Role of Oxygen and Fluorine in Passivation of the GaSb(111) Surface Depending on Its Termination
Abstract
:1. Introduction
2. Computational Details
3. Results and Discussion
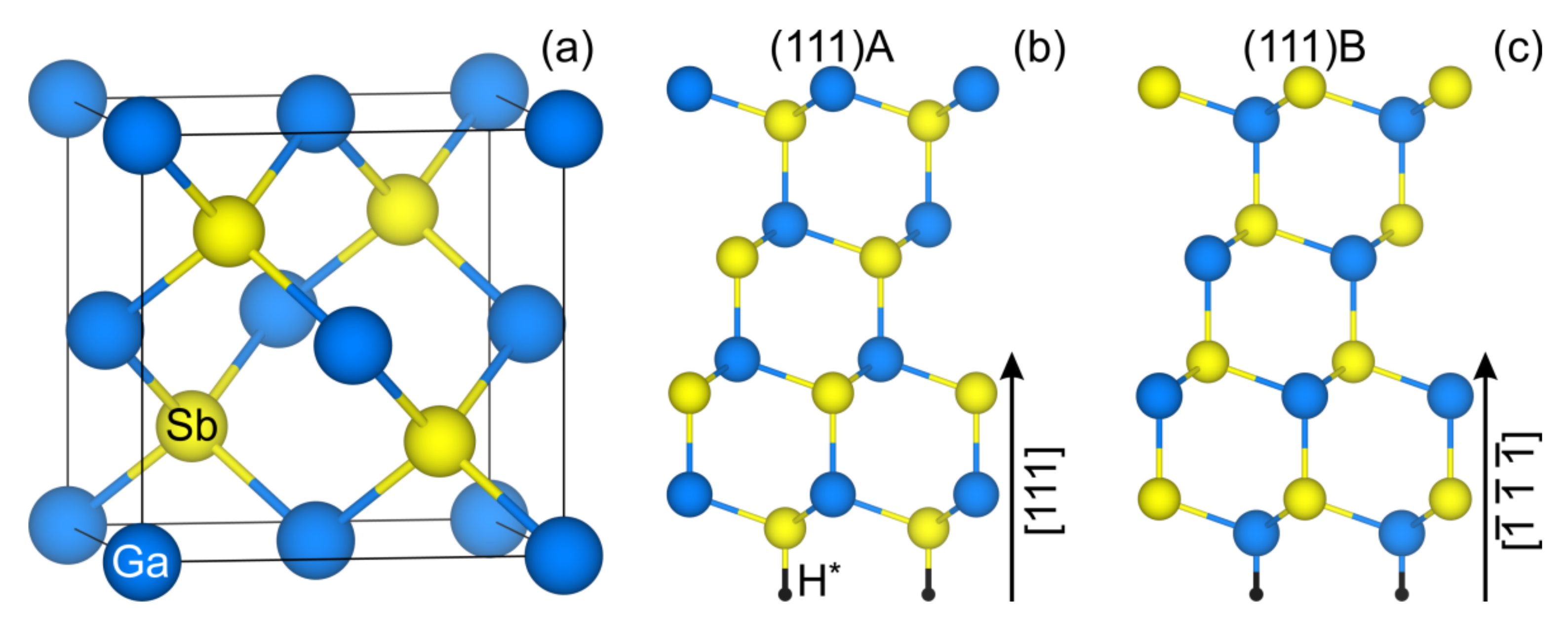
3.1. Atomic and Electronic Structure of Clean GaSb(111) Surface
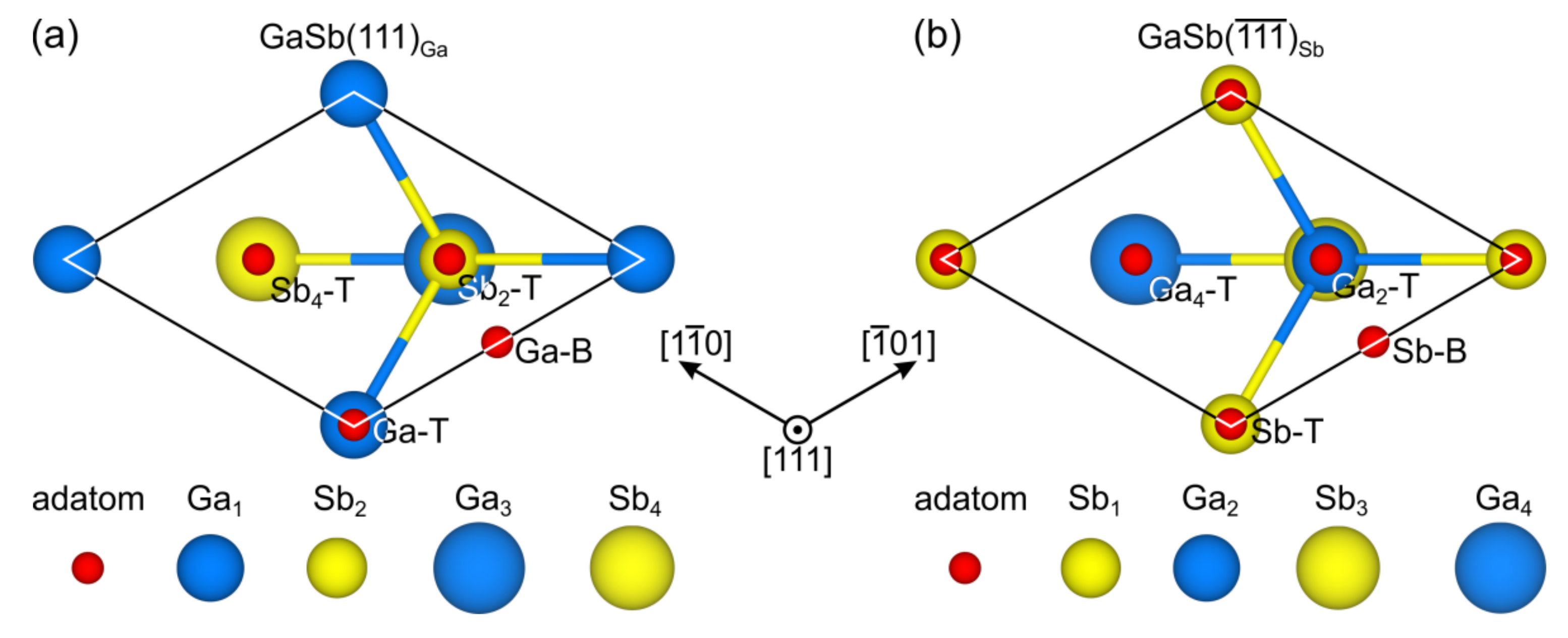
3.2. Oxygen and Fluorine Adsorption on the GaSb(111)A Surface
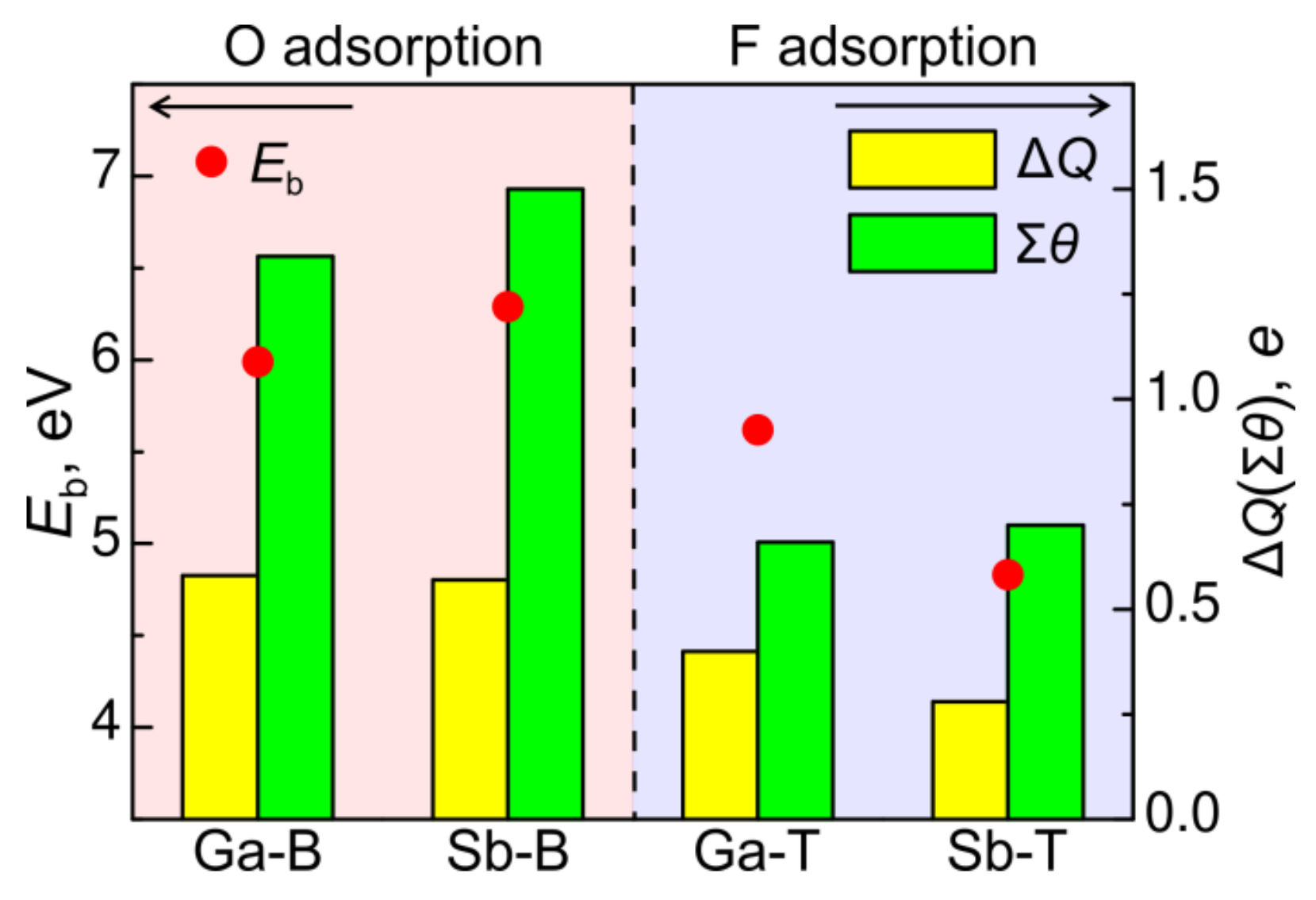
3.2.1. Oxygen
3.2.2. Fluorine
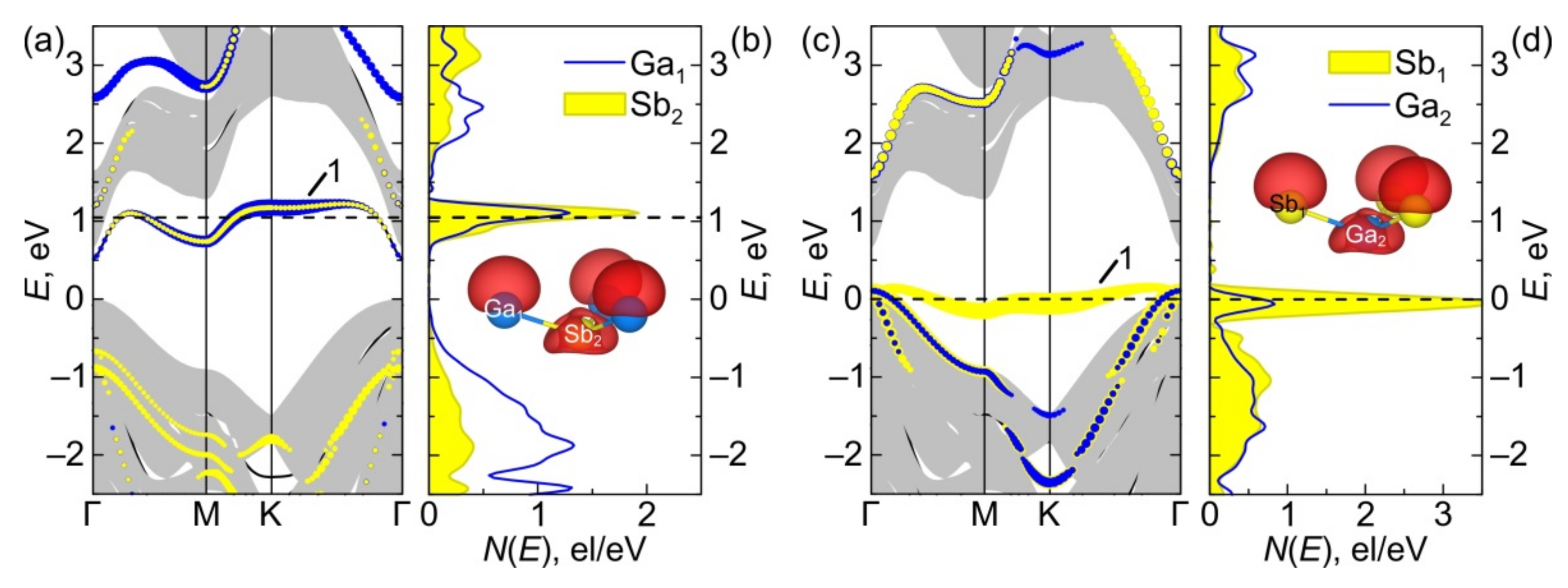
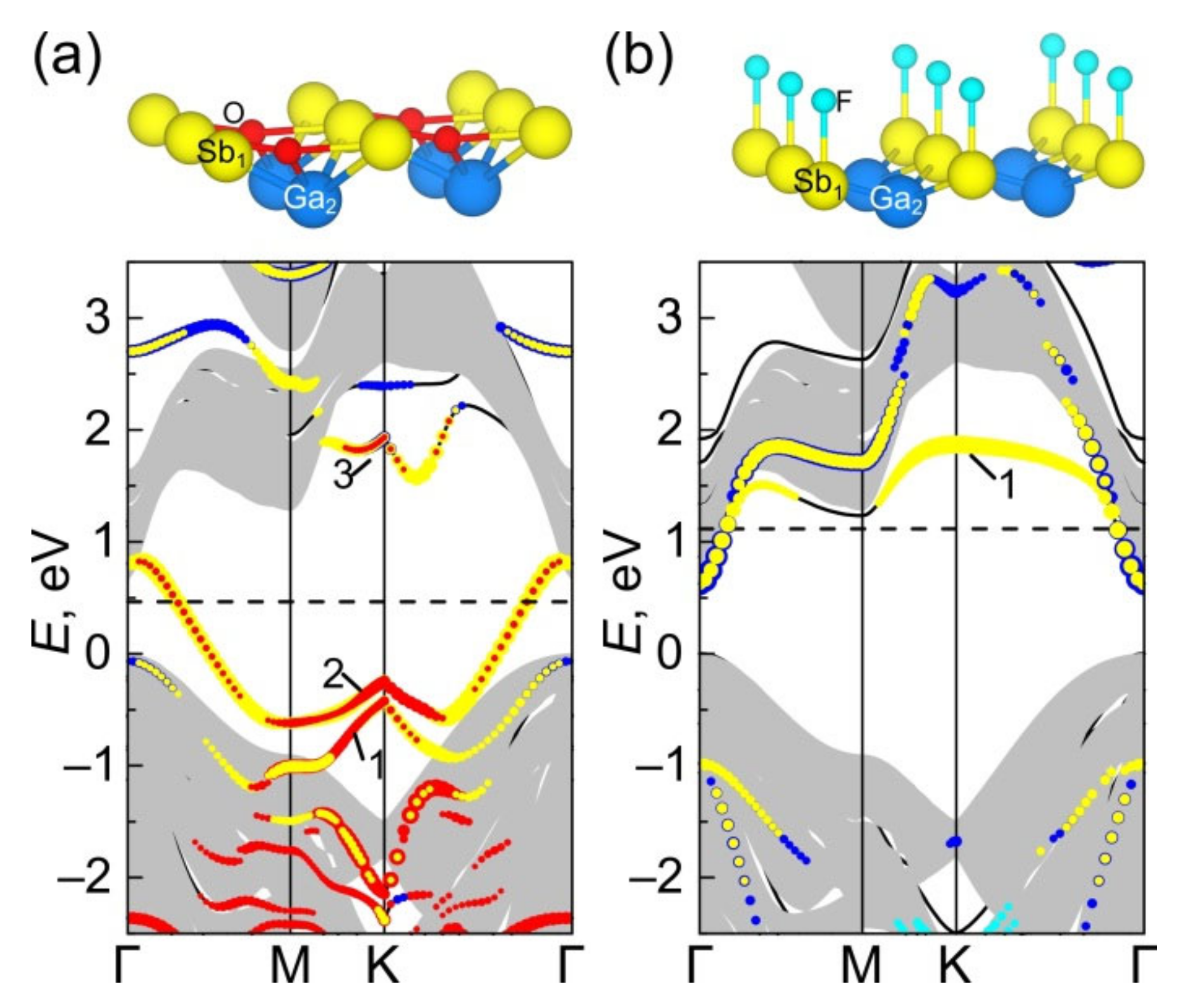
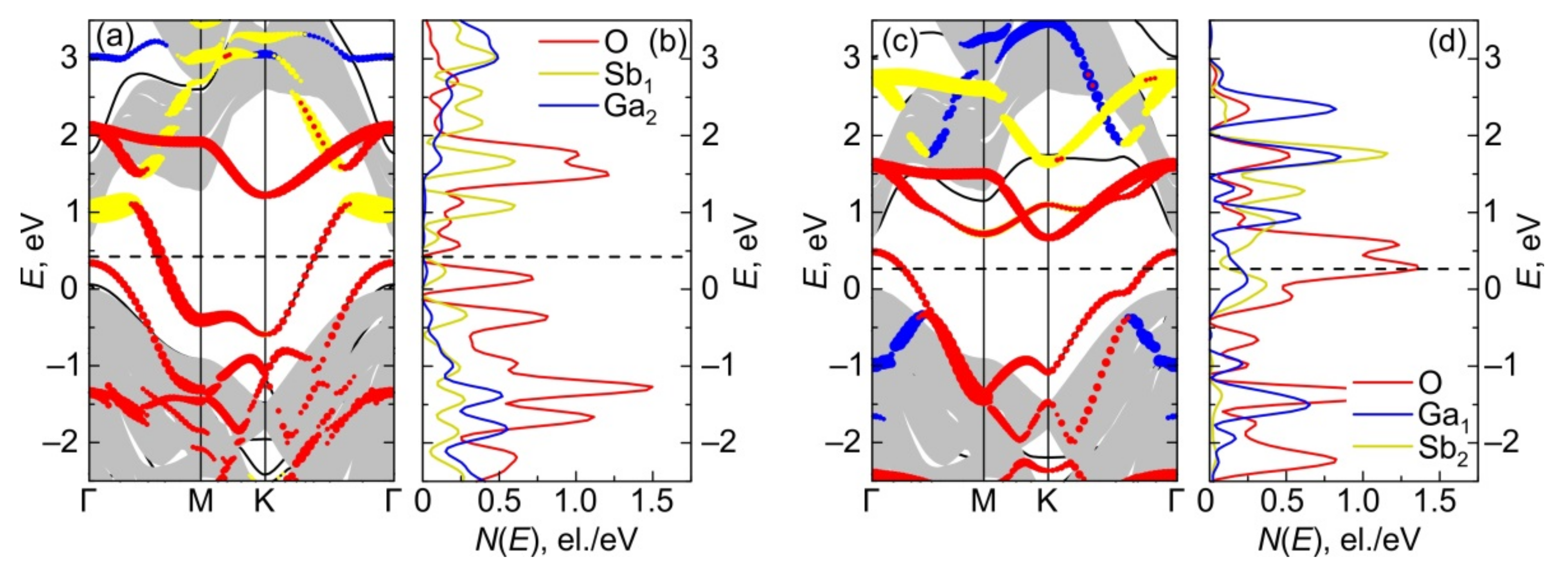
3.2.3. Electronic Energy Spectra
3.3. Adsorption on the GaSb(111)B Surface
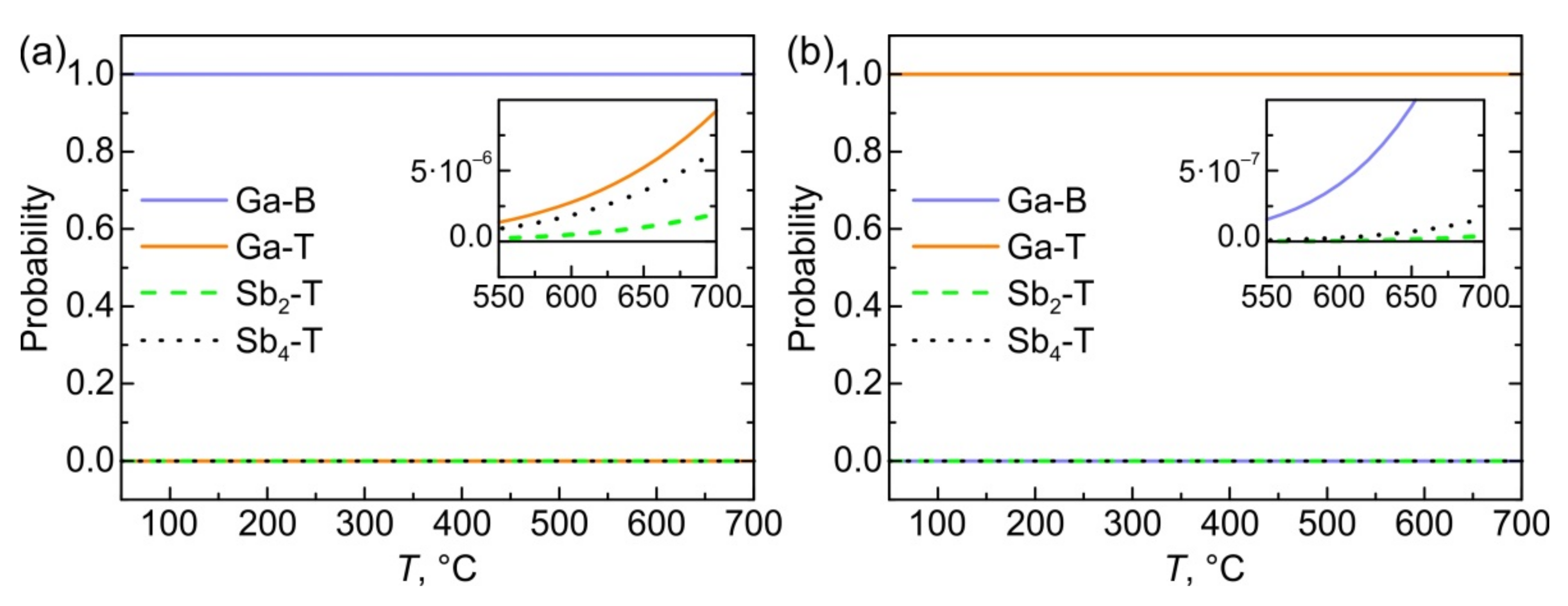
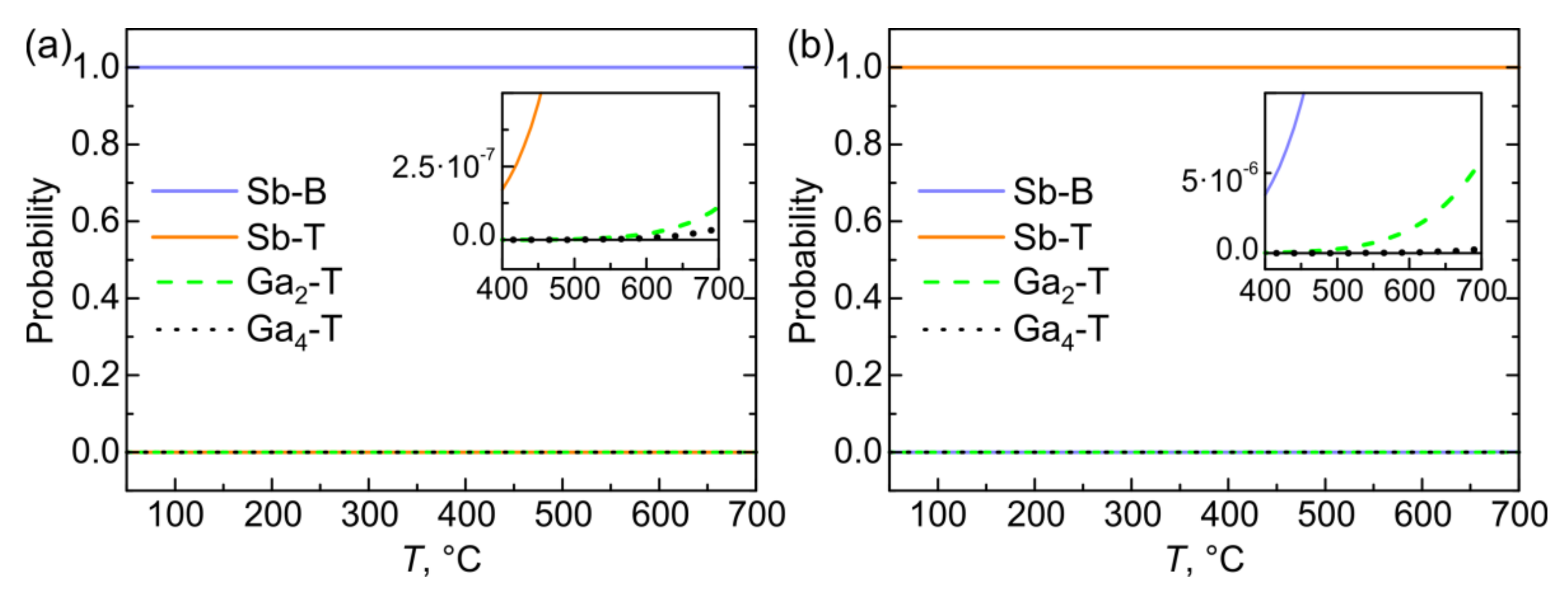
3.4. Oxygen Concentration
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sze, S.M.; Kwok, K.N. Physics of Semiconductor Device, 3rd ed.; Wiley-Interscience: Hoboken, NJ, USA, 2007; pp. 1–815. [Google Scholar]
- Alferov, Z.I. The history and future of semiconductor heterostructures. Semiconductors 1998, 32, 1–14. [Google Scholar] [CrossRef]
- Alferov, Z.I. The double heterostructure: Concept and its applications in physics, electronics and technology. Int. J. Mod. Phys. B 2002, 16, 647–675. [Google Scholar] [CrossRef]
- Oktyabrsky, S.; Ye, P.D. Fundamentals of III–V Semiconductor MOSFETs; Springer: New York, NY, USA, 2010; pp. 1–445. [Google Scholar]
- Hinkle, C.L.; Vogel, E.M.; Ye, P.D.; Wallace, R.M. Interfacial chemistry of oxides on InxGa(1–x)As and implications for MOSFET applications. Curr. Opin. Solid State Mater. Sci. 2011, 15, 188–207. [Google Scholar] [CrossRef]
- Berchenko, N.N.; Medvedev, Y.V. The chemistry of the compound semiconductor–intrinsic insulator interface. Russ. Chem. Rev. 1994, 63, 623–639. [Google Scholar] [CrossRef]
- Aksenov, M.S.; Kokhanovskii, A.Y.; Polovodov, P.A.; Devyatova, S.F.; Golyashov, V.A.; Kozhukhov, A.S.; Prosvirin, I.P.; Khandarkhaeva, S.E.; Gutakovskii, A.K.; Valisheva, N.A.; et al. InAs-based metal-oxide-semiconductor structure formation in low-energy Townsend discharge. Appl. Phys. Lett. 2015, 107, 173501. [Google Scholar] [CrossRef]
- Ahrenkiel, R.K.; Kazmenski, L.L.; Jamjoum, O.; Russel, P.E.; Ireland, P.J.; Wagner, R.S. Properties of plasma oxyfluorides grown on GaAs. Thin Solid Films 1982, 95, 327–331. [Google Scholar] [CrossRef]
- Lucovsky, G.; Bauer, R.S. Local atomic order in native III–V oxides. J. Vac. Sci. Technol. 1980, 17, 946–951. [Google Scholar] [CrossRef]
- Valisheva, N.A.; Tereshchenko, O.E.; Prosvirin, I.P.; Levtsova, T.A.; Rodjakina, E.E.; Kovchavcev, A.V. Composition and morphology of fluorinated anodic oxides on InAs(111)A surface. Appl. Surf. Sci. 2010, 256, 5722–5726. [Google Scholar] [CrossRef]
- Valisheva, N.A.; Aksenov, M.S.; Golyashov, V.A.; Levtsova, T.A.; Kovchavtsev, A.P.; Gutakovskii, A.K.; Khandarkhaeva, S.E.; Kalinkin, A.V.; Prosvirin, I.P.; Bukhtiyarov, V.I.; et al. Oxide-free InAs(111)A interface in metal-oxide-semiconductor structure with very low density of states prepared by anodic oxidation. Appl. Phys. Lett. 2014, 105, 161601. [Google Scholar] [CrossRef]
- Levtsova, T.A.; Kokovkin, V.V.; Valisheva, N.A. Electrochemical oxidation of indium arsenide in ammonia-glycol electrolyte with ammonium fluoride additive. Russ. J. Appl. Chem. 2011, 84, 1738–1743. [Google Scholar] [CrossRef]
- Bazovkin, V.M.; Valisheva, N.A.; Guzev, A.A.; Efimov, V.M.; Kovchavtsev, A.P.; Kuryshev, G.L.; Lee, I.I.; Stroganov, A.S. 1x384 hybrid linear infrared focal plane arrays on InAs MOS structure for spectrometric applications. Proc. SPIE 2014, 05126, 118–128. [Google Scholar] [CrossRef]
- Ahrenkiel, K.; Kazmenski, L.L.; Ireland, P.J.; Jamjoum, O.; Russell, P.E.; Dunlavy, D.; Wagner, R.S.; Pattillo, S.; Jervis, T. Reduction of surface states on GaAs by the plasma growth of oxyfluorides. J. Vac. Sci. Technol. 1982, 21, 434–437. [Google Scholar] [CrossRef]
- Mönch, W. Semiconductor Surfaces and Interfaces, 3rd ed.; Springer: Berlin, Germany, 2001; pp. 1–548. [Google Scholar]
- Miyamura, S.; Kasai, Y.; Yamamura, Y.; Inokuma, T.; Iiyama, K.; Takamiya, S. Oxygen and sulfur adsorption effects on electronic states of GaAs(100) surfaces studied with discrete variational Xα method. Jpn. J. Appl. Phys. 2003, 42, 7244–7249. [Google Scholar] [CrossRef]
- Scarrozza, M.; Pourtois, G.; Houssa, M.; Caymax, M.; Meuris, M.; Heyns, M.M.; Stesmans, A. Adsorption of molecular oxygen on the reconstructed β2(2 × 4)-GaAs(001) surface: A first-principles study. Surf. Sci. 2019, 603, 203–208. [Google Scholar] [CrossRef]
- Wang, W.; Lee, G.; Huang, M.; Wallace, R.M.; Cho, K. First-principles study of GaAs(001)-β2(2 × 4) surface oxidation. Microelectron. Eng. 2011, 88, 3419–3423. [Google Scholar] [CrossRef]
- Lee, S.M.; Lee, S.H.; Scheffler, M. Adsorption and diffusion of a Cl adatom on the GaAs(001)-c(8 × 2) ζ surface. Phys. Rev. B 2004, 69, 125317. [Google Scholar] [CrossRef] [Green Version]
- Bakulin, A.V.; Eremeev, S.V.; Tereshchenko, O.E.; Kulkova, S.E. Chlorine adsorption on the InAs(001) surface. Semiconductors 2011, 45, 21–29. [Google Scholar] [CrossRef]
- Eremeev, S.V.; Valisheva, N.A.; Tereshchenko, O.E.; Kulkova, S.E. Change in the electronic properties of an InAs(111)A surface at oxygen and fluorine adsorption. Semiconductors 2012, 46, 49–55. [Google Scholar] [CrossRef]
- Bakulin, A.V.; Kulkova, S.E.; Eremeev, S.V.; Tereshchenko, O.E. Early stages of halogen adsorption on cation-rich InAs(001): Surface etching mechanism. J. Phys. Chem. C 2014, 118, 10097–10105. [Google Scholar] [CrossRef]
- Bakulin, A.; Kulkova, S.; Tereshchenko, O.E.; Shaposhnikov, A.; Smolin, I. The peculiarities of halogens adsorption on A3B5(001) surface. IOP Conf. Ser. Mater. Sci. Eng. 2015, 77, 012002. [Google Scholar] [CrossRef]
- Bakulin, A.V.; Kulkova, S.E. Halogen adsorption at an As-stabilized β2–GaAs(001)–(2 × 4) surface. Semiconductors 2016, 50, 171–179. [Google Scholar] [CrossRef]
- Bakulin, A.V.; Kulkova, S.E.; Aksenov, M.S.; Valisheva, N.A. Fluorine and oxygen adsorption and their coadsorption on the (111) surface of InAs and GaAs. J. Phys. Chem. C 2016, 120, 17491–17500. [Google Scholar] [CrossRef]
- Valisheva, N.A.; Bakulin, A.V.; Aksenov, M.S.; Khandarkhaeva, S.E.; Kulkova, S.E. Passivation mechanism of the native oxide/InAs interface by fluorine. J. Phys. Chem. C 2017, 121, 20744–20750. [Google Scholar] [CrossRef]
- Fuks, A.A.; Bakulin, A.V.; Kulkova, S.E.; Valisheva, N.A.; Postnikov, A.V. Effect of oxygen and fluorine absorption on the electronic structure of the InSb(111) surface. Semiconductors 2020, 54, 1–10. [Google Scholar] [CrossRef]
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Kresse, G.; Hafner, J. Ab initio molecular dynamics for liquid metals. Phys. Rev. B 1993, 47, 558–561. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [Green Version]
- Vurgaftman, I.; Meyer, J.R.; Ram-Mohan, L.R. Band parameters for III–V compound semiconductors and their alloys. J. Appl. Phys. 2001, 89, 5815–5875. [Google Scholar] [CrossRef] [Green Version]
- Momma, K.; Izumi, F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Cryst. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- Kumar, V.N.; Arivanandhan, M.; Rajesh, G.; Koyama, T.; Momose, Y.; Sakata, K.; Ozawa, T.; Okano, Y.; Inatomi, Y.; Hayakawa, Y. Investigation of directionally solidified InGaSb ternary alloys from Ga and Sb faces of GaSb(111) under prolonged microgravity at the International Space Station. NPJ Microgravity 2016, 2, 16026. [Google Scholar] [CrossRef] [Green Version]
- Shiraishi, K. A new slab model approach for electronic structure calculation of polar semiconductor surface. J. Phys. Soc. Jpn. 1990, 59, 3455–3458. [Google Scholar] [CrossRef]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillonin-zone integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Ferreira, L.G.; Marques, M.; Teles, L.K. Approximation to density functional theory for the calculation of band gaps of semiconductors. Phys. Rev. B 2008, 78, 125116. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, L.G.; Marques, M.; Teles, L.K. Slater half-occupation technique revisited: The LDA-1/2 and GGA-1/2 approaches for atomic ionization energies and band gaps in semiconductors. AIP Adv. 2011, 1, 032119. [Google Scholar] [CrossRef] [Green Version]
- Pela, R.R.; Marques, M.; Teles, L.K. Comparing LDA-1/2, HSE03, HSE06 and G0W0 approaches for band gap calculations of alloys. J. Phys. Condens. Matter 2015, 27, 505502. [Google Scholar] [CrossRef] [PubMed]
- Xue, K.H.; Yuan, J.H.; Fonseca, L.R.C.; Miao, X.S. Improved LDA-1/2 method for band structure calculations in covalent semiconductors. Comput. Mater. Sci. 2018, 153, 493–505. [Google Scholar] [CrossRef] [Green Version]
- Krukau, A.V.; Vydrov, O.A.; Izmaylov, A.F.; Scuseria, G.E. Influence of the exchange screening parameter on the performance of screened hybrid functionals. J. Chem. Phys. 2006, 125, 224106. [Google Scholar] [CrossRef]
- Becke, A.D.; Johnson, E.R. A simple effective potential for exchange. J. Chem. Phys. 2006, 124, 221101. [Google Scholar] [CrossRef]
- Manz, T.A.; Limas, N.G. Introducing DDEC6 atomic population analysis: Part 1. Charge partitioning theory and methodology. RSC Adv. 2016, 6, 47771–47801. [Google Scholar] [CrossRef] [Green Version]
- Limas, N.G.; Manz, T.A. Introducing DDEC6 atomic population analysis: Part 2. Computed results for a wide range of periodic and nonperiodic materials. RSC Adv. 2016, 6, 45727–45747. [Google Scholar] [CrossRef]
- Tang, W.; Sanville, E.; Henkelman, G. A grid-based Bader analysis algorithm without lattice bias. J. Phys. Condens. Matter 2009, 21, 084204. [Google Scholar] [CrossRef] [PubMed]
- Lozovoi, A.Y.; Paxton, A.T.; Finnis, M.W. Structural and chemical embrittlement of grain boundaries by impurities: A general theory and first-principles calculations for copper. Phys. Rev. B 2006, 74, 155416. [Google Scholar] [CrossRef] [Green Version]
- Kulkov, S.S.; Eremeev, S.V.; Kulkova, S.E. Hydrogen adsorption on low-index surfaces of B2 titanium alloys. Phys. Solid State 2009, 51, 1281–1289. [Google Scholar] [CrossRef]
- Mäkelä, J.; Jahanshah Rad, Z.S.; Lehtiö, J.P.; Kuzmin, M.; Punkkinen, M.P.J.; Laukkanen, P.; Kokko, K. Crystalline and oxide phases revealed and formed on InSb(111)B. Sci. Rep. 2018, 8, 14382. [Google Scholar] [CrossRef] [PubMed]


| Surface | Δ12, % | Δ23, % | Δ34, % | Δ45, % | Δχ, eV1/2 |
|---|---|---|---|---|---|
| GaSb(111)A | −6.0 | +1.5 | −1.7 | +0.4 | 0.24 |
| InSb(111)A [27] | −5.5 | +2.4 | −3.3 | +0.4 | 0.27 |
| GaAs(111)A [25] | −5.4 | +2.0 | −1.0 | − | 0.37 |
| InAs(111)A [25] | −3.8 | +2.0 | −1.0 | − | 0.40 |
| GaSb(111)B | +3.4 | −0.3 | +0.6 | −0.1 | 0.24 |
| Surface | Position | Ga-B | Ga-T | Sb2-T | Sb4-T |
|---|---|---|---|---|---|
| O/GaSb(111)A | Eb, eV | 5.99 | 5.11 | 4.98 | 5.08 |
| MC, eV | −0.39 | −0.12 | −1.08 | −0.05 | |
| CC, eV | 6.38 | 5.23 | 6.06 | 5.13 | |
| ΔQ, e | 0.58 (1.05) | 0.45 (0.62) | 0.61 (1.09) | 0.65 (1.12) | |
| h, Å | 0.25 | 1.76 | −0.23 | −1.00 | |
| d(O–Ga1), Å | 2.22 × 2 | 1.76 | 2.56 × 3 | 2.73 × 3 | |
| d(O–Sb2), Å | 2.05 | 3.83 × 3 | 1.98 | 2.55 × 3 | |
| θ(O–Ga1), e | 0.33 × 2 | 0.78 | 0.20 × 3 | 0.12 × 3 | |
| θ(O–Sb2), e | 0.68 | 0.02 × 3 | 0.81 | 0.30 × 3 | |
| F/GaSb(111)A | Eb, eV | 4.43 | 5.62 | 4.19 | 4.31 |
| MC, eV | −0.01 | −0.02 | −0.15 | −0.05 | |
| CC, eV | 6.00 | 5.13 | 5.13 | 5.13 | |
| ΔQ, e | 0.48 (0.75) | 0.40 (0.65) | 0.41 (0.72) | 0.57 (0.78) | |
| h, Å | 1.12 | 1.81 | 1.61 | 0.94 | |
| d(F–Ga1), Å | 2.49 × 2 | 1.81 | 3.01 × 3 | 2.72 × 3 | |
| d(F–Sb2), Å | 2.48 | 3.71 × 3 | 2.11 | 3.02 × 3 | |
| θ(F–Ga1), e | 0.15 × 2 | 0.60 | 0.05 × 3 | 0.10 × 3 | |
| θ(F–Sb2), e | 0.28 | 0.02 × 3 | 0.55 | 0.10 × 3 |
| Surface | Position | Sb-B | Sb-T | Ga2 | Ga4 |
|---|---|---|---|---|---|
| O/GaSb(111)B | Eb, eV | 6.29 | 5.45 | 5.04 | 4.95 |
| MC, eV | −0.23 | −0.01 | −0.71 | −0.24 | |
| CC, eV | 6.52 | 5.46 | 5.75 | 5.19 | |
| ΔQ, e | 0.57 (1.15) | 0.40 (0.75) | 0.52 (1.05) | 0.63 (1.08) | |
| h, Å | −0.21 | 1.86 | 0.13 | −0.22 | |
| d(O–Sb1), Å | 2.22 × 2 | 1.86 | 2.55 × 3 | 2.56 × 3 | |
| d(O–Ga2), Å | 1.94 | 3.85 × 3 | 1.92 | 2.83 × 3 | |
| θ(O–Sb1), e | 0.49 × 2 | 0.94 | 0.29 × 3 | 0.31 × 3 | |
| θ(O–Ga2), e | 0.52 | 0.01 × 3 | 0.57 | 0.11 × 3 | |
| F/GaSb(111)B | Eb, eV | 4.04 | 4.83 | 3.82 | 3.56 |
| MC, eV | −0.34 | −0.02 | −0.37 | −0.13 | |
| CC, eV | 4.38 | 4.85 | 4.19 | 4.85 | |
| ΔQ, e | 0.29 (0.77) | 0.28 (0.65) | 0.38 (0.68) | 0.50 (0.74) | |
| h, Å | −0.09 | 1.95 | 1.75 | 1.10 | |
| d(F–Sb1), Å | 2.26 × 2 | 1.95 | 3.09 × 3 | 2.78 × 3 | |
| d(F–Ga2), Å | 2.51 | 3.73 × 3 | 1.92 | 3.02 × 3 | |
| θ(F–Sb1), e | 0.39 × 2 | 0.67 | 0.07 × 3 | 0.15 × 3 | |
| θ(F–Ga2), e | 0.15 | 0.01 × 3 | 0.49 | 0.05 × 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bakulin, A.V.; Chumakova, L.S.; Korchuganov, A.V.; Kulkova, S.E. Role of Oxygen and Fluorine in Passivation of the GaSb(111) Surface Depending on Its Termination. Crystals 2022, 12, 477. https://doi.org/10.3390/cryst12040477
Bakulin AV, Chumakova LS, Korchuganov AV, Kulkova SE. Role of Oxygen and Fluorine in Passivation of the GaSb(111) Surface Depending on Its Termination. Crystals. 2022; 12(4):477. https://doi.org/10.3390/cryst12040477
Chicago/Turabian StyleBakulin, Alexander V., Lora S. Chumakova, Aleksandr V. Korchuganov, and Svetlana E. Kulkova. 2022. "Role of Oxygen and Fluorine in Passivation of the GaSb(111) Surface Depending on Its Termination" Crystals 12, no. 4: 477. https://doi.org/10.3390/cryst12040477
APA StyleBakulin, A. V., Chumakova, L. S., Korchuganov, A. V., & Kulkova, S. E. (2022). Role of Oxygen and Fluorine in Passivation of the GaSb(111) Surface Depending on Its Termination. Crystals, 12(4), 477. https://doi.org/10.3390/cryst12040477






