Seed-Borne Probiotic Yeasts Foster Plant Growth and Elicit Health Protection in Black Gram (Vigna mungo L.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation of Seed-Borne Yeasts
2.2. Morphological and Biochemical Characterization of the Seed-Borne Yeast Isolates
2.3. Molecular Characterization of Selected Seed-Borne Yeasts Isolates
2.4. Functional Characterization of Seed-Borne Yeasts for Plant Growth Promotion Activity
2.4.1. IAA Production
2.4.2. Phosphate Solubilization
2.4.3. Zinc Solubilization
2.4.4. 1-Aminocyclopropane-1-carboxylate Deaminase (ACCD) Activity
2.4.5. Biological Nitrogen Fixation Potential
2.4.6. Hydrogen Cyanide (HCN) Production
2.4.7. Siderophore Production
2.5. Screening of Yeast Isolates for Antagonistic Activity against Phytopathogenic Fungi by Dual Culture Plate Method
2.6. Test for Compatibility of Seed-Borne Yeast Isolates with Rhizobium sp. BMBS1
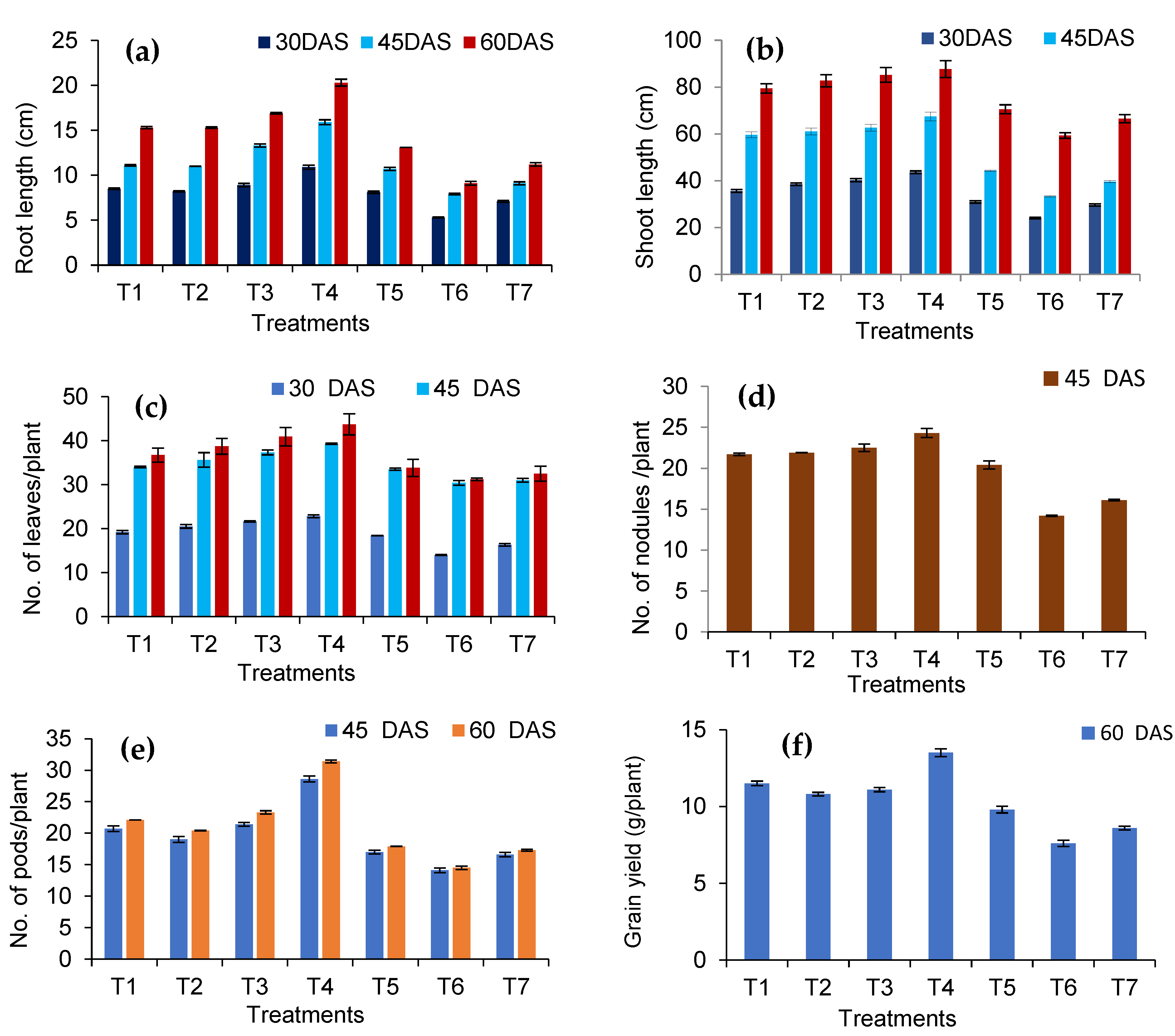
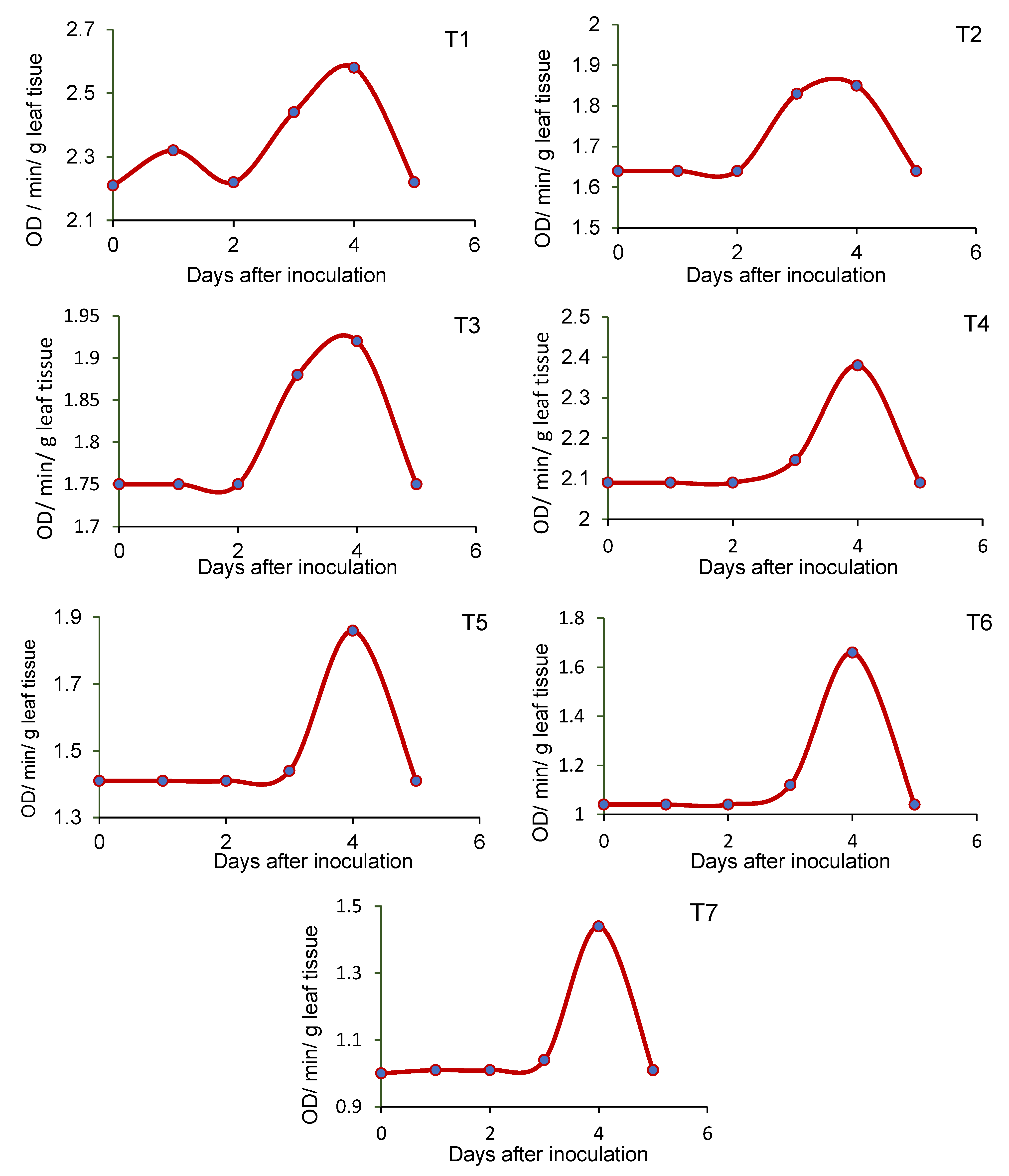
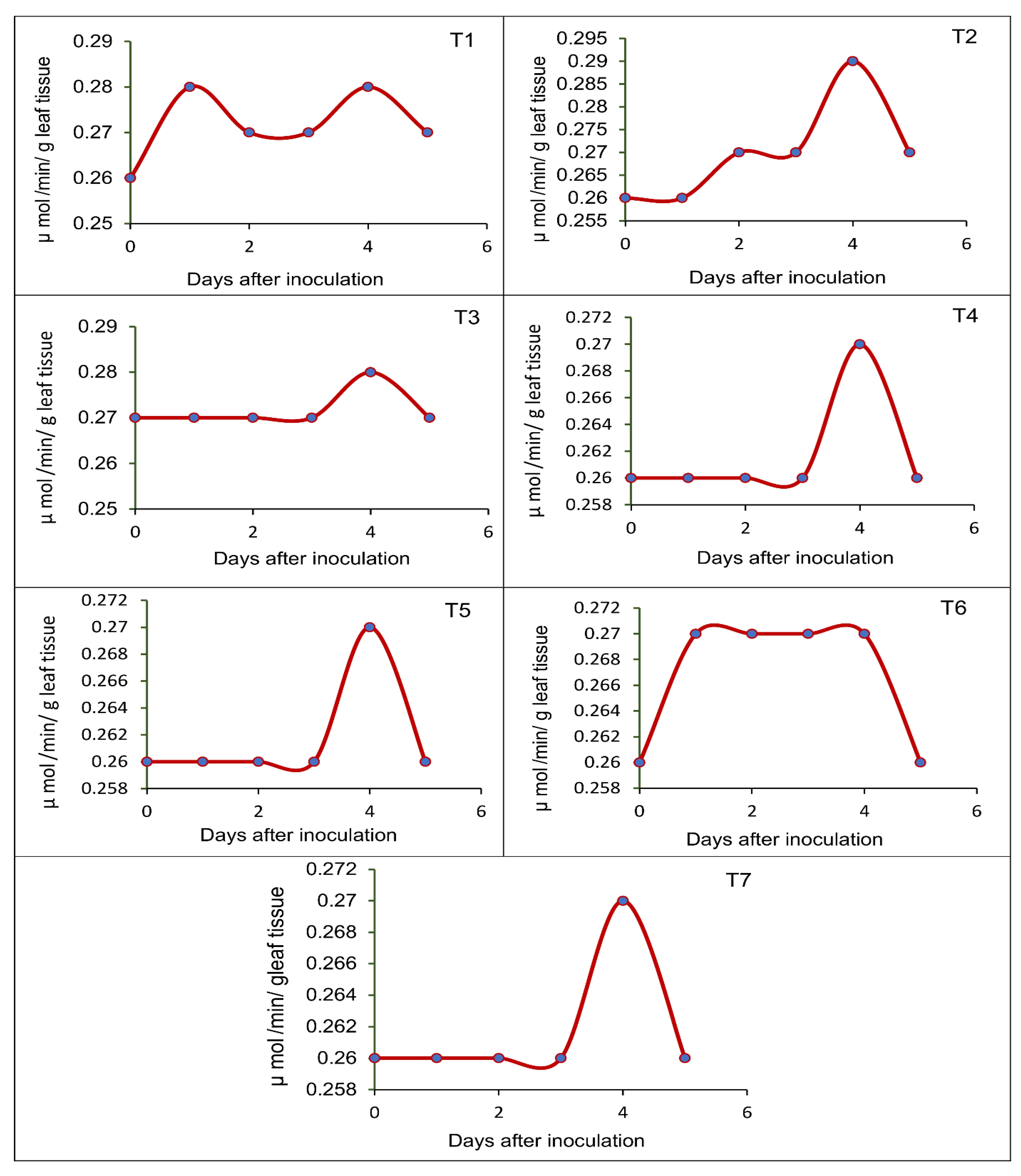
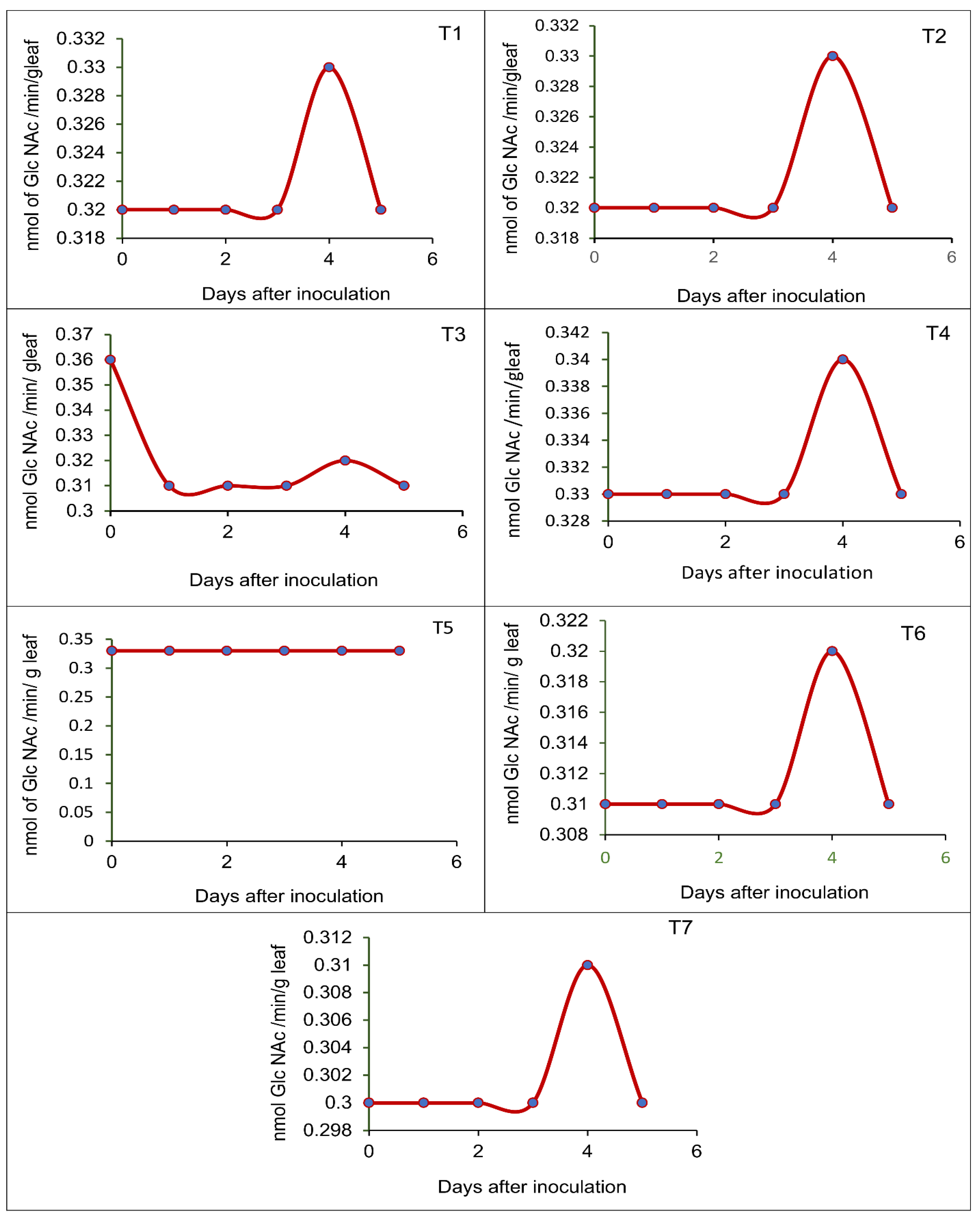
2.7. Pot Culture Assay to Assess the Potential of the Seed-Borne Yeast Isolates in the Induction of Systemic Resistance against Rhizoctonia Solani MTCC 4633
- T1—Issatchenkia terricola GRY4 + Rhizobium sp. BMBS 1 + Rhizoctonia solani MTCC 4633
- T2—Pichia kudriavzevii POY5 + Rhizobium sp. BMBS 1 + Rhizoctonia solani MTCC 4633
- T3—Issatchenkia terricola GRY4 + Rhizoctonia solani MTCC 4633
- T4—Pichia kudriavzevii POY5 + Rhizoctonia solani MTCC 4633
- T5—Rhizobium sp. BMBS1 + Rhizoctonia solani MTCC 4633
- T6—Rhizoctonia solani MTCC 4633, and
- T7—Uninoculated control
2.8. Effect of Seed-Borne Yeasts on the Induction of Defense-Related Enzymes in Blackgram upon Challenge Inoculation
2.9. Statistical Analysis
3. Results
3.1. Morphological and Biochemical Characterization of the Seed-Borne Yeast Isolates
3.2. Selection of Efficient Seed-Borne Yeasts through Evaluation of Different Plant Growth Promotion Traits
3.3. Extracellular Enzyme Production and Antagonistic Activity against Phytopathogenic Fungi of the Seed-Borne Yeast Isolates
3.4. Reflections of the Seed-Borne Yeast Isolates on Growth and Yield of Black gram
3.5. Effect of Seed-Borne Yeast Isolates on the Induction of Defense-Related Enzymes in Black Gram upon Challenge Inoculation
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yang, J.; Kloepper, J.W.; Ryu, C.M. Rhizosphere bacteria help plants tolerate abiotic stress. Trends Plant Sci. 2009, 14, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Viveros, O.; Jorquera, M.A.; Crowley, D.E.; Gajardo, G.; Mora, M.L. Mechanisms and practical considerations involved in plant growth promotion by rhizobacteria. J. Soil Sci. Plant Nutr. 2010, 10, 293–319. [Google Scholar] [CrossRef]
- Kim, Y.C.; Glick, B.; Bashan, Y.; Ryu, C.M. Enhancement of plant drought tolerance by microbes. In Plant Responses to Drought Stress; Aroca, R., Ed.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 383–413. [Google Scholar]
- Cloete, K.J.; Valentine, A.J.; Stander, M.A.; Blomerus, L.M.; Botha, A. Evidence of symbiosis between the soil Yeast Cryptococcus laurentii and a Sclerophyllous Medicinal Shrub, Agathosma betulina(Berg.). Pillans. Microb Ecol. 2009, 57, 624–632. [Google Scholar] [CrossRef] [PubMed]
- Botha, A. The importance and ecology of yeasts in soil. Soil Biol. Biochem. 2011, 43, 1–8. [Google Scholar] [CrossRef]
- Doty, S.L. Symbiotic Plant-Bacterial Endospheric Interactions. Microorganisms 2018, 6, 28. [Google Scholar] [CrossRef]
- Joubert, P.M.; Doty, S.L. Endophytic yeasts, biology, ecology and applications. In Endophytes of Forest Trees; Pirttila, A.M., Frank, A.C., Eds.; Springer International Publishing AG: Cham, Switzerland, 2018; pp. 3–14. [Google Scholar]
- El-Tarabily, K.A. Suppression of Rhizoctonia solani diseases of sugar beet by antagonistic and plant growth-promoting yeasts. J Appl. Microbiol. 2004, 96, 69–75. [Google Scholar] [CrossRef]
- El-Tarabily, K.A.; Sivasithamparam, K. Potential of yeasts as bio-control agents of soil-borne fungal plant pathogens and as plant growth promoters. Mycoscience 2006, 47, 25–35. [Google Scholar] [CrossRef]
- Sansone, G.; Rezza, I.; Calvente, V.; Benuzzi, D.; Tosetti, M.I.S.D. Control of Botrytis cinerea strains resistant to iprodione in apple with rhodotorulic acid and yeasts. Postharvest Biol. Tech. 2005, 35, 245–251. [Google Scholar] [CrossRef]
- Nassar, A.H.; El-Tarabily, K.A.; Sivasithamparam, K. Promotion of plant growth by an auxin-producing isolate of the yeast Williopsis saturnus endophytic in maize (Zea mays L.) roots. Biol. Fertil. Soils 2005, 42, 97–108. [Google Scholar] [CrossRef]
- Falih, A.M.; Wainwright, M. Nitrification, S-oxidation and P-solubilization by the soil yeast Williopsis californica and by Saccharomyces cerevisiae. Mycol. Res. 1995, 99, 200–204. [Google Scholar] [CrossRef]
- Alonso, L.M.; Kleiner, D.; Ortega, E. Spores of the mycorrhizal fungus Glomus mossae host yeasts that solubilize phosphate and accumulate polyphosphates. Mycorrhiza 2008, 18, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Vassileva, M.; Azcon, R.; Barea, J.M.; Vassilev, N. Rock phosphate solubilization by free and encapsulated cells of Yarowia lipolytica. Process. Biochem. 2000, 35, 693–697. [Google Scholar] [CrossRef]
- Nakamura, Y.; Fukuhara, H.; Sano, K. Secreted phytase activities of yeasts. Biosci. Biotechnol. Biochem. 2000, 64, 841–844. [Google Scholar] [CrossRef]
- Amprayn, K.O.; Rose, M.T.; Kecskes, M.; Pereg, L.; Nguyen, H.T.; Kennedy, I.R. Plant growth promoting characteristics of soil yeast (Candida tropicalis H.Y.) and its effectiveness for promoting rice growth. Appl. Soil Ecol. 2012, 61, 295–299. [Google Scholar] [CrossRef]
- De Tenorio, D.A.; de Medeiros, E.V.; Lima, C.S.; da Silva, J.M.; de Barros, J.A.; Neves, R.P.; Laranjeira, D. Biological control of Rhizoctonia solani in cowpea plants using yeast. Trop. Plant Pathol. 2019, 44, 113–119. [Google Scholar] [CrossRef]
- Ferraz, P.; Cássio, F.; Lucas, C. Potential of yeasts as biocontrol agents of the phytopathogen causing cacao Witches’ Broom Disease. Is microbial warfare a solution. Front. Microbiol. 2019, 10, 1766. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.F.; Sun, P.F.; Lu, H.Y.; Wei, J.Y.; Xiao, H.S.; Fang, W.T.; Cheng, B.Y.; Chou, J.Y. Plant growth promoting traits of yeasts isolated from the phyllosphere and rhizosphere of Drosera spathulata Lab. Fungal Biol. 2016, 120, 433–448. [Google Scholar] [CrossRef]
- Ignatova, L.V.; Brazhnikova, Y.V.; Berzhanova, R.Z.; Mukasheva, T.D. Plant growth-promoting and antifungal activity of yeasts from dark chestnut soil. Microbiol. Res. 2015, 175, 78–83. [Google Scholar] [CrossRef]
- Sarabia, M.; Cazares, S.; Gonzalez-Rodríguez, A.; Mora, F.; Carreón-Abud, Y.; Larsen, J. Plant growth promotion traits of rhizosphere yeasts and their response to soil characteristics and crop cycle in maize agroecosystems. Rhizosphere 2018, 6, 67–73. [Google Scholar] [CrossRef]
- Fernandez-San Millan, A.; Farran, I.; Larraya, L.; Ancin, M.; Arregui, L.M.; Veramendi, J. Plant growth-promoting traits of yeasts isolated from Spanish vineyards: Benefits for seedling development. Microbiol. Res. 2020, 237, 126480. [Google Scholar] [CrossRef]
- Kaewwongwal, A.; Kongjaimun, A.; Somta, P.; Chankaew, S.; Yimram, T.; Srinives, P. Genetic diversity of the black gram [Vigna mungo (L.) Hepper] gene pool as revealed by S.S.R. markers. Breed. Sci. 2015, 565, 127–137. [Google Scholar] [CrossRef]
- Kaur, S.; Dhillon, G.S.; Brar, S.K.; Vallad, G.E.; Chand, R.; Chauhan, V.B. Emerging phytopathogen Macrophomina phaseolina, biology, economic importance and current diagnostic trends. Crit. Rev. Microbiol. 2012, 38, 136–151. [Google Scholar] [CrossRef] [PubMed]
- Ajayi-Oyetunde, O.; Bradley, C.A. Rhizoctonia solani: Taxonomy, population biology and management of Rhizoctonia seedling disease of soybean. Plant Pathol. 2018, 67, 3–17. [Google Scholar] [CrossRef]
- Pandey, A.K.; Burlakoti, R.R.; Rathore, A.; Nair, R.M. Morphological and molecular characterization of Macrophomina phaseolina isolated from three legume crops and evaluation of mungbean genotypes for resistance to dry root rot. Crop Prot. 2020, 127, 104962. [Google Scholar] [CrossRef]
- Kurtzman, C.P.; Fell, J.W.; Boekhout, T. The Yeasts; Elsevier: Amsterdam, The Netherlands, 2011; p. 11. [Google Scholar]
- Gerhardt, P. Manual of Methods for General Bacteriology; American Society for Microbiology: Washington, DC, USA, 1981. [Google Scholar]
- Kurtzman, C.P.; Fell, J.W.; Boekhout, T.; Robert, V. Methods for isolation, phenotypic characterization and maintenance of yeasts. In The Yeasts, a Taxonomic Study; Kurtzman, C.P., Fell, J.W., Boekhout, T., Eds.; Elsevier: Amsterdam, The Netherlands, 2011; pp. 87–110. [Google Scholar]
- Kreger-van Rij, N.J.W. The Yeasts: A Taxonomic Study; Elsevier: Amsterdam, The Netherlands, 1984. [Google Scholar]
- Wickerham, L.J.; Burton, I.C.N. Carbon assimilation tests for the classification of yeasts. J. Bacteriol. 1948, 56, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Beisher, L. Microbiology in Practice, a Self-Instructional Laboratory Course; Harper Collins, Inc.: New York, NY, USA, 1991; pp. 53–131. [Google Scholar]
- Simmons, J.S. A culture medium for differentiating organisms of typhoid-colon aerogenes groups and for isolation of certain fungi. J. Infect. Dis. 1926, 39, 209. [Google Scholar] [CrossRef]
- Zitomer, S.W.; Eveleigh, D.E. Cellulase screening by iodine staining: An artifact. Enzym. Microb. Technol. 1987, 9, 214–216. [Google Scholar] [CrossRef]
- Hsu, S.C.; Lockwood, J.L. Powdered chitin agar as a selective medium for enumeration of actinomycetes in water and soil. Appl. Microbiol. 1975, 29, 422–426. [Google Scholar] [CrossRef]
- Tsuchida, O.; Yamagata, Y.; Ishizuka, T.; Arai, T.; Yamada, J.; Takeuchi, M.; Ichishima, E. An alkaline proteinase of an alkalophilic Bacillus sp. Curr. Microbiol. 1986, 14, 7–12. [Google Scholar] [CrossRef]
- Gordon, S.A.; Weber, R.P. Colorimetric estimation of indole acetic acid. Plant Physiol. 1951, 26, 192–195. [Google Scholar] [CrossRef]
- Murphy, J.; Riley, J.P. A modified single solution method for the determination of phosphate in natural waters. Anal. Chim. Acta. 1962, 27, 31–36. [Google Scholar] [CrossRef]
- Bunt, J.S.; Rovira, A.D. Microbiological studies of some sub antartic soils. J. Soil Sci. 1955, 6, 119–128. [Google Scholar] [CrossRef]
- Penrose, D.M.; Glick, B.R. Methods for isolating and characterizing A.C.C. deaminase-containing plant growth-promoting rhizobacteria. Plant Physiol. 2003, 118, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Bergersen, F.J. The quantitative relationship between nitrogen fixation and the acetylene-reduction assay. Aust. J. Biol. Sci. 1970, 23, 1015–1026. [Google Scholar] [CrossRef]
- Bakker, A.W.; Schippers, B. Microbial cyanide production in the rhizosphere in relation to potato yield reduction and Pseudomonas spp-mediated plant growth-stimulation. Soil Biol. Biochem. 1987, 19, 451–457. [Google Scholar] [CrossRef]
- Milagres, A.M.F.; Napoleao, D.; Machuca, A. Detection of siderophore production from several fungi and bacteria by a modification of chrome azurol S (C.A.S.) agar plate assay. J. Microbiol. Methods 1999, 37, 1–6. [Google Scholar] [CrossRef]
- Schwyn, B.; Neilands, J. Universal chemical assay for the detection and determination of siderophores. Anal. Biochem. 1987, 160, 47–56. [Google Scholar] [CrossRef]
- Rabindran, R.; Vidhyasekaran, P. Development of a formulation of Pseudomonas fluorescens PfALR2 for management of rice sheath blight. Crop Prot. 1996, 15, 715–721. [Google Scholar] [CrossRef]
- Anandraj, B.; Delapierre, A.L.R. Studies on influence of bioinoculants (Pseudomonas flourescens, Rhizobium sp., and Bacillus megaterium) in green gram. J. Bischi. Tech. 2010, 1, 95–99. [Google Scholar]
- Castillo, F.J.; Penel, C.; Greppin, H. Peroxidase release induced by ozone in Sedum album leaves. Plant Physiol. 1984, 74, 846–851. [Google Scholar] [CrossRef]
- Mayer, A.M.; Harel, E.; Ben-Shau, l.R. Assay of catechol oxidase-a critical comparison of methods. Phytochemistry 1966, 5, 783–789. [Google Scholar] [CrossRef]
- Chaparro-Giraldo, A.; Barata, R.M.; Chabregas, S.M.; Azevedo, A.; Silva-Filho, M.C. Soybean leghemoglobin targeted to potato chloroplasts influences growth and development of transgenic plants. Plant Cell Rep. 2000, 19, 961–965. [Google Scholar] [CrossRef] [PubMed]
- Boller, T.; Mauch, F. Colorimetric assay for chitinase. In Methods in Enzymology; Wood, W.A., Kellogg, S.T., Eds.; Academic Press: Cambridge, MA, USA, 1988; p. 161. [Google Scholar]
- I.B.M. Corp. Released 2019. IBM SPSS Statistics for Windows, Version 26.0; I.B.M. Corp: Armonk, NY, USA, 2019. [Google Scholar]
- Ebabhi, A.M.; Adekunle, A.A.; Okunowa, W.O.; Osuntoki, A.A. Isolation and characterization of yeast strains from local food crops. J. Yeast Fungal Res. 2013, 4, 38–43. [Google Scholar]
- Nutaratat, P.; Srisuk, N.; Arunrattiyakorn, P.; Limtong, S. Plant growth-promoting traits of epiphytic and endophytic yeasts isolated from rice and sugar cane leaves in Thailand. Fungal Biol. 2014, 118, 683–694. [Google Scholar] [CrossRef]
- Romero, M.C.; Gatti, M.E.; Córdoba, S.; Cazau, M.C.; Arambarri, A.M. Physiological and morphological characteristics of yeasts isolated from waste oil effluents. World J. Microbiol. Biotechnol. 2000, 16, 683–686. [Google Scholar] [CrossRef]
- Okerentugba, P.O.; Ataikiru, T.L.; Ichor, T. Isolation and characterization of hydrocarbon utilizing yeast isolates from palm wine. Am. J. Mol. Biol. 2016, 6, 63–70. [Google Scholar] [CrossRef]
- Reis, V.R.; Bassi, A.P.; da Silva, J.C.; Ceccato-Antonini, S.R. Characteristics of Saccharomyces cerevisiae yeasts exhibiting rough colonies and pseudohyphal morphology with respect to alcoholic fermentation. Braz. J. Microbiol. 2013, 44, 1121–1131. [Google Scholar] [CrossRef]
- Ghosh, S.K. Study of yeast flora from fruit of Syzygium cumini (Linn.) skeel. Agric. Biol. J. N. Am. 2011, 2, 1166–1170. [Google Scholar] [CrossRef]
- Avila, C.L.S.; Martins, C.E.C.B.; Schwan, R.F. Identification and characterization of yeasts in sugarcane silages. J. Appl. Microbiol. 2010, 10, 1677–1686. [Google Scholar]
- Artnarong, S.; Masniyom, P.; Maneesri, J. Isolation of yeast and acetic acid bacteria from palmyra palm fruit pulp (Borassus flabellifer Linn.). Int. Food Res. J. 2016, 23, 1308–1314. [Google Scholar]
- Khamna, S.; Yokota, A.; Peberdy, J.F.; Lumyong, S. Indole3-acetic acid production by Streptomyces sp. isolated from some Thai medicinal plant rhizosphere soils. Eur. J. Biosci. 2010, 4, 23–32. [Google Scholar] [CrossRef]
- Nutaratat, P.; Amsri, W.; Srisuk, N.; Arunrattiyakorn, P.; Limtong, S. Indole-3-acetic acid production by newly isolated red yeast Rhodosporidium paludigenum. J. Gen. Appl. Microbiol. 2015, 61, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Xin, G.; Glawe, D.; Doty, S.L. Characterization of three endophytic, indole-3-acetic acid-producing yeasts occurring in Populus trees. Mycol. Res. 2009, 113, 973–980. [Google Scholar] [CrossRef]
- Kumla, J.; Nundaeng, S.; Suwannarach, N.; Lumyong, S. Evaluation of multifarious plant growth promoting trials of yeast isolated from the Soil of Assam Tea (Camellia sinensis var. assamica) plantations in Northern Thailand. Microorganisms 2020, 8, 1168. [Google Scholar]
- Srinivasan, R.; Krishnan, S.R.; Ragunath, K.S.; Ponni, K.K.; Balaji, G.; Prabhakaran, N.; Chelliappan, B.; Narayanan, R.L.; Gracy, M.; Latha, K. Prospects of utilizing a multifarious yeast (MSD1), isolated from South Indian coast as an Agricultural input. Biocatal. Agric. Biotechnol. 2022, 39, 1022–1032. [Google Scholar] [CrossRef]
- Perez-Montano, F.; Alias-Villegas, C.; Bellogín, R.A.; del Cerro, P.; Espuny, M.R.; Jiménez-Guerrero, I.; López-Baena, F.J.; Ollero, F.J.; Cubo, T. Plant growth promotion in cereal and leguminous agricultural important plants: From microorganism capacities to crop production. Microbiol. Res. 2014, 169, 325–336. [Google Scholar] [CrossRef]
- Zajc, J.; Gostincar, C.; Cernosa, A.; Gunde-Cimerman, N. Stress-tolerant yeasts: Opportunistic pathogenicity versus biocontrol potential. Genes 2019, 10, 42. [Google Scholar] [CrossRef]
- Pretscher, J.; Fischkal, T.; Branscheidt, S.; Jager, L.; Kahl, S.; Schlander, M.; Thines, E.; Claus, H. Yeasts from different habitats and their potential as biocontrol agents. Fermentation 2018, 4, 31. [Google Scholar] [CrossRef]
- Junker, K.; Chailyan, A.; Hesselbart, A.; Forster, J.; Wendland, J. Multi-omics characterization of the necrotrophic mycoparasite Saccharomycopsis schoenii. PLoS Pathog. 2019, 15, e1007692. [Google Scholar] [CrossRef]
- Sripodok, C.; Thammasittirong, S.; Thammasittirong, A. Antifungal activity of soil yeast (Lachancea kluyveri SP132) against rice pathogenic fungi and its plant growth promoting activity. J. Int. Soc. Southeast Asian Agric. Sci. 2019, 25, 55–65. [Google Scholar]
- Bar Shimon, M.; Yehuda, H.; Cohen, L.; Weiss, B.; Kobeshnikov, A.; Daus, A.; Goldway, M.; Wisniewski, M.; Droby, S. Characterization of extracellular lytic enzymes produced by the yeast biocontrol agent Candida oleophila. Curr. Genet. 2004, 45, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Chandarana, K.A.; Pramanik, R.S.; Amaresan, N. Interaction between ciliate and plant growth promoting bacteria influences the root structure of rice plants, soil PLFAs and respiration properties. Rhizosphere 2022, 21, 1004–1066. [Google Scholar] [CrossRef]
- Patten, C.L.; Glick, B.R. Role of Pseudomonas putida indoleacetic acid in development of the host plant root system. Appl. Environ. Microbiol. 2002, 68, 3795–3801. [Google Scholar] [CrossRef] [PubMed]
- Remans, R.; Beebe, S.; Blair, M.; Manrique, G.; Tovar, E.; Rao, I.; Croonenborghs, A.; Torres-Gutierrez, R.; El-Howeity, M.; Michiels, J.; et al. Physiological and genetic analysis of root responsiveness to auxin-producing plant growth-promoting bacteria in common bean (Phaseolus vulgaris L.). Plant Soil. 2008, 302, 149–161. [Google Scholar] [CrossRef]
- Nakayan, P.; Hameed, A.; Singh, S.; Young, L.; Hung, M.; Young, C. Phosphate-solubilizing soil yeast Meyerozyma guilliermondii CC1 improves maize (Zea mays L.) productivity and minimizes requisite chemical fertilization. Plant Soil 2013, 373, 301–315. [Google Scholar] [CrossRef]
- Sun, P.F.; Fang, W.T.; Shin, L.Y.; Wei, J.Y.; Fu, S.F.; Chou, J.Y. Indole-3-Acetic Acid-producing yeasts in the phyllosphere of the carnivorous plant Drosera indica L. PLoS ONE 2014, 9, e114196. [Google Scholar] [CrossRef]
- Nandakumar, R.; Babu, S.; Viswanathan, R.; Raguchander, T.; Samiyappan, R. Induction of systemic resistance in rice against sheath blight disease by Pseudomonas fluorescens. Soil Biol. Biochem. 2001, 33, 603–612. [Google Scholar] [CrossRef]
- Mohamaddi, M.; Kazemi, H. Changes in peroxidase and polyphenol oxidase activities in susceptible and resistant wheat heads inoculated with Fusarium graminearum and induced resistance. Plant Sci. 2002, 162, 491–498. [Google Scholar] [CrossRef]
- Palma, J.M.; Mateos, R.M.; López-Jaramillo, J.; Rodríguez-Ruiz, M.; González-Gordo, S.; Lechuga-Sancho, A.M.; Corpas, F.J. Plant catalases as NO and H2S targets. Redox Biol. 2020, 34, 101525. [Google Scholar] [CrossRef]
- Thanuja, K.G.; Annadurai, B.; Thankappan, S.; Uthandi, S. Non-rhizobial endophytic (N.R.E.) yeasts assist nodulation of Rhizobium in root nodules of blackgram (Vigna mungo L.). Arch. Microbiol. 2020, 10, 2739–2749. [Google Scholar] [CrossRef]
- Annadurai, B.; Thangappan, S.; Kennedy, Z.J.; Uthandi, S. Co- inoculant response of plant growth promoting non-rhizobial endophytic yeast Candida tropicalis VYW1 and Rhizobium sp. VRE1 for enhanced plant nutrition, nodulation, growth and soil nutrient status in Mungbean (Vigna mungo L.,). Symbiosis 2021, 83, 115–128. [Google Scholar] [CrossRef]
- Attia, M.S.; El-Sayyad, G.S.; El-kodous, M.A.; El-Batal, A.I. The effective antagonistic potential of plant growth-promoting rhizobacteria against Alternaria solani causing early blight disease in tomato plant. Sci. Hortic. 2020, 266, 109289. [Google Scholar] [CrossRef]
- Shaikh, S.S.; Wani, S.J.; Sayyed, R.Z.; Thakur, R. Gulati, A. Production, purification and kinetics of chitinase of Stenotrophomonas maltophilia isolated from rhizospheric soil, Indian. J. Exp. Biol. 2021, 56, 274–278. [Google Scholar]
- Sayyed, R.Z.; Seifi, S.; Patel, P.R.; Shaikh, S.S.; Jadhav, H.P.; Enshasy, H.E. Siderophore production in groundnut rhizosphere isolate, Achromobacter sp. RZS2 influenced by physicochemical factors and metal ions. Environ. Sustain. 2019, 1, 295–301. [Google Scholar] [CrossRef]
- Sagar, A.; Sayyed, R.Z.; Ramteke, P.W.; Sharma, S.; Marraiki, N.; Elgorban, A.M.; Syed, A. ACC deaminase and antioxidant enzymes producing halophilic Enterobacter sp. PR14 promotes the growth of rice and millets under salinity stress. Physiol. Mol. Biol. Plants. 2020, 26, 1847–1854. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Awan, S.A.; Ikram, R.; Rizwan, M.; Akhtar, N.; Yasmin, H.; Sayyed, R.Z.; Ali, S.; Ilyas, N. Effects of 24-Epibrassinolide regulated antioxidants and osmolyte defense and endogenous hormones in two wheat varieties under drought stress. Physiol. Planta. 2020, 172, 696–706. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Prasad, P.; Das, S.N.; Kalam, S.; Sayyed, R.Z.; Reddy, M.S.; El Enshasy, H. Plant Growth Promoting Rhizobacteria (PGPR) as Green bioinoculants: Recent developments, constraints, and prospects. Sustainability. 2021, 13, 1140. [Google Scholar] [CrossRef]
- Hamid, B.; Zaman, M.; Farooq, S.; Fatima, S.; Sayyed, R.Z.; Baba, Z.A.; Sheikh, T.A.; Reddy, M.S.; El Enshasy, H.; Gafur, A.; et al. Bacterial plant biostimulants: A sustainable way towards improving growth, productivity, and health of crops. Sustainability. 2021, 21, 2856. [Google Scholar] [CrossRef]
- Kusale, S.P.; Attar, Y.C.; Sayyed, R.Z.; Malek, R.A.; Ilyas, N.; Suriani, N.L.; Khan, N.; Enshasy, H.E. Production of plant beneficial and antioxidants metabolites by Klebsiella variicola under salinity stress. Molecules. 2021, 26, 1894. [Google Scholar] [CrossRef]

| Seed-Borne Yeast Isolates | IAA Production (µg/mL) | IAA without Tryptophan (µg/mL) | Siderophore Production | HCN Production | ACCD Activity (nmol α-Ketobutyrate Released /min/mg Protein) |
|---|---|---|---|---|---|
| POY1 | 6.81 ± 1.838 ij | 5.59 ± 0.259 d | − | + | ND |
| POY2 | 13.54 ± 2.590 defg | 10.03 ± 0.059 a | − | − | ND |
| POY3 | 15.69 ± 1.928 bcde | 6.89 ± 0.021 c | − | − | ND |
| POY4 | 18.22 ± 2.943 abc | 3.25 ± 0.026 f | + | − | ND |
| POY5 | 21.62 ± 0.061 a | 9.50 ± 0.214 ab | + | + | 2.69 ± 0.014 |
| TOY1 | 13.93 ± 2.333 def | 9.42 ± 0.733 ab | − | + | ND |
| TOY2 | 15.23 ± 3.570 cde | 2.33 ± 0.087 g | − | − | ND |
| TOY3 | 13.83 ± 2.673 def | 2.36 ± 0.063 g | − | − | ND |
| TOY4 | 6.32 ± 3.452 j | 4.81 ± 0.013 e | + | + | ND |
| TOY5 | 10.16 ± 2.836 ghi | 3.17 ± 0.016 f | − | + | ND |
| GRY1 | 13.11 ± 2.614 efg | 2.61 ± 0.004 g | − | − | ND |
| GRY2 | 16.81 ± 5.108 bcd | 2.44 ± 0.060 g | − | − | ND |
| GRY3 | 12.43 ± 3.051 bcd | 2.61 ± 0.075 fg | − | − | ND |
| GRY4 | 18.81 ± 4.644 ab | 7.44 ± 0.200 c | + | + | 2.4 ± 0.002 |
| GRY5 | 10.45 ± 1.055 fgh | 2.33 ± 0.009 g | − | + | ND |
| BGY1 | 16.81 ± 5.108 bcd | 2.36 ± 0.034 g | − | − | ND |
| BGY2 | 11.18 ± 0.932 fg | 9.25 ± 0.075 b | + | − | ND |
| BGY3 | 12.59 ± 3.505 efg | 4.25 ± 0.122 e | − | − | ND |
| BGY4 | 12.43 ± 3.172 efg | 2.28 ± 0.076 g | − | + | ND |
| BGY5 | 7.17 ± 0.867 hij | 9.06 ± 0.097 b | − | − | N.D. |
| SEd | 2.06 | 0.08 | 1.07 | ||
| CD (0.05) | 4.14 | 0.16 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bright, J.P.; Karunanadham, K.; Maheshwari, H.S.; Karuppiah, E.A.A.; Thankappan, S.; Nataraj, R.; Pandian, D.; Ameen, F.; Poczai, P.; Sayyed, R.Z. Seed-Borne Probiotic Yeasts Foster Plant Growth and Elicit Health Protection in Black Gram (Vigna mungo L.). Sustainability 2022, 14, 4618. https://doi.org/10.3390/su14084618
Bright JP, Karunanadham K, Maheshwari HS, Karuppiah EAA, Thankappan S, Nataraj R, Pandian D, Ameen F, Poczai P, Sayyed RZ. Seed-Borne Probiotic Yeasts Foster Plant Growth and Elicit Health Protection in Black Gram (Vigna mungo L.). Sustainability. 2022; 14(8):4618. https://doi.org/10.3390/su14084618
Chicago/Turabian StyleBright, Jeberlin Prabina, Kumutha Karunanadham, Hemant S. Maheshwari, Eraivan Arutkani Aiyanathan Karuppiah, Sugitha Thankappan, Rajinimala Nataraj, Durga Pandian, Fuad Ameen, Peter Poczai, and Riyaz Z. Sayyed. 2022. "Seed-Borne Probiotic Yeasts Foster Plant Growth and Elicit Health Protection in Black Gram (Vigna mungo L.)" Sustainability 14, no. 8: 4618. https://doi.org/10.3390/su14084618
APA StyleBright, J. P., Karunanadham, K., Maheshwari, H. S., Karuppiah, E. A. A., Thankappan, S., Nataraj, R., Pandian, D., Ameen, F., Poczai, P., & Sayyed, R. Z. (2022). Seed-Borne Probiotic Yeasts Foster Plant Growth and Elicit Health Protection in Black Gram (Vigna mungo L.). Sustainability, 14(8), 4618. https://doi.org/10.3390/su14084618








