Emerging Concern with Imminent Therapeutic Strategies for Treating Resistance in Biofilm
Abstract
:1. Introduction
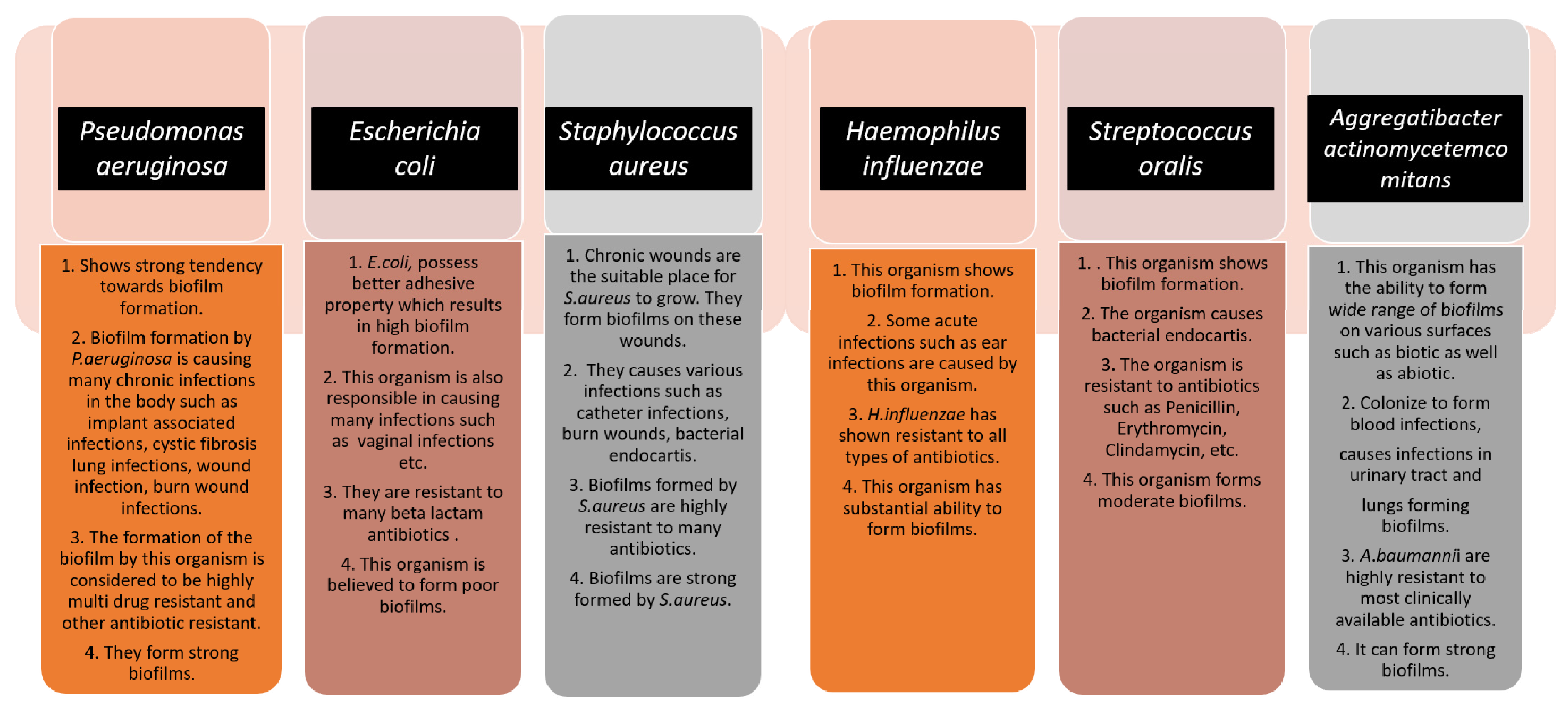
2. Pathogen
2.1. Bacterial Type and Importance of One Health Approach
2.2. Genetic Regulation of Biofilm Formation
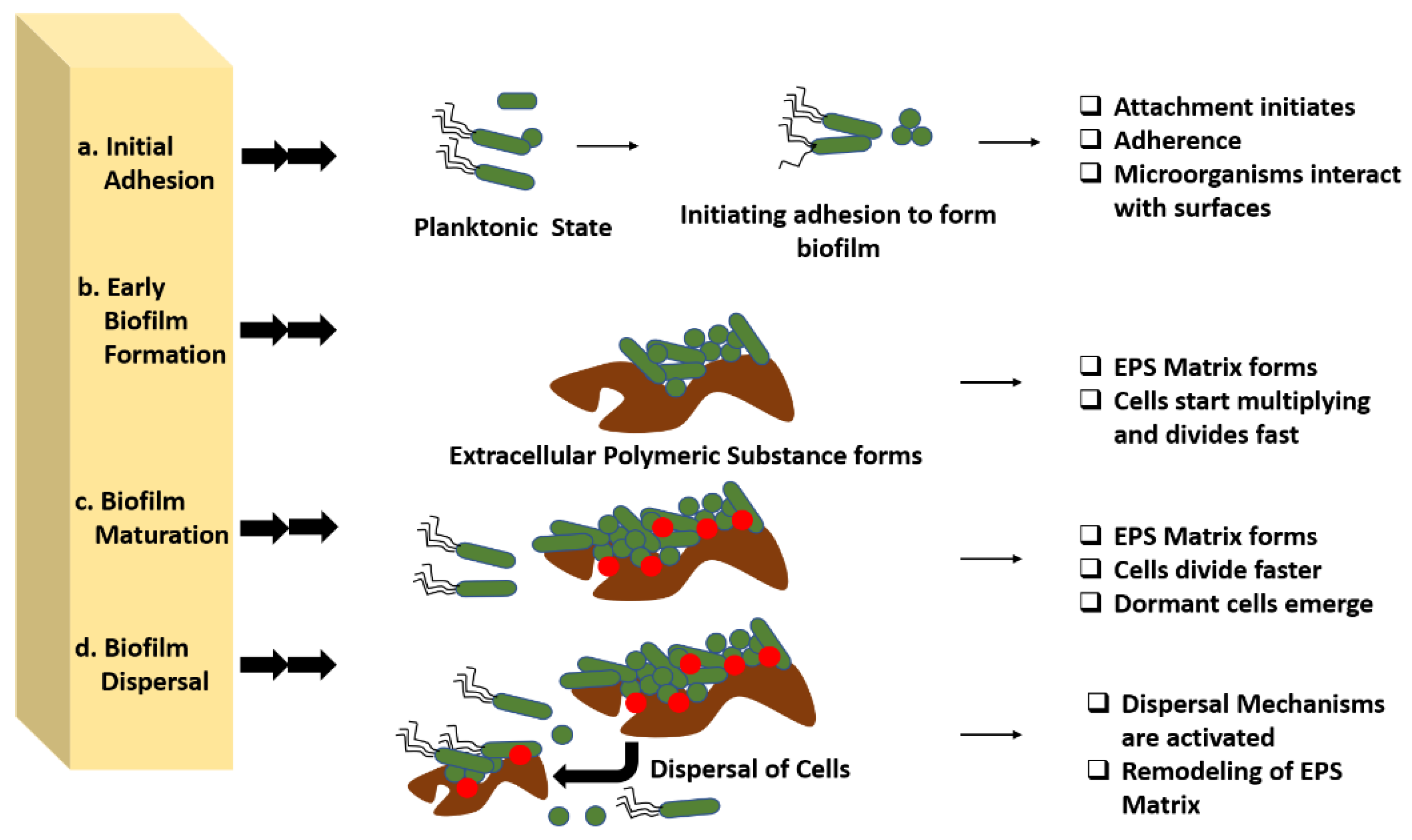
3. Biofilm Development
- I.
- sRNAs functioning by base-pairing with other RNAs;
- II.
- Protein-binding sRNAs mimicking the protein binding regions found in various mRNAs to oppose and sequester their cognate regulatory proteins.
3.1. Initial Adhesion
3.2. Early Biofilm Formation
3.3. Biofilm Maturation
3.4. Dispersal
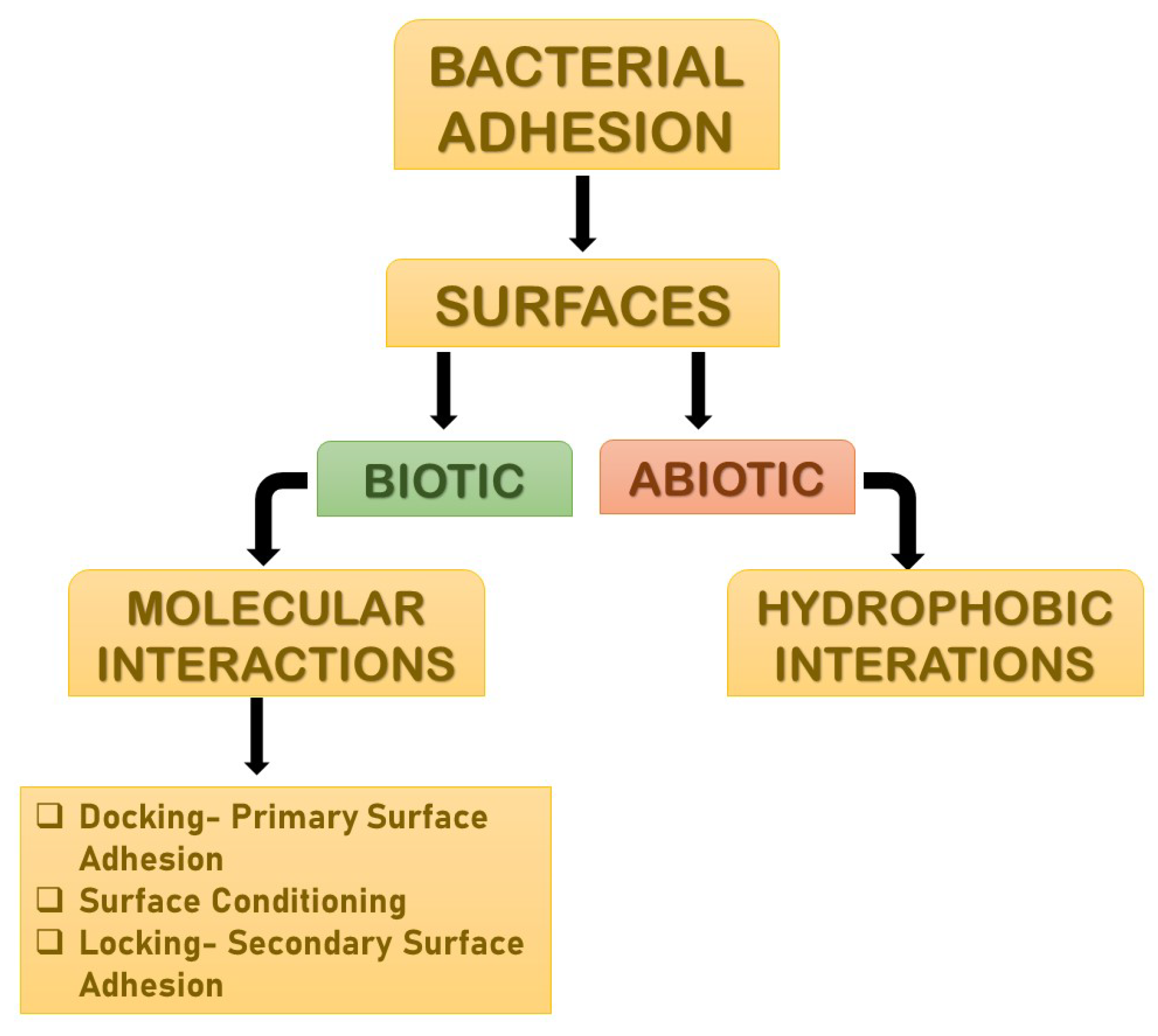
4. Molecular Interactions
4.1. Primary Bacterial Adhesion: Docking
4.2. Surface Conditioning
4.3. Secondary Bacterial Adhesion: Locking
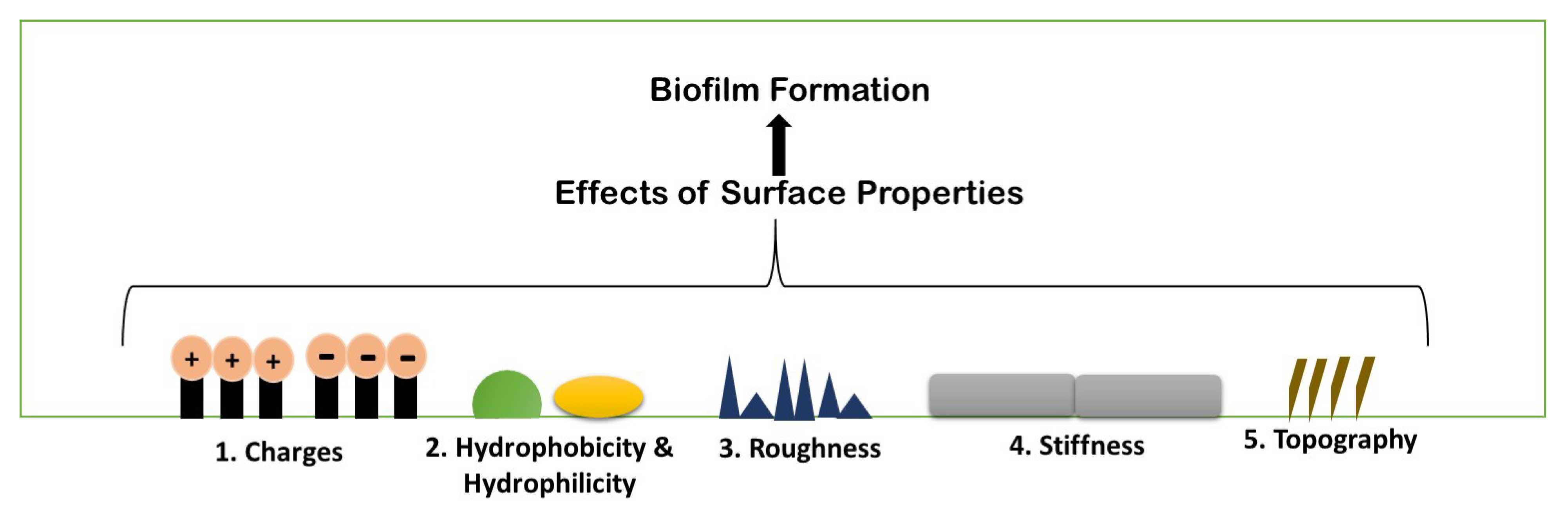
5. Physio-Chemical Surface Properties Controlling Biofilm Formation
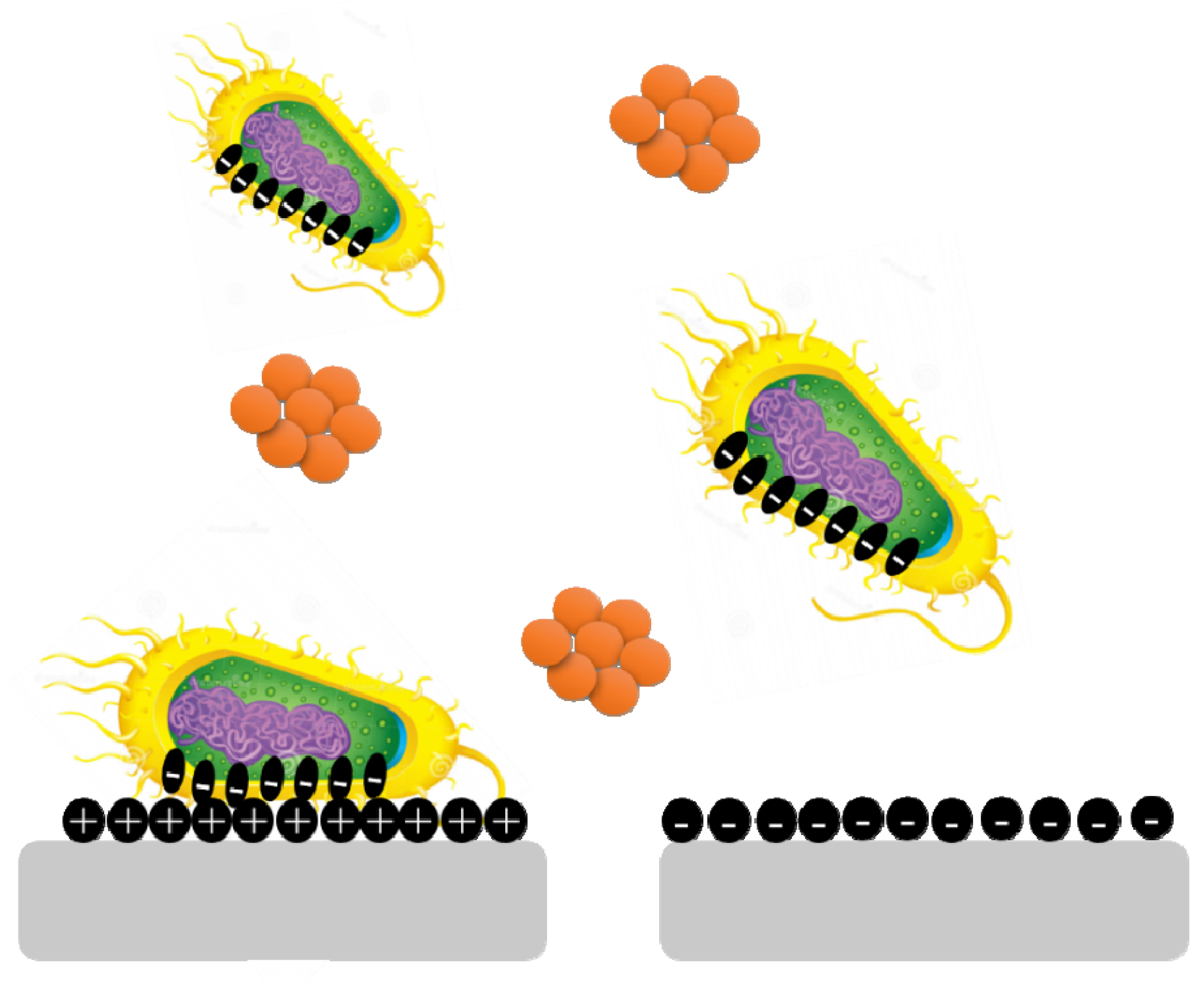
5.1. Surface Charges
5.2. Hydrophobicity and Hydrophilicity
5.3. Roughness and Topography
5.4. Stiffness
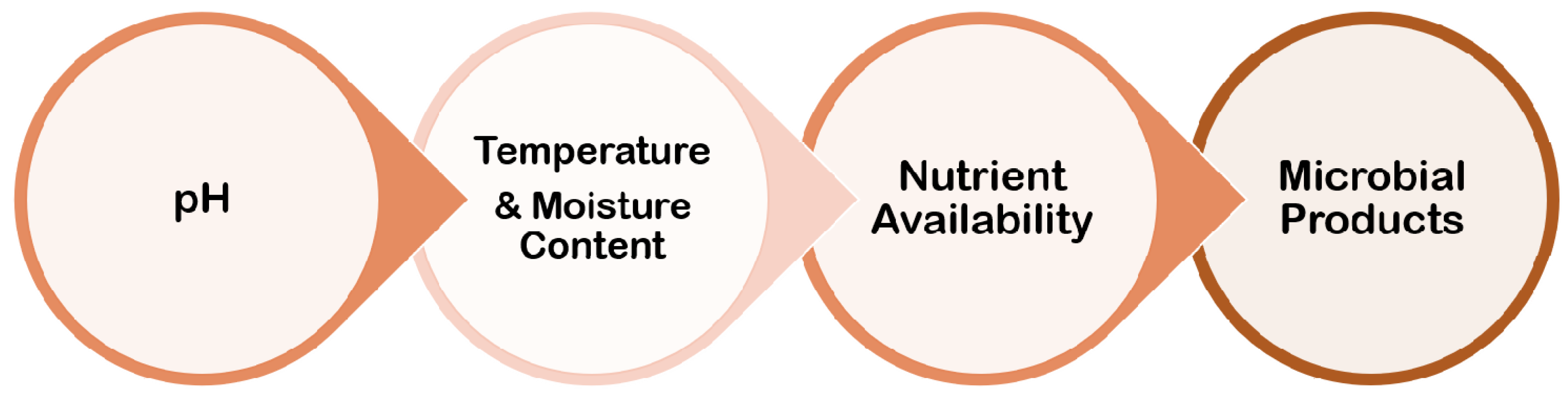
6. Environmental Conditions
6.1. pH
6.2. Temperature and Moisture Content
6.3. Nutrient Availability
6.4. Microbial Products
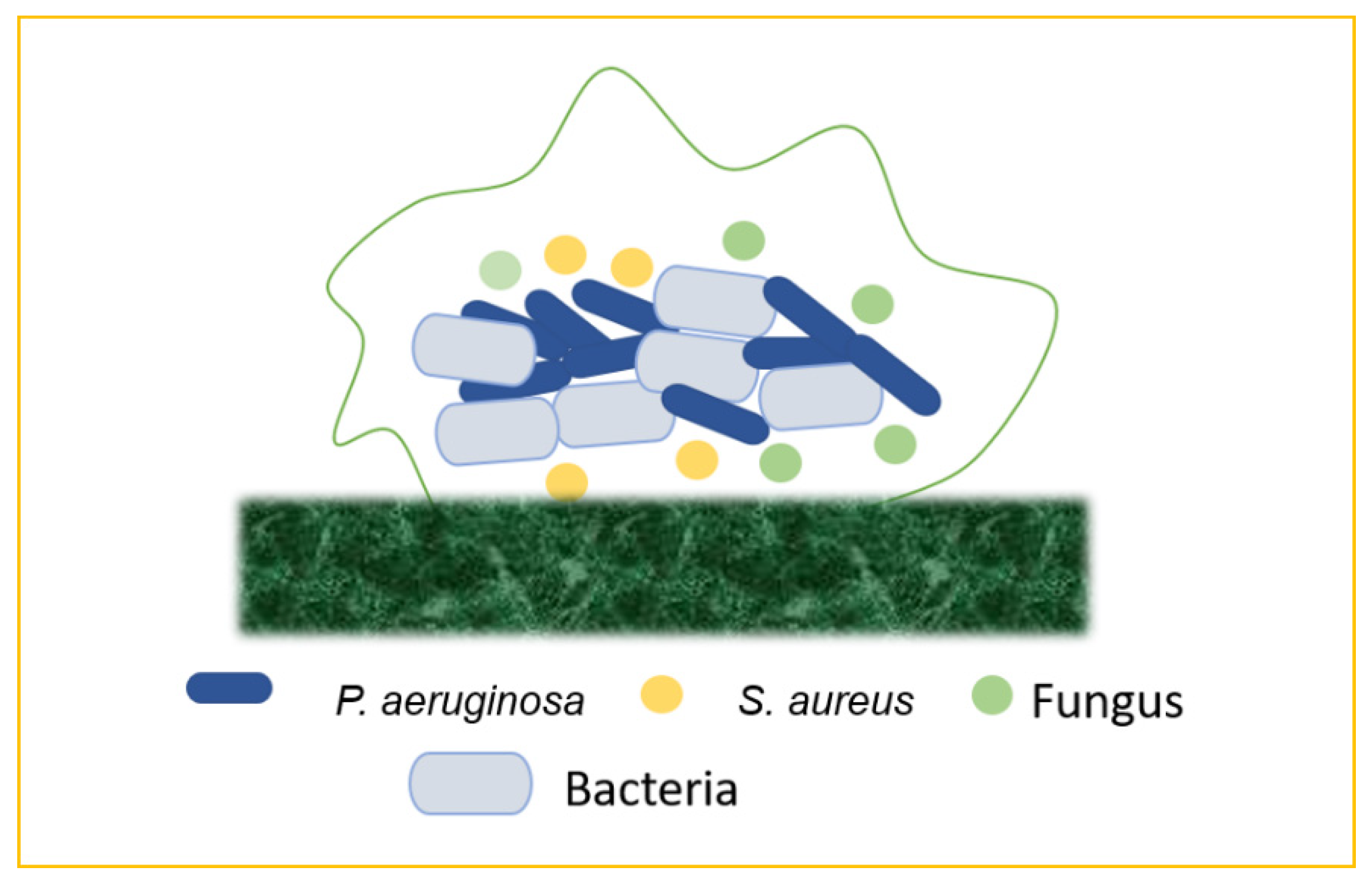
7. Emergence of Mixed Biofilm
7.1. Bacteria–Bacteria Mixed Biofilms
7.2. Bacteria–Fungi Mixed Biofilms
7.3. Bacteria–Virus Mixed Biofilms
8. Biofilm-Associated Human Diseases
9. Therapeutic Approaches to Combat Biofilm Development
10. Prevalence of Antibiotic Resistance in Biofilm
- I.
- The penetration of the antibiotic is either slow or incomplete.
- II.
- Concentration gradients of a metabolic source or product results in zones of bacteria that are slow or do not develop.
- III.
- Some cells show signs of an adaptive stress response.
- IV.
- Only a small percentage of cells mature into a highly protected persisted state [54].
11. Drug Delivery Strategies to Combat Biofilm-Associated Diseases
12. Future Perspective
13. Concluding Remarks
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Glossary
| Biofilm | A biofilm is a complex microbiome structure composed of different bacterial colonies or single types of cells that adhere to the surface. These cells, which are embedded in extracellular polymeric substances, a matrix made up of eDNA, proteins, and polysaccharides, demonstrated high levels of antibiotic resistance. |
| Biofilm Development Process | Biofilm formation is commonly thought to occur in four stages: bacterial attachment to a surface, microcolony formation, biofilm maturation, and bacterial detachment (also known as dispersal) to colonize new areas. |
| Adhesion | A biofilm is a collection of bacteria that are attached to a substrate and which is made up of many bacteria that are co-adhered by physical appendages and extracellular polymeric substances. The microbes themselves, as well as a substrate, are required for biofilm growth. This process is known as adhesion. |
| Molecular Interactions | Biofilms are held together with the help of various molecular interactions. |
| Surface Conditioning | Conditioning films are formed as a result of macromolecule adsorption on the substrate, altering the adhesion conditions for bacteria to this surface. This is known as surface conditioning. |
| Antibiotic Resistance | Antibiotic resistance occurs when germs such as bacteria and fungi develop the ability to resist drugs that are designed to kill them. This means that the germs are not killed and can continue to multiply. |
| Biofilm Resistance | Bacteria gain additional resistance power from the biofilm matrix, allowing them to not only tolerate harsh conditions, but also resist antibiotics, known as biofilm resistance |
| Resistomes | The resistomes are the accumulation of all the antibiotic resistance genes. |
| One Health Approach | One Health refers to the collaborative efforts of multiple disciplines working on a local, national, and global scale to achieve optimal health for people, animals, and the environment. |
References
- Brown, D. The Discovery of Water Channels (Aquaporins). Ann. Nutr. Metab. 2017, 1, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Del Pozo, J.L. Biofilm-related disease. Expert Rev. Anti. Infect. Ther. 2018, 16, 51–65. [Google Scholar] [CrossRef] [PubMed]
- Jamal, M.A.W.; Andleeb, S.; Jalil, F.; Imran, M.; Nawaz, M.A.; Hussain, T.; Ali, M.; Rafiq, M.; Kamil, M.A. Bacterial biofilm and associated infections. J. Chin. Med. Assoc. 2018, 81, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Davies, J.D.D. Origins and evolution of antibiotic resistance. Microbiol. Mol. Biol. Rev. 2010, 74, 417–433. [Google Scholar] [CrossRef] [Green Version]
- Prestinaci, F.P.P.; Pantosti, A. Antimicrobial resistance: A global multifaceted phenomenon. Pathog. Glob. Health 2015, 109, 309–318. [Google Scholar] [CrossRef] [Green Version]
- Tagliabue, A.R.R. Changing Priorities in Vaccinology: Antibiotic Resistance Moving to the Top. Front. Immunol. 2018, 9, 1068. [Google Scholar] [CrossRef]
- Aslam, B.W.W.; Arshad, M.I.; Khurshid, M.; Muzammil, S.; Rasool, M.H.; Nisar, M.A.; Alvi, R.F.; Aslam, M.A.; Qamar, M.U.; Salamat, M.K.F.; et al. Antibiotic resistance: A rundown of a global crisis. Infect. Drug Resist. 2018, 11, 1645–1658. [Google Scholar] [CrossRef] [Green Version]
- Sinclair, J.R. Importance of a One Health approach in advancing global health security and the Sustainable Development Goals. Rev. Sci. Tech. 2019, 38, 145–154. [Google Scholar] [CrossRef]
- Thambirajoo, M.; Maarof, M.; Lokanathan, Y.; Katas, H.; Ghazalli, N.F.; Tabata, Y.; Fauzi, M.B. Potential of Nanoparticles Integrated with Antibacterial Properties in Preventing Biofilm and Antibiotic Resistance. Antibiotics 2021, 10, 1338. [Google Scholar] [CrossRef]
- Donlan, R.M. Biofilm Formation: A Clinically Relevant Microbiological Process. Clin. Infect. Dis. 2001, 33, 1387–1392. [Google Scholar] [CrossRef] [Green Version]
- Donlan, R.M. Biofilms: Microbial Life on Surfaces. Emerg. Infect. Dis. 2002, 8, 881–890. [Google Scholar] [CrossRef] [PubMed]
- Madigan, M.T.; Martinko, J.M.; Dunlap, P.V.; Clark, D.P. Brock Biology of Microorganisms; Prentice Hall/Pearson Education: Hoboken, NJ, USA, 2003. [Google Scholar]
- Dumaru, R.B.R.; Shrestha, L.B. Study of biofilm formation and antibiotic resistance pattern of gram-negative Bacilli among the clinical isolates at BPKIHS, Dharan. BMC Res. Notes 2019, 12, 38. [Google Scholar] [CrossRef] [PubMed]
- Subh, L.C.W.; Pinkvos, T.J.; Behrens, P.; Brandenburg, K.; Gutsmann, T.; Stiesch, M.; Doll, K.; Winkel, A. Synthetic anti-endotoxin peptides interfere with Gram-positive and Gram-negative bacteria, their adhesion and biofilm formation on titanium. J. Appl. Microbiol. 2020, 129, 1272–1286. [Google Scholar] [CrossRef] [PubMed]
- Muhsin, J.; Ufaq, T.; Tahir, H.; Saadia, A. Bacterial Biofilm: Its Composition, Formation and Role in Human Infections. J. Microbiol. Biotechnol. 2015, 4, 1–14. [Google Scholar]
- Fleming, D.; Rumbaugh, K.P. Approaches to Dispersing Medical Biofilms. Microorganisms 2017, 5, 15. [Google Scholar] [CrossRef]
- Roy, P.K.H.A.; Mizan, M.F.R.; Hossain, M.I.; Ashrafudoulla, M.; Toushik, S.H.; Nahar, S.; Kim, Y.K.; Ha, S.D. Effects of environmental conditions (temperature, pH, and glucose) on biofilm formation of Salmonella enterica serotype Kentucky and virulence gene expression. Poult. Sci. 2021, 100, 101209. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, Y.; Ge, Y.; Zhu, X.; Pan, J. Regulatory Mechanisms and Promising Applications of Quorum Sensing-Inhibiting Agents in Control of Bacterial Biofilm Formation. Front. Microbiol. 2020, 11, 589640. [Google Scholar] [CrossRef]
- Pena, R.T.; Blasco, L.; Ambroa, A.; González-Pedrajo, B.; Fernández-García, L.; López, M.; Bleriot, I.; Bou, G.; García-Contreras, R.; Wood, T.K.; et al. Relationship Between Quorum Sensing and Secretion Systems. Front. Microbiol. 2019, 10, 1100. [Google Scholar] [CrossRef] [Green Version]
- Wolska, K.I.; Grudniak, A.M.; Rudnicka, Z.; Markowska, K. Genetic control of bacterial biofilms. J. Appl. Genet. 2016, 57, 225–238. [Google Scholar] [CrossRef] [Green Version]
- Larson, E.L.G.-D.C.; Lee, L.V.; Della-Latta, P.; Kain, D.J.; Keswick, B.H. Microbial flora of hands of homemakers. Am. J. Infect. Control 2003, 31, 72–79. [Google Scholar] [CrossRef]
- Lutz, J.K.; Lee, J. Prevalence and antimicrobial-resistance of Pseudomonas aeruginosa in swimming pools and hot tubs. Int. J. Environ. Res. Public Health 2011, 8, 554–564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Podschun, R. UUKs. as nosocomial pathogens: Epidemiology, taxonomy, typing methods, and pathogenicity factors. Clin. Microbiol. Rev. 1998, 11, 589–603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, C.H.S.P.; Moi, S.H.; Chuang, L.Y. Biofilm Formation in Acinetobacter Baumannii: Genotype-Phenotype Correlation. Molecules 2019, 24, 1849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koo, H.; Allan, R.N.; Howlin, R.P.; Stoodley, P.; Hall-Stoodley, L. Targeting microbial biofilms: Current and prospective therapeutic strategies. Nat. Rev. Microbiol. 2017, 15, 740–755. [Google Scholar] [CrossRef] [PubMed]
- Sauer, K.C.A.; Ehrlich, G.D.; Costerton, J.W.; Davies, D.G. Pseudomonas aeruginosa displays multiple phenotypes during development as a biofilm. J. Bacteriol. 2002, 184, 1140–1154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muhammad, M.H.; Idris, A.L.; Fan, X.; Guo, Y.; Yu, Y.; Jin, X.; Qiu, J.; Guan, X.; Huang, T. Beyond Risk: Bacterial Biofilms and Their Regulating Approaches. Front. Microbiol. 2020, 11, 928. [Google Scholar] [CrossRef] [PubMed]
- Chambers, J.R.; Sauer, K. Small RNAs and their role in biofilm formation. Trends Microbiol. 2013, 21, 39–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomason, M.K.; Storz, G. Bacterial antisense RNAs: How many are there and what are they doing? Annu. Rev. Genet. 2010, 44, 167–188. [Google Scholar] [CrossRef] [Green Version]
- Pearce, D.; Bazin, M.J.; Lynch, J.M. The rhisosphere as a biofilm. In Microbial Biofilms; Lappin-Scott, H.M., Costerton, J.W., Eds.; Cambridge University Press: Cambridge, UK, 1995; pp. 207–220. [Google Scholar]
- Kostakioti, M.; Hadjifrangiskou, M.; Hultgren, S.J. Bacterial Biofilms: Development, Dispersal, and Therapeutic Strategies in the Dawn of the Postantibiotic Era. Cold Spring Harb. Perspect. Med. 2013, 3, a010306. [Google Scholar] [CrossRef] [Green Version]
- Marshall, K.C. Mechanisms of bacterial adhesion at solid-water interfaces. In Bacterial Adhesion: Mechanisms and Physiological Significance; Springer: Boston, MA, USA, 1985; pp. 133–161. [Google Scholar]
- Marshall, K.C.; Stout, R.; Mitchell, R.I. Selective Sorption of Bacteria from Seawater. II. Mechanism of the Initial Events in the Sorption of Marine Bacteria to Surfaces. Can. J. Microbiol. 1971, 68, 337–348. [Google Scholar]
- O’Toole, G.A.; Kolter, R. Flagellar and twitching motility are necessary for Pseudomonas aeruginosa biofilm development. Mol. Microbiol. 1998, 30, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Dunne, W.M., Jr. Bacterial adhesion: Seen any good biofilms lately? Clin. Microbiol. Rev. 2002, 15, 155–166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- An, Y.H.; Dickinson, R.B.; Doyle, R.J. Mechanisms of Bacterial Adhesion and Pathogenesis of Implant and Tissue Infections. In Handbook of Bacterial Adhesion: Principles, Methods, and Applications; Humana Press: Totowa, NJ, USA, 2000. [Google Scholar]
- Song, F.K.H.; Ren, D. Effects of Material Properties on Bacterial Adhesion and Biofilm Formation. J. Dent. Res. 2015, 94, 1027–1034. [Google Scholar] [CrossRef] [PubMed]
- Mazumder, S.F.J.; Dietrich, A.M.; Puri, I.K. Role of hydrophobicity in bacterial adherence to carbon nanostructures and biofilm formation. Biofouling 2010, 26, 333–339. [Google Scholar] [CrossRef]
- Rao, Y.; Shang, W.; Yang, Y.; Zhou, R.; Rao, X. Fighting Mixed-Species Microbial Biofilms With Cold Atmospheric Plasma. Front. Microbiol. 2020, 11, 1000. [Google Scholar] [CrossRef]
- Kvich, L.; Burmølle, M.; Bjarnsholt, T.; Lichtenberg, M. Do Mixed-Species Biofilms Dominate in Chronic Infections?–Need for in situ Visualization of Bacterial Organization. Front. Cell. Infect. Microbiol. 2020, 10, 396. [Google Scholar] [CrossRef]
- Batoni, G.; Maisetta, G.; Esin, S. Therapeutic Potential of Antimicrobial Peptides in Polymicrobial Biofilm-Associated Infections. Int. J. Mol. Sci. 2021, 22, 482. [Google Scholar] [CrossRef]
- Metcalf, D.G.; Bowler, P.G. Biofilm delays wound healing: A review of the evidence. Burn. Trauma 2013, 1, 2321–3868. [Google Scholar] [CrossRef] [Green Version]
- Allison, D.L.; Willems, H.M.E.; Jayatilake, J.A.M.S.; Bruno, V.M.; Peters, B.M.; Shirtliff, M.E. Candida-bacteria interactions: Their impact on human disease. Microbiol. Spectr. 2016, 4, 103–136. [Google Scholar] [CrossRef] [Green Version]
- Neu, U.; Mainou, B.A. Virus interactions with bacteria: Part ners in the infectious dance. PLoS Pathog. 2020, 16, e1008234. [Google Scholar] [CrossRef]
- Anselme, K.D.P.; Popa, A.M.; Giazzon, M.; Liley, M.; Ploux, L. The interaction of cells and bacteria with surfaces structured at the nanometre scale. Acta Biomater. 2010, 6, 3824–3846. [Google Scholar] [CrossRef] [PubMed]
- Krasowska, A.; Sigler, K. How microorganisms use hydrophobicity and what does this mean for human needs? Front. Cell. Infect. Microbiol. 2014, 4, 112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olivares, E.; Badel-Berchoux, S.; Provot, C.; Prévost, G.; Bernardi, T.; Jehl, F. Clinical Impact of Antibiotics for the Treatment of Pseudomonas aeruginosa Biofilm Infections. Front. Microbiol. 2020, 10, 2894. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Li, X.; Yu, C.; Wang, Y. Promising Therapeutic Strategies Against Microbial Biofilm Challenges. Front. Cell. Infect. Microbiol. 2020, 10, 359. [Google Scholar] [CrossRef] [PubMed]
- Bi, Y.; Xia, G.; Shi, C.; Wan, J.; Liu, L.; Chen, Y.; Wu, Y.; Zhang, W.; Zhou, M.; He, H.; et al. Therapeutic strategies against bacterial biofilms. Fundam. Res. 2021, 1, 193–212. [Google Scholar] [CrossRef]
- Weldrick, P.J.; Hardman, M.J.; Paunov, V.N. Enhanced clearing of wound-related pathogenic bacterial biofilms using protease-functionalized antibiotic nanocarriers ACS. Appl. Mater. Interfaces 2019, 11, 43902–43919. [Google Scholar] [CrossRef]
- Wasfi, R.; Hamed, S.; Amer, M.A.; Fahmy, L.I. Proteus mirabilis Biofilm: Development and Therapeutic Strategies. Front. Cell. Infect. Microbiol. 2020, 10, 414. [Google Scholar] [CrossRef]
- Machado, D.; Castro, J.; Palmeira-De-Oliveira, A.; de Oliveira, J.M.; Cerca, N. Bacterial Vaginosis Biofilms: Challenges to Current Therapies and Emerging Solutions. Front. Microbiol. 2016, 6, 1528. [Google Scholar] [CrossRef] [Green Version]
- Panda, S.K.; Buroni, S.; Tiwari, V.; da Silva, L.C.N. Editorial: Insights into New Strategies to Combat Biofilms. Front. Microbiol. 2021, 12, 742647. [Google Scholar] [CrossRef]
- Renner, L.D.; Weibel, D.B. Physicochemical regulation of biofilm formation. MRS Bull. 2011, 36, 347–355. [Google Scholar] [CrossRef] [Green Version]
- Jones, E.M.; Cochrane, C.A.; Percival, S.L. The Effect of pH on the Extracellular Matrix and Biofilms. Adv. Wound Care 2015, 4, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, R.; Santhakumari, S.; Poonguzhali, P.; Geetha, M.; Dyavaiah, M.; Xiangmin, L. Bacterial Biofilm Inhibition: A Focused Review on Recent Therapeutic Strategies for Combating the Biofilm Mediated Infections. Front. Microbiol. 2021, 12, 676458. [Google Scholar] [CrossRef] [PubMed]
- Fernando, S.; Gunasekara, T.; Holton, J. Antimicrobial Nanoparticles: Applications and mechanisms of action. Sri Lankan J. Infect. Dis. 2018, 8, 2. [Google Scholar] [CrossRef]
- Raghunath, A.; Perumal, E. Metal oxide nanoparticles as antimicrobial agents: A promise for the future. Int. J. Antimicrob. Agents 2017, 49, 137–152. [Google Scholar] [CrossRef] [PubMed]
- García-Pinel, B.; Porras-Alcalá, C.; Ortega-Rodríguez, A.; Sarabia, F.; Prados, J.; Melguizo, C.; López-Romero, J.M. Lipid-Based Nanoparticles: Application and Recent Advances in Cancer Treatment. Nanomaterials 2019, 9, 638. [Google Scholar] [CrossRef] [Green Version]
- Mi, G.J.; Shi, D.; Wang, M.; Webster, T.J. Reducing Bacterial Infections and Biofilm Formation Using Nanoparticles and Nanostructured Antibacterial Surfaces. Adv. Healtc. Mater. 2018, 7, e1800103. [Google Scholar] [CrossRef]
- Salgar-Chaparro, S.J.L.K.; Pojtanabuntoeng, T.; Darwin, A.; Machuca, L.L. Nutrient Level Determines Biofilm Characteristics and Subsequent Impact on Microbial Corrosion and Biocide Effectiveness. Appl. Environ. Microbiol. 2020, 86, e02885-19. [Google Scholar] [CrossRef] [Green Version]
- Costerton, J.W.; Cheng, K.J.; Geesey, G.G.; Ladd, T.I.; Nickel, J.C.; Dasgupta, M.; Marrie, T.J. Bacterial biofilms in nature and disease. Annu. Rev. Microbiol. 1987, 41, 435–464. [Google Scholar] [CrossRef]
- Costerton, J.W.; Lewandowski, Z.; Caldwell, D.E.; Korber, D.R.; Lappin-Scott, H.M. Microbial biofilms. Annu. Rev. Microbiol. 1995, 49, 711–745. [Google Scholar] [CrossRef]
- Davies, D. Understanding biofilm resistance to antibacterial agents. Nat. Rev. Drug Discov. 2003, 2, 114–122. [Google Scholar] [CrossRef]
- Stewart, P.S. Mechanisms of antibiotic resistance in bacterial biofilms. Int. J. Med. Microbiol. 2002, 292, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.T.; Yu, F.P.; McFeters, G.A.; Stewart, P.S. Nonuniform spatial patterns of respiratory activity within biofilms during disinfection. Appl. Environ. Microbiol. 1995, 61, 2252–2256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, D.; Cha, C.J. Antibiotic resistome from the One-Health perspective: Understanding and controlling antimicrobial resistance transmission. Exp. Mol. Med. 2021, 53, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Miquel, S.; Lagrafeuille, R.; Souweine, B.; Forestier, C. Anti-biofilm Activity as a Health Issue. Front. Microbiol. 2016, 7, 592. [Google Scholar] [CrossRef]
- Busscher, H.J.; van der Mei, H.C.; Subbiahdoss, G.; Jutte, P.C.; van den Dungen, J.J.; Zaat, S.A.; Schultz, M.J.; Grainger, D.W. Biomaterial-associated infection: Locating the finish line in the race for the surface. Sci. Transl. Med. 2012, 4, 153rv10. [Google Scholar] [CrossRef] [Green Version]
- Schütz, C.A.; Juillerat-Jeanneret, L.; Mueller, H.; Lynch, I.; Riediker, M. NanoImpactNet Consortium. Therapeutic nanoparticles in clinics and under clinical evaluation. Nanomedicine 2013, 8, 449–467. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pandey, R.P.; Mukherjee, R.; Chang, C.-M. Emerging Concern with Imminent Therapeutic Strategies for Treating Resistance in Biofilm. Antibiotics 2022, 11, 476. https://doi.org/10.3390/antibiotics11040476
Pandey RP, Mukherjee R, Chang C-M. Emerging Concern with Imminent Therapeutic Strategies for Treating Resistance in Biofilm. Antibiotics. 2022; 11(4):476. https://doi.org/10.3390/antibiotics11040476
Chicago/Turabian StylePandey, Ramendra Pati, Riya Mukherjee, and Chung-Ming Chang. 2022. "Emerging Concern with Imminent Therapeutic Strategies for Treating Resistance in Biofilm" Antibiotics 11, no. 4: 476. https://doi.org/10.3390/antibiotics11040476






