Decreased Irradiance and Nutrient Enrichment Mitigate the Negative Effect of Ocean Warming on Growth and Biochemical Compositions of a Canopy-Forming Marine Macroalga
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Specimens
2.2. Experimental Design
2.3. Measurements of SGR and Biochemical Compositions
2.4. Statistical Analysis
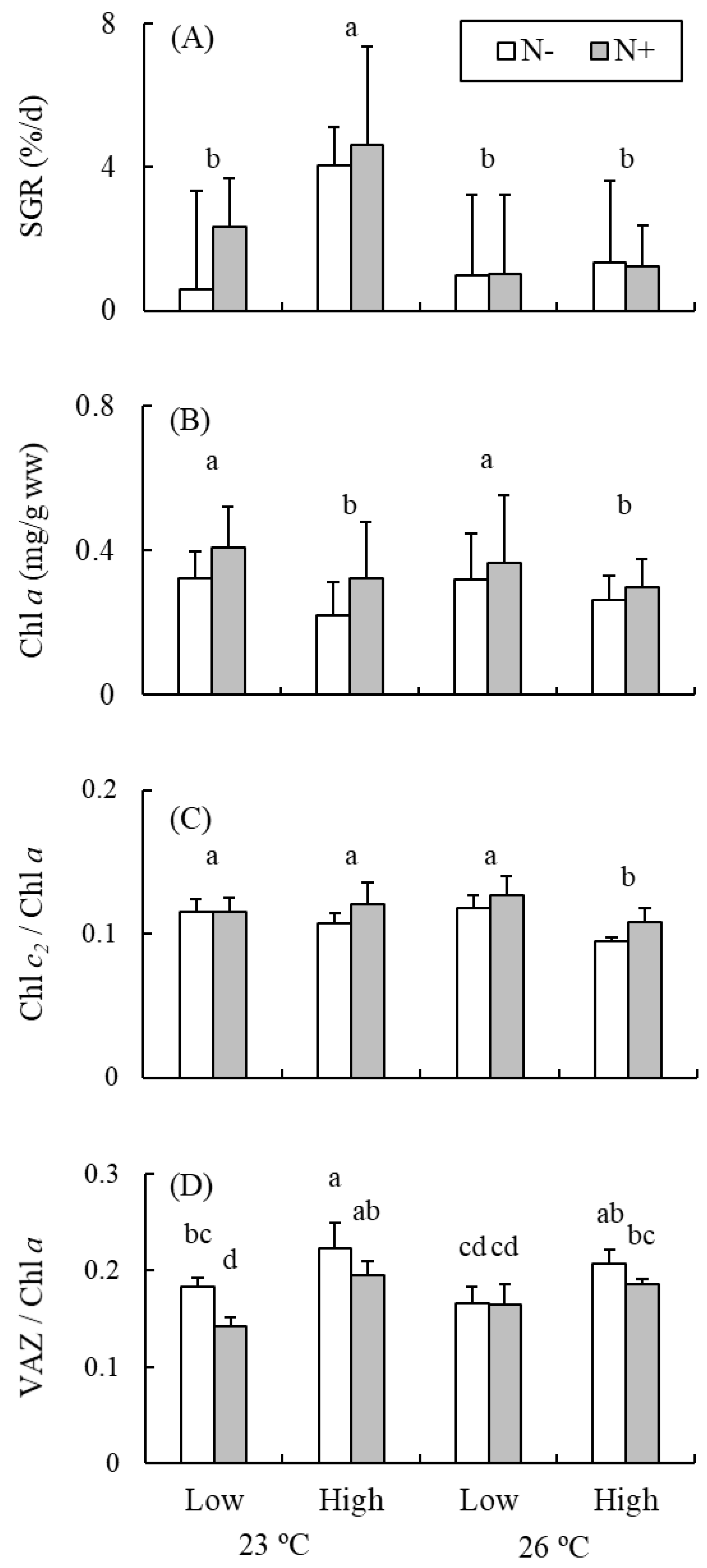
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zandalinas, S.I.; Fritschi, F.B.; Mittler, R. Global Warming, Climate Change, and Environmental Pollution: Recipe for a Multifactorial Stress Combination Disaster. Trends Plant Sci. 2021, 26, 588–599. [Google Scholar] [CrossRef] [PubMed]
- Nunes, L.J.; Meireles, C.I.; Gomes, C.J.P.; Ribeiro, N.M.A. Forest Contribution to Climate Change Mitigation: Management Oriented to Carbon Capture and Storage. Climate 2020, 8, 21. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, K.; Yoshida, G.; Hori, M.; Umezawa, Y.; Moki, H.; Kuwae, T. Macroalgal metabolism and lateral carbon flows can create significant carbon sinks. Biogeosciences 2020, 17, 2425–2440. [Google Scholar] [CrossRef]
- Lobell, D.B.; Sibley, A.; Ortiz-Monasterio, J.I. Extreme heat effects on wheat senescence in India. Nat. Clim. Chang. 2012, 2, 186–189. [Google Scholar] [CrossRef]
- Frölicher, T.L.; Fischer, E.; Gruber, N. Marine heatwaves under global warming. Nature 2018, 560, 360–364. [Google Scholar] [CrossRef] [PubMed]
- Smale, D.A. Impacts of ocean warming on kelp forest ecosystems. New Phytol. 2019, 225, 1447–1454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perkins-Kirkpatrick, S.E.; Lewis, S.C. Increasing trends in regional heatwaves. Nat. Commun. 2020, 11, 3357. [Google Scholar] [CrossRef]
- Ordóñez, R.A.; Savin, R.; Cossani, C.M.; Slafer, G. Yield response to heat stress as affected by nitrogen availability in maize. Field Crop. Res. 2015, 183, 184–203. [Google Scholar] [CrossRef]
- Thomas, M.K.; Aranguren-Gassis, M.; Kremer, C.T.; Gould, M.R.; Anderson, K.; Klausmeier, C.A.; Litchman, E. Temperature-nutrient interactions exacerbate sensitivity to warming in phytoplankton. Glob. Chang. Biol. 2017, 23, 3269–3280. [Google Scholar] [CrossRef]
- Endo, H.; Gao, X. A New Classification Tool and a Systematic Review of Macroalgal Studies Disentangle the Complex Interactive Effects of Warming and Nutrient Enrichment on Primary Production. Front. Mar. Sci. 2022, 9, 1–9. [Google Scholar] [CrossRef]
- Murata, N.; Takahashi, S.; Nishiyama, Y.; Allakhverdiev, S.I. Photoinhibition of photosystem II under environmental stress. Biochim. Biophys. Acta (BBA) Bioenerg. 2007, 1767, 414–421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balfagón, D.; Sengupta, S.; Gómez-Cadenas, A.; Fritschi, F.B.; Azad, R.K.; Mittler, R.; Zandalinas, S.I. Jasmonic Acid Is Required for Plant Acclimation to a Combination of High Light and Heat Stress. Plant Physiol. 2019, 181, 1668–1682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, C.J.; Saunders, M.I.; Possingham, H.P.; Richardson, A.J. Interactions between global and local stressors of ecosystems determine management effectiveness in cumulative impact mapping. Divers. Distrib. 2014, 20, 538–546. [Google Scholar] [CrossRef] [Green Version]
- Côté, I.M.; Darling, E.; Brown, C. Interactions among ecosystem stressors and their importance in conservation. Proc. R. Soc. B Biol. Sci. 2016, 283, 20152592. [Google Scholar] [CrossRef] [PubMed]
- Coleman, M.A.; Wernberg, T. Forgotten underwater forests: The key role of fucoids on Australian temperate reefs. Ecol. Evol. 2017, 7, 8406–8418. [Google Scholar] [CrossRef] [Green Version]
- Eger, A.M.; Marzinelli, E.; Gribben, P.; Johnson, C.R.; Layton, C.; Steinberg, P.D.; Wood, G.; Silliman, B.R.; Vergés, A. Playing to the Positives: Using Synergies to Enhance Kelp Forest Restoration. Front. Mar. Sci. 2020, 7, 544. [Google Scholar] [CrossRef]
- Filbee-Dexter, K.; Wernberg, T. Substantial blue carbon in overlooked Australian kelp forests. Sci. Rep. 2020, 10, 12341. [Google Scholar] [CrossRef]
- Endo, H.; Suehiro, K.; Gao, X.; Agatsuma, Y. Interactive effects of elevated summer temperature, nutrient availability, and irradiance on growth and chemical compositions of juvenile kelp, Eisenia bicyclis. Phycol. Res. 2017, 65, 118–126. [Google Scholar] [CrossRef]
- Gao, X.; Endo, H.; Nagaki, M.; Agatsuma, Y. Interactive effects of nutrient availability and temperature on growth and survival of different size classes of Saccharina japonica (Laminariales, Phaeophyceae). Phycologia 2017, 56, 253–260. [Google Scholar] [CrossRef]
- Steen, H. Interspecific competition between Enteromorpha (Ulvales: Chlorophyceae) and Fucus (Fucales: Phaeophyceae) germlings: Effects of nutrient concentration, temperature, and settlement density. Mar. Ecol. Prog. Ser. 2004, 278, 89–101. [Google Scholar] [CrossRef] [Green Version]
- Steen, H.; Rueness, J. Comparison of survival and growth in germlings of six fucoid species (Fucales, Phaeophyceae) at two different temperature and nutrient levels. Sarsia 2004, 89, 175–183. [Google Scholar] [CrossRef]
- Hwang, R.-L.; Tsai, C.-C.; Lee, T.-M. Assessment of temperature and nutrient limitation on seasonal dynamics among species of Sargassum from a coral reef in southern taiwan. J. Phycol. 2004, 40, 463–473. [Google Scholar] [CrossRef]
- Piñeiro-Corbeira, C.; Barreiro, R.; Franco, J.N.; Cremades, J.; Cunha, J.; Arenas, F. Unexpected nutrient influence on the thermal ecophysiology of seaweeds that recently followed opposite abundance shifts. Mar. Environ. Res. 2019, 151, 104747. [Google Scholar] [CrossRef] [PubMed]
- Endo, H.; Sugie, T.; Yonemori, Y.; Nishikido, Y.; Moriyama, H.; Ito, R.; Okunishi, S. Vegetative Reproduction Is More Advantageous Than Sexual Reproduction in a Canopy-Forming Clonal Macroalga under Ocean Warming Accompanied by Oligotrophication and Intensive Herbivory. Plants 2021, 10, 1522. [Google Scholar] [CrossRef]
- Endo, H.; Inomata, E.; Gao, X.; Kinoshita, J.; Sato, Y.; Agatsuma, Y. Heat Stress Promotes Nitrogen Accumulation in Meristems via Apical Blade Erosion in a Brown Macroalga with Intercalary Growth. Front. Mar. Sci. 2020, 7, 7. [Google Scholar] [CrossRef]
- Mabin, C.; Johnson, C.; Wright, J. Physiological response to temperature, light, and nitrates in the giant kelp Macrocystis pyrifera, from Tasmania, Australia. Mar. Ecol. Prog. Ser. 2019, 614, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Pintó-Marijuan, M.; Munné-Bosch, S. Photo-oxidative stress markers as a measure of abiotic stress-induced leaf senescence: Advantages and limitations. J. Exp. Bot. 2014, 65, 3845–3857. [Google Scholar] [CrossRef] [Green Version]
- Macintyre, H.L.; Kana, T.M.; Anning, T.; Geider, R.J. Review Photoacclimation of Photosynthesis Irradiance Response Curves and Photosynthetic Pigments In Microalgae And Cyanobacteria. J. Phycol. 2002, 38, 17–38. [Google Scholar] [CrossRef] [Green Version]
- Endo, H.; Okumura, Y.; Sato, Y.; Agatsuma, Y. Interactive effects of nutrient availability, temperature, and irradiance on photosynthetic pigments and color of the brown alga Undaria pinnatifida. J. Appl. Phycol. 2017, 29, 1683–1693. [Google Scholar] [CrossRef]
- Goss, R.; Lepetit, B. Biodiversity of NPQ. J. Plant Physiol. 2015, 172, 13–32. [Google Scholar] [CrossRef]
- Gerard, V.A. The role of nitrogen nutrition in high-temperature tolerance of the kelp, Laminaria saccharina (Chromophyta). J. Phycol. 1997, 33, 800–810. [Google Scholar] [CrossRef]
- Hay, K.B.; Millers, K.A.; Poore, A.G.B.; Lovelock, C.E. The use of near infrared reflectance spectrometry for characterization of brown algal tissue1. J. Phycol. 2010, 46, 937–946. [Google Scholar] [CrossRef]
- Flukes, E.; Wright, J.T.; Johnson, C.R. Phenotypic plasticity and biogeographic variation in physiology of habitat-forming seaweed: Response to temperature and nitrate. J. Phycol. 2015, 51, 896–909. [Google Scholar] [CrossRef] [PubMed]
- Kay, L.M.; Schmidt, A.L.; Wilson, K.L.; Lotze, H.K. Interactive effects of increasing temperature and nutrient loading on the habitat-forming rockweed Ascophyllum nodosum. Aquat. Bot. 2016, 133, 70–78. [Google Scholar] [CrossRef]
- Endo, H.; Suehiro, K.; Kinoshita, J.; Agatsuma, Y. Combined Effects of Temperature and Nutrient Enrichment on Palatability of the Brown Alga Sargassum yezoense (Yamada) Yoshida & T. Konno. Am. J. Plant Sci. 2015, 6, 275–282. [Google Scholar] [CrossRef] [Green Version]
- Gao, X.; Endo, H.; Nagaki, M.; Agatsuma, Y. Growth and survival of juvenile sporophytes of the kelp Ecklonia cava in response to different nitrogen and temperature regimes. Fish. Sci. 2016, 82, 623–629. [Google Scholar] [CrossRef]
- Franco, J.N.; Tuya, F.; Bertocci, I.; Rodríguez, L.; Martínez, B.; Pinto, I.S.; Arenas, F. The ‘golden kelp’ Laminaria ochroleuca under global change: Integrating multiple eco-physiological responses with species distribution models. J. Ecol. 2017, 106, 47–58. [Google Scholar] [CrossRef] [Green Version]
- Kokubu, Y.; Rothäusler, E.; Filippi, J.-B.; Durieux, E.D.; Komatsu, T. Revealing the deposition of macrophytes transported offshore: Evidence of their long-distance dispersal and seasonal aggregation to the deep sea. Sci. Rep. 2019, 9, 4331. [Google Scholar] [CrossRef]
- Gouvêa, L.P.; Assis, J.; Gurgel, C.F.; Serrao, E.; Silveira, T.C.; Santos, R.; Duarte, C.M.; Peres, L.M.; Carvalho, V.F.; Batista, M.; et al. Golden carbon of Sargassum forests revealed as an opportunity for climate change mitigation. Sci. Total Environ. 2020, 729, 138745. [Google Scholar] [CrossRef]
- Abe, H.; Komatsu, T.; Kokubu, Y.; Natheer, A.; Rothausler, E.A.; Shishido, H.; Yoshizawa, S.; Ajisaka, T. Invertebrate Fauna Associated with Floating Sargassum horneri (Fucales: Sargassaceae) in the East China Sea. Species Divers. 2013, 18, 75–85. [Google Scholar] [CrossRef] [Green Version]
- Yamasaki, M.; Aono, M.; Ogawa, N.; Tanaka, K.; Imoto, Z.; Nakamura, Y. Drifting algae and fish: Implications of tropical Sargassum invasion due to ocean warming in western Japan. Estuar. Coast. Shelf Sci. 2014, 147, 32–41. [Google Scholar] [CrossRef]
- Pang, S.J.; Shan, T.F.; Zhang, Z.H.; Sun, J.Z. Cultivation of the intertidal brown alga Hizikia fusiformis (Harvey) Okamura: Mass production of zygote-derived seedlings under commercial cultivation conditions, a case study experience. Aquac. Res. 2008, 39, 1408–1415. [Google Scholar] [CrossRef]
- Li, J.; Liu, Y.; Liu, Y.; Wang, Q.; Gao, X.; Gong, Q. Effects of temperature and salinity on the growth and biochemical composition of the brown alga Sargassum fusiforme (Fucales, Phaeophyceae). J. Appl. Phycol. 2019, 31, 3061–3068. [Google Scholar] [CrossRef]
- Liu, L.; Lin, L. Effect of Heat Stress on Sargassum fusiforme Leaf Metabolome. J. Plant Biol. 2020, 63, 229–241. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, H. Studies on the Population Dynamics and Genetic Structure of the Kelp Eisenia bicyclis in the Earthquake-Subsided Rocky Shore. Ph.D. Thesis, Tohoku University, Sendai, Japan, 2018. [Google Scholar]
- The physical science basis. In Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change (IPCC); Cambridge University Press: New York, NY, USA, 2013.
- Kokubu, S.; Nishihara, G.; Watanabe, Y.; Tsuchiya, Y.; Amamo, Y.; Terada, R. The effect of irradiance and temperature on the photosynthesis of a native alga Sargassum fusiforme (Fucales) from Kagoshima, Japan. Phycolpgia 2015, 54, 235–247. [Google Scholar] [CrossRef]
- Baba, M. Effect of Temperature and Irradiance on Germling Growth In Eight Sargassaceous Species. Mar. Eoc. Res. Inst. 2007, 10, 9–20. (In Japanese) [Google Scholar]
- Tatewaki, M. Formation of a Crustaceous Sporophyte with Unilocular Sporangia in Scytosiphon lomentaria. Phycologia 1966, 6, 62–66. [Google Scholar] [CrossRef]
- Schaffelke, B.; Klumpp, D. Nutrient-limited growth of the coral reef macroalga Sargassum baccularia and experimental growth enhancement by nutrient addition in continuous flow culture. Mar. Ecol. Prog. Ser. 1998, 164, 199–211. [Google Scholar] [CrossRef]
- Endo, H.; Suehiro, K.; Kinoshita, J.; Gao, X.; Agatsuma, Y. Combined Effects of Temperature and Nutrient Availability on Growth and Phlorotannin Concentration of the Brown Alga Sargassum patens (Fucales; Phaeophyceae). Am. J. Plant Sci. 2013, 4, 14–20. [Google Scholar] [CrossRef] [Green Version]
- Zapata, M.; Rodriguez, F.; Garrido, J. Separation of chlorophylls and carotenoids from marine phytoplankton:a new HPLC method using a reversed phase C8 column and pyridine-containing mobile phases. Mar. Ecol. Prog. Ser. 2000, 195, 29–45. [Google Scholar] [CrossRef] [Green Version]
- Osanai, T.; Oikawa, A.; Shirai, T.; Kuwahara, A.; Iijima, H.; Tanaka, K.; Ikeuchi, M.; Kondo, A.; Saito, K.; Hirai, M.Y. Capillary electrophoresis-mass spectrometry reveals the distribution of carbon metabolites during nitrogen starvation in Synechocystis sp. PCC 6803. Environ. Microbiol. 2013, 16, 512–524. [Google Scholar] [CrossRef] [PubMed]
- Baird, A.H.; Madin, J.; Álvarez-Noriega, M.; Fontoura, L.; Kerry, J.T.; Kuo, C.-Y.; Precoda, K.; Torres-Pulliza, D.; Woods, R.M.; Zawada, K.J.A.; et al. A decline in bleaching suggests that depth can provide a refuge from global warming in most coral taxa. Mar. Ecol. Prog. Ser. 2018, 603, 257–264. [Google Scholar] [CrossRef] [Green Version]

| Source | df | MS | F | p | |
|---|---|---|---|---|---|
| Temperature (T) | 1 | 36.920 | 8.628 | 0.005 | ** |
| Nutrient (N) | 1 | 3.710 | 0.866 | 0.358 | |
| Irradiance (I) | 1 | 29.960 | 7.001 | 0.012 | * |
| T × I | 1 | 20.240 | 4.730 | 0.036 | * |
| T × N | 1 | 4.110 | 0.961 | 0.333 | |
| I × N | 1 | 1.200 | 0.281 | 0.599 | |
| T × N × I | 1 | 0.800 | 0.187 | 0.668 |
| Source | df | MS | F | p | |
|---|---|---|---|---|---|
| Chl a | |||||
| Temperature (T) | 1 | 0.001 | 0.005 | 0.829 | |
| Nutrient (N) | 1 | 0.055 | 3.879 | 0.056 | |
| Irradiance (I) | 1 | 0.074 | 5.239 | 0.027 | * |
| T × I | 1 | 0.003 | 0.208 | 0.651 | |
| T × N | 1 | 0.008 | 0.540 | 0.467 | |
| I × N | 1 | <0.001 | 0.001 | 0.973 | |
| T× N × I | 1 | 0.001 | 0.055 | 0.816 | |
| Chl c2 /Chl a | |||||
| Temperature (T) | 1 | <0.001 | 0.952 | 0.335 | |
| Nutrient (N) | 1 | 0.001 | 9.593 | 0.004 | ** |
| Irradiance (I) | 1 | 0.002 | 15.402 | <0.001 | *** |
| T × I | 1 | 0.001 | 11.380 | 0.002 | ** |
| T × N | 1 | 0.001 | 0.677 | 0.415 | |
| I× N | 1 | <0.001 | 2.405 | 0.129 | |
| T × N × I | 1 | <0.001 | 0.817 | 0.371 | |
| Fuco/Chl a | |||||
| Temperature (T) | 1 | <0.001 | 0.094 | 0.761 | |
| Nutrient (N) | 1 | <0.001 | 0.151 | 0.700 | |
| Irradiance (I) | 1 | <0.001 | 0.142 | 0.708 | |
| T × I | 1 | 0.001 | 0.671 | 0.418 | |
| T× N | 1 | <0.001 | 0.134 | 0.716 | |
| I× N | 1 | 0.001 | 0.675 | 0.416 | |
| T × N× I | 1 | <0.001 | <0.001 | 0.985 | |
| VAZ/Chl a | |||||
| Temperature (T) | 1 | 0.003 | 0.483 | 0.491 | |
| Nutrient (N) | 1 | 0.187 | 26.901 | <0.001 | *** |
| Irradiance (I) | 1 | 0.547 | 78.877 | <0.001 | *** |
| T × I | 1 | 0.019 | 2.673 | 0.110 | |
| T × N | 1 | 0.054 | 7.734 | 0.008 | ** |
| I × N | 1 | <0.001 | 0.024 | 0.877 | |
| T × N × I | 1 | 0.036 | 5.165 | 0.029 | * |
| Source | df | MS | F | p | |
|---|---|---|---|---|---|
| Carbon % | |||||
| Temperature (T) | 1 | 40.829 | 9.413 | 0.004 | ** |
| Nutrient (N) | 1 | 2.517 | 0.580 | 0.451 | |
| Irradiance (I) | 1 | 192.871 | 44.469 | <0.001 | *** |
| T × I | 1 | 0.041 | 0.009 | 0.923 | |
| T × N | 1 | 25.426 | 5.863 | 0.020 | * |
| I × N | 1 | 1.350 | 0.311 | 0.580 | |
| T × N × I | 1 | 2.403 | 0.554 | 0.461 | |
| Nitrogen % | |||||
| Temperature (T) | 1 | <0.001 | 0.032 | 0.859 | |
| Nutrient (N) | 1 | 0.859 | 174.838 | <0.001 | *** |
| Irradiance (I) | 1 | 0.001 | 0.184 | 0.670 | |
| T × I | 1 | 0.001 | 0.233 | 0.632 | |
| T × N | 1 | <0.001 | 0.002 | 0.964 | |
| I × N | 1 | 0.038 | 7.772 | 0.008 | ** |
| T × N × I | 1 | 0.010 | 1.961 | 0.169 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Charan, H.; Inomata, E.; Endo, H.; Sato, Y.; Okumura, Y.; Aoki, M.N. Decreased Irradiance and Nutrient Enrichment Mitigate the Negative Effect of Ocean Warming on Growth and Biochemical Compositions of a Canopy-Forming Marine Macroalga. J. Mar. Sci. Eng. 2022, 10, 479. https://doi.org/10.3390/jmse10040479
Charan H, Inomata E, Endo H, Sato Y, Okumura Y, Aoki MN. Decreased Irradiance and Nutrient Enrichment Mitigate the Negative Effect of Ocean Warming on Growth and Biochemical Compositions of a Canopy-Forming Marine Macroalga. Journal of Marine Science and Engineering. 2022; 10(4):479. https://doi.org/10.3390/jmse10040479
Chicago/Turabian StyleCharan, Harshna, Eri Inomata, Hikaru Endo, Yoichi Sato, Yutaka Okumura, and Masakazu N. Aoki. 2022. "Decreased Irradiance and Nutrient Enrichment Mitigate the Negative Effect of Ocean Warming on Growth and Biochemical Compositions of a Canopy-Forming Marine Macroalga" Journal of Marine Science and Engineering 10, no. 4: 479. https://doi.org/10.3390/jmse10040479
APA StyleCharan, H., Inomata, E., Endo, H., Sato, Y., Okumura, Y., & Aoki, M. N. (2022). Decreased Irradiance and Nutrient Enrichment Mitigate the Negative Effect of Ocean Warming on Growth and Biochemical Compositions of a Canopy-Forming Marine Macroalga. Journal of Marine Science and Engineering, 10(4), 479. https://doi.org/10.3390/jmse10040479







