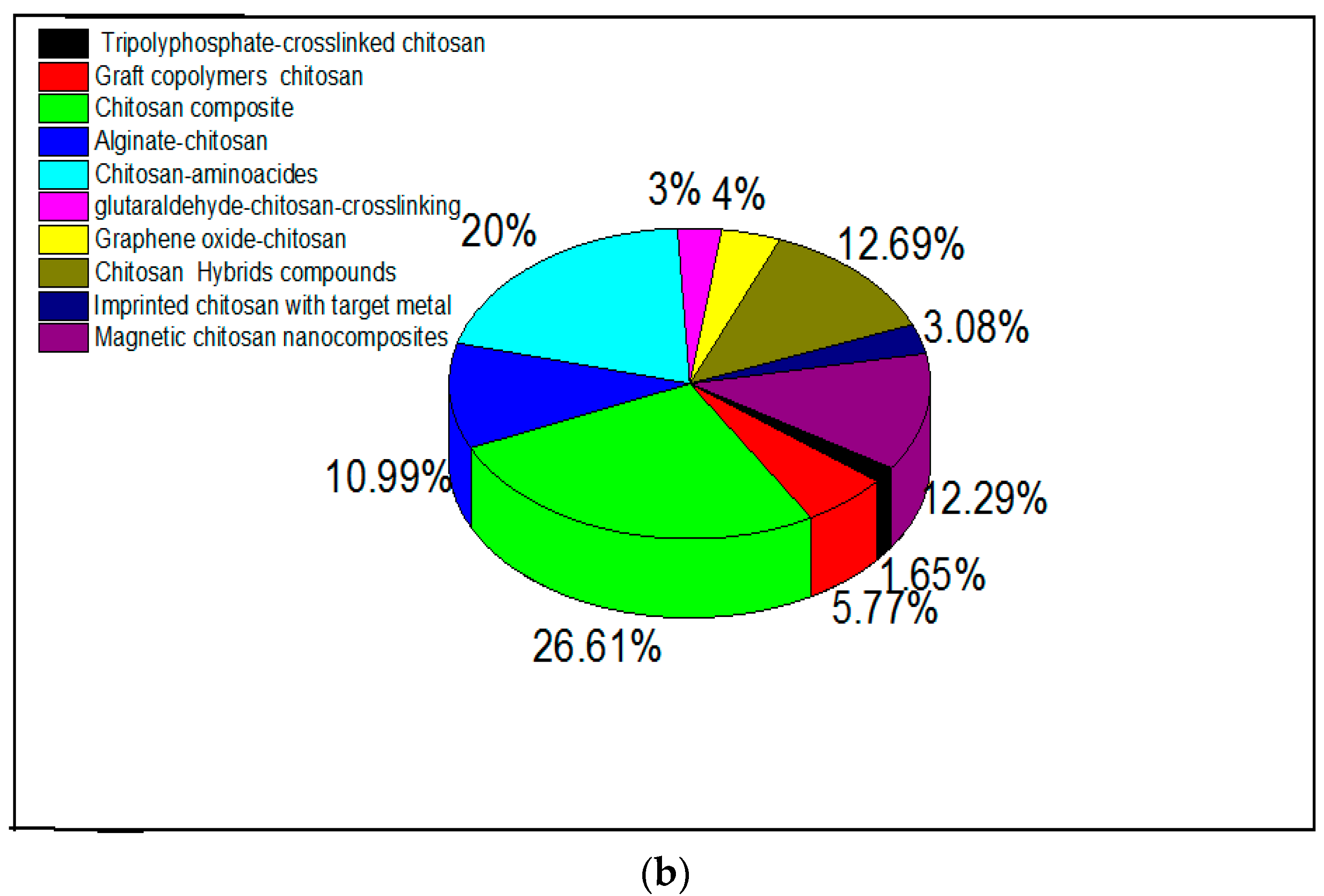
Amino-polysaccharide chitosan and polycarboxylic biopolymer alginate (containing guluronic and mannuronic acid groups) have been modified in research either by cross-linking to improve chemical stability [54] or through functionalization by grafting specific reactive groups [95,96,97]. Modifications of chitosan and alginate are realized by physical and chemical methods, and expand their applications [98]. Researchers have confirmed that a chemical modification is necessary to overcome chemical resistance and the weak physical strength of chitosan, and that cross-linking reinforces the chemical stability of the compound [98]. Research interest in alginate and chitosan-based adsorbents for the removal of metal ions from 2015 is shown in Figure 1a,b, respectively. There has been an increasing number of publications in this field, many of which discuss related modifications.
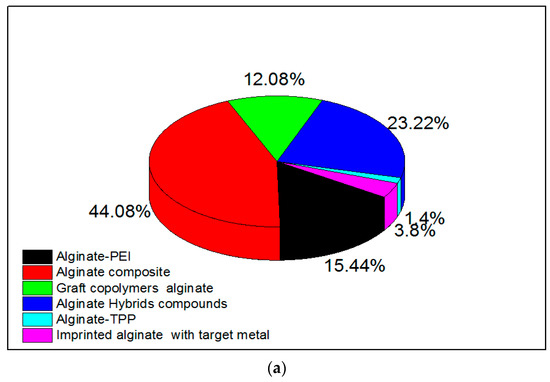
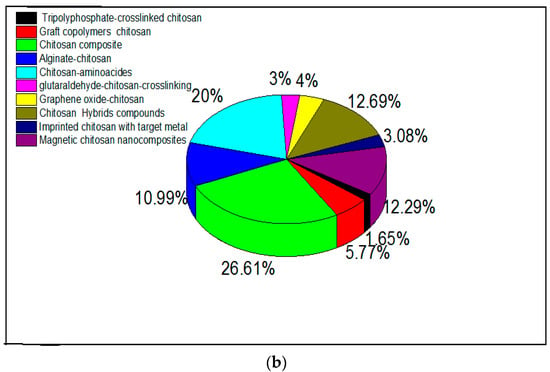
Figure 1.
Research interest in (a) alginate-based and (b) chitosan-based adsorbents for metal ions. Data were obtained from ScienceDirect using different keywords which are represented in the figure.
4.2. Physical State of Chitosan and Alginate and Related Effects on Adsorption Properties
Physical modifications give rise to varied shapes depending on the technique used. A number of techniques have been used to mold chitosan and alginate into different homogeneous or heterogeneous morphologies of different sizes. The forms include powder, flakes, wires, gel and gel beads, hydrogel, polymers which are soluble in water, sponges, membranes, fibers, grains, and resins. The size of the particles is a key parameter in controlling their sorption performance. The adsorption capacity varies randomly due to variations in particle size. The physical structure of chitosan and alginate, with larger surface areas, greter porosity and smaller size, can speed up the adsorption process, thereby shortening the time required for adsorption equilibrium [99]. Diverse forms of alginate and chitosan, like beads, films, powders, and nanofibers are obtained through physical or chemical modifications, as described below.
4.3. Grafting or Functionalization
Grafting or functionalization is the addition of chemical groups in the structure of the polysaccharide by covalent bonds, which has become a common means of modifying the structural properties of parent materials to improve their adsorption capacity and selectivity toward a target metal [100]. This type of modification can cause an increase in the number of functional sites involved in metal complexation [101]. The removal efficiency of metal ions by materials grafted on chitosan and alginate compared to their natural forms is directly related to the added function. Thus, modifications might increase the efficiency, or decrease efficiency but improve other properties.
4.4. Difference between Functionalization, Copolymerization and Grafting
There is a difference between functionalization, copolymerization, and grafting. The functionalization of polymers is the process of introducing new chemical groups into the structure of the polymer to create a product with specific properties. Copolymerization (or grafting of polymers) is the formation of a copolymer from the copolymerization of at least two types of monomers which are chemically different, called co-monomers. It is therefore formed with at least two repeating units, and a copolymer is distinct from a homopolymer. The advantage of copolymers such as chitosan-cyclodextrins and alginate-bentonite lies in their physicochemical and mechanical properties, which are intermediate with those obtained by the corresponding homopolymers. Creating composite materials by combining polysaccharides with other polymers that contain functional groups is a cost-effective approach that can improve the elimination of a wide range of pollutants [102]. Uniting monomers into a chain is a process known as polymerization, which yields polymers. When different monomers are united, the product is known as a copolymer and the process as copolymerization. Depending on the alignment sequence of different monomers in a polymer chain, copolymers have diverse properties [103]. Graft copolymerization is a common method to improve adsorption capacity and increase the chelating or complexing properties by introducing functional groups into the initial structure of chitosan. During grafting, side chains are covalently bonded to the key polymer backbone to give a branched copolymer structure [101]. According to Benamer et al. (2011), graft copolymerization onto cross-linked chitosan beads with glutaraldehyde is essential to augment the percentage of adsorption sites. The adsorption capacity of the grafted copolymer shows a better ability to adsorb Cd(II) and Pb(II) ions than unmodified chitosan, and as such, the adsorption capacity of the graft copolymer increases with increasing degree of grafting [104]. For example, Mishra and Sharma (2011) synthesized chitosan-graft-c-cyclodextrin (Ch-g-c-CD) for Cd(II) removal, achieving better adsorption, i.e., 833.33 mg/g, which was better the raw form [105].
4.5. Cross-Linking
Crosslinking agents (crosslinkers) are bifunctional with different forms such as straight chains, branch chains, and rings. Crosslinking leads to the formation of a three-dimensional network by a chemical or physical path. Links between macromolecular chains are created. Crosslinking can be accomplished by chemical methods (using crosslinking agents; crosslinking corresponds to the creation of covalent bonds between linear chains) or physical methods (resulting from non-covalent linkages between polymer chains by heat curing, electron-beam, or ultraviolet irradiation processes) to enable chitosan to be stable in acidic solution for metal sorption. Physical crosslinking is weaker than chemical crosslinking since it relates to physical attractive forces such as ionic interactions, hydrophobic and hydrogen bonding. Well-known crosslinking agents for chitosan and alginate are ethylene glycol diglycidyl ether (EGDE), epichlorohydrin (ECH), polyethyleneimine (PEI), triphosphate (TPP) and glutaraldehyde (GLA). According to research, crosslinking is the most popular modification method in the preparation of chitosan and alginate-based adsorbents. Cross-linkers are bridges between different polymer chains which combine with the functional groups of chitosan; hence, a cross-linker requires at least two functional groups per molecule [86]. For example, PEI is used extensively as a crosslinking and grafting agent for the adsorption of Au(III) [106], Cr(VI) [107], La(III) and Tb(III) [108], Cu(II) [109], Pb(II), Cu(II), Cd(II), Zn(II) and Ni(II) [110].
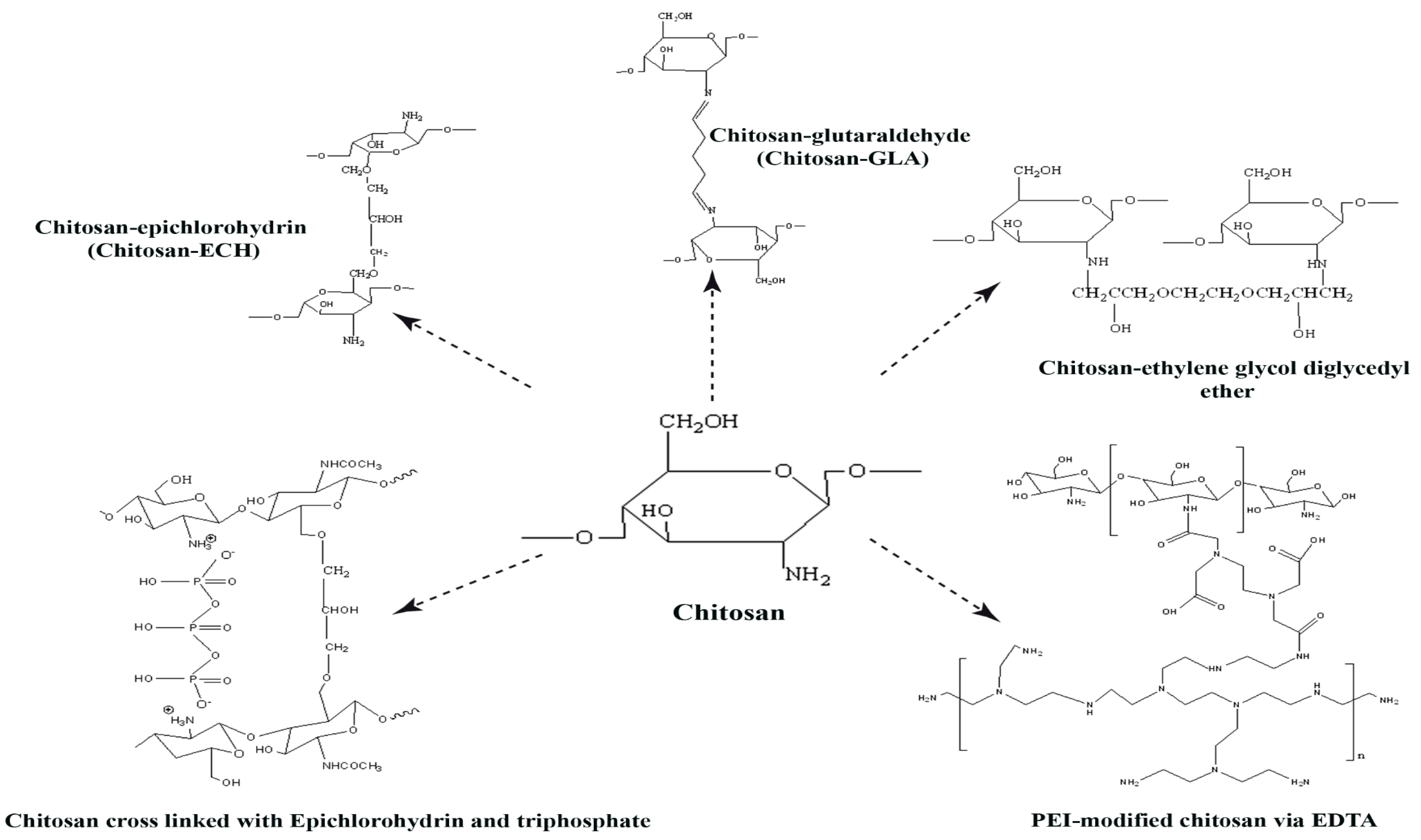
In addition, some polymers like cyclodextrins can be used for the grafting of other functions to the structure of chitosan or the incorporation of two properties by coupling. A chitosan rich in –OH and –NH2 must be grafted with –COOH to improve adsorption in acidic solutions. For example, a new method for the modification of β-cyclodextrins using chitosan (molecularly imprinted polymers (MPI)) was used with success by Ahmed et al. (2017) for the selective separation of Remazol Red 3BS in a trichromatic mixture. This new polymer had a regeneration efficiency for four cycles [111]. Zhao et al. (2018) chemically incorporated β-cyclodextrins into chitosan by way of esterification using citric acid, obtaining a biosorbent with a high capacity to remove reactive dyes from wastewater [112]. Figure 2 summarizes crosslinking methods.
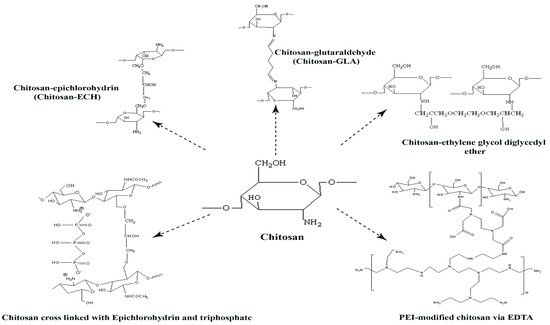
Figure 2.
Schematic representation of chitosan and the possibility of cross-linked chitosan; chitosan-glutaraldehyde(chitosan-GLA), chitosan-epichlorohydrin (chitosan-ECH), chitosan-ethylene glycol diglycidyl ether (chitosan-EGDE), cross-linking of chitosan with epichlorohydrin and triphosphate and polyethyleneimine (PEI)-modified chitosan via ethylenediaminetetraacetic acid (EDTA).
Various cross-linking methods are used for alginate hydrogels [113,114]. Lee et al. (2013) reported that alginate beads are not limited to spherical shapes. By varying the falling distance of an alginate solution, bead formation can be tear-, egg-, or spherically-shaped. The authors showed that the shape of Ca(II)-alginate beads depend on the impact force of the droplet when it hits the surface of the gelation bath [115]. The physical form or diameters of alginate beads plays a vital role in the increase of the adsorption efficiency and qmax of pollutants. Park et al. (2004) compared the adsorption capacity of prepared beads, capsules and alginate gel-coated cotton or paper for the elimination of lead ions. The authors showed that the uptake of lead was 1540 mg/g and 450 mg/g using alginate capsules of 3.5–4.0 mm and 2.5–3.0 mm of dry sodium alginate respectively. With the given adsorbent sizes, equilibrium was reached after 3 h, and decreased with a reduction in the size of adsorbents. Equilibrium was reached rapidly within 10 min, which was faster than other adsorbents, and lead uptake capacity was also slightly greater than that of alginate beads. Matrix alginate gel adsorbents can be used in a dried state, which makes them easy to handle. The dried form can be used in industry for water treatment, including in water purifier systems. The authors of [115] demonstrated the potential industrial applications of biomaterials for the elimination of various heavy metals [10]. The high binding capacity of capsules may have been due to the new structural characteristics of the beads in capsule form. The question of what caused the changes in the structures of alginate and chitosan remains unanswered. Generally, the chemical modification of a product results in new characteristics of the texture of the material. To increase the range of its applications, certain objectives must be met: 1. Clear visual improvement of adsorption capacity compared to base materials; 2. Increasing the pH range for the application of the material; 3. Strengthen stability; 4. Increased fixation ability; and 5. Improved fixation selectivity.
5. Criteria for Choosing the Best Adsorbent/Biosorbent
The choice between adsorption or biosorption is based on selecting the most promising adsorbent/biosorbent type from a wide range of naturally available materials. Several researchers have modified chitosan and alginate; this is necessary because natural forms are not selective for some heavy metals, and are difficult to use in polluted water. The results after modification are satisfactory if there is an improvement in the pH range and reduction of equilibrium time. However, there are no clear criteria for the best adsorbent, because adsorption capacity depends on several parameters. These parameters are directly linked to the behavior of the metal in aqueous solution, and the adsorption of two adsorbents can only be compared with the same equilibrium concentrations (C0, mmol/L or mg/L), preparations and methods of agitation, and sorption mechanisms.
Comparisons of the adsorption performance also depend on the effluent, i.e., its composition, concentration, competition between pollutants in the complex solution and the method used to decontaminate water and wastewater (discontinuous method “batch”, continuous method “column”). Thus, a direct comparison of data obtained using different biomaterials based on alginate, chitosan, other material, or even the same material, e.g., chitosan–chitosan, for the elimination of the same pollutants is not possible because the experimental conditions will not be the same e.g., the origin of the chitosan, degree of desaytelation, method of preparation, and condition related to adsorption, etc. However, we can compare the statistics of the aforementioned research to get an idea of the efficacy of adsorbents and justify the modifications made. In addition, due to the rarity of price information, it is difficult to make a clear comparison of manufacturing expenses. The performance of adsorbents can be compared and classified according to their efficiency and porous structure. Additionally, the time to reach adsorption equilibrium should be as short as possible. Therefore, adsorbents with high surface area, porosity, and fast adsorption kinetics are preferable for removing pollutants [5].
Generally, an adsorbent that is effective in the treatment of various pollutants should meet several requirements: 1. Effective at removing a variety of pollutants; 2. Acceptable sorption rate; 3. High selectivity at different concentrations to examine the removal efficiency for one metal versus another (in bimetallic and multimetallic solutions); 4. Particle size offering a large surface area and a high level of porosity; 5. Adequate physical, mechanical and thermal resistance; 6. Regenerative power if necessary (with most properties remaining stable after several cycles); 7. Tolerance to a large variety of operating parameters (strong acidic or basic medium and temperature); 8. Basic structure rich in active functions; and 9. Inexpensive base material and modification process.
6. Mechanisms of Sorption/Biosorption, Relationship between the Mechanisms and pH
After selecting the most promising adsorbents, the next step is to identify the adsorption mechanisms. A large number of papers devoted to the elimination of pollutants by modified chitosan and alginate adsorbents have focused on performance and descriptions of sorption parameters, while only a few have reported on the sorption mechanisms and the positive input of unmodified materials in these mechanisms.
Pollutant adsorption/sorption on solid/gel adsorbents involves three main steps: 1. Transport of the pollutant from the bulk solution to the sorbent surface (mass transport); 2. Adsorption on the particle surface (transport film); and 3. Transport within the sorbent particle (slow intraparticle after fast adsorption). Kinetics and isotherms provide information about the sorption mechanism, how the pollutant is bound in the sorbent, whether single- or multi-layer absorption occurs, and whether the particles are strong or weak and temperature-sensitive. This type of information is essential for understanding the sorption process and for choosing a simple desorption strategy and chemical agent that will achieve the desired desorption operation.
Due to the complexity of the materials and their specific properties (such as the presence of complexing chemical groups, small surface area, low porosity, heterogeneous or homogeneous surface area and swelling phenomenon), the sorption mechanism differs from traditional adsorbents. These mechanisms are complex, because of the presence of different interactions. In addition, a wide range of chemical structures vary with a change in pH, salt concentration, and the presence of ligands.
pH can affect the adsorption properties of chitosan and alginate and the mechanisms of metal ions in solution. Metal complexation by chitosan and alginate may involve various mechanisms; often, two different mechanisms of chelation and ion exchange are present. Sorption efficiency is affected by pH and other parameters. Amine sites (-NH2, -NH, etc) are the most important reactive groups to trap metal ions, although hydroxyl groups are responsible for metal ion binding through chelating mechanisms. Acidic solutions are protonated and possess electrostatic properties. Thus, it is possible to adsorb metal ions through anion exchange mechanisms. Several single or mixed interactions exist, including: 1. Chelation interaction (coordination) at amino groups in pendant fashion or in combination with vicinal hydroxyl groups; 2. Phenomena of complexation (electrostatic attraction) in acidic media; and 3. Ion exchange with protonated amino groups by proton or anion exchange, where the counter ion is exchanged for the metal anion. Physical adsorption plays no role in the interaction between cross-linked chitosan or alginate beads and contaminants, since the beads have a small surface area. pH can also affect the speciation of metal ions, and a change in speciation can lead to interactions that turn the chelating mechanism into an electrostatic attraction mechanism.
7. Proposed Mechanism for Sorption by Chitosan-Acids Amine-Based Materials
After the desired modifications of the parent materials (chitosan or natural alginate) have been performed, the mechanism between adsorbates and adsorbent varies according to the physical and chemical forces which occur during the adsorption of the target pollutant [98]. The presence of ligands on the chitosan or alginate may play an important role in the adsorption mechanism and, depending on the crosslinking agent, can vary the mechanisms. Thus, the adsorption mechanisms are complex, because not only the chitosan or alginate plays a role, but also the pre-matrix, types of modification, choice of grafting and crosslinking agent. Knowing this, cross-linking offers other possibilities for sorption, e.g., other reactions like complexation, attraction electrostatic, and ion-exchange. In this case, it is essential to note that different types of interactions can act at the same time and in a competitive manner [45]. Sometimes, physical adsorption and chemical interactions are involved in the adsorption process, such as, ion-exchange, acid–base interactions, and hydrogen bonding due to the amino groups.
In crosslinked materials, both physical adsorption in the polymer structure and chemisorption of the pollutant via hydrogen bonding, acid-base interactions, complexation, and ion exchange are involved in the sorption process. In most cases, a combination of these interactions is proposed for adsorption mechanisms, even though the efficiency and the selectivity of the adsorbents are attributed to the chemical network.
The grafting of chitosan and alginate is a common way to enhance the adsorption properties, improve the target metal selectivity, improve the chelating or complexation properties and introduce functional groups into the chitosan [47,73,84,116,117,118,119]. The adsorption of pollutants such as dyes or metal ions on the active groups of chitosan, alginate or chitosan- and alginate-based materials (such as –OH, –COOH and –NH2) depends on the molecular structure of the sorbent compounds. The structure varies according to the modification protocol and the percentage of the grafting or crosslinking agent in the polymer matrix. Crosslinking agents bind to metal ions by various methods, such as: (1) coordination bonds; (2) ion exchange; (3) complexation; (4) electrostatic interaction; (4) acid-base interactions; and (5) hydrogen bonds. Crini confirmed that the nature of the bonds between pollutants and material surfaces depend on the extent of the acid–base interactions; for weak acid–base interactions, only hydrogen bonds are formed, while for strong acid–bases, interactions may gradually change to a chemical complexation [45,120]. When regenerating the loaded sorbent, the co-existence of interactions between the pollutants and the sorbent matrix must to be taken into account. Typically, a combination of these interactions has been proposed to clarify the mechanisms of adsorption of chitosan, alginate and their derivatives.
Biosorbents based on chitosan and alginate have functional groups, such as carboxyl, hydroxyl and amine groups, which serve as active sites to bind heavy metals, either by ion exchange (where –COOH groups are involved) or by a complexation mechanism (where –COOH groups, –OH, –SH and –NH2 may be involved) [121,122,123]. The high percentage of nitrogen can activate chelation mechanisms, thereby increasing the sorption efficiency. Thus, a high percentage of –S and –O and the increase of –N offer advantages in terms of the mechanisms of sorption, as summarized in Table 2.
Often, the efficiency of chitosan-based material sorption is due to the coordination between –N and M2+, and/or the ion-exchange between the internal or external cross-linking center ions Na+/Ca++ and M2+. The presence of Na+ and Ca2+ cations on the surface of the materials has a positive impact on the mechanisms involved. Some examples of the possible interactions between metals and sorbents are dependent on the structure of the sorbents. Table 2 summarizes the possible interactions with pollutants, depending on the type of pollutant, type of modification and the structure of the biosorbent. For example, for cyclodextrin-based adsorbents, organic beads containing amine functions grafted onto cyclodextrins formed an inclusion complex with the pollutant through host–guest interactions [45]. The presence of hydrophobic pollutant–pollutant interactions can clarify some important properties of adsorption [124,125]. However, recent studies have shown that, in general, the binding mechanism of a specific pollutant plays a dominant role in sorption. For example, in grafted or coated silica-based adsorbents containing biopolymers, although the mineral matrix may participate in the sorption mechanisms, the interactions between the polymer and the pollutant mostly explain the mechanism [126,127].

Table 2.
Sorption features acquired with the addition of a new function.
Table 2.
Sorption features acquired with the addition of a new function.
| Adsorbent | Functions (Natural and Added Functions) | Features Acquired with the Addition of a New Function in the Mechanism | Source |
|---|---|---|---|
| Ca(II)–alginate–grafted–amino–carbamate | –O Adding –N function | Adding the –N function causes ion-exchange complex formation between U(VI) ions. | [126] |
| Chitosan functionalization with amino acids | –O Increasing percent of –N | Interaction with Cu(II) occurs with –OH and –NH2 groups belonging to both chitosan and amino acids incorporated into the matrix, uptake of copper occurs via chelation, ion exchange or complexation. | [128] |
| Glutaraldehyde–crosslinked–chitosan | –O –N | Increasing the percentage of glutaraldehyde in the chitosan matrix increases the efficacy of Au(III) due to various mechanisms which occr together, like electrostatic attraction, chelation, and reduction of Au(III). | [129] |
| Tripolyphosphate (TPP)- chitosan-ethylene glycol diglycidyl ether (EGDE) | –O –N | The increase in the amount of Cr(VI) adsorption compared to natural chitosan due to ion exchange. | [130] |
| Chitosan-cross-linked- ethylene glycol diglycidyl ether and modified through the reaction with ethylenediamine and 3-amino-1,2,4-triazole-5-thiol | –O –N Adding of –S function | The complex mechanism between Ag(I) and the donor atoms (N and/or S) on modified chitosan. Also, the presence of free lone pairs of electrons on –N or –S atoms that are suitable for coordination with Ag(I) resulting in the corresponding chitosan modified–metal complex. | [131,132] |
| Xanthation-alginate CS2 | –O Presence of C=S group | Complexation through carboxylate groups and ion exchange between metals and Ca(II) are the dominant mechanism in the case of alginate, but in the case of alginate modifier, the most remarkable result deals with the increasing the possibility of the ion-exchange mechanism during interaction of Xan-Alg2 with Cd(II). | [133] |
| Alginate-polyethyleneimine composite hydrogel | –O Adding –N | Chelation mechanism between the Cd(II), Pb(II) and the amine groups of the alginate/PEI hydrogel. | [118] |
| Biobased amphoteric aerogel derived from amine -modified clay-enriched chitosan/alginate | –O –N | The adsorption mechanism was the result of electrostatic interactions. | [134] |
8. Amino Acid-Grafted-Chitosan and -Alginate
Recently, research interest in amino acid-grafted-chitosan and -alginate for the removal of pollutants from water and wastewater has grown [12]. The graft method endows the chitosan substrate with a variety of additional functions and properties resulting from the nature of the grafted amino acid. Therefore, amino acid-grafted-cgfgsgfhitosan or -alginate can be designed for the adsorption of specific pollutants, with good adsorption capacity and enhanced selectivity.
According to the classification of Nieboer and Richardson,(1980), Pletnev and Zernov, and Hard & Soft Acid-Base theory [135,136,137], the metals involved in pollution belong to class B and to the intermediate class, as follows: Hg(II), Pb(II), Cu(II), Ni(II), Zn(II) and Cd(II), but U(VI) belongs to class A. The listed metals are sensitive to the amine function –NH2 and form bonds. Wang and Zhuang (2018) confirmed that amino groups play a major role in the adsorption process [98]. This means that the addition of amine functions is an effective way to improve the adsorption and structural properties of alginate and chitosan; these functions are selective, and are responsible for the fixation of the majority of heavy metals.
The choice of grafted molecules/functions gives chitosan substrates new properties resulting from the nature of the grafted amino acid. Consequently, chitosan grafted with amino acids can be designed to trap specific pollutants, with good adsorption capacity and augmented selectivity of adsorption [12]. Increasing the percentage of nitrogen in the polysaccharide structure yields better results compared to unmodified chitosan. Fujiwara et al. [138] used cross-linked and lysine-modified chitosan. Other modifications to chitosan with a series of amino acids have been made by Ishii et al. [13] and Ramesh et al. [139], who successfully modified chitosan with glycine. With regard to alginate, modifications have been carried out by Guibal et al. with polyethyleneimine (PEI) and urea, and biuret with the aim of increasing the rate of the amine function in the structure of the new adsorbents. The –NH2 function is known for its metallic chelation characteristics [140,141].
8.1. Functionalization of Chitosan by Amine Functions
Chitosan has a large number of amino and hydroxyl groups and can be used as an adsorbent to purify effluents containing heavy metals, radioactive metals and dyes [142,143]. It is widely accepted that this function plays an important role in the process of adsorption/sorption/biosorption. The addition of amine functions is an effective method to enhance adsorption and strengthen the structural properties of chitosan; these functions are selective, and are responsible for the fixing of most heavy metals via several possible mechanisms. The presence of more available and useful functional groups enhances the selective adsorption of adsorbates by specific sites for each pollutant, thus increasing the adsorption capacity and selectivity [93,144].
The presence of amino groups makes the polymer a cationic polyelectrolyte (pKa = 6.5) a few such compounds may be found in nature [145]—with an adsorption capacity over a wide pH range. Chitosan is the only pseudo-natural cationic polymer among other natural polysaccharides. Thus, it has unique properties which contribute to its rich potential applications, i.e., low toxicity and good chemical reactivity [84]. Furthermore, due to the presence of the amino and hydroxyl groups, chitosan can be easily modified by chemical methods to yield modified chitosan. In recent years, many studies have shown the value of chitosan grafted with amino acids for the removal of various pollutants in water and wastewater [13,84,138,139,146]. The conjugation of amino acid moieties with chitosan has emerged as an alternative to increasing the adsorption capacity of chitosan-based materials [84,147]. The grafted molecules confer new properties on the chitosan substrate, resulting from the nature of the grafted amino acids. Therefore, amino acid-grafted chitosan can be designed to trap specific pollutants, with good adsorption capacity and increased adsorption selectivity. Increasing the percentage of nitrogen in the polysaccharide structure, in most cases, ensures better results compared to unmodified chitosan. Fujiwara et al. [138] used cross-linked chitosan modified with lysine. The authors studied the adsorption of Pt(IV), Pd(II), and Au(III) from aqueous solutions by comparing the efficiency of crosslinked chitosan resin (CCR, crosslinked with EDGE and PEI) with that of l-lysine modified crosslinked chitosan resin (LMCCR). Lysine grafting enhanced adsorption compared to classic crosslinking with a crosslinking agent, because the grafting of N-groups increased the number of sorption sites in LMCCR which are responsible for the fixation of several types of metals. Also, the higher surface area and ion exchange capacity of LMCCR compared to that of CCR enhanced the adsorption capacity, increased the density of sorption sites and changed the sorption sites to increase the sorption capacity [138]. Other modifications of chitosan with a series of amino acids have been made by Ishii et al. and Ramesh et al. [13,139], who successfully modified chitosan with glycine.
Negm et al. grafted glycine into chitosan to increase the adsorption sites for metal ions. The resulting material was applied to the treatment of industrial wastewater containing copper and cobalt ions. The adsorption capacity augmented with increasing numbers of amine groups. However, according to the pseudo-second-order equation (PSO), at a pH of 7, the maximum adsorption capacity of the modified chitosan, chitosan-glycine, was 82.9 mg/g for Co(II) and 165.91 for Cu(II). These values changed to 234.9 mg Co(II)/g and 281.28 mg Cu(II)/g when the pH increased to 9 [148]. Raw chitosan was more effective than the proposed modification, with a adsorption capacity of 115.4 mg/g for Co(II) and 196.08 mg/g for Cu(II) at pH 7; the capacity increased when the pH increased to 9 [148]. The authors noted that the percentage removal of metal ions on the modified chitosan was lower than that of raw chitosan. They attributed this to intermolecular hydrogen bonding between the substitution groups and unit monomer of chitosan, which led to a decrease in the number of free active groups such as –OH and –NH2 on the chitosan [148]. Raw chitosan with available free active groups had a higher adsorption capacity than its derivatives [148].
Karthik and Meenakshi synthesized new adsorbents based on polyaniline and chitosan (polyaniline grafted chitosan, PGC) for the elimination of Pb(II) and Cd(II). The maximum adsorptive capacity of Pb(II) was 16.07 mg/g and 14.33 mg/g for Cd(II) at an initial pH of 6. A TGA (thermo-gravimetric analysis) and DSC (differential scanning calorimetry) indicated that polyaniline grafted chitosan (PGC) exhibited greater thermal stability than chitosan, which was considered to be a positive contribution [149]. According to Beppu et al., the improved qmax for copper removal after histidine immobilization in the chitosan matrix was due to the highly porous chitosan structure after grafting [150]. Researchers have also fabricated chitosan membranes functionalized with l-aspartic acid, l-glutamic acid, l-histidine, and l-taurine, and confirmed that the modification improved the sorption efficiency [151].
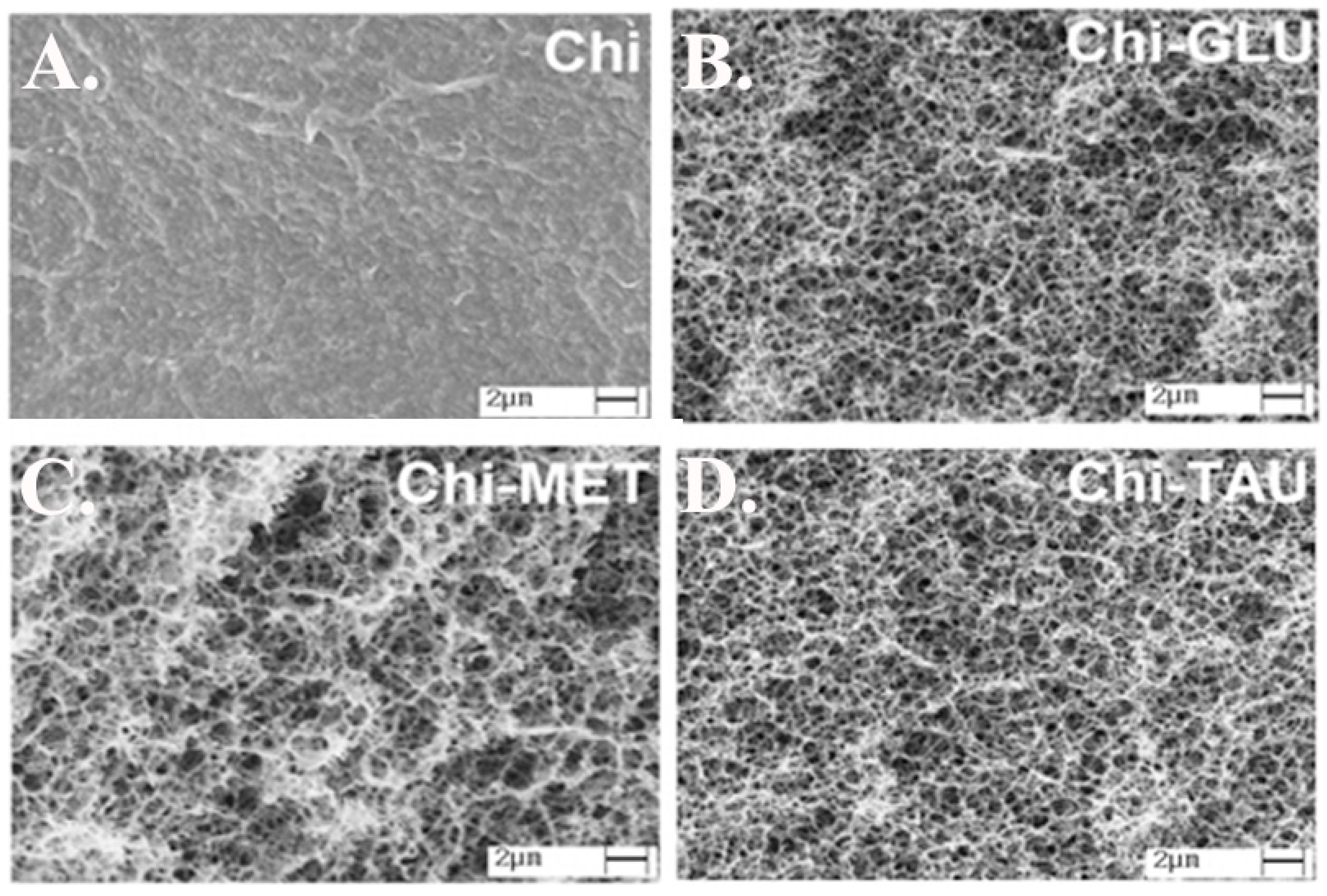
Abu El-Soad et al. worked on the adsorption of copper by amino acid-functionalized chitosan. Beads of chitosan were conjugated with glutamic (GLU), methionine (MET) and taurine (TAU) amino acid with the aim of increasing the uptake of Cu(II) in an aqueous media. The amino acid conjugations increased the Qmax from 82.55 mg/g prior to amino acid conjugation to as high as 146.79 mg/g for Chi–TAU, 152.51 mg/g for Chi–GLU and 170.3 mg/g for Chi–MET [128]. These results were attributed to the introduction of additional amino functions and new carboxylate and amino acid residues into the chitosan backbone, which can also be explored for applications requiring amino acids [128]. Figure 3 shows the change in structure of chitosan after modification.
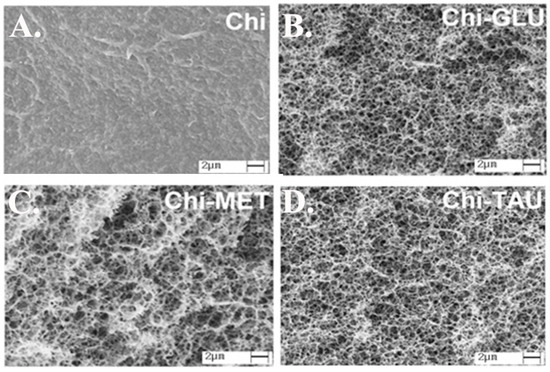
Figure 3.
Improvement of the structure of chitosan after functionalization by adding amino acids: (A) chitosan, (B) chitosan- glutamic acid (GLU), (C) chitosan-methionine (MET), and (D) chitosan-taurine (TAU). Reproduced from [128].
Various modified biosorbents have shown minor adsorption efficiency toward some metal ions compared to the original biosorbent. For example, Negm et al. confirmed that chitosan had the highest adsorption efficiency in an alkaline medium, i.e., approximately of 100% for copper and cobalt ions, while in a neutral medium, it exhibited the lowest metal removal efficiency. Glycine-modified chitosan showed moderate to high metal elimination efficiency in either media compared to natural chitosan [148].
The elimination efficiency of chitosan-glycine for copper and cobalt ions at a pH of 9 decreased by less than 5%, while chloroacetic acid-modified chitosan showed low adsorption efficiency toward these ions. This was attributed to the elevated amine function percentage in the alkaline medium. Moreover, the precipitation process occurred in an alkaline medium, which significantly increased the efficiency. This weak sorption for chloroacetic acid-modified chitosan was due to the link created between ClCH2– and H2N– groups in the chemical structure of the novel material. The linkage decreased the available –NH2, which was responsible for metal ion adsorption. The metal removal efficiency increased with chloroacetic acid-modified chitosan in an alkaline medium compared to a neutral one, and was attributed to the breakdown of–CH2Cl and –NH2 linkages [148].
In order to augment the Ni(II) adsorption capacity of natural chitosan beads (CB), Eser et al. chemically modified beads with histidine (HIS-ECH-CB) using epichlorohydrin (ECH) as cross-linking agent. According to the authors, the functionalized chitosan with the histidine showed an adsorption capacity of 55.6 mg/g under optimal conditions, i.e., 303 K and pH 5, although the researchers did not mention the sorption capacity of natural beads of chitosan [152].
8.2. Functionalization of Alginate by Adding Amine Functions
Researchers confirmed that alginate is useful for sequestering positively charged heavy metal ions such as Pb(II), Cd(II), Cu(II), Cr(III) and Zn(II) [73,153,154,155,156]. Benettayeb et al. confirmed that the addition of amine functions in the alginate structure by simple grafting of urea and biuret enhanced the adsorption properties of various metal ions, like Pb(II), Cu(II) and Cd(II) [73], Ni(II), Zn(II) and Hg(II) [74,75]. Such grafting can improve the selectivity and introduce new reaction possibilities in the sorption process. These researchers confirmed that for the adsorption of metals ions Cd(II), Pb(II) and Cu(II), the choice of the grafting of –NH2 function in alginate was not random, because, according to the Nieboer and Richardson (1980) and Pearson [135,136] classification, these metals are class B and intermediate class, the addition of this function improves the adsorption efficiency [73]. These authors confirmed that Pb(II) has an affinity for oxygen, and several researchers have reached efficiencies of more than 99%.
Polyethyleneimine (PEI) is known for its metal chelating properties due to the presence of a large number of amine groups in each molecule. It is often used to modify the sorbent surface area to increase sorption capacity [146,154]. Navarro et al. confirmed that Ca(II)-alginate (CA) does not contain active binding sites for the sorption of Cr(VI). CA-PEI is a novel adsorbent for sequestering Cr(VI) ions from aqueous solutions. The authors mentioned that the simplicity of its synthesis and the requirement of only inexpensive and nontoxic reagents, especially organic solvents, is one of its practical advantages associated with its use. Additionally, protonated amino groups of PEI served as binding sites for negatively charged Cr(VI) ions [156]. Bertagnolli et al. modified alginate beads with glutaraldehyde-crosslinked PEI (PEI-alginate) and alginate-carbon disulfide-grafted PEI (PEI-CS2-alginate), which were applied for the effective removal of Cd(II), Cu(II) and Pb(II) [141]. According to the researchers, the addition of PEI should improve the sorption properties and selectivity to alkali and alkaline earth metal ions, and reduce the influence of anions [141]. In addition, it was assumed that this type of reaction of PEI with carbon disulfide would form dithiocarbamate moieties with strong reactivity for metal ions [141,157,158]. Figure 4 shows the change in the structure of the alginate after the addition of an amine function.
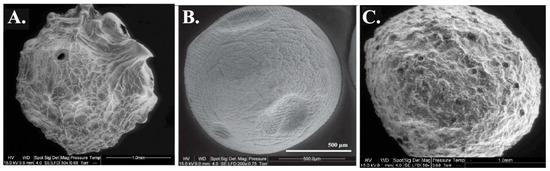
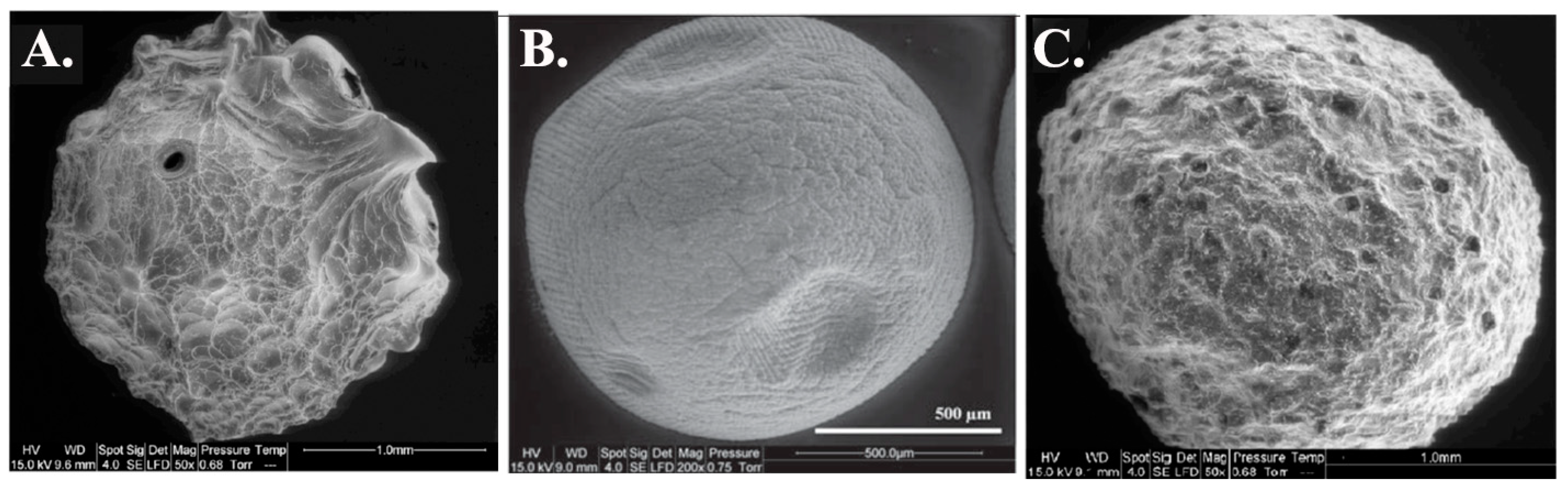
Figure 4.
Morphological change of alginate and alginate-based adsorbents (grafting of amine function)—ESEM Images. (A). Alginate beads, (B). Alginate-urea, and (C). Alginate-PEI, from [75].
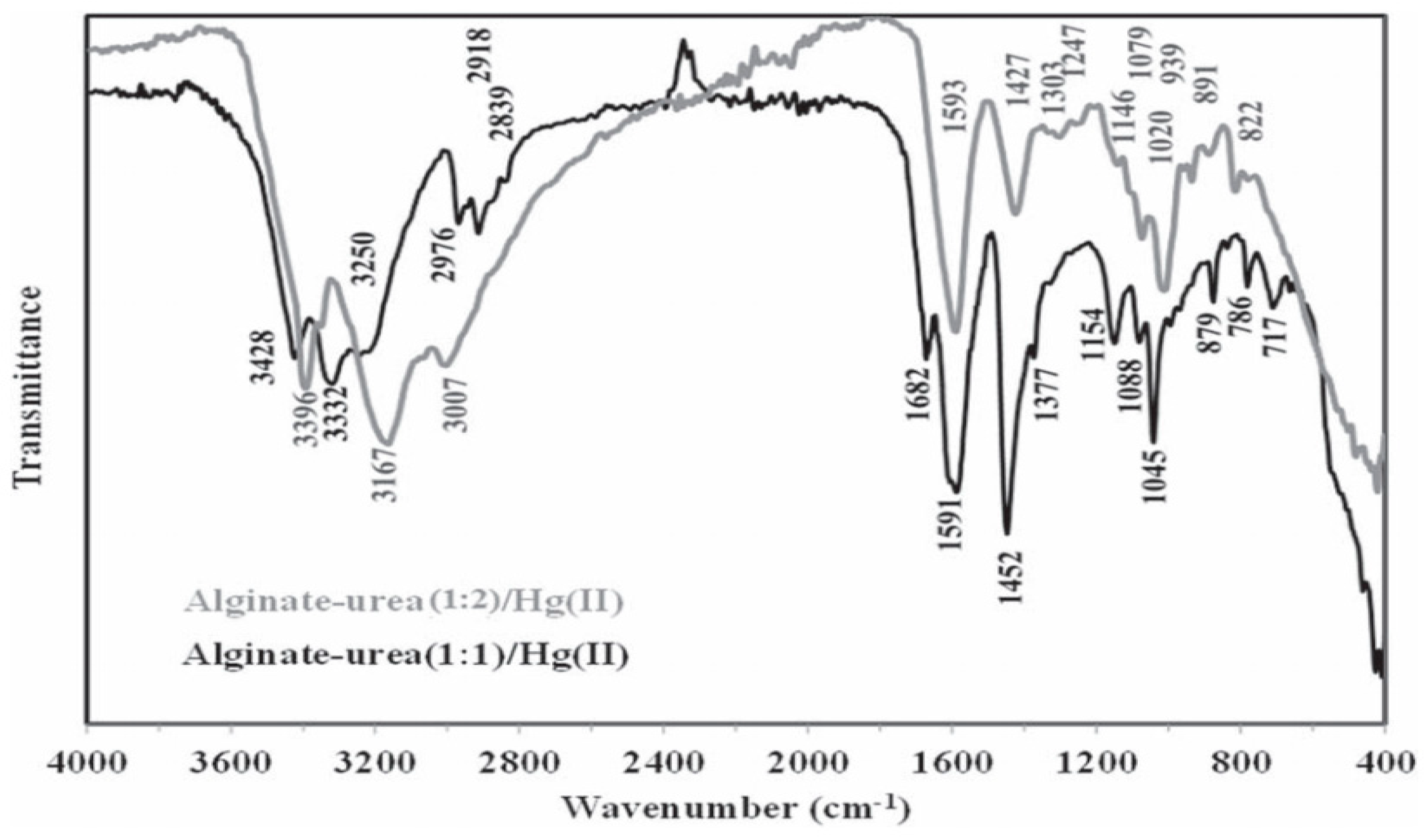
In addition, in the work carried out by Benettayeb et al., [75] an FTIR analysis confirmed the success of the grafting reaction of urea into the alginate, and even led to proposals about the reaction involved during this process. According to this analysis (see Figure 5), the peaks of primary amine appeared at 1146 cm−1, while the N-H symmetric and asymmetric stretching vibrations appeared at around 3500 and 3200 cm−1 (replaced by a large –OH band in the alginate). Also, other specific bands identified at 1745–1400 cm−1 confirmed the presence of different forms of amine groups in the alginate–urea structure after the grafting reaction. Appendix A Table A1 summarizes some of the new properties that appeared in the structure of chitosan and alginate after modification that did not exist in the unmodified parent compounds, as well as their contributions to the adsorption process.
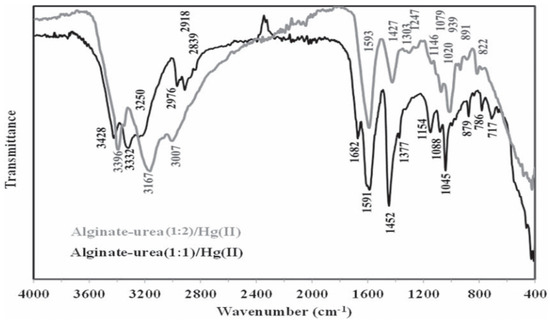
Figure 5.
Change in alginate structure after grafting two ratios of urea, (1:1) and (1:2), in order to incorporate amine functions [75].
9. Conclusions
Generally, the chemical modification of a product implies bestowing new characteristics upon its texture and widening its range of applications. In our case, study of the functionalization of alginate and chitosan had to meet the following objectives: 1. Significant visual improvement in the adsorption capacity compared to the base materials; 2. Increase in the pH range for the application of materials; 3. Enhanced stability; 4. Improved fixing capacities; and 5. Improved fixation selectivity. Therefore, chemical modification may be used to achieve the following objectives: Improve the existing properties, for example, the resistance of the ionic gel by additional covalent cross-linking; enhance biodegradation; improve the adsorption properties and the selectivity for each metal; and introduce completely new properties that do not exist in the unmodified parent alginate and chitosan.
Author Contributions
Conceptualization, A.B., S.G. and F.Z.S.; formal analysis, A.B., F.Z.S., S.G. and M.U.; resources, A.B., S.G. and F.Z.S.; writing—original draft preparation, A.B., S.G., M.U. and F.Z.S.; writing—review and editing, A.B., S.G., M.U., C.H.C., I.S. and M.S.; supervision, S.G., C.H.C., M.S. and I.S.; project administration, S.G. and A.B. All authors have read and agreed to the published version of the manuscript.
Funding
This review article received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
This work was carried out in the “Laboratoire de Génie Chimique et de Catalyse hétérogène, LGCCO”, at USTO-MB (Laboratoire 4216), Algeria. This laboratory is still under construction, although it was founded in 1986 by Pr. KESSAS Rachid. I thank all the members for this work.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A

Table A1.
Contributions acquired during modification of chitosan and alginate in recent studies.
Table A1.
Contributions acquired during modification of chitosan and alginate in recent studies.
| Sorbent | Metals | Contributions Acquired during Modification | Active Site Responsible for Fixing Metal | Reason for Increase or Decrease in qmax | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Before Modification | After Modification | ||||||||||
| Chitosan beads Chitosan-GLA beads Chitosan-alginate beads | Pb(II) | 1. New active site for Pb(II) adsorption 2. Same fixed situation pH profile up to 5 | –NH2 –OH | –OH –NH2 –COOH | –COOH and –NH2 are invloved in the adsorption of lead ions, which are not involved during the formation of the polyelectrolyte complex. | ||||||
| Chitosan beads Chitosan-GLA beads Chitosan-EGDE beads Chitosan-ECH beads | Cu(II) | 1. Chitosan beads dissolved in EDTA therefore difficult to reuse 2. Cross-linked chitosan does not dissolve in EDTA and desorption succeeds 3. Chitosan soluble in 5% (v/v) acetic acid and cross-linking chitosan insoluble | –COOH –OH | –OH –NH –OH –NH –OH –NH2 | When GLA and EDGA react with –NH2 the capacity decreases compared to normal chitosan while ECH reacts with –OH, which stabilizes the sorption capacity compared to the other two | ||||||
| Alginate beads Alginate-urea Alginate-biuret | Cd(II) Hg(II) Ni(II) Cu(II) Pb(II) Zn(II) | 1. New active site for sorption of heavy metals ions 2. Adsorbent resists different pH and temperature | –OH –COOH | –OH –COOH –NH2 | The increase in sorption efficiency compared to natural alginate is due to the presence of the –N and –O atom at the same time | ||||||
| Alginate ALG/PEI composite hydrogel | Cu(II) Pb(II) | 1. Adsorption capacity of the ALG/PEI composite hydrogel was remarkably improved after the incorporation of PEI | –OH –COOH | –OH –COOH –NH2 | The increase in sorption efficiency compared to natural alginate is due to the presence of the –N and –O atom at the same time | ||||||
| Alginate Alginate-EDTA | Cd(II) | 1. Mix the structural properties of alginate and EDTA to ameliorate the affinity of alginate vis-a-vis Cd(II) | –COOH –OH | –COOH –NH | Improve the adsorption and binding selectivity, due to the presence of different active groups | ||||||
| Mechanisms | Operating Conditions | qm (mg/g) | Isotderm Model | Mode | Ref. | ||||||
| t (min) | pH | T (°C) | rpm | Adsorbent Dose | Column | Batch | |||||
| The COO− ions formed due to the dissociation of carboxylate groups attract the positively charged of lead ions while the lone pair electrons on –N atom of –NH2 groups form complex with lead ions. So, chitosan beads with amine groups form complexation with Pb(II) ions | 100 | 4.5 | Room Temp. | 400 | 0.5 g | 34.98 14.24 60.27 | Langmuir | / | Batch | [117] | |
| / | 60 | 6.0 | Room Temp. | 500 | 0.01 g | 80.71 59.67 62.47 45.94 | Langmuir | / | Batch | [47] | |
| 6–8 h | 4.5–5.5 | 20 °C | 150 | 1 g/L | exceeded 200 | Langmuir/Sips | / | Batch | [73,74,75] | |
| 24 h | 5.5 | 25 °C | 200 | 0.5 g/L | 322.6 344.8 | Langmuir | / | Batch | [118] | |
| 6 h | 5.5 | 288–303 K | / | 100 mg | 177.3 | / | / | Batch | [119] | |
References
- Jehan, S.; Khan, S.; Khattak, S.A.; Muhammad, S.; Rashid, A.; Muhammad, N. Hydrochemical properties of drinking water and their sources apportionment of pollution in Bajaur agency, Pakistan. Meas. J. Int. Meas. Confed. 2019, 139, 249–257. [Google Scholar] [CrossRef]
- Singh, N.B.; Nagpal, G.; Agrawal, S. Rachna Water purification by using Adsorbents: A Review. Environ. Technol. Innov. 2018, 11, 187–240. [Google Scholar] [CrossRef]
- Mousazadeh, M.; Niaragh, E.K.; Usman, M.; Khan, S.U.; Sandoval, M.A. A critical review of state-of-the-art electrocoagulation technique applied to COD-rich industrial wastewaters. Environ. Sci. Pollut. Res. 2021, 28, 43143–43172. [Google Scholar] [CrossRef] [PubMed]
- Usman, M.; Waseem, M.; Mani, N.; Andiego, G. Optimization of soil aquifer treatment by chemical oxidation with hydrogen peroxide addition. Pollution 2018, 4, 369–379. [Google Scholar]
- Gupta, V.K.; Carrott, P.J.M.; Ribeiro Carrott, M.M.L. Suhas Low-Cost adsorbents: Growing approach to wastewater treatmenta review. Crit. Rev. Environ. Sci. Technol. 2009, 39, 783–842. [Google Scholar] [CrossRef]
- Usman, M. New Applications of Fine-Grained Iron Oxyhydroxides as Cost-Effective Arsenic Adsorbents in Water Treatment. Ph.D. Thesis, Technische Universität Hamburg, Hamburg, Germany, 2020. [Google Scholar]
- Khan, S.U.; Farooqi, I.H.; Usman, M.; Basheer, F. Energy Efficient Rapid Removal of Arsenic in an Electrocoagulation Reactor with Hybrid Fe/Al Electrodes: Process Optimization Using CCD and Kinetic Modeling. Water 2020, 12, 2876. [Google Scholar] [CrossRef]
- Tzu, T.W. Sorption of Pb(II), Cd(II), and Ni(II) Toxic Metal Ions by Alginate-Bentonite. J. Environ. Prot. 2013, 04, 51–55. [Google Scholar] [CrossRef]
- Wu, F.C.; Tseng, R.L.; Juang, R.S. A review and experimental verification of using chitosan and its derivatives as adsorbents for selected heavy metals. J. Environ. Manag. 2010, 91, 798–806. [Google Scholar] [CrossRef]
- Park, H.G.; Chae, M.Y. Novel type of alginate gel-based adsorbents for heavy metal removal. J. Chem. Technol. Biotechnol. 2004, 79, 1080–1083. [Google Scholar] [CrossRef]
- Papageorgiou, S.K.; Kouvelos, E.P.; Katsaros, F.K. Calcium alginate beads from Laminaria digitata for the removal of Cu+2 and Cd+2 from dilute aqueous metal solutions. Desalination 2008, 224, 293–306. [Google Scholar] [CrossRef]
- Arica, M.Y.; Bayramoǧlu, G.; Yilmaz, M.; Bektaş, S.; Genç, Ö. Biosorption of Hg2+, Cd2+, and Zn2+ by Ca-alginate and immobilized wood-rotting fungus Funalia trogii. J. Hazard. Mater. 2004, 109, 191–199. [Google Scholar] [CrossRef]
- Ishii, H.; Minegishi, M.; Lavitpichayawong, B.; Mitani, T. Synthesis of chitosan-amino acid conjugates and their use in heavy metal uptake. Int. J. Biol. Macromol. 1995, 17, 21–23. [Google Scholar] [CrossRef]
- Hamza, M.F.; Aly, M.M.; Abdel-Rahman, A.A.H.; Ramadan, S.; Raslan, H.; Wang, S.; Vincent, T.; Guibal, E. Functionalization of magnetic chitosan particles for the sorption of U(VI), Cu(II) and Zn(II)-hydrazide derivative of glycine-grafted chitosan. Materials 2017, 10, 539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamza, M.F.; Wei, Y.; Benettayeb, A.; Wang, X.; Guibal, E. Efficient removal of uranium, cadmium and mercury from aqueous solutions using grafted hydrazide-micro-magnetite chitosan derivative. J. Mater. Sci. 2019, 55, 4193–4212. [Google Scholar] [CrossRef]
- Hamza, M.F.; Mubark, A.E.; Wei, Y.Y.; Vincent, T.; Guibal, E.; Lu, S.; Salih, K.A.M.; Mira, H.; Dhmees, A.S.; Fujita, T.; et al. As(V) sorption from aqueous solutions using quaternized algal/polyethyleneimine composite beads. Gels 2020, 6, 137396. [Google Scholar] [CrossRef] [PubMed]
- Aslam, M.M.A.; Kuo, H.W.; Den, W.; Usman, M.; Sultan, M.; Ashraf, H. Functionalized carbon nanotubes (Cnts) for water and wastewater treatment: Preparation to application. Sustainability 2021, 13, 5717. [Google Scholar] [CrossRef]
- Schindler, P.W.; Stumm, W. The Surface Chemistry of Oxides, Hydroxides, and Oxide Minerals. In Aquatic Surface Chemistry: Chemical Processes at the Particle-Water Interface; John Wiley and Sons: New York, NY, USA, 1987. [Google Scholar]
- Gaya, U.I.; Otene, E.; Abdullah, A.H. Adsorption of aqueous Cd(II) and Pb(II) on activated carbon nanopores prepared by chemical activation of doum palm shell. Springerplus 2015, 4, 458. [Google Scholar] [CrossRef] [Green Version]
- Bohli, T. Comparative Study of Bivalent Cationic Metals Adsorption Pb(II), Cd(II), Ni(II) and Cu(II) on Olive Stones Chemically Activated Carbon. J. Chem. Eng. Process Technol. 2013, 4, 1000158. [Google Scholar] [CrossRef] [Green Version]
- Abudaia, J.A.; Sulyman, M.O.; Elazaby, K.Y.; Ben-Ali, S.M. Adsorption of Pb (II) and Cu (II) from Aqueous Solution onto Activated Carbon Prepared from Dates Stones. Int. J. Environ. Sci. Dev. 2013, 4, 191–195. [Google Scholar] [CrossRef] [Green Version]
- Zhang, K.; Cheung, W.H.; Valix, M. Roles of physical and chemical properties of activated carbon in the adsorption of lead ions. Chemosphere 2005, 60, 1129–1140. [Google Scholar] [CrossRef]
- Usman, M.; Katsoyiannis, I.; Mitrakas, M.; Zouboulis, A.; Ernst, M. Performance evaluation of small sized powdered ferric hydroxide as arsenic adsorbent. Water 2018, 10, 957. [Google Scholar] [CrossRef] [Green Version]
- Usman, M.; Katsoyiannis, I.; Rodrigues, J.H.; Ernst, M. Arsenate removal from drinking water using by-products from conventional iron oxyhydroxides production as adsorbents coupled with submerged microfiltration unit. Environ. Sci. Pollut. Res. 2020, 28, 59063–59075. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Usman, M.; Zarebanadkouki, M.; Waseem, M.; Katsoyiannis, I.A.; Ernst, M. Mathematical modeling of arsenic(V) adsorption onto iron oxyhydroxides in an adsorption-submerged membrane hybrid system. J. Hazard. Mater. 2020, 400, 123221. [Google Scholar] [CrossRef] [PubMed]
- Usman, M.; Belkasmi, A.I.; Katsoyiannis, I.A.; Ernst, M. Pre-deposited dynamic membrane adsorber formed of microscale conventional iron oxide-based adsorbents to remove arsenic from water: Application study and mathematical modeling. J. Chem. Technol. Biotechnol. 2021, 96, 1504–1514. [Google Scholar] [CrossRef]
- Pawluk, K.; Fronczyk, J.; Garbulewski, K. Removal of dissolved metals from road runoff using zeolite PRBs. Chem. Eng. Trans. 2013, 32, 331–336. [Google Scholar]
- Balintova, M.; Holub, M.; Singovszka, E. Study of iron, copper and zinc removal from acidic solutions by sorption. Chem. Eng. Trans. 2012, 28, 175–180. [Google Scholar]
- Fronczyk, J.; Pawluk, K.; Garbulewski, K. Multilayer PRBs-Effective technology for protection of groundwater environment in traffic infrastructure. Chem. Eng. Trans. 2012, 28, 67–72. [Google Scholar]
- Wyszkowski, M.; Radziemska, M. Effects of chromium(III and VI) on spring barley and maize biomass yield and content of nitrogenous compounds. J. Toxicol. Environ. Health-Part A Curr. Issues 2010, 73, 1274–1282. [Google Scholar] [CrossRef]
- Payne, K.B.; Abdel-Fattah, T.M. Adsorption of divalent lead ions by zeolites and activated carbon: Effects of pH, temperature, and ionic strength. J. Environ. Sci. Health-Part A Toxic/Hazardous Subst. Environ. Eng. 2004, 39, 2275–2291. [Google Scholar] [CrossRef]
- Li, Q.; Zhai, J.; Zhang, W.; Wang, M.; Zhou, J. Kinetic studies of adsorption of Pb(II), Cr(III) and Cu(II) from aqueous solution by sawdust and modified peanut husk. J. Hazard. Mater. 2007, 141, 163–167. [Google Scholar] [CrossRef]
- Salim, M.; Munekage, Y.; Naing, K.M. Arsenic(III) removal from contaminated water using silica ceramic: A batch adsorption study. J. Appl. Sci. 2007, 7, 2314–2320. [Google Scholar] [CrossRef]
- Salim, M.; Munekage, Y. Removal of arsenic from aqueous solution using silica ceramic: Adsorption kinetics and equilibrium studies. Int. J. Environ. Res. 2009, 3, 13–22. [Google Scholar] [CrossRef]
- Sulaymon, A.H.; Mohammed, A.A.; Al-Musawi, T.J. Competitive biosorption of lead, cadmium, copper, and arsenic ions using algae. Environ. Sci. Pollut. Res. 2013, 20, 3011–3023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaewsarn, P. Biosorption of copper(II) from aqueous solutions by pre-treated biomass of marine algae Padina sp. Chemosphere 2002, 47, 1081–1085. [Google Scholar] [CrossRef]
- Shamshad, I.; Khan, S.; Waqas, M.; Ahmad, N.; Ur-Rehman, K.; Khan, K. Removal and bioaccumulation of heavy metals from aqueous solutions using freshwater algae. Water Sci. Technol. 2015, 71, 38–44. [Google Scholar] [CrossRef]
- Bayramoǧlu, G.; Tuzun, I.; Celik, G.; Yilmaz, M.; Arica, M.Y. Biosorption of mercury(II), cadmium(II) and lead(II) ions from aqueous system by microalgae Chlamydomonas reinhardtii immobilized in alginate beads. Int. J. Miner. Process. 2006, 81, 35–43. [Google Scholar] [CrossRef]
- Sheng, P.X.; Ting, Y.P.; Chen, J.P.; Hong, L. Sorption of lead, copper, cadmium, zinc, and nickel by marine algal biomass: Characterization of biosorptive capacity and investigation of mechanisms. J. Colloid Interface Sci. 2004, 275, 131–141. [Google Scholar] [CrossRef]
- O’Connell, D.W.; Birkinshaw, C.; O’Dwyer, T.F. Heavy metal adsorbents prepared from the modification of cellulose: A review. Bioresour. Technol. 2008, 99, 6709–6724. [Google Scholar] [CrossRef]
- Benguella, B.; Benaissa, H. Cadmium removal from aqueous solutions by chitin: Kinetic and equilibrium studies. Water Res. 2002, 36, 2463–2474. [Google Scholar] [CrossRef]
- Synowiecki, J.; Al-Khateeb, N.A. Production, Properties, and Some New Applications of Chitin and Its Derivatives. Crit. Rev. Food Sci. Nutr. 2003, 43, 145–171. [Google Scholar] [CrossRef]
- Kumar, M.R. A review of chitin and chitosan applications. React. Funct. Polym. 2000, 46, 1–27. [Google Scholar] [CrossRef]
- Bailey, S.E.; Olin, T.J.; Bricka, R.M.; Adrian, D.D. A review of potentially low-cost sorbents for heavy metals. Water Res. 1999, 33, 2469–2479. [Google Scholar] [CrossRef]
- Crini, G. Recent developments in polysaccharide-based materials used as adsorbents in wastewater treatment. Prog. Polym. Sci. 2005, 30, 38–70. [Google Scholar] [CrossRef]
- Rorrer, G.L.; Hsien, T.Y.; Way, J.D. Synthesis of Porous-Magnetic Chitosan Beads for Removal of Cadmium Ions from Waste Water. Ind. Eng. Chem. Res. 1993, 32, 2170–2178. [Google Scholar] [CrossRef]
- Wan Ngah, W.S.; Endud, C.S.; Mayanar, R. Removal of copper(II) ions from aqueous solution onto chitosan and cross-linked chitosan beads. React. Funct. Polym. 2002, 50, 181–190. [Google Scholar] [CrossRef]
- Liu, D.; Li, Z.; Zhu, Y.; Li, Z.; Kumar, R. Recycled chitosan nanofibril as an effective Cu(II), Pb(II) and Cd(II) ionic chelating agent: Adsorption and desorption performance. Carbohydr. Polym. 2014, 111, 469–476. [Google Scholar] [CrossRef]
- Babel, S.; Kurniawan, T.A. Low-cost adsorbents for heavy metals uptake from contaminated water: A review. J. Hazard. Mater. 2003, 97, 219–243. [Google Scholar] [CrossRef]
- Varma, A.J.; Deshpande, S.V.; Kennedy, J.F. Metal complexation by chitosan and its derivatives: A review. Carbohydr. Polym. 2004, 55, 77–93. [Google Scholar] [CrossRef]
- Ibáñez, J.P.; Umetsu, Y. Uptake of trivalent chromium from aqueous solutions using protonated dry alginate beads. Hydrometallurgy 2004, 72, 327–334. [Google Scholar] [CrossRef]
- Papageorgiou, S.K.; Katsaros, F.K.; Kouvelos, E.P.; Nolan, J.W.; Le Deit, H.; Kanellopoulos, N.K. Heavy metal sorption by calcium alginate beads from Laminaria digitata. J. Hazard. Mater. 2006, 137, 1765–1772. [Google Scholar] [CrossRef]
- Kaçar, Y.; Arpa, Ç.; Tan, S.; Denizli, A.; Genç, Ö.; Arica, M.Y. Biosorption of Hg(II) and Cd(II) from aqueous solutions: Comparison of biosorptive capacity of alginate and immobilized live and heat inactivated Phanerochaete chrysosporium. Process Biochem. 2002, 37, 601–610. [Google Scholar] [CrossRef]
- Cataldo, S.; Muratore, N.; Orecchio, S.; Pettignano, A. Enhancement of adsorption ability of calcium alginate gel beads towards Pd(II) ion. A kinetic and equilibrium study on hybrid Laponite and Montmorillonite-alginate gel beads. Appl. Clay Sci. 2015, 118, 162–170. [Google Scholar] [CrossRef]
- Cataldo, S.; Gianguzza, A.; Pettignano, A. Sorption of Pd(II) ion by calcium alginate gel beads at different chloride concentrations and pH. A kinetic and equilibrium study Sorption of Pd(II) ion by calcium alginate gel beads. Arab. J. Chem. 2016, 9, 656–667. [Google Scholar] [CrossRef] [Green Version]
- Gotoh, T.; Matsushima, K.; Kikuchi, K.I. Preparation of alginate-chitosan hybrid gel beads and adsorption of divalent metal ions. Chemosphere 2004, 55, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Justi, K.C.; Fávere, V.T.; Laranjeira, M.C.M.; Neves, A.; Peralta, R.A. Kinetics and equilibrium adsorption of Cu(II), Cd(II), and Ni(II) ions by chitosan functionalized with 2[-bis-(pyridylmethyl)aminomethyl]-4-methyl-6- formylphenol. J. Colloid Interface Sci. 2005, 291, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.S.; Lingamdinne, L.P.; Yang, J.K.; Chang, Y.Y.; Koduru, J.R. Fabrication of chitosan/graphene oxide-gadolinium nanorods as a novel nanocomposite for arsenic removal from aqueous solutions. J. Mol. Liq. 2020, 320, 114410. [Google Scholar] [CrossRef]
- Boddu, V.M.; Abburi, K.; Talbott, J.L.; Smith, E.D.; Haasch, R. Removal of arsenic (III) and arsenic (V) from aqueous medium using chitosan-coated biosorbent. Water Res. 2008, 42, 633–642. [Google Scholar] [CrossRef] [PubMed]
- Benettayeb, A.; Haddou, B. New biosorbents based on the seeds, leaves and husks powder of Moringa oleifera for the effective removal of various toxic pollutants. Int. J. Environ. Anal. Chem. 2021, 1–26. [Google Scholar] [CrossRef]
- Allouche, F.N.; Guibal, E.; Mameri, N. Preparation of a new chitosan-based material and its application for mercury sorption. Colloids Surfaces A Physicochem. Eng. Asp. 2014, 446, 224–232. [Google Scholar] [CrossRef]
- Campos, K.; Guibal, E.; Peirano, F.; Ly, M.; Maldonado, H. Mercury sorption on chitosan. In Advanced Materials Research; Trans Tech Publications Ltd.: Baech, Switzerland, 2007; Volume 20–21, pp. 635–638. [Google Scholar]
- Imam, E.A.; El-Tantawy El-Sayed, I.; Mahfouz, M.G.; Tolba, A.A.; Akashi, T.; Galhoum, A.A.; Guibal, E. Synthesis of A-aminophosphonate functionalized chitosan sorbents: Effect of methyl vs phenyl group on uranium sorption. Chem. Eng. J. 2018, 352, 1022–1034. [Google Scholar] [CrossRef]
- Guo, X.; Yang, H.; Liu, Q.; Liu, J.; Chen, R.; Zhang, H.; Yu, J.; Zhang, M.; Li, R.; Wang, J. A chitosan-graphene oxide/ZIF foam with anti-biofouling ability for uranium recovery from seawater. Chem. Eng. J. 2020, 382, 122850. [Google Scholar] [CrossRef]
- Benettayeb, A.; Morsli, A.; Elwakeel, K.Z.; Hamza, M.F.; Guibal, E. Recovery of Heavy Metal Ions Using Magnetic Glycine-Modified Chitosan—Application to Aqueous Solutions and Tailing Leachate. Appl. Sci. 2021, 11, 8377. [Google Scholar] [CrossRef]
- Piron, E.; Accominotti, M.; Domard, A. Interaction between chitosan and uranyl ions. Role of physical and physicochemical parameters on the kinetics of sorption. Langmuir 1997, 13, 1653–1658. [Google Scholar] [CrossRef]
- Schmuhl, R.; Krieg, H.M.; Keizer, K. Adsorption of Cu(II) and Cr(VI) ions by chitosan: Kinetics and equilibrium studies. Water SA 2001, 27, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.; Turhan, M.; Gunasekaran, S. Selected properties of pH-sensitive, biodegradable chitosan-poly(vinyl alcohol) hydrogel. Polym. Int. 2004, 53, 911–918. [Google Scholar] [CrossRef]
- Barros Júnior, L.M.; Macedo, G.R.; Duarte, M.M.L.; Silva, E.P.; Lobato, A.K.C.L. Biosorption of cadmium using the fungus aspergillus niger. Braz. J. Chem. Eng. 2003, 20, 229–239. [Google Scholar] [CrossRef]
- Burke, A.; Yilmaz, E.; Hasirci, N.; Yilmaz, O. Iron(III) ion removal from solution through adsorption on chitosan. J. Appl. Polym. Sci. 2002, 84, 1185–1192. [Google Scholar] [CrossRef]
- Yang, T.C.; Zall, R.R. Absorption of Metals by Natural Polymers Generated from Seafood Processing Wastes. Ind. Eng. Chem. Prod. Res. Dev. 1984, 23, 168–172. [Google Scholar] [CrossRef]
- Arica, M.Y.; Arpa, Ç.; Ergene, A.; Bayramoǧlu, G.U.; Genç, Ö. Ca-alginate as a support for Pb(II) and Zn(II) biosorption with immobilized Phanerochaete chrysosporium. Carbohydr. Polym. 2003, 52, 167–174. [Google Scholar] [CrossRef]
- Benettayeb, A.; Guibal, E.; Morsli, A.; Kessas, R. Chemical modification of alginate for enhanced sorption of Cd(II), Cu(II) and Pb(II). Chem. Eng. J. 2017, 316, 704–714. [Google Scholar] [CrossRef]
- Benettayeb, A.; Guibal, E.; Bhatnagar, A.; Morsli, A.; Kessas, R. Effective removal of nickel (II) and zinc (II) in mono-compound and binary systems from aqueous solutions by application of alginate-based materials. Int. J. Environ. Anal. Chem. 2021, 1–22. [Google Scholar] [CrossRef]
- Benettayeb, A.; Morsli, A.; Guibal, E.; Kessas, R. New derivatives of urea-grafted alginate for improving the sorption of mercury ions in aqueous solutions New derivatives of urea-grafted alginate for improving the sorption of mercury ions in aqueous solutions. Materi. Res. Express 2021, 7, 035303. [Google Scholar]
- Basu, H.; Singhal, R.K.; Saha, S.; Pimple, M.V. Chitosan impregnated Ca-alginate: A new hybrid material for removal of uranium from potable water. J. Radioanal. Nucl. Chem. 2017, 314, 1905–1914. [Google Scholar] [CrossRef]
- Shim, J.; Kumar, M.; Mukherjee, S.; Goswami, R. Sustainable removal of pernicious arsenic and cadmium by a novel composite of MnO2 impregnated alginate beads: A cost-effective approach for wastewater treatment. J. Environ. Manag. 2019, 234, 8–20. [Google Scholar] [CrossRef] [PubMed]
- Dewangan, T.; Tiwari, A.; Bajpai, A.K. Removal of arsenic ions from aqueous solutions by adsorption onto biopolymeric crosslinked calcium alginate beads. Toxicol. Environ. Chem. 2009, 91, 1055–1067. [Google Scholar] [CrossRef]
- Sen, S.; Dutta, A.; Ponnala, R.; Kamila, B.; Baltrenas, P.; Baltrenaite, E.; Dutta, S. Removal of hexavalent chromium from synthetic wastewater using alginate immobilized cyanobacteria: Experiment and mathematical modeling. Environ. Eng. Sci. 2020, 37, 283–294. [Google Scholar] [CrossRef]
- Zeng, H.; Wang, F.; Xu, K.; Zhang, J.; Li, D. Preparation of manganese sludge strengthened chitosan-alginate hybrid adsorbent and its potential for As(III) removal. Int. J. Biol. Macromol. 2020, 149, 1222–1231. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, H.; Hu, X.; Feng, H.; Xiong, W.; Guo, W.; Zhou, J.; Mosa, A.; Peng, Y. Multicavity triethylenetetramine-chitosan/alginate composite beads for enhanced Cr(VI) removal. J. Clean. Prod. 2019, 231, 733–745. [Google Scholar] [CrossRef]
- Yi, X.; He, J.; Guo, Y.; Han, Z.; Yang, M.; Jin, J.; Gu, J.; Ou, M.; Xu, X. Encapsulating Fe3O4 into calcium alginate coated chitosan hydrochloride hydrogel beads for removal of Cu (II) and U (VI) from aqueous solutions. Ecotoxicol. Environ. Saf. 2018, 147, 699–707. [Google Scholar] [CrossRef]
- Kuczajowska-Zadrożna, M.; Filipkowska, U.; Jóźwiak, T. Adsorption of Cu (II) and Cd (II) from aqueous solutions by chitosan immobilized in alginate beads. J. Environ. Chem. Eng. 2020, 8, 103878. [Google Scholar] [CrossRef]
- Wang, J.; Chen, C. Chitosan-based biosorbents: Modification and application for biosorption of heavy metals and radionuclides. Bioresour. Technol. 2014, 160, 129–141. [Google Scholar] [CrossRef] [PubMed]
- Skjåk-Bræk, G.; Grasdalen, H.; Smidsrød, O. Inhomogeneous polysaccharide ionic gels. Carbohydr. Polym. 1989, 10, 31–54. [Google Scholar] [CrossRef]
- Habuda-Stanić, M.; Ravančić, M.; Flanagan, A. A Review on Adsorption of Fluoride from Aqueous Solution. Materials 2014, 7, 6317–6366. [Google Scholar] [CrossRef] [PubMed]
- Joseph, O. Etude du Potentiel D’utilisation de Résidus Agricoles Haïtiens pour le Traitement par Biosorption D’effluents Pollués. Ph.D. Thesis, Institut National des Sciences Appliquées, Bellignat, France, 2009. [Google Scholar]
- Chiou, M.S.; Ho, P.Y.; Li, H.Y. Adsorption of anionic dyes in acid solutions using chemically cross-linked chitosan beads. Dye. Pigment. 2004, 60, 69–84. [Google Scholar] [CrossRef]
- Crini, G. Non-conventional low-cost adsorbents for dye removal: A review. Bioresour. Technol. 2006, 97, 1061–1085. [Google Scholar] [CrossRef]
- Pillai, C.K.S.; Paul, W.; Sharma, C.P. Chitin and chitosan polymers: Chemistry, solubility and fiber formation. Prog. Polym. Sci. 2009, 34, 641–678. [Google Scholar] [CrossRef]
- Abegunde, S.M.; Idowu, K.S.; Adejuwon, O.M.; Adeyemi-Adejolu, T. A review on the influence of chemical modification on the performance of adsorbents. Resour. Environ. Sustain. 2020, 1, 100001. [Google Scholar] [CrossRef]
- Yu, J.; Zheng, J.; Lu, Q.; Yang, S.; Wang, X.; Zhang, X.; Yang, W. Reusability and selective adsorption of Pb2+ on chitosan/P(2-acrylamido-2-methyl-1-propanesulfonic acid-co-acrylic acid) hydrogel. Iran. Polym. J. 2016, 25, 1009–1019. [Google Scholar] [CrossRef]
- Zhang, H.; Dang, Q.; Liu, C.; Cha, D.; Yu, Z.; Zhu, W.; Fan, B. Uptake of Pb(II) and Cd(II) on Chitosan Microsphere Surface Successively Grafted by Methyl Acrylate and Diethylenetriamine. ACS Appl. Mater. Interfaces 2017, 9, 11144–11155. [Google Scholar] [CrossRef]
- Bolto, B.A. Soluble polymers in water purification. Prog. Polym. Sci. 1995, 20, 987–1041. [Google Scholar] [CrossRef]
- Chassary, P.; Vincent, T.; Sanchez Marcano, J.; MacAskie, L.E.; Guibal, E. Palladium and platinum recovery from bicomponent mixtures using chitosan derivatives. Hydrometallurgy 2005, 76, 131–147. [Google Scholar] [CrossRef]
- Azarova, Y.A.; Pestov, A.V.; Ustinov, A.Y.; Bratskaya, S.Y. Application of chitosan and its N-heterocyclic derivatives for preconcentration of noble metal ions and their determination using atomic absorption spectrometry. Carbohydr. Polym. 2015, 134, 680–686. [Google Scholar] [CrossRef]
- Pestov, A.V.; Privar, Y.O.; Ustinov, A.Y.; Voit, A.V.; Azarova, Y.A.; Mekhaev, A.V.; Bratskaya, S.Y. Effect of polymer backbone chemical structure on metal ions binding by imidazolylmethyl derivatives. Chem. Eng. J. 2016, 283, 323–329. [Google Scholar] [CrossRef]
- Wang, J.; Zhuang, S. Removal of various pollutants from water and wastewater by modified chitosan adsorbents. Crit. Rev. Environ. Sci. Technol. 2017, 47, 2331–2386. [Google Scholar] [CrossRef]
- Zhang, L.; Zeng, Y.; Cheng, Z. Removal of heavy metal ions using chitosan and modified chitosan: A review. J. Mol. Liq. 2016, 214, 175–191. [Google Scholar] [CrossRef]
- Glass, S.; Mantel, T.; Appold, M.; Sen, S.; Usman, M.; Ernst, M.; Filiz, V. Amine-Terminated PAN Membranes as Anion-Adsorber Materials. Chem. Ing. Tech. 2021, 93, 1396–1400. [Google Scholar] [CrossRef]
- Sutirman, Z.A.; Sanagi, M.M.; Abd Karim, K.J.; Naim, A.A.; Wan Ibrahim, W.A. Chitosan-based adsorbents for the removal of metal ions from aqueous solutions. Malays. J. Anal. Sci. 2018, 22, 839–850. [Google Scholar] [CrossRef]
- Gomez-Maldonado, D.; Erramuspe, I.B.V.; Peresin, M.S. Natural polymers as alternative adsorbents and treatment agents for water remediation. BioResources 2019, 14, 10093–10160. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Rawlins, J.W.; Ray, P. Polymer Grafting and Crosslinking; John Wiley & Sons: Hoboken, NJ, USA, 2008; ISBN 9780470404652. [Google Scholar]
- Benamer, S.; Mahlous, M.; Tahtat, D.; Nacer-Khodja, A.; Arabi, M.; Lounici, H.; Mameri, N. Radiation synthesis of chitosan beads grafted with acrylic acid for metal ions sorption. Radiat. Phys. Chem. 2011, 80, 1391–1397. [Google Scholar] [CrossRef]
- Mishra, A.K.; Sharma, A.K. Synthesis of γ-cyclodextrin/chitosan composites for the efficient removal of Cd(II) from aqueous solution. Int. J. Biol. Macromol. 2011, 49, 504–512. [Google Scholar] [CrossRef] [PubMed]
- Bediako, J.K.; Lin, S.; Sarkar, A.K.; Zhao, Y.; Choi, J.W.; Song, M.H.; Wei, W.; Reddy, D.H.K.; Cho, C.W.; Yun, Y.S. Benignly-fabricated crosslinked polyethylenimine/calcium-alginate fibers as high-performance adsorbents for effective recovery of gold. J. Clean. Prod. 2020, 252, 119389. [Google Scholar] [CrossRef]
- Yan, Y.; An, Q.; Xiao, Z.; Zheng, W.; Zhai, S. Flexible core-shell/bead-like alginate@PEI with exceptional adsorption capacity, recycling performance toward batch and column sorption of Cr(VI). Chem. Eng. J. 2017, 313, 475–486. [Google Scholar] [CrossRef]
- Wei, Y.; Salih, K.A.M.; Hamza, M.F.; Fujita, T.; Rodríguez-Castellón, E.; Guibal, E. Synthesis of a new phosphonate-based sorbent and characterization of its interactions with lanthanum (Iii) and terbium (iii). Polymers 2021, 13, 1513. [Google Scholar] [CrossRef]
- Pan, Y.; Zhao, H. A Green Method to Fabricate Polyethyleneimine-Alginate Multilayer Coatings Modified Polyurethane Foam for Cu2+ Adsorption from Aqueous Solution. Polym. Technol. Mater. 2019, 58, 384–393. [Google Scholar] [CrossRef]
- Demey, H.; Vincent, T.; Guibal, E. A Novel Algal-Based Sorbent for Heavy Metal Removal. Chem. Eng. J. 2017, 332, 582–595. [Google Scholar] [CrossRef]
- Ahmed, M.B.; Zhou, J.L.; Ngo, H.H.; Guo, W.; Thomaidis, N.S.; Xu, J. Progress in the biological and chemical treatment technologies for emerging contaminant removal from wastewater: A critical review. J. Hazard. Mater. 2017, 323, 274–298. [Google Scholar] [CrossRef]
- Zhao, J.; Zou, Z.; Ren, R.; Sui, X.; Mao, Z.; Xu, H.; Zhong, Y.; Zhang, L.; Wang, B. Chitosan adsorbent reinforced with citric acid modified β-cyclodextrin for highly efficient removal of dyes from reactive dyeing effluents. Eur. Polym. J. 2018, 108, 212–218. [Google Scholar] [CrossRef]
- Laus, R.; Costa, T.G.; Szpoganicz, B.; Fávere, V.T. Adsorption and desorption of Cu(II), Cd(II) and Pb(II) ions using chitosan crosslinked with epichlorohydrin-triphosphate as the adsorbent. J. Hazard. Mater. 2010, 183, 233–241. [Google Scholar] [CrossRef]
- Zhao, F.; Yang, Z.; Wei, Z.; Spinney, R.; Sillanpää, M.; Tang, J.; Tam, M.; Xiao, R. Polyethylenimine-modified chitosan materials for the recovery of La(III) from leachates of bauxite residue. Chem. Eng. J. 2020, 388, 124307. [Google Scholar] [CrossRef]
- Lee, B.B.; Ravindra, P.; Chan, E.S. Size and shape of calcium alginate beads produced by extrusion dripping. Chem. Eng. Technol. 2013, 36, 1627–1642. [Google Scholar] [CrossRef]
- Jayakumar, R.; Prabaharan, M.; Reis, R.L.; Mano, J.F. Graft copolymerized chitosan-Present status and applications. Carbohydr. Polym. 2005, 62, 142–158. [Google Scholar] [CrossRef] [Green Version]
- Ngah, W.S.W.; Fatinathan, S. Pb(II) biosorption using chitosan and chitosan derivatives beads: Equilibrium, ion exchange and mechanism studies. J. Environ. Sci. 2010, 22, 338–346. [Google Scholar] [CrossRef]
- Godiya, C.B.; Liang, M.; Sayed, S.M.; Li, D.; Lu, X. Novel alginate/polyethyleneimine hydrogel adsorbent for cascaded removal and utilization of Cu2+ and Pb2+ ions. J. Environ. Manag. 2019, 232, 829–841. [Google Scholar] [CrossRef]
- Wang, M.; Wang, Z.; Zhou, X.; Li, S. Efficient removal of heavy metal ions in wastewater by using a novel alginate-EDTA hybrid aerogel. Appl. Sci. 2019, 9, 547. [Google Scholar] [CrossRef] [Green Version]
- Crini, G.; Badot, P.M. Application of chitosan, a natural aminopolysaccharide, for dye removal from aqueous solutions by adsorption processes using batch studies: A review of recent literature. Prog. Polym. Sci. 2008, 33, 399–447. [Google Scholar] [CrossRef]
- Gadd, G.M. Biosorption: Critical review of scientific rationale, environmental importance and significance for pollution treatment. J. Chem. Technol. Biotechnol. 2009, 84, 13–28. [Google Scholar] [CrossRef]
- Li, W.W.; Yu, H.Q. Insight into the roles of microbial extracellular polymer substances in metal biosorption. Bioresour. Technol. 2014, 160, 15–23. [Google Scholar] [CrossRef]
- Liu, H.; Fang, H.H.P. Characterization of electrostatic binding sites of extracellular polymers by linear programming analysis of titration data. Biotechnol. Bioeng. 2002, 80, 806–811. [Google Scholar] [CrossRef]
- Delval, F.; Crini, G.; Vebrel, J.; Knorr, M.; Sauvin, G.; Conte, E. Starch-Modified Filters Used for the Removal of Dyes from Waste Water. Macromol. Symp. 2003, 203, 165–172. [Google Scholar] [CrossRef]
- Crini, G. Studies on adsorption of dyes on beta-cyclodextrin polymer. Bioresour. Technol. 2003, 90, 193–198. [Google Scholar] [CrossRef]
- Phan, T.N.T.; Bacquet, M.; Morcellet, M. The removal of organic pollutants from water using new silica-supported β-cyclodextrin derivatives. React. Funct. Polym. 2002, 52, 117–125. [Google Scholar] [CrossRef]
- Phan, T.N.T.; Bacquet, M.; Morcellet, M. Synthesis and characterization of silica gels functionalized with monochlorotriazinyl β-cyclodextrin and their sorption capacities towards organic compounds. J. Incl. Phenom. 2000, 38, 345–359. [Google Scholar] [CrossRef]
- Mahl, C.R.A.; Taketa, T.B.; Bataglioli, R.A.; de Arruda, E.J.; Beppu, M.M. Chitosan Functionalization with Amino Acids Yields to Higher Copper Ions Adsorption Capacity. J. Polym. Environ. 2018, 26, 4338–4349. [Google Scholar] [CrossRef]
- Bui, T.H.; Lee, W.; Jeon, S.B.; Kim, K.W.; Lee, Y. Enhanced Gold(III) adsorption using glutaraldehyde-crosslinked chitosan beads: Effect of crosslinking degree on adsorption selectivity, capacity, and mechanism. Sep. Purif. Technol. 2020, 248, 116989. [Google Scholar] [CrossRef]
- Nitsae, M.; Madjid, A.; Hakim, L.; Sabarudin, A. Preparation of chitosan beads using tripolyphosphate and ethylene glycol diglycidyl ether as crosslinker for Cr(VI) adsorption. Chem. Chem. Technol. 2016, 10, 105–114. [Google Scholar] [CrossRef]
- Elwakeel, K.Z.; El-Sayed, G.O.; Darweesh, R.S. Fast and selective removal of silver(I) from aqueous media by modified chitosan resins. Int. J. Miner. Process. 2013, 120, 26–34. [Google Scholar] [CrossRef]
- Wang, L.; Xing, R.; Liu, S.; Yu, H.; Qin, Y.; Li, K.; Feng, J.; Li, R.; Li, P. Recovery of silver (I) using a thiourea-modified chitosan resin. J. Hazard. Mater. 2010, 180, 577–582. [Google Scholar] [CrossRef]
- Córdova, B.M.; Venâncio, T.; Olivera, M.; Huamani-Palomino, R.G.; Valderrama, A.C. Xanthation of alginate for heavy metal ions removal. Characterization of xanthate-modified alginates and its metal derivatives. Int. J. Biol. Macromol. 2021, 169, 130–142. [Google Scholar] [CrossRef]
- Khan, M.N.; Chowdhury, M.; Rahman, M.M. Biobased amphoteric aerogel derived from amine-modified clay-enriched chitosan/alginate for adsorption of organic dyes and chromium (VI) ions from aqueous solution. Mater. Today Sustain. 2021, 13, 100077. [Google Scholar] [CrossRef]
- Pearson, R.G. Hard and soft acids and bases-the evolution of a chemical concept. Coord. Chem. Rev. 1990, 100, 403–425. [Google Scholar] [CrossRef]
- Nieboer, E.; Richardson, D.H.S. The replacement of the nondescript term “heavy metals” by a biologically and chemically significant classification of metal ions. Environ. Pollution. Ser. B Chem. Phys. 1980, 1, 3–26. [Google Scholar] [CrossRef]
- Pletnev, I.V.; Zernov, V.V. Classification of metal ions according to their complexing properties: A data-driven approach. Anal. Chim. Acta 2002, 455, 131–142. [Google Scholar] [CrossRef]
- Fujiwara, K.; Ramesh, A.; Maki, T.; Hasegawa, H.; Ueda, K. Adsorption of platinum (IV), palladium (II) and gold (III) from aqueous solutions onto l-lysine modified crosslinked chitosan resin. J. Hazard. Mater. 2007, 146, 39–50. [Google Scholar] [CrossRef]
- Ramesh, P.; Subbiahpandi, A.; Thirumurugan, P.; Perumal, P.T.; Ponnuswamy, M.N. 3-[4-(2,4-Dichlorophenyl)-3,5-dicyano-6-methoxypyridin-2-yl]indole. Acta Cryst. 2008, 64, o1892. [Google Scholar]
- Bertagnolli, C.; Grishin, A.; Vincent, T.; Guibal, E. Synthesis and Application of a Novel Sorbent (Tannic Acid-Grafted-Polyethyleneimine Encapsulated in Alginate Beads) for Heavy Metal Removal. Sep. Sci. Technol. 2015, 50, 2897–2906. [Google Scholar] [CrossRef]
- Bertagnolli, C.; Grishin, A.; Vincent, T.; Guibal, E. Recovering Heavy Metal Ions from Complex Solutions Using Polyethylenimine Derivatives Encapsulated in Alginate Matrix. Ind. Eng. Chem. Res. 2016, 55, 2461–2470. [Google Scholar] [CrossRef]
- Liu, B.; Wang, D.; Yu, G.; Meng, X. Adsorption of heavy metal ions, dyes and proteins by chitosan composites and derivatives-A review. J. Ocean Univ. China 2013, 12, 500–508. [Google Scholar] [CrossRef]
- Yong, S.K.; Bolan, N.S.; Lombi, E.; Skinner, W.; Guibal, E. Sulfur-containing chitin and chitosan derivatives as trace metal adsorbents: A review. Crit. Rev. Environ. Sci. Technol. 2013, 43, 1741–1794. [Google Scholar] [CrossRef]
- Yu, J.; Zheng, J.; Lu, Q.; Yang, S.; Zhang, X.; Wang, X.; Yang, W. Selective adsorption and reusability behavior for Pb2+ and Cd2+ on chitosan/poly(ethylene glycol)/poly(acrylic acid) adsorbent prepared by glow-discharge electrolysis plasma. Colloid Polym. Sci. 2016, 294, 1585–1598. [Google Scholar] [CrossRef]
- Miretzky, P.; Cirelli, A.F. Hg(II) removal from water by chitosan and chitosan derivatives: A review. J. Hazard. Mater. 2009, 167, 10–23. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, M.; Sastre, A.; Guibal, E. Pd and Pt recovery using chitosan gel beads. I. Influence of the drying process on diffusion properties. Sep. Sci. Technol. 2002, 37, 2143–2166. [Google Scholar] [CrossRef]
- Casettari, L.; Vllasaliu, D.; Lam, J.K.W.; Soliman, M.; Illum, L. Biomedical applications of amino acid-modified chitosans: A review. Biomaterials 2012, 33, 7565–7583. [Google Scholar] [CrossRef] [PubMed]
- Negm, N.A.; El Sheikh, R.; El-Farargy, A.F.; Hefni, H.H.H.; Bekhit, M. Treatment of industrial wastewater containing copper and cobalt ions using modified chitosan. J. Ind. Eng. Chem. 2015, 21, 526–534. [Google Scholar] [CrossRef]
- Karthik, R.; Meenakshi, S. Removal of Pb(II) and Cd(II) Ions from Aqueous Solution Using Polyaniline Grafted Chitosan; Elsevier B.V.: Amsterdam, The Netherlands, 2015; Volume 263, ISBN 9145124523. [Google Scholar]
- Beppu, M.M.; Arruda, E.J.; Vieira, R.S.; Santos, N.N. Adsorption of Cu(II) on porous chitosan membranes functionalized with histidine. J. Memb. Sci. 2004, 240, 227–235. [Google Scholar] [CrossRef]
- Boggione, M.J.; Mahl, C.R.A.; Beppu, M.M.; Farruggia, B. Synthesis and characterization of chitosan membranes functionalized with amino acids and copper for adsorption of endoglucanase. Powder Technol. 2017, 315, 250–257. [Google Scholar] [CrossRef]
- Eser, A.; Nüket Tirtom, V.; Aydemir, T.; Becerik, S.; Dinçer, A. Removal of nickel(II) ions by histidine modified chitosan beads. Chem. Eng. J. 2012, 210, 590–596. [Google Scholar] [CrossRef]
- Jang, L.K.; Nguyen, D.; Geesey, G.G. An equilibrium model for absorption of multiple divalent metals by alginate gel under acidic conditions. Water Res. 1999, 33, 2826–2832. [Google Scholar] [CrossRef]
- Jang, L.K.; Nguyen, D.; Geesey, G.G. Selectivity of alginate gel for Cu over Zn when acidic conditions prevail. Water Res. 1999, 33, 2817–2825. [Google Scholar] [CrossRef]
- Ibáez, J.P.; Umetsu, Y. Potential of protonated alginate beads for heavy metals uptake. Hydrometallurgy 2002, 64, 89–99. [Google Scholar] [CrossRef]
- Navarro, R.R.; Furukawa, M.; Matsumura, M. Hybrid properties of alginate-PEI adsorbent for chromium (VI) removal from aqueous solutions. Sep. Sci. Technol. 2006, 41, 3619–3637. [Google Scholar] [CrossRef]
- Jones, M.M.; Burka, L.T.; Hunter, M.E.; Basinger, M.; Campo, G.; Weaver, A.D. Dithiocarbamate chelating agents for toxic heavy metals. J. Inorg. Nucl. Chem. 1980, 42, 775–778. [Google Scholar] [CrossRef]
- Wang, S.; Vincent, T.; Faur, C.; Guibal, E. Alginate and algal-based beads for the sorption of metal cations: Cu(II) and pb(II). Int. J. Mol. Sci. 2016, 17, 1453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).