Chemical Doping of a Silica Matrix with a New Organic Dye from the Group of Heterocyclic Compounds—Chemical, Optical and Surface Characteristics
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis
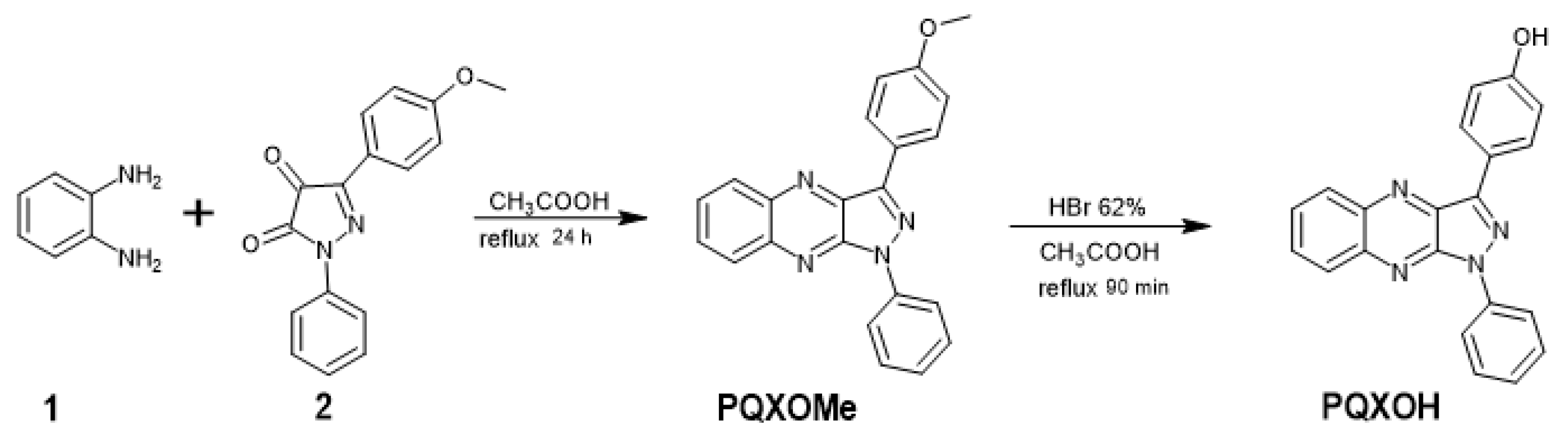
2.2.1. Synthesis of Organic Day PQXOH
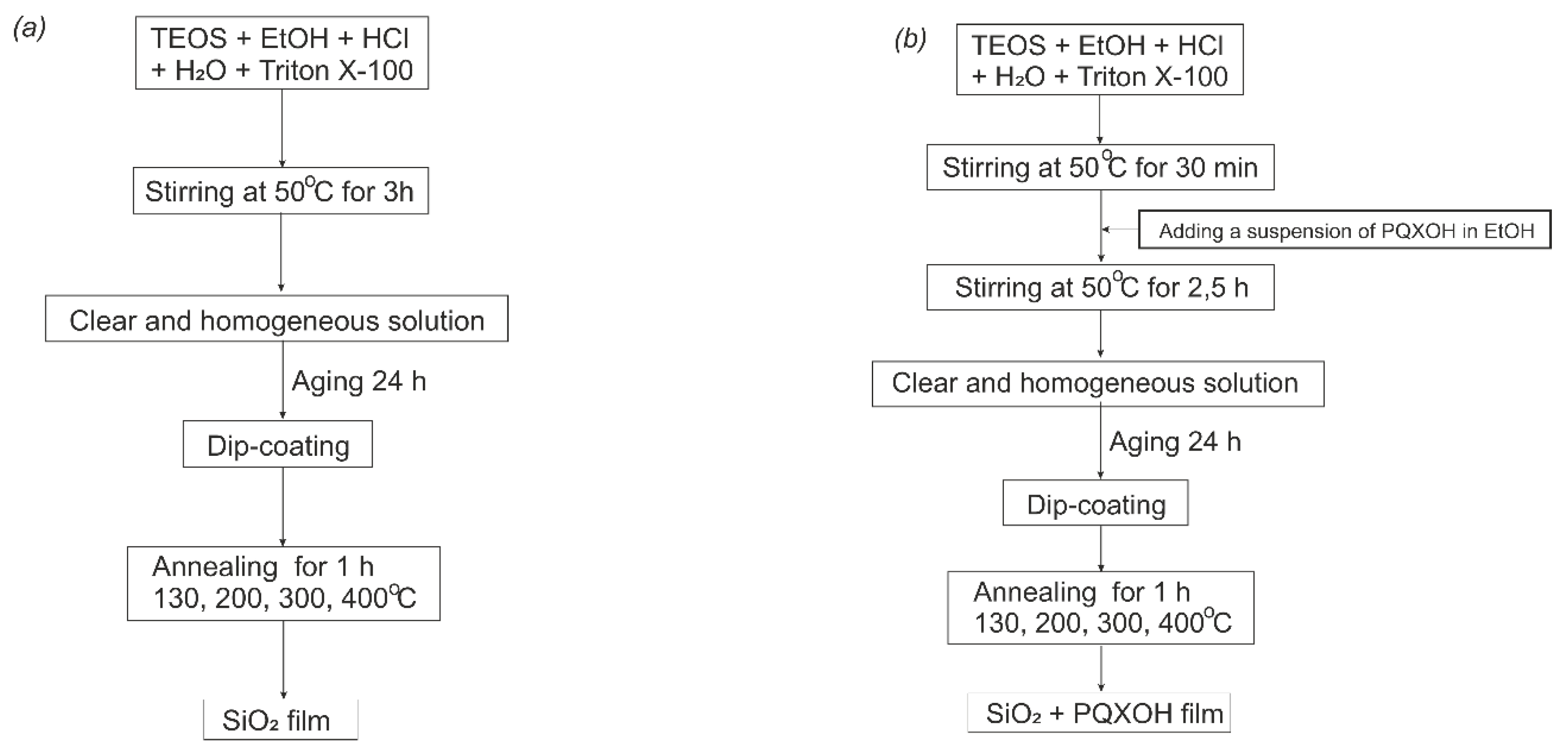
2.2.2. Synthesis of Sols
2.2.3. Films Fabrication
2.3. Equipment and Methods
3. Results
3.1. The Synthesis of PQXOH and Their Photophysics Properties
3.1.1. Synthetic Procedures
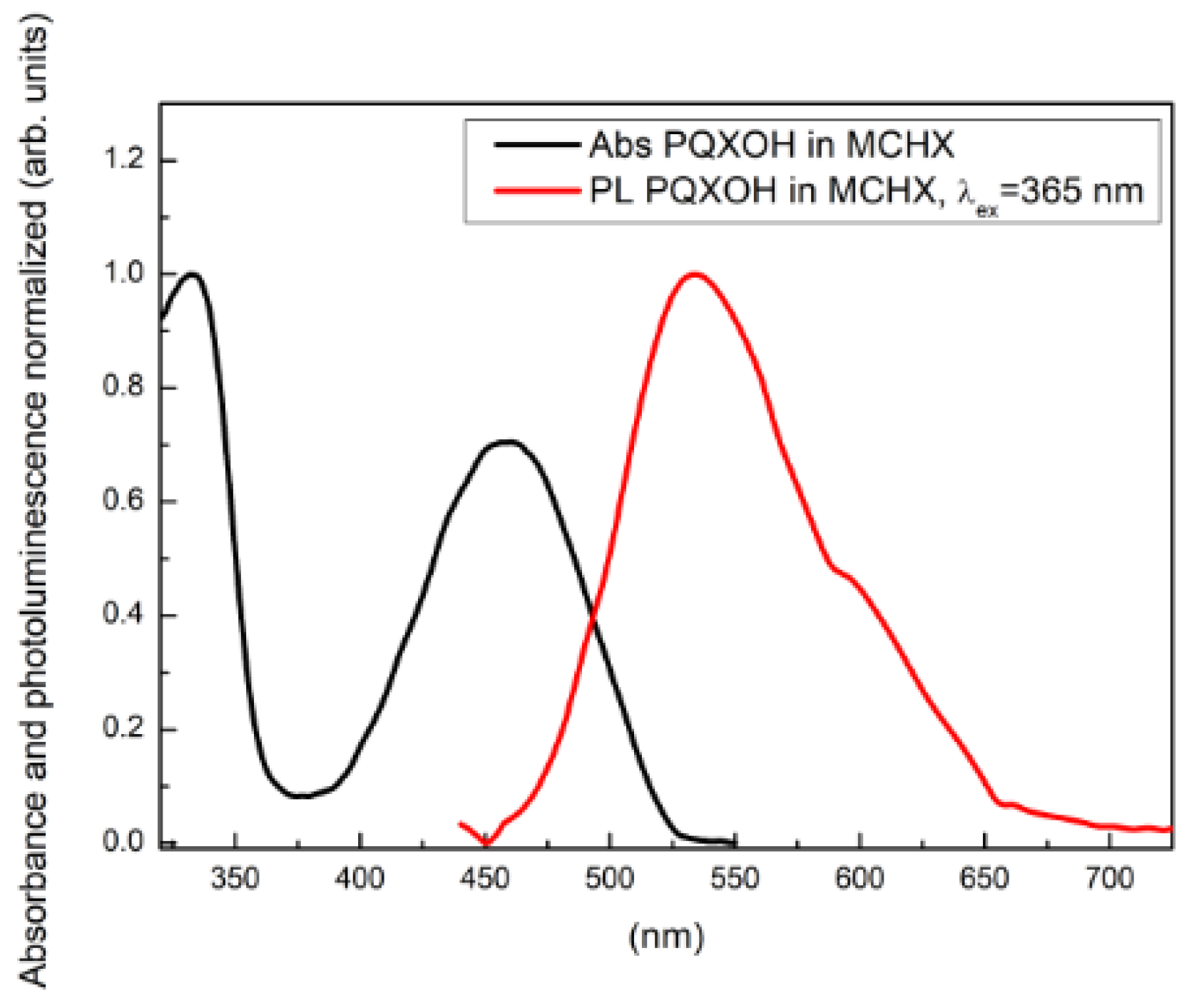
3.1.2. The Photophysical Properties of PQXOH
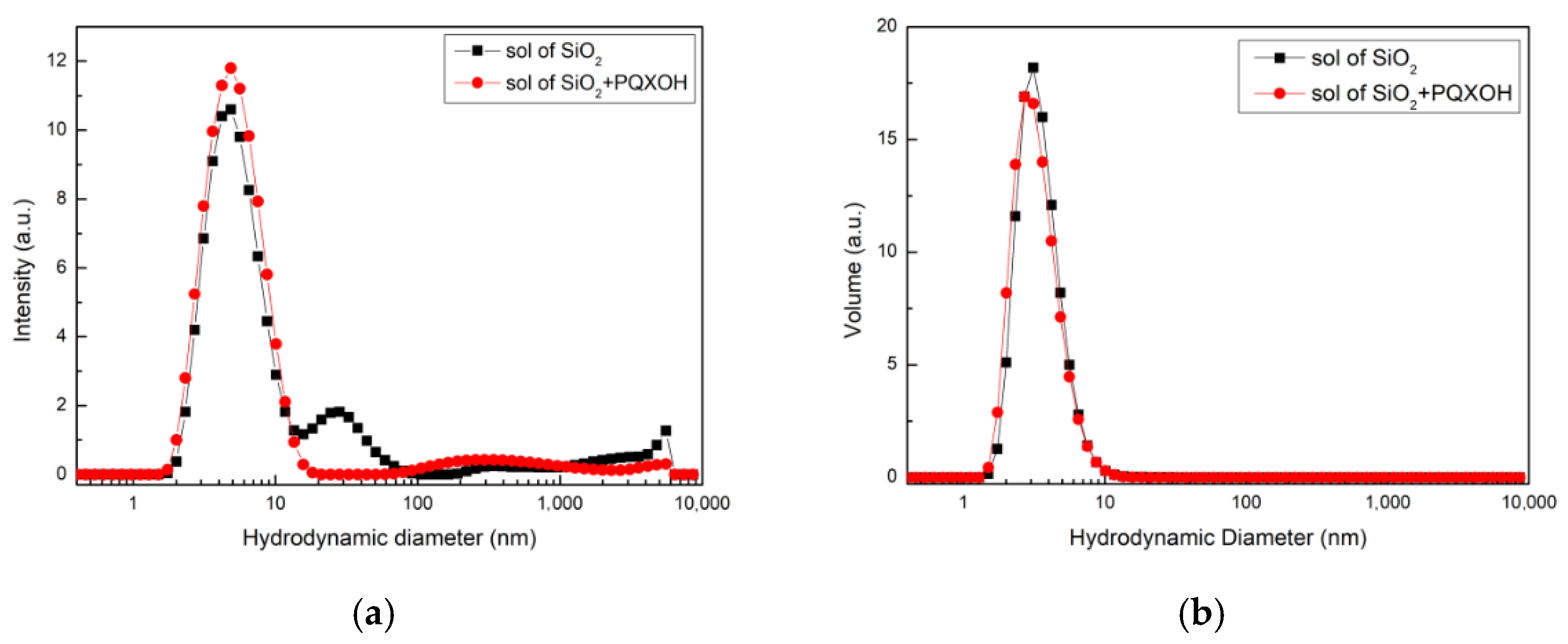
3.2. Characteristics of the Sol with PQXOH Impregnated
3.3. Technological Characteristics of the Produced Films
3.4. Optical Properties of Composite Layers
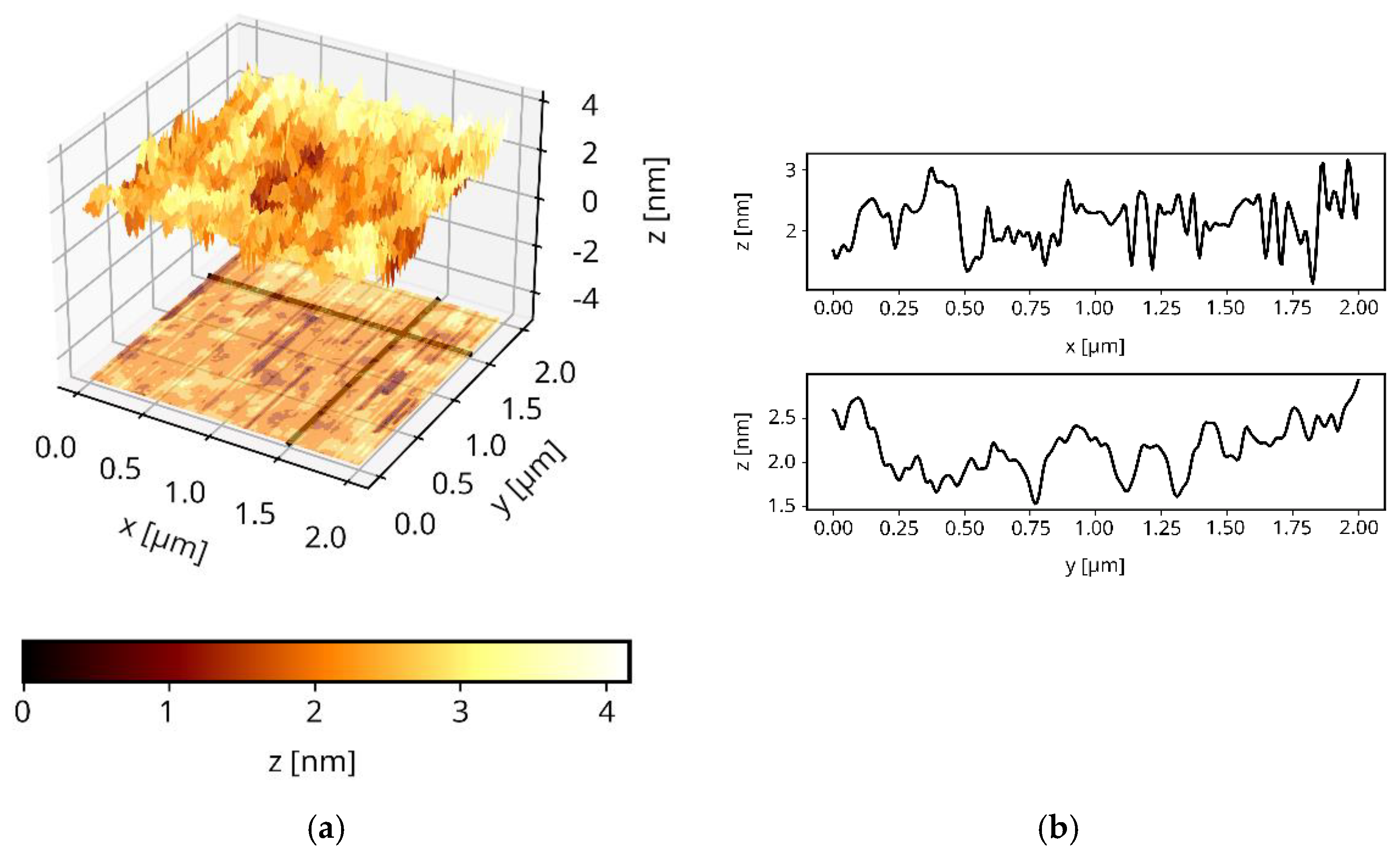
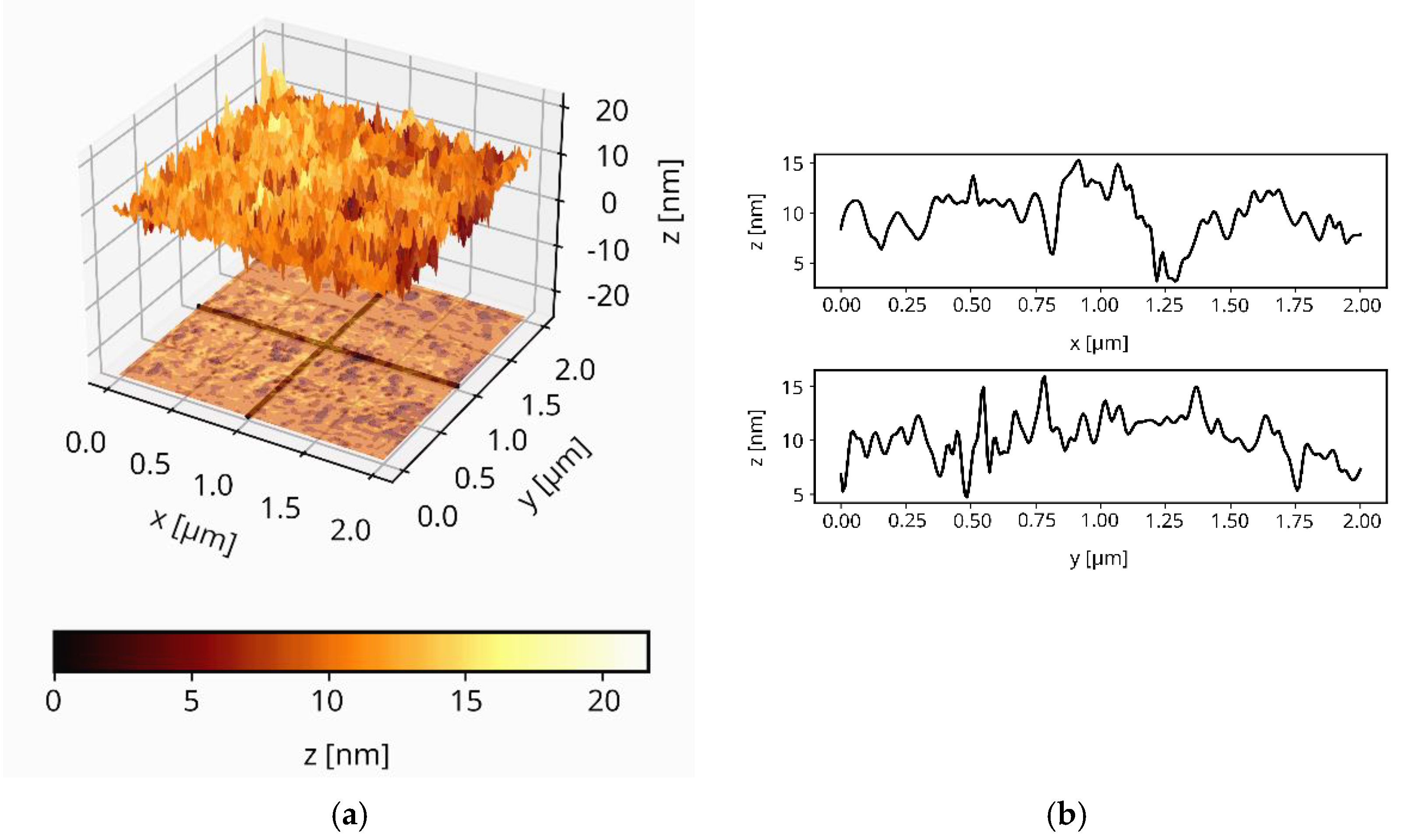
3.5. The Surface Morphology
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Marks-Bielska, R.; Bielski, S.; Pik, K.; Kurowska, K. The importance of renewable energy sources in Poland’s energy mix. Energies 2020, 13, 4624. [Google Scholar] [CrossRef]
- Dincer, I.; Bicer, Y. Photonic Energy Production. In Comprehensive Energy Systems; Elsevier Inc.: Amsterdam, The Netherlands, 2018; Volume 3–5, pp. 707–754. [Google Scholar] [CrossRef]
- Ramalingam, K.; Indulkar, C. Solar Energy and Photovoltaic Technology. In Distributed Generation Systems: Design, Operation and Grid Integration; Elsevier: Amsterdam, The Netherlands, 2017; pp. 69–147. [Google Scholar] [CrossRef]
- Best Research—Cell Efficiency Chart. Available online: https://www.nrel.gov/pv/cell-efficiency.html (accessed on 18 February 2022).
- Parida, B.; Iniyan, S.; Goic, R. A review of solar photovoltaic technologies. Renew. Sustain. Energy Rev. 2011, 15, 1625–1636. [Google Scholar] [CrossRef]
- Richardson, B.J.; Zhu, L.; Yu, Q. Design and development of plasmonic nanostructured electrodes for ITO-free organic photovoltaic cells on rigid and highly-flexible substrates. Nanotechnology 2017, 28, 165401. [Google Scholar] [CrossRef] [PubMed]
- Hong, L.; Yao, H.; Cui, Y.; Ge, Z.; Hou, J. Recent advances in high-efficiency organic solar cells fabricated by eco-compatible solvents at relatively large-area scale. APL Mater. 2020, 8, 120901. [Google Scholar] [CrossRef]
- Duan, L.; Uddin, A. Progress in Stability of Organic Solar Cells. Adv. Sci. 2020, 7, 1903259. [Google Scholar] [CrossRef]
- Hermann, A.M. Luminescent solar concentrators—A review. Sol. Energy 1982, 29, 323–329. [Google Scholar] [CrossRef]
- Aghaei, M.; Ebadi, H.; de Oliveira, A.K.V.; Vaezi, S.; Eskandari, A.; Castañón, J.M. New concepts and applications of solar PV systems. In Photovoltaic Solar Energy Conversion; Elsevier: Amsterdam, The Netherlands, 2020; pp. 349–390. [Google Scholar] [CrossRef]
- Moraitis, P.; Schropp, R.E.I.; van Sark, W.G.J.H.M. Nanoparticles for Luminescent Solar Concentrators—A review. Opt. Mater. 2018, 84, 636–645. [Google Scholar] [CrossRef]
- Meinardi, F.; Bruni, F.; Brovelli, S. Luminescent solar concentrators for building-integrated photovoltaics. Nat. Rev. Mater. 2017, 21, 17072. [Google Scholar] [CrossRef]
- Patrizi, B.; Iagatti, A.; Abbondanza, L.; Bussotti, L.; Zanardi, S.; Salvalaggio, M.; Fusco, R.; Foggi, P. Ultrafast Intramolecular and Solvation Dynamics in 4,7-Bis(4,5-dibutylbenzo[1,2- b:4,3- b′]bisthiophene[1,2- b:4,3- b′]bisthiophen-2-yl)-2,1,3-benzothiadiazole. J. Phys. Chem. C 2019, 123, 5840–5852. [Google Scholar] [CrossRef]
- Coropceanu, I.; Bawendi, M.G. Core/shell quantum dot based luminescent solar concentrators with reduced reabsorption and enhanced efficiency. Nano Lett. 2014, 14, 4097–4101. [Google Scholar] [CrossRef]
- Davis, N.J.L.K.; MacQueen, R.W.; Jones, S.T.E.; Orofino-Pena, C.; Cortizo-Lacalle, D.; Taylor, R.G.D.; Credgington, D.; Skabara, P.J.; Greenham, N.C. Star-shaped fluorene-BODIPY oligomers: Versatile donor-acceptor systems for luminescent solar concentrators. J. Mater. Chem. C 2017, 5, 1952–1962. [Google Scholar] [CrossRef]
- Papucci, C.; Charaf, R.; Coppola, C.; Sinicropi, A.; di Donato, M.; Taddei, M.; Foggi, P.; Battisti, A.; de Jong, B.; Zani, L.; et al. Luminescent solar concentrators with outstanding optical properties by employment of D–A–D quinoxaline fluorophores. J. Mater. Chem. C 2021, 9, 15608–15621. [Google Scholar] [CrossRef]
- Papucci, C.; Dessì, A.; Coppola, C.; Sinicropi, A.; Santi, G.; Di Donato, M.; Taddei, M.; Foggi, P.; Zani, L.; Reginato, G.; et al. Benzo[1,2-d:4,5-d′]bisthiazole fluorophores for luminescent solar concentrators: Synthesis, optical properties and effect of the polymer matrix on the device performances. Dye. Pigment. 2021, 188, 109207. [Google Scholar] [CrossRef]
- Bünzli, J.C.G.; Chauvin, A.S. Lanthanides in solar energy conversion. In Handbook on the Physics and Chemistry of Rare Earths; Elsevier: Amsterdam, The Netherlands, 2014; Volume 44, pp. 169–281. [Google Scholar] [CrossRef]
- Meinardi, F.; Colombo, A.; Velizhanin, K.A.; Simonutti, R.; Lorenzon, M.; Beverina, L.; Viswanatha, R.; Klimov, V.; Brovelli, S. Large-area luminescent solar concentrators based on Stokes-shift-engineered nanocrystals in a mass-polymerized PMMA matrix. Nat. Photonics 2014, 8, 392–399. [Google Scholar] [CrossRef]
- Lucarelli, J.; Lessi, M.; Manzini, C.; Minei, P.; Bellina, P.; Pucci, A. N-alkyl diketopyrrolopyrrole-based fluorophores for luminescent solar concentrators: Effect of the alkyl chain on dye efficiency. Dye. Pigment. 2016, 135, 154–162. [Google Scholar] [CrossRef]
- Wu, J.; Xin, W.; Wu, Y.; Zhan, Y.; Li, J.; Wang, J.; Huang, S.; Wang, X. Solid-state photoluminescent silicone-carbon dots/dendrimer composites for highly efficient luminescent solar concentrators. Chem. Eng. J. 2021, 422, 130158. [Google Scholar] [CrossRef]
- Fiorini, V.; Monti, N.; Vigarani, G.; Santi, G.; Fasulo, F.; Massi, M.; Giorgini, L.; Muñoz-García, A.B.; Pavone, M.; Pucci, A.; et al. Colourless luminescent solar concentrators based on Iridium(III)-Phosphors. Dye. Pigment. 2021, 193, 109532. [Google Scholar] [CrossRef]
- Yzeiri, X.; Calamante, M.; Dessì, A.; Franchi, D.; Pucci, A.; Ventura, F.; Reginato, G.; Zani, L.; Mordini, A. Synthesis and spectroscopic characterization of thienopyrazine-based fluorophores for application in luminescent solar concentrators (LSCs). Molecules 2021, 26, 5428. [Google Scholar] [CrossRef]
- de Clercq, D.M.; Chan, S.V.; Hardy, J.; Price, M.B.; Davis, N.J.L.K. Reducing reabsorption in luminescent solar concentrators with a self-assembling polymer matrix. J. Lumin. 2021, 236, 118095. [Google Scholar] [CrossRef]
- Wojtasik, K. Properties and application of 1H-pyrazolo[3,4-b]quinoxaline derivatives. Przem. Chem. 2018, 97, 1046–1050. [Google Scholar] [CrossRef]
- Uchacz, T.; Wojtasik, K.; Szlachcic, P.; Gondek, E.; Pokladko-Kowar, M.; Danel, A.; Stadnicka, K. The photophysical properties of 1H-pyrazolo[3,4-b]quinoxalines derivatives and their possible optoelectronic application. Opt. Mater. 2018, 80, 87–97. [Google Scholar] [CrossRef]
- Zoombelt, A.P.; Fonrodona, M.; Wienk, M.M.; Sieval, A.B.; Hummelen, J.C.; Janssen, R.A.J. Photovoltaic performance of an ultrasmall band gap polymer. Org. Lett. 2009, 11, 903–906. [Google Scholar] [CrossRef] [PubMed]
- Akel, S.; Sharif, M.A.; Al-Esseili, R.; Al-Wahish, M.A.; Hodali, H.A.; Müller-Buschbaum, P.; Schmidt-Mende, L.; Al-Hussein, M. Photovoltaic cells based on ternary P3HT:PCBM: Ruthenium(II) complex bearing 8-(diphenylphosphino)quinoline active layer. Colloids Surf. A Physicochem. Eng. Asp. 2021, 622, 126685. [Google Scholar] [CrossRef]
- Gondek, E.; Danel, A.; Kityk, I.V. 6-N,N-diarylsubstituted 1H-pyrazolo[3,4-b]quinoxalines-novel materials for single-layered photovoltaic devices. J. Mater. Sci. Mater. Electron. 2009, 20, 461–468. [Google Scholar] [CrossRef]
- Gondek, E.; Kityk, I.V.; Danel, A. Photovoltaic effect in single layer 1H-pyrazolo[3,4-b]quinoline and 1H-pyrazolo[3,4-b]quioxaline/poly(3-decylthiophene) polymer cells. Z. Naturforsch. Sect. A J. Phys. Sci. 2009, 64, 632–638. [Google Scholar] [CrossRef]
- Karasiński, P.; Skolik, M. Sol-gel derived antireflective structures for applications in silicon solar cells. Electron. Technol. Conf. 2016 2016, 10175, 1017517. [Google Scholar] [CrossRef]
- Esposito, S. “Traditional” Sol-Gel Chemistry as a Powerful Tool for the Preparation of Supported Metal and Metal Oxide Catalysts. Materials 2019, 12, 668. [Google Scholar] [CrossRef]
- Catauro, M.; Ciprioti, S.V. Characterization of Hybrid Materials Prepared by Sol-Gel Method for Biomedical Implementations. A Critical Review. Materials 2021, 14, 1788. [Google Scholar] [CrossRef]
- Levy, D.; Zayat, M. The Sol-Gel Handbook; Wiley Blackwell: Hoboken, NJ, USA, 2015. [Google Scholar] [CrossRef]
- Reisfeld, R. Luminescent Solar Concentrators and the Ways to Increase Their Efficiencies. In The Sol-Gel Handbook; Wiley Blackwell: Hoboken, NJ, USA, 2015; pp. 1281–1308. [Google Scholar] [CrossRef]
- Braun, S.; Rappoport, S.; Zusman, R.; Avnir, D.; Ottolenghi, M. Biochemically active sol-gel glasses: The trapping of enzymes. Mater. Lett. 1990, 10, 1–5. [Google Scholar] [CrossRef]
- Zaggout, F.R.; Qaraman, A.E.F.; Aziz, H.A.E. Behavior of α-Naphtholphthalein as analytical pH-Indicator entrapped into sol gel matrix. J. Dispers. Sci. Technol. 2008, 29, 58–62. [Google Scholar] [CrossRef]
- Klein, L.; Aparicio, M.; Jitianu, A. Handbook of Sol-Gel Science and Technology—Processing, Characterization and Applications; Springer: New York, NY, USA, 2018. [Google Scholar]
- Avnir, D.; Ottolenghi, M.; Braun, S.; Lev, O.; Levy, D. Organically Doped Sol-Gel Porous Glasses: Chemical Sensors, Enzymatic Sensors, Electrooptical Materials, Luminescent Materials and Photochromic Materials. In Sol-Gel Optics; Springer: New York, NY, USA, 1994; pp. 539–582. [Google Scholar] [CrossRef]
- Sachs, F.; Becherescu, P. Ueber Ketopyrazolone. II. 1.3-Diphenylpyrazolindion-(4.5). Ber. Dtsch. Chem. Ges. 1903, 36, 1132–1138. [Google Scholar] [CrossRef]
- Pettit, G.R.; Piatak, D.M.; Gavrilov, B.G.; Mal, N.A.; Zapiski, U. Hydrogen Bromide-Acetic Acid Demethylation of 2,3-Dimethoxy-6-bromobenzoic Acid. An Example of Concomitant Bromine Migration1,2. J. Org. Chem. 1960, 25, 721–725. [Google Scholar] [CrossRef]
- Karasiński, P.; Jaglarz, J.; Reben, M.; Skoczek, E.; Mazur, J. Porous silica xerogel films as antireflective coatings—Fabrication and characterization. Opt. Mater. 2011, 33, 1989–1994. [Google Scholar] [CrossRef]
- Avnir, D.; Kaufman, V.R.; Reisfeld, R. Organic fluorescent dyes trapped in silica and silica-titania thin films by the sol-gel method. Photophysical, film and cage properties. J. Non. Cryst. Solids 1985, 74, 395–406. [Google Scholar] [CrossRef]
- Dahn, H.; Nussbaum, J. Groupes de départ inhabituels lors de cyclisations dans la série des quinoxalines, II [1]. Helv. Chim. Acta 1969, 52, 1661–1671. [Google Scholar] [CrossRef]
- Della Sala, P.; Buccheri, N.; Sanzone, A.; Sassi, M.; Neri, P.; Talotta, C.; Rocco, A.; Pinchetti, V.; Beverina, L.; Brovelli, S. First demonstration of the use of very large Stokes shift cycloparaphenylenes as promising organic luminophores for transparent luminescent solar concentrators. Chem. Commun. 2019, 55, 3160–3163. [Google Scholar] [CrossRef]
- Fu, J.; Shang, H.; Li, Z.; Wang, W.; Chen, D. Thermal annealing effects on the stress stability in silicon dioxide films grown by plasma-enhanced chemical vapor deposition. Microsyst. Technol. 2017, 23, 2753–2757. [Google Scholar] [CrossRef]
- Ramalla, I.; Gupta, R.K.; Bansal, K. Effect on superhydrophobic surfaces on electrical porcelain insulator, improved technique at polluted areas for longer life and reliability. Int. J. Eng. Technol. 2015, 4, 509. [Google Scholar] [CrossRef]
- Martins, O.; Almeida, R.M. Sintering anomaly in silica-titania sol-gel films. J. Sol-Gel Sci. Technol. 2000, 19, 651–655. [Google Scholar] [CrossRef]
- Zięba, M.; Wojtasik, K.; Tyszkiewicz, C.; Gondek, E.; Nizioł, J.; Suchanek, K.; Wojtasik, M.; Pakieła, W.; Karasiński, P. High refractive index silica-titania films fabricated via the sol–gel method and dip-coating technique—Physical and chemical characterization. Materials 2021, 14, 7125. [Google Scholar] [CrossRef] [PubMed]
- Asthana, Y.; Hong, L.Y.; Kim, D.P.; Lee, T.S.; Sung, Y.M. Formulation of thermally cured organic-inorganic superhydrophilic coating for antifogging optical application. Mol. Cryst. Liq. Cryst. 2007, 463, 117–129. [Google Scholar] [CrossRef]
- O’Sullivan, M.; Odewunmi, N.A.; Cullen, M.; Sorour, A.A.; Duffy, B.; Gasem, Z.M.; Oubaha, M. The Role of the Chelating Agent on the Structure and Anticorrosion Performances of an Organosilane—Zirconium Sol-Gel Coatings. Adv. Mater. Phys. Chem. 2018, 8, 89–104. [Google Scholar] [CrossRef][Green Version]
- Kermadi, S.; Agoudjil, N.; Sali, S.; Zougar, L.; Boumaour, M.; Broch, L.; En Naciri, A.; Placido, F. Microstructure and optical dispersion characterization of nanocomposite sol-gel TiO2-SiO2 thin films with different compositions. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 145, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Aizawa, M.; Nosaka, Y.; Fujii, N. FT-IR liquid attenuated total reflection study of TiO2SiO2 sol-gel reaction. J. Non. Cryst. Solids 1991, 128, 77–85. [Google Scholar] [CrossRef]
- Shajari-Ghasemkheili, S.; Sarraf-Mamoory, R. Development of a transparent silica-titania-methyl siliconate nanocoating with photocatalytic-hydrophobic properties aided by response surface method. Mater. Res. Express 2019, 6, 106430. [Google Scholar] [CrossRef]
- Karasiński, P.; Domanowska, A.; Gondek, E.; Sikora, A.; Tyszkiewicz, C.; Skolik, M. Homogeneity of sol-gel derived silica-titania waveguide films—Spectroscopic and AFM studies. Opt. Laser Technol. 2020, 121, 105840. [Google Scholar] [CrossRef]
- di Nunzio, M.R.; Gutiérrez, M.; Douhal, A. Electronic and Molecular Motions in Silica-Material Hosts. In Chemistry of Silica and Zeolite-Based Materials—Synthesis, Characterization and Applications; Douhal, A., Anpo, M., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2019; pp. 273–294. [Google Scholar] [CrossRef]
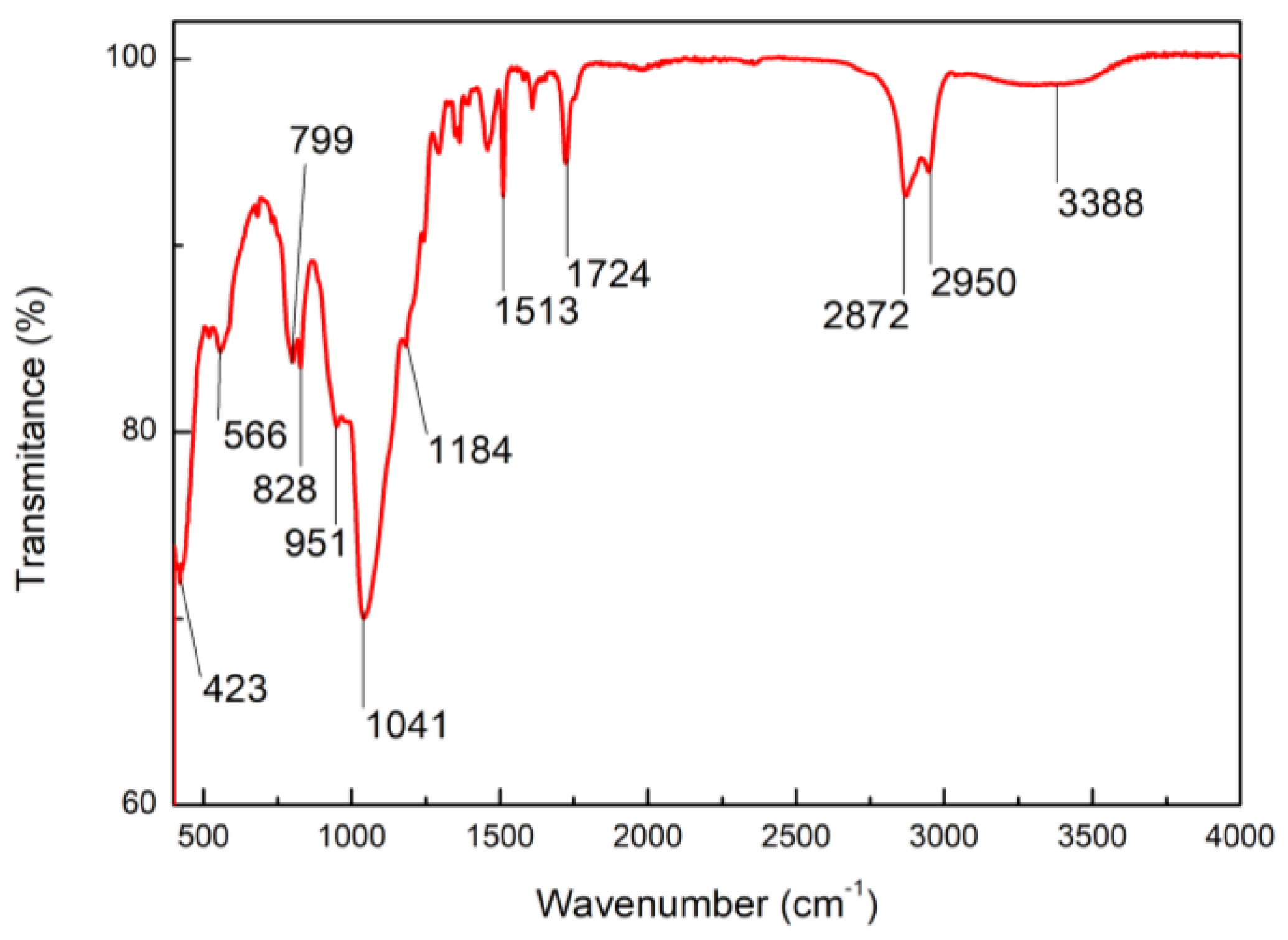
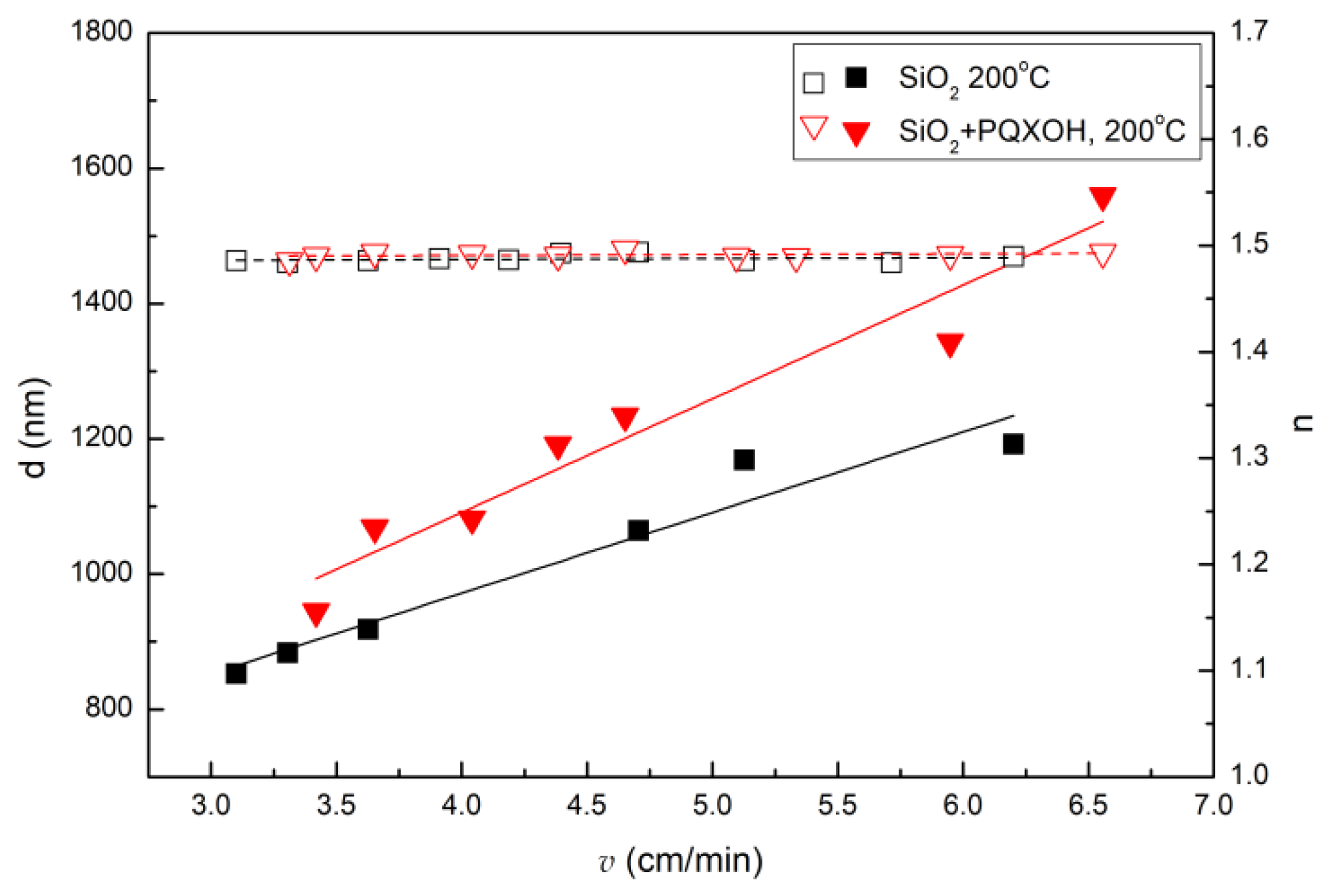
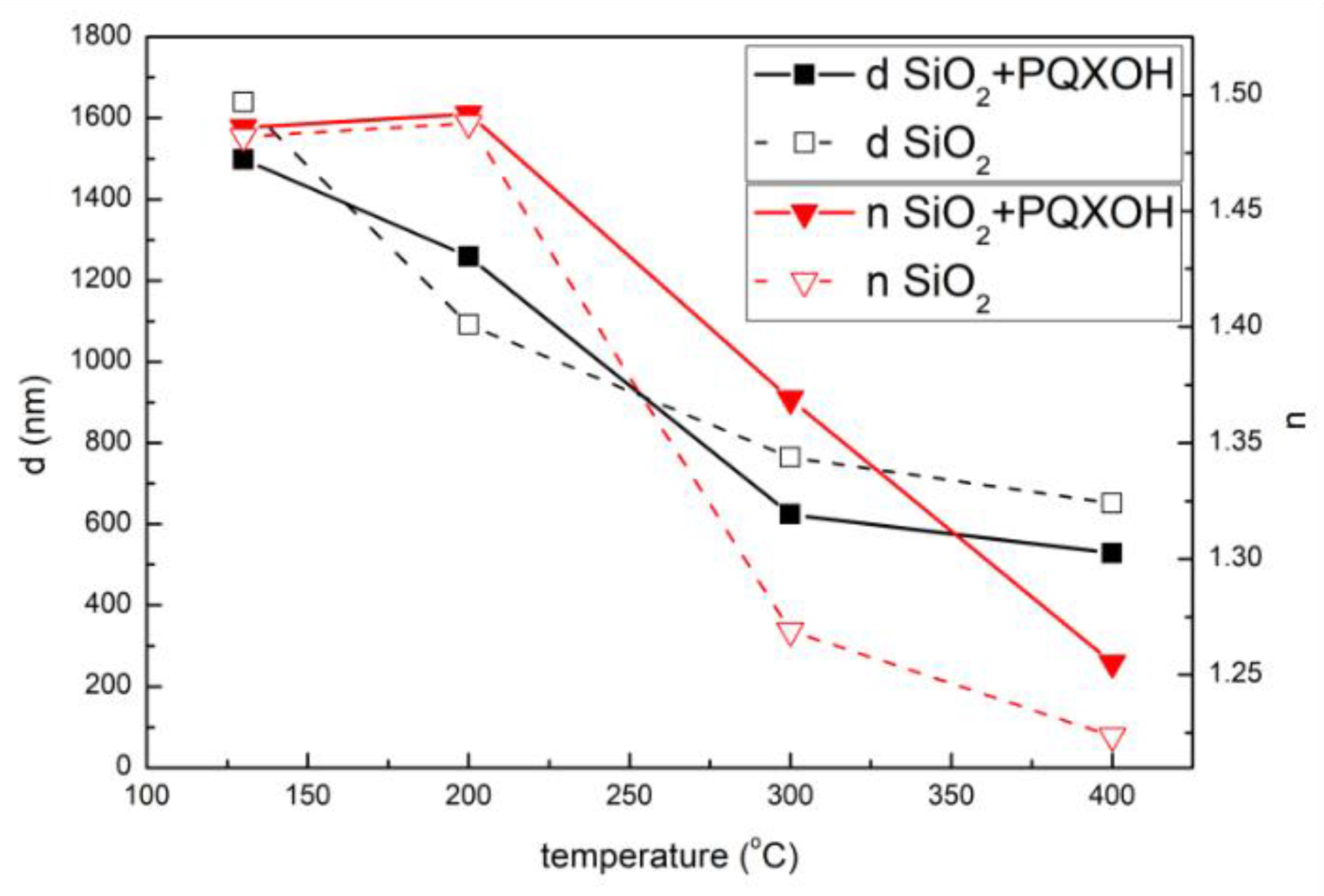
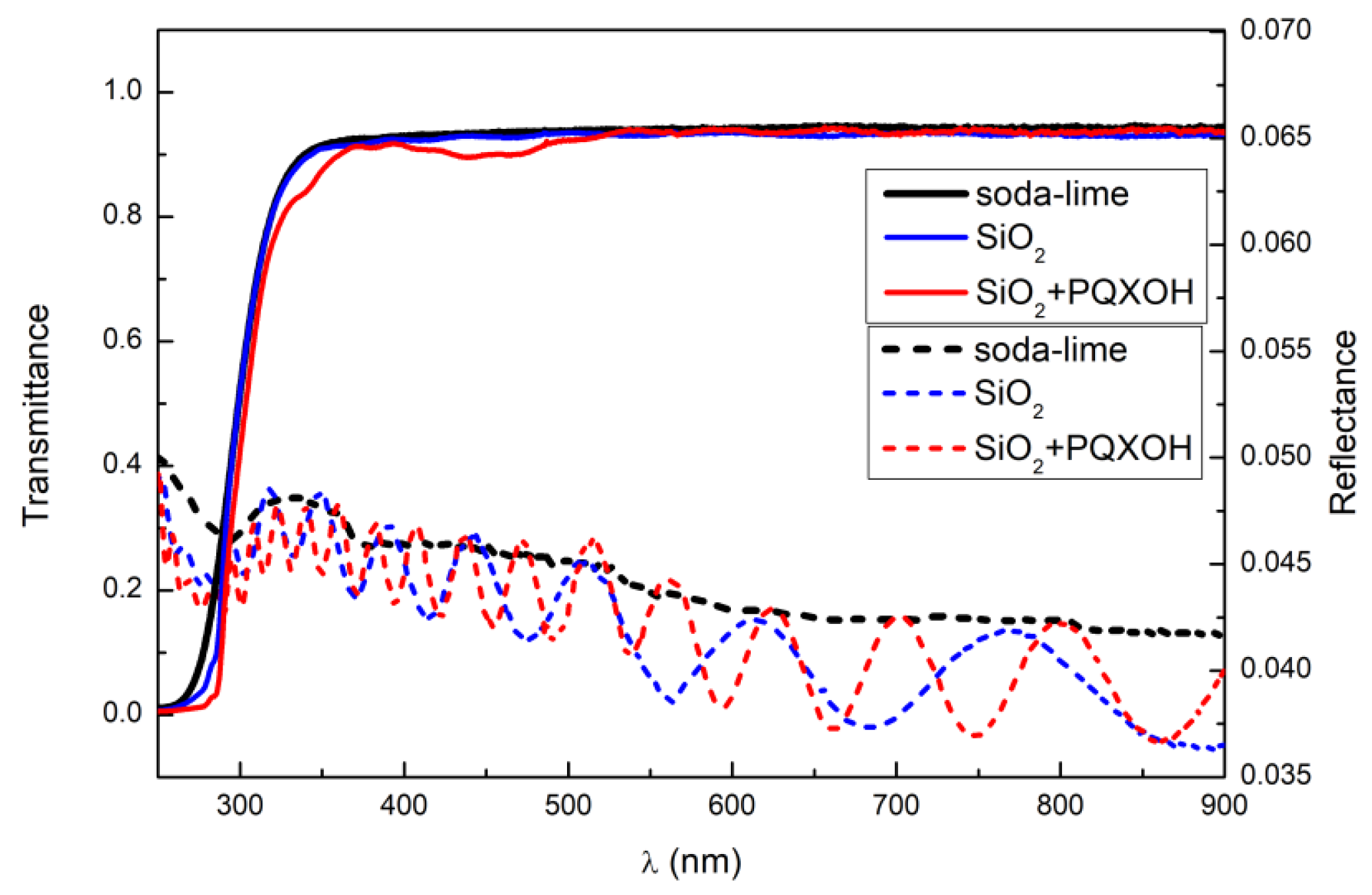

| Sol | TEOS | EtOH | HCl | H2O | Triton X-100 | PQXOH |
|---|---|---|---|---|---|---|
| A | 1 | 3.89 | 0.02 | 2.44 | 0.22 | - |
| B | 1 | 3.89 | 0.02 | 2.44 | 0.22 | 0.17% |
| Compound | Solvent | λAbs (nm) | λPL (nm) (λex = 365 nm) | SS [nm (eV)] |
|---|---|---|---|---|
| PQXOH | MCHX | 458 | 534 | 76 (0.39) |
| THF | 455 | 570 | 115 (0.55) | |
| CAN | 455 | 586 | 131 (0.61) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wojtasik, K.; Zięba, M.; Wojtasik, M.; Tyszkiewicz, C.; Pokladko-Kowar, M.; Gondek, E.; Danel, A.; Karasiński, P. Chemical Doping of a Silica Matrix with a New Organic Dye from the Group of Heterocyclic Compounds—Chemical, Optical and Surface Characteristics. Crystals 2022, 12, 478. https://doi.org/10.3390/cryst12040478
Wojtasik K, Zięba M, Wojtasik M, Tyszkiewicz C, Pokladko-Kowar M, Gondek E, Danel A, Karasiński P. Chemical Doping of a Silica Matrix with a New Organic Dye from the Group of Heterocyclic Compounds—Chemical, Optical and Surface Characteristics. Crystals. 2022; 12(4):478. https://doi.org/10.3390/cryst12040478
Chicago/Turabian StyleWojtasik, Katarzyna, Magdalena Zięba, Michał Wojtasik, Cuma Tyszkiewicz, Monika Pokladko-Kowar, Ewa Gondek, Andrzej Danel, and Paweł Karasiński. 2022. "Chemical Doping of a Silica Matrix with a New Organic Dye from the Group of Heterocyclic Compounds—Chemical, Optical and Surface Characteristics" Crystals 12, no. 4: 478. https://doi.org/10.3390/cryst12040478
APA StyleWojtasik, K., Zięba, M., Wojtasik, M., Tyszkiewicz, C., Pokladko-Kowar, M., Gondek, E., Danel, A., & Karasiński, P. (2022). Chemical Doping of a Silica Matrix with a New Organic Dye from the Group of Heterocyclic Compounds—Chemical, Optical and Surface Characteristics. Crystals, 12(4), 478. https://doi.org/10.3390/cryst12040478








