Protective Effect of a Cocoa-Enriched Diet on Oxidative Stress Induced by Intensive Acute Exercise in Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Nutritional and Exercise Intervention
2.3. Sample Collection
2.4. Peritoneal Macrophages Isolation and ROS Production
2.5. Plasma Cortisol and Noradrenaline (NA) Concentration
2.6. Serum Immunoglobulins Concentration
2.7. Statistical Analysis
3. Results
3.1. Exercise Performance
3.2. Organ Weight and Plasma Cortisol and NA Concentrations
3.3. Hemograme
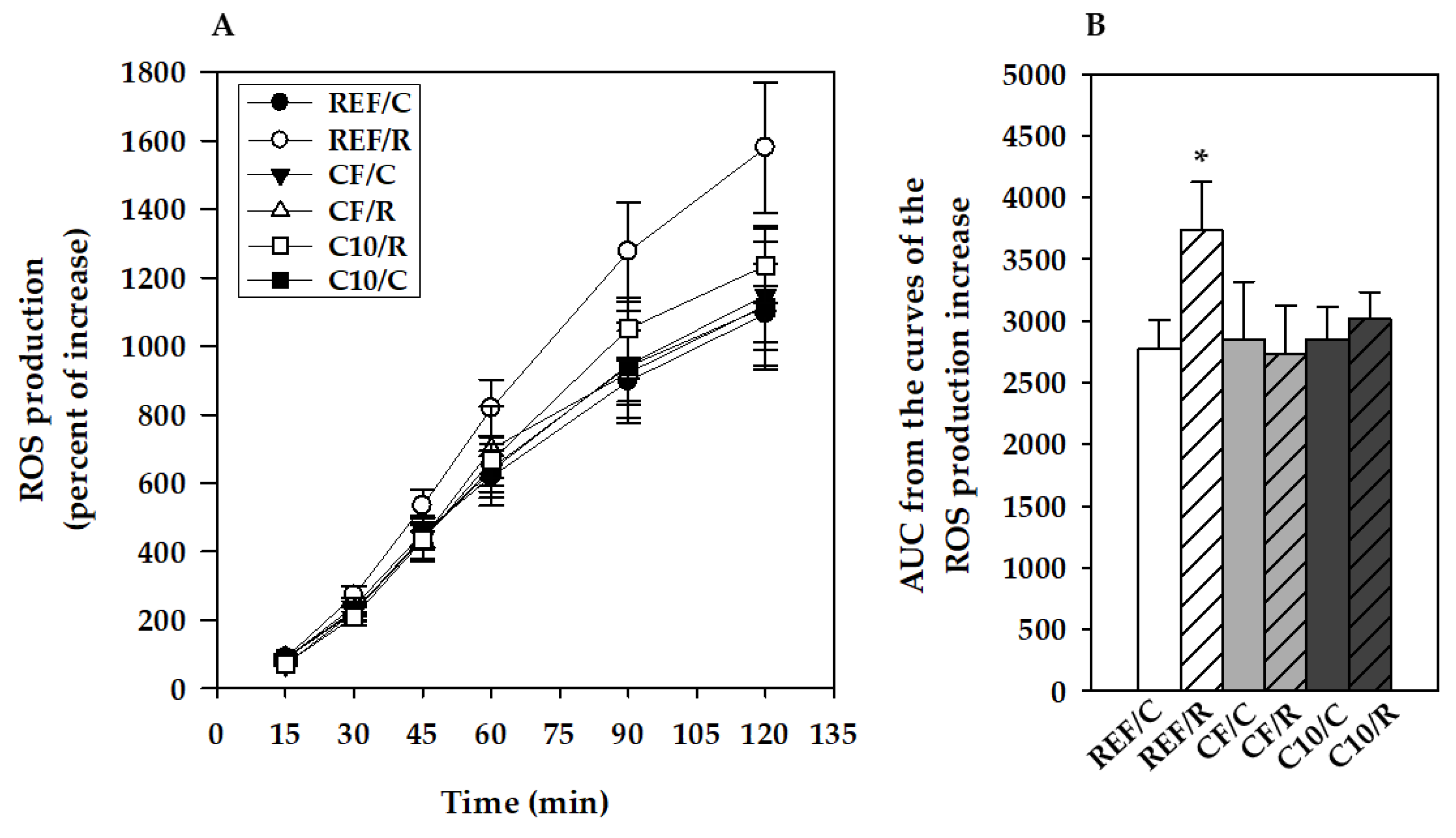
3.4. ROS Production by Peritoneal Macrophages
3.5. Serum Immunoglobulins
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Keen, C.L. Chocolate: Food as Medicine/Medicine as Food. J. Am. Coll. Nutr. 2001, 20, 436S–439S. [Google Scholar] [CrossRef] [PubMed]
- Allgrove, J.; Farrell, E.; Gleeson, M.; Williamson, G.; Cooper, K. Regular dark chocolate consumption’s reduction of oxidative stress and increase of free-fatty-acid mobilization in response to prolonged cycling. Int. J. Sport Nutr. Exerc. Metab. 2011, 21, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Ludovici, V.; Barthelmes, J.; Nägele, M.P.; Enseleit, F.; Ferri, C.; Flammer, A.J.; Ruschitzka, F.; Sudano, I. Cocoa, Blood Pressure, and Vascular Function. Front. Nutr. 2017, 4, 36. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Cano, F.J.; Massot-Cladera, M.; Franch, A.; Castellote, C.; Castell, M. The effects of cocoa on the immune system. Front. Pharmacol. 2013, 4, 71. [Google Scholar] [CrossRef] [PubMed]
- De Feo, M.; Paladini, A.; Ferri, C.; Carducci, A.; Del Pinto, R.; Varrassi, G.; Grassi, D. Anti-inflammatory and anti-nociceptive effects of cocoa: A review on future perspectives in treatment of pain. Pain Ther. 2020, 9, 231–240. [Google Scholar] [CrossRef]
- Andújar, I.; Recio, M.C.; Giner, R.M.; Ríos, J.L. Cocoa polyphenols and their potential benefits for human health. Oxid. Med. Cell. Longev. 2012, 2012, 906252. [Google Scholar] [CrossRef]
- Hüttemann, M.; Lee, I.; Malek, M.H. (−)-Epicatechin maintains endurance training adaptation in mice after 14 days of detraining. FASEB J. 2012, 26, 1413–1422. [Google Scholar] [CrossRef]
- Copp, S.W.; Inagaki, T.; White, M.J.; Hirai, D.M.; Ferguson, S.K.; Holdsworth, C.T.; Sims, G.E.; Poole, D.C.; Musch, T.I. (−)-Epicatechin administration and exercising skeletal muscle vascular control and microvascular oxygenation in healthy rats. Am. J. Physiol.-Heart Circ. Physiol. 2013, 304, H206–H214. [Google Scholar] [CrossRef]
- Ali, D.; Bustami, M.; Alhussainy, T.; Al-Hanbali, M.; Abdel-Malek, S.; Al-Hanbali, R.; Alhussainy, T.; Qadan, F.; Matalka, K.Z. Epicatechin suppresses IL-6, IL-8 and enhances IL-10 production with NF-kappaB nuclear translocation in whole blood stimulated system. Neuroendocrinol. Lett. 2009, 30, 131–138. [Google Scholar]
- Shay, J.; Elbaz, H.A.; Lee, I.; Zielske, S.P.; Malek, M.H.; Hüttemann, M. Molecular mechanisms and therapeutic effects of (−)-epicatechin and other polyphenols in cancer, inflammation, diabetes, and neurodegeneration. Oxid. Med. Cell. Longev. 2015, 2015, 181260. [Google Scholar] [CrossRef]
- Massot-Cladera, M.; Franch, À.; Pérez-Cano, F.J.; Castell, M. Cocoa and cocoa fibre differentially modulate IgA and IgM production at mucosal sites. Br. J. Nutr. 2016, 115, 1539–1546. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Camps-Bossacoma, M.; Pérez-Cano, F.J.; Franch, À.; Castell, M. Theobromine is responsible for the effects of cocoa on the antibody immune status of rats. J. Nutr. 2018, 148, 464–471. [Google Scholar] [CrossRef] [PubMed]
- Pingitore, A.; Pace, G.; Lima, P.; Mastorci, F.; Quinones, A.; Iervasi, G.; Vassalle, C. Exercise and oxidative stress: Potential effects of antioxidant dietary strategies in sports. Nutrition 2015, 31, 916–922. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef] [PubMed]
- Valacchi, G.; Virgili, F.; Cervellati, C.; Pecorelli, A. OxInflammation: From subclinical condition to pathological biomarker. Front. Physiol. 2018, 9, 858. [Google Scholar] [CrossRef] [PubMed]
- Dalleau, S.; Baradat, M.; Guéraud, F.; Huc, L. Cell death and diseases related to oxidative stress:4-hydroxynonenal (HNE) in the balance. Cell Death Differ. 2013, 20, 1615–1630. [Google Scholar] [CrossRef] [PubMed]
- Higgins, M.R.; Izadi, A.; Kaviani, M. Antioxidants and exercise performance: With a focus on vitamin e and c supplementation. Int. J. Environ. Res. Public Health 2020, 17, 8452. [Google Scholar] [CrossRef]
- Niemeijer, A.; Lund, H.; Stafne, S.N.; Ipsen, T.; Goldschmidt, C.L.; Jørgensen, C.T.; Juhl, C.B. Adverse events of exercise therapy in randomised controlled trials: A systematic review and meta-Analysis. Br. J. Sports Med. 2020, 54, 1073–1080. [Google Scholar] [CrossRef]
- Russomanno, G.; Corbi, G.; Manzo, V.; Ferrara, N.; Rengo, G.; Puca, A.A.; Latte, S.; Carrizzo, A.; Calabrese, M.C.; Andriantsitohaina, R.; et al. The anti-ageing molecule sirt1 mediates beneficial effects of cardiac rehabilitation. Immun. Ageing 2017, 14, 7. [Google Scholar] [CrossRef]
- Dhapare, S.; Li, H.; Sakagami, M. Salvianolic acid B as an anti-emphysema agent II: In vivo reversal activities in two rat models of emphysema. Pulm. Pharmacol. Ther. 2018, 53, 52–60. [Google Scholar] [CrossRef]
- Ramiro-Puig, E.; Castell, M. Cocoa: Antioxidant and immunomodulator. Br. J. Nutr. 2009, 101, 931–940. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.M.; Murphy, E.A.; Carmichael, M.D.; Davis, B. Quercetin increases brain and muscle mitochondrial biogenesis and exercise tolerance. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 296, R1071–R1077. [Google Scholar] [CrossRef] [PubMed]
- Nim Kim, E.; Hee Lim, J.; Young Kim, M.; Hyun Ban, T.; Jang, I.A.; Eun Yoon, H.; Whee Park, C.; Sik Chang, Y.; Soon Choi, B. Resveratrol, an Nrf2 activator, ameliorates aging-related progressive rena linjury. Aging 2018, 10, 83–99. [Google Scholar] [CrossRef] [PubMed]
- Cavarretta, E.; Peruzzi, M.; Del Vescovo, R.; Di Pilla, F.; Gobbi, G.; Serdoz, A.; Ferrara, R.; Schirone, L.; Sciarretta, S.; Nocella, C.; et al. Dark chocolate intake positively modulates redox status and markers of muscular damage in elite football athletes: A randomized controlled study. Oxid. Med. Cell. Longev. 2018, 2018, 4061901. [Google Scholar] [CrossRef]
- Estruel-Amades, S.; Camps-Bossacoma, M.; Massot-Cladera, M.; Pérez-Cano, F.J.; Castell, M. Alterations in the innate immune system due to exhausting exercise in intensively trained rats. Sci. Rep. 2020, 10, 967. [Google Scholar] [CrossRef]
- Estruel-Amades, S.; Ruiz-Iglesias, P.; Périz, M.; Franch, À.; Pérez-Cano, F.J.; Camps-Bossacoma, M.; Castell, M. Changes in lymphocyte composition and functionality after intensive training and exhausting exercise in rats. Front. Physiol. 2019, 10, 1491. [Google Scholar] [CrossRef]
- Ruiz-Iglesias, P.; Estruel-Amades, S.; Camps-Bossacoma, M.; Massot-Cladera, M.; Castell, M.; Pérez-Cano, F.J. Alterations in the mucosal immune system by a chronic exhausting exercise in Wistar rats. Sci. Rep. 2020, 10, 17950. [Google Scholar] [CrossRef]
- Ruiz-Iglesias, P.; Gorgori-González, A.; Massot-Cladera, M.; Castell, M.; Pérez-Cano, F.J. Does flavonoid consumption improve exercise performance? Is it related to changes in the immune system and inflammatory biomarkers? A systematic review of clinical studies since 2005. Nutrients 2021, 13, 1132. [Google Scholar] [CrossRef]
- Sánchez, D.; Quiñones, M.; Moulay, L.; Muguerza, B.; Miguel, M.; Aleixandre, A. Soluble fiber-enriched diets improve inflammation and oxidative stress biomarkers in Zucker fatty rats. Pharmacol. Res. 2011, 64, 31–35. [Google Scholar] [CrossRef]
- Lecumberri, E.; Mateos, R.; Izquierdo-Pulido, M.; Rupérez, P.; Goya, L.; Bravo, L. Dietary fibre composition, antioxidant capacity and physico-chemical properties of a fibre-rich product from cocoa (Theobroma cacao L.). Food Chem. 2007, 104, 948–954. [Google Scholar] [CrossRef]
- Massot-Cladera, M.; Costabile, A.; Childs, C.E.; Yaqoob, P.; Franch, À.; Castell, M.; Pérez-Cano, F.J. Prebiotic effects of cocoa fibre on rats. J. Funct. Foods 2015, 19, 341–352. [Google Scholar] [CrossRef]
- Costa, R.J.S.; Snipe, R.M.J.; Kitic, C.M.; Gibson, P.R. Systematic review: Exercise-induced gastrointestinal syndrome—implications for health and intestinal disease. Aliment. Pharmacol. Ther. 2017, 46, 246–265. [Google Scholar] [CrossRef] [PubMed]
- Estruel-Amades, S.; Massot-Cladera, M.; Garcia-Cerdà, P.; Pérez-Cano, F.J.; Franch, Á.; Castell, M.; Camps-Bossacoma, M. Protective effect of hesperidin on the oxidative stress induced by an exhausting exercise in intensively trained rats. Nutrients 2019, 11, 783. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Iglesias, P.; Massot-Cladera, M.; Rodríguez-Lagunas, M.J.; Franch, À.; Camps-Bossacoma, M.; Castell, M.; Pérez-Cano, F.J. A cocoa diet can partially attenuate the alterations in microbiota and mucosal immunity induced by a single session of intensive exercise in rats. Front. Nutr. in press.
- Lalanza, J.F.; Sanchez-Roige, S.; Cigarroa, I.; Gagliano, H.; Fuentes, S.; Armario, A.; Capdevila, L.; Escorihuela, R.M. Long-term moderate treadmill exercise promotes stress-coping strategies in male and female rats. Sci. Rep. 2015, 5, 16166. [Google Scholar] [CrossRef]
- Ramos-Romero, S.; Pérez-Cano, F.J.; Ramiro-Puig, E.; Franch, A.; Castell, M. Cocoa intake attenuates oxidative stress associated with rat adjuvant arthritis. Pharmacol. Res. 2012, 66, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Massot-Cladera, M.; Franch, A.; Castellote, C.; Castell, M.; Pérez-Cano, F.J.; Franch, À.; Castellote, C.; Castell, M.; Pérez-Cano, F.J. Cocoa flavonoid-enriched diet modulates systemic and intestinal immunoglobulin synthesis in adult Lewis rats. Nutrients 2013, 5, 3272–3286. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Romero, S.; Pérez-Cano, F.J.; Pérez-Berezo, T.; Castellote, C.; Franch, A.; Castell, M. Effect of a cocoa flavonoid-enriched diet on experimental autoimmune arthritis. Br. J. Nutr. 2012, 107, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Camps-Bossacoma, M.; Abril-Gil, M.; Saldaña-Ruiz, S.; Franch, À.; Pérez-Cano, F.J.; Castell, M. Cocoa diet prevents antibody synthesis and modifies lymph node composition and functionality in a rat oral sensitization model. Nutrients 2016, 8, 242. [Google Scholar] [CrossRef]
- Abril-Gil, M.; Massot-Cladera, M.; Pérez-Cano, F.J.; Castellote, C.; Franch, À.; Castell, M. A diet enriched with cocoa prevents IgE synthesis in a rat allergy model. Pharmacol. Res. 2012, 65, 603–608. [Google Scholar] [CrossRef]
- Pérez-Berezo, T.; Ramírez-Santana, C.; Franch, A.; Ramos-Romero, S.; Castellote, C.; Pérez-Cano, F.J.; Castell, M. Effects of a cocoa diet on an intestinal inflammation model in rats. Exp. Biol. Med. 2012, 237, 1181–1188. [Google Scholar] [CrossRef]
- Sperkowska, B.; Murawska, J.; Przybylska, A.; Gackowski, M.; Kruszewski, S.; Durmowicz, M.; Rutkowska, D. Cardiovascular effects of chocolate and wine—narrative review. Nutrients 2021, 13, 4269. [Google Scholar] [CrossRef] [PubMed]
- Decroix, L.; Tonoli, C.; Lespagnol, E.; Balestra, C.; Descat, A.; Drittij-Reijnders, M.J.; Blackwell, J.R.; Stahl, W.; Jones, A.M.; Weseler, A.R.; et al. One-week cocoa flavanol intake increases prefrontal cortex oxygenation at rest and during moderate-intensity exercise in normoxia and hypoxia. J. Appl. Physiol. 2018, 125, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Shaw, K.; Singh, J.; Sirant, L.; Neary, P.; Chilibeck, P.D. Effect of Dark Chocolate Supplementation on Tissue Oxygenation, Metabolism, and Performance in Trained Cyclists at Altitude. Int. J. Sport Nutr. Exerc. Metab. 2020, 30, 420–426. [Google Scholar] [CrossRef] [PubMed]
- Decroix, L.; Soares, D.D.; Meeusen, R.; Heyman, E.; Tonoli, C. Cocoa Flavanol Supplementation and Exercise: A Systematic Review. Sports Med. 2018, 48, 867–892. [Google Scholar] [CrossRef]
- Bowtell, J.; Kelly, V. Fruit-Derived Polyphenol Supplementation for Athlete Recovery and Performance. Sports Med. 2019, 49, 3–23. [Google Scholar] [CrossRef]
- Lü, J.M.; Lin, P.H.; Yao, Q.; Chen, C. Chemical and molecular mechanisms of antioxidants: Experimental approaches and model systems. J. Cell. Mol. Med. 2010, 14, 840–860. [Google Scholar] [CrossRef]
- de Carvalho, F.G.; Fisher, M.G.; Thornley, T.T.; Roemer, K.; Pritchett, R.; de Freitas, E.C.; Pritchett, K. Cocoa flavanol effects on markers of oxidative stress and recovery after muscle damage protocol in elite rugby players. Nutrition 2019, 62, 47–51. [Google Scholar] [CrossRef]
- Ramiro-Puig, E.; Urpí-Sardà, M.; Pérez-Cano, F.J.; Franch, A.; Castellote, C.; Andrés-Lacueva, C.; Izquierdo-Pulido, M.; Castell, M. Cocoa-enriched diet enhances antioxidant enzyme activity and modulates lymphocyte composition in thymus from young rats. J. Agric. Food Chem. 2007, 55, 6431–6438. [Google Scholar] [CrossRef]
- Haramizu, S.; Ota, N.; Hase, T.; Murase, T. Catechins attenuate eccentric exercise-induced inflammation and loss of force production in muscle in senescence-accelerated mice. J. Appl. Physiol. 2011, 111, 1654–1663. [Google Scholar] [CrossRef]
- Vargas-Mendoza, N.; Angeles-Valencia, M.; Morales-González, Á.; Madrigal-Santillán, E.O.; Morales-Martínez, M.; Madrigal-Bujaidar, E.; Álvarez-González, I.; Gutiérrez-Salinas, J.; Esquivel-Chirino, C.; Chamorro-Cevallos, G.; et al. Oxidative stress, mitochondrial function and adaptation to exercise: New perspectives in nutrition. Life 2021, 11, 1269. [Google Scholar] [CrossRef]
- Nogueira, L.; Ramirez-Sanchez, I.; Perkins, G.A.; Murphy, A.; Taub, P.R.; Ceballos, G.; Villarreal, F.J.; Hogan, M.C.; Malek, M.H. (−)-Epicatechin enhances fatigue resistance and oxidative capacity in mouse muscle. J. Physiol. 2011, 589, 4615–4631. [Google Scholar] [CrossRef] [PubMed]
- Sachdeva, A.K.; Kuhad, A.; Chopra, K. Epigallocatechin gallate ameliorates behavioral and biochemical deficits in rat model of load-induced chronic fatigue syndrome. Brain Res. Bull. 2011, 86, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Pence, B.D.; Bhattacharya, T.K.; Park, P.; Rytych, J.L.; Allen, J.M.; Sun, Y.; McCusker, R.H.; Kelley, K.W.; Johnson, R.W.; Rhodes, J.S.; et al. Long-term supplementation with EGCG and beta-alanine decreases mortality but does not affect cognitive or muscle function in aged mice. Exp. Gerontol. 2017, 98, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Ciliberti, M.G.; Albenzio, M.; De Palo, P.; Santillo, A.; Caroprese, M. Nexus Between Immune Responses and Oxidative Stress: The Role of Dietary Hydrolyzed Lignin in ex vivo Bovine Peripheral Blood Mononuclear Cell Response. Front. Vet. Sci. 2020, 7, 9. [Google Scholar] [CrossRef]
- Simpson, R.J.; Kunz, H.; Agha, N.; Graff, R. Exercise and the regulation of immune functions. In Progress in Molecular Biology and Translational Science; Elsevier Inc.: Amsterdam, The Netherlands, 2015; Volume 135, pp. 355–380. [Google Scholar]
- Krüger, K.; Mooren, F.C. T cell homing and exercise. Exerc. Immunol. Rev. 2007, 13, 37–54. [Google Scholar]
- Hackney, A.C. Stress and the neuroendocrine system: The role of exercise as a stressor and modifier of stress. Expert Rev. Endocrinol. Metab. 2006, 1, 783–792. [Google Scholar] [CrossRef]
- Tsang, C.; Hodgson, L.; Bussu, A.; Farhat, G.; Al-Dujaili, E. Effect of polyphenol-rich dark chocolate on salivary cortisol and mood in adults. Antioxidants 2019, 8, 149. [Google Scholar] [CrossRef]
- Hernández-González, T.; González-Barrio, R.; Escobar, C.; Madrid, J.A.; Periago, M.J.; Collado, M.C.; Scheer, F.A.J.L.; Garaulet, M. Timing of chocolate intake affects hunger, substrate oxidation, and microbiota: A randomized controlled trial. FASEB J. 2021, 35, e21649. [Google Scholar] [CrossRef]
- Mercer, K.W.; Densmore, J.J. Hematologic disorders in the athlete. Clin. Sports Med. 2005, 24, 599–621. [Google Scholar] [CrossRef]
- Chang, C.W.; Chen, C.Y.; Yen, C.C.; Wu, Y.T.; Hsu, M.C. Repressed exercise-induced hepcidin levels after Danggui Buxue Tang supplementation in male recreational runners. Nutrients 2018, 10, 1318. [Google Scholar] [CrossRef]
- Mairbäurl, H.; Bogdanova, A.; Lombardi, G. Red blood cells in sports: Effects of exercise and training on oxygen supply by red blood cells. Front. Physiol. 2013, 4, 332. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Qi, R.; Wu, H.; Shi, W.; Xu, Y.; Li, M. Reduction of hemoglobin, not iron, inhibited maturation of red blood cells in male rats exposed to high intensity endurance exercises. J. Trace Elem. Med. Biol. 2019, 52, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.E.; Garbutt, G.; Lopes, P.; Tunstall Pedoe, D. Effects of prolonged strenuous exercise (marathon running) on biochemical and haematological markers used in the investigation of patients in the emergency department. Br. J. Sports Med. 2004, 38, 292–294. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Nakaji, S.; Mutsuo, Y.; Liu, Q.; Kurakake, S.; Okamura, N.; Kumae, T.; Umeda, T.; Sugawara, K. Impact of a Competitive Marathon Race on Systemic Cytokine and Neutrophil Responses. Med. Sci. Sports Exerc. 2003, 35, 348–355. [Google Scholar] [CrossRef]
- Zhu, Q.Y.; Schramm, D.D.; Gross, H.B.; Holt, R.R.; Kim, S.H.; Yamaguchi, T.; Kwik-Uribe, C.L.; Keen, C.L. Influence of cocoa flavanols and procyanidins on free radical-induced human erythrocyte hemolysis. Clin. Dev. Immunol. 2005, 12, 27–34. [Google Scholar] [CrossRef]
- Yan Zhu, Q.; Holt, R.R.; Lazarus, S.A.; Orozco, T.J.; Keen, C.L. Inhibitory Effects of Cocoa Flavanols and Procyanidin Oligomers on Free Radical-Induced Erythrocyte Hemolysis. Exp. Biol. Med. 2002, 227, 321–329. [Google Scholar]
- Mckune, A.J.; Smith, L.L.; Semple, S.J. Influence of ultra-endurance exercise on immunoglobulin isotypes and subclasses. Br. J. Sports Med. 2005, 39, 665–670. [Google Scholar] [CrossRef]
- Hejazi, K.; Reza, S.; Hosseini, A. Effect of Selected Exercise on Serum Immunoglobulin (IgA, IgG, and IgM) In Middle-Endurance Elite Runners Effects of Hydrotherapy on postural control and electromyography parameters in men with chronic non-specific low back pain View project Effect of Int. Int. J. Sport Stud. 2012, 2, 509–514. [Google Scholar]
- Vahid, I.; Valiollah, S.; Mehdi, A. The effects of physical activity on homoral immune system (IgA, IgG, IgM). Procedia-Soc. Behav. Sci. 2009, 1, 2718–2721. [Google Scholar] [CrossRef][Green Version]
- Suzuki, K. Effects of exercise on antibody production. World J. Immunol. 2015, 5, 160. [Google Scholar] [CrossRef]
- Tijardović, M.; Marijančević, D.; Bok, D.; Kifer, D.; Lauc, G.; Gornik, O.; Keser, T. Intense Physical Exercise Induces an Anti-inflammatory Change in IgG N-Glycosylation Profile. Front. Physiol. 2019, 10, 1522. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruiz-Iglesias, P.; Massot-Cladera, M.; Rodríguez-Lagunas, M.J.; Franch, À.; Camps-Bossacoma, M.; Pérez-Cano, F.J.; Castell, M. Protective Effect of a Cocoa-Enriched Diet on Oxidative Stress Induced by Intensive Acute Exercise in Rats. Antioxidants 2022, 11, 753. https://doi.org/10.3390/antiox11040753
Ruiz-Iglesias P, Massot-Cladera M, Rodríguez-Lagunas MJ, Franch À, Camps-Bossacoma M, Pérez-Cano FJ, Castell M. Protective Effect of a Cocoa-Enriched Diet on Oxidative Stress Induced by Intensive Acute Exercise in Rats. Antioxidants. 2022; 11(4):753. https://doi.org/10.3390/antiox11040753
Chicago/Turabian StyleRuiz-Iglesias, Patricia, Malén Massot-Cladera, Maria J. Rodríguez-Lagunas, Àngels Franch, Mariona Camps-Bossacoma, Francisco J. Pérez-Cano, and Margarida Castell. 2022. "Protective Effect of a Cocoa-Enriched Diet on Oxidative Stress Induced by Intensive Acute Exercise in Rats" Antioxidants 11, no. 4: 753. https://doi.org/10.3390/antiox11040753
APA StyleRuiz-Iglesias, P., Massot-Cladera, M., Rodríguez-Lagunas, M. J., Franch, À., Camps-Bossacoma, M., Pérez-Cano, F. J., & Castell, M. (2022). Protective Effect of a Cocoa-Enriched Diet on Oxidative Stress Induced by Intensive Acute Exercise in Rats. Antioxidants, 11(4), 753. https://doi.org/10.3390/antiox11040753










