Acute Stress Response of Sheep to Shearing Procedures: Dynamic Change of Cortisol Concentration and Protein Electrophoretic Pattern
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Design
2.2. Sampling Procedures and Laboratory Analysis
2.3. Statistical Analysis
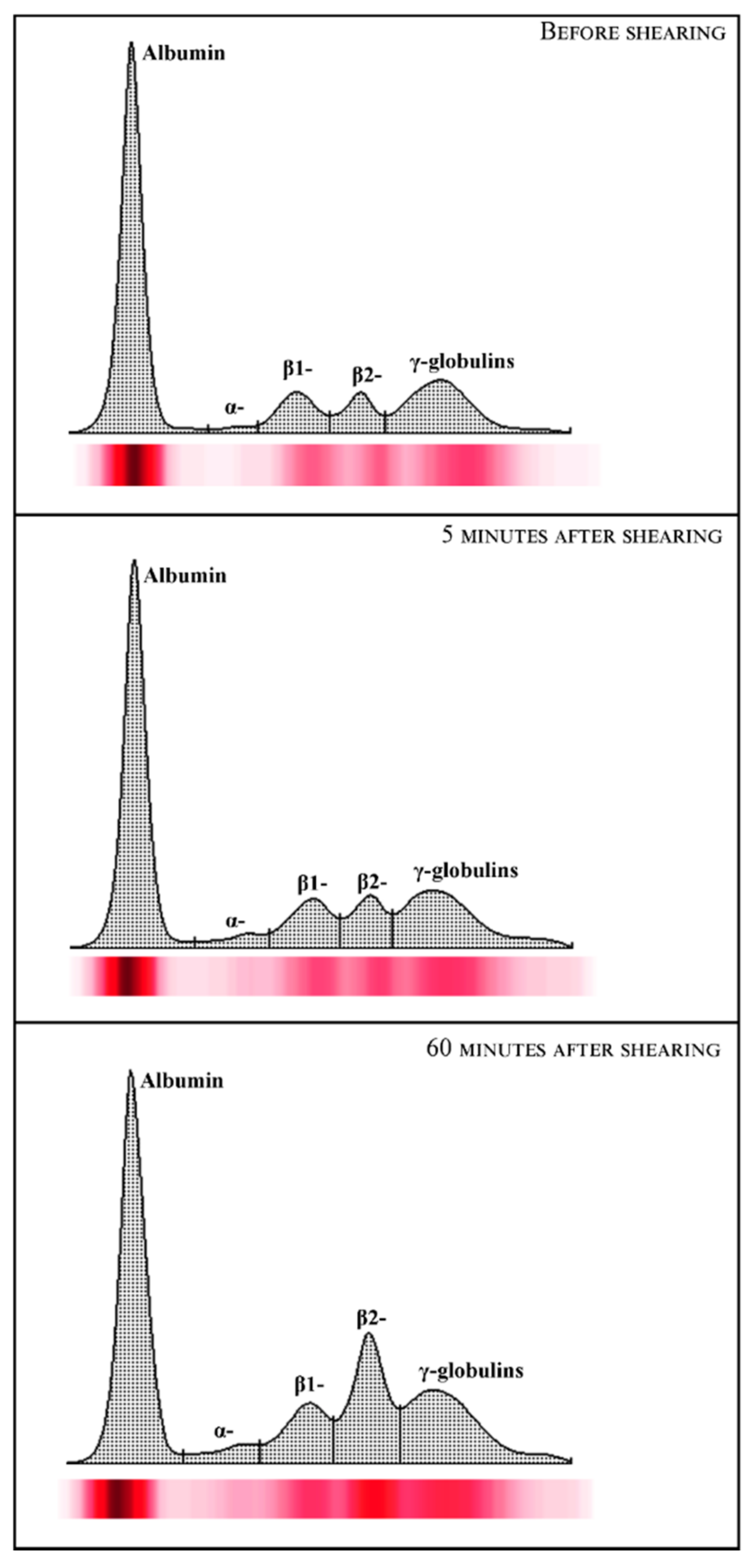
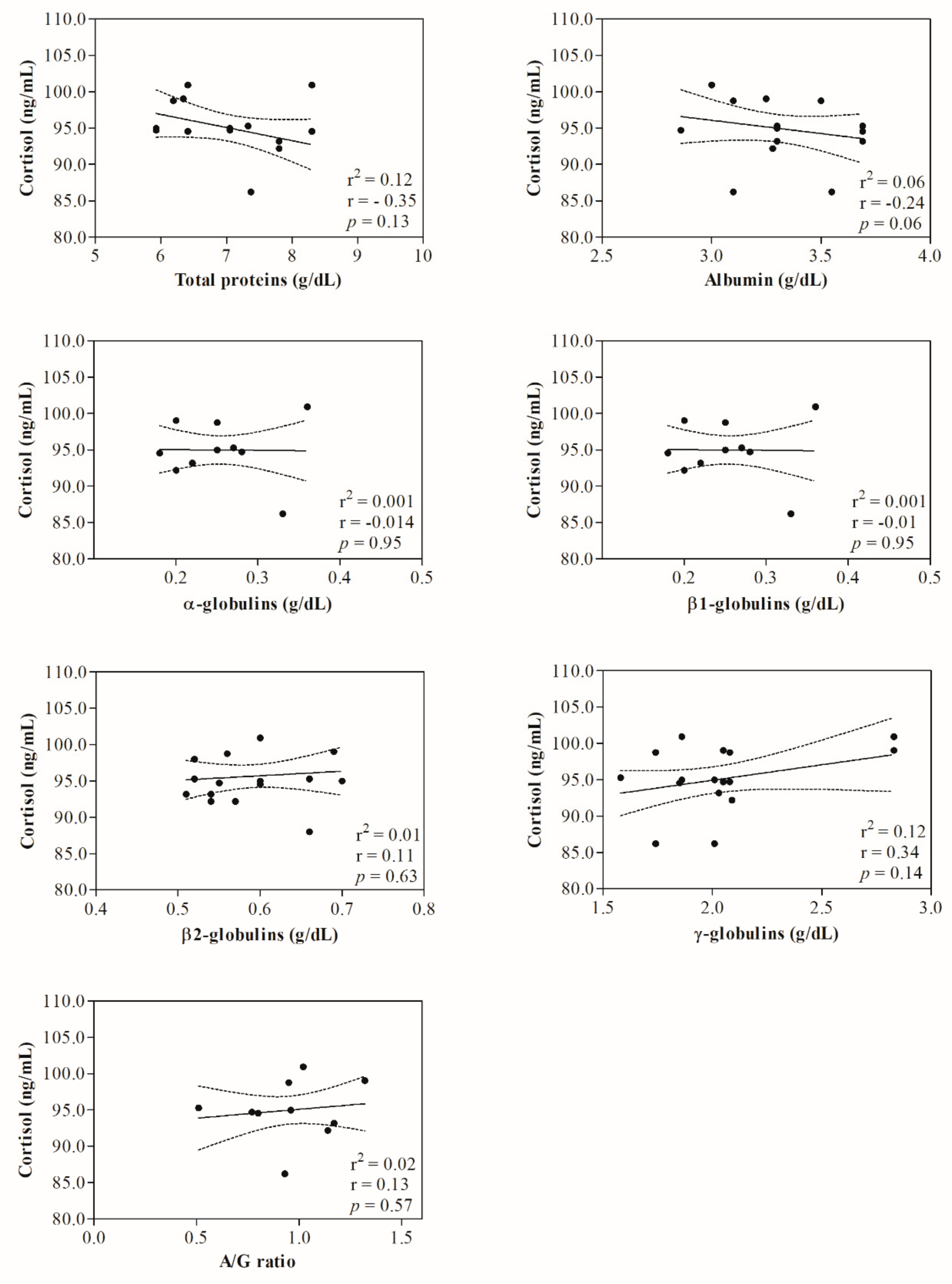
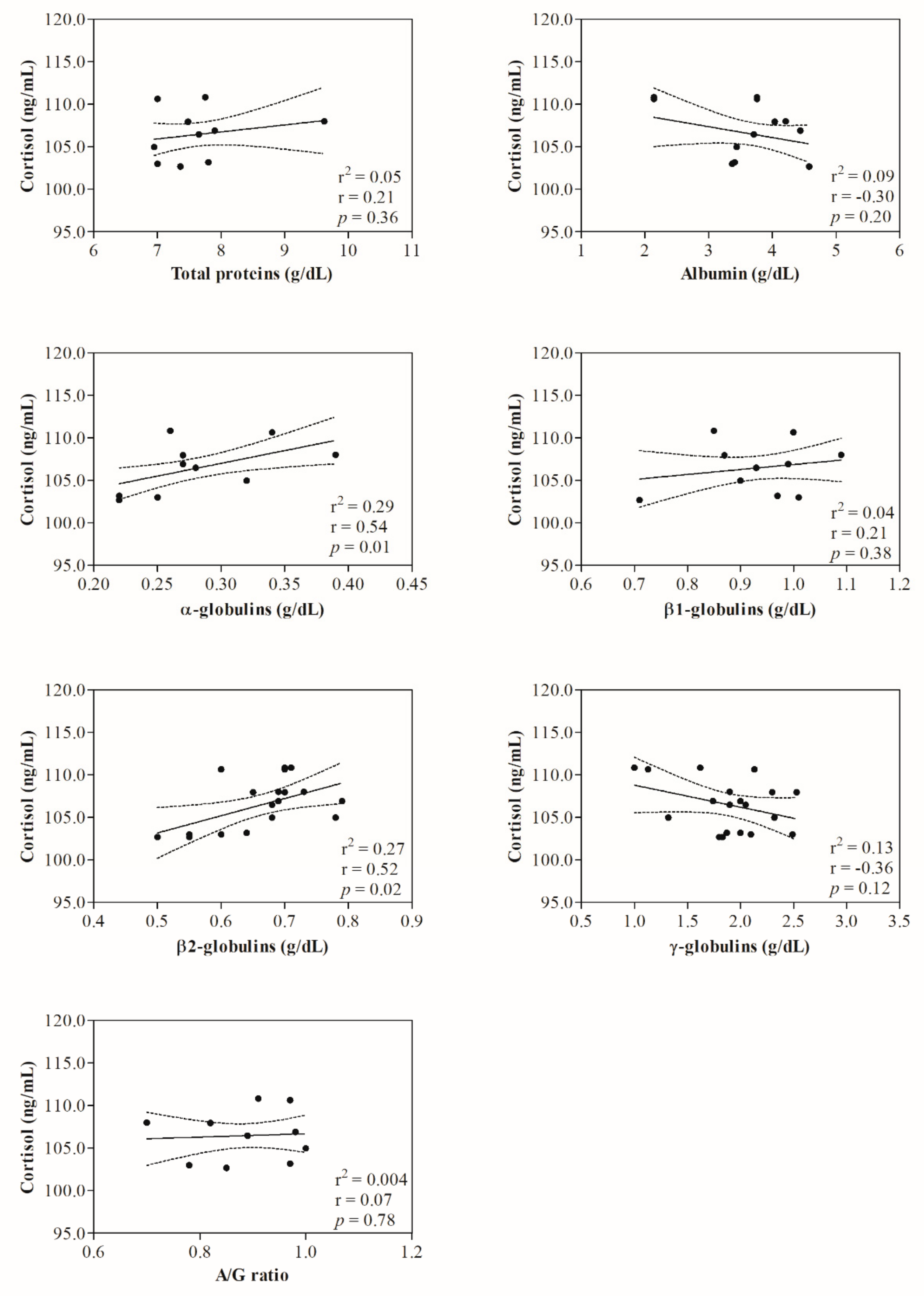
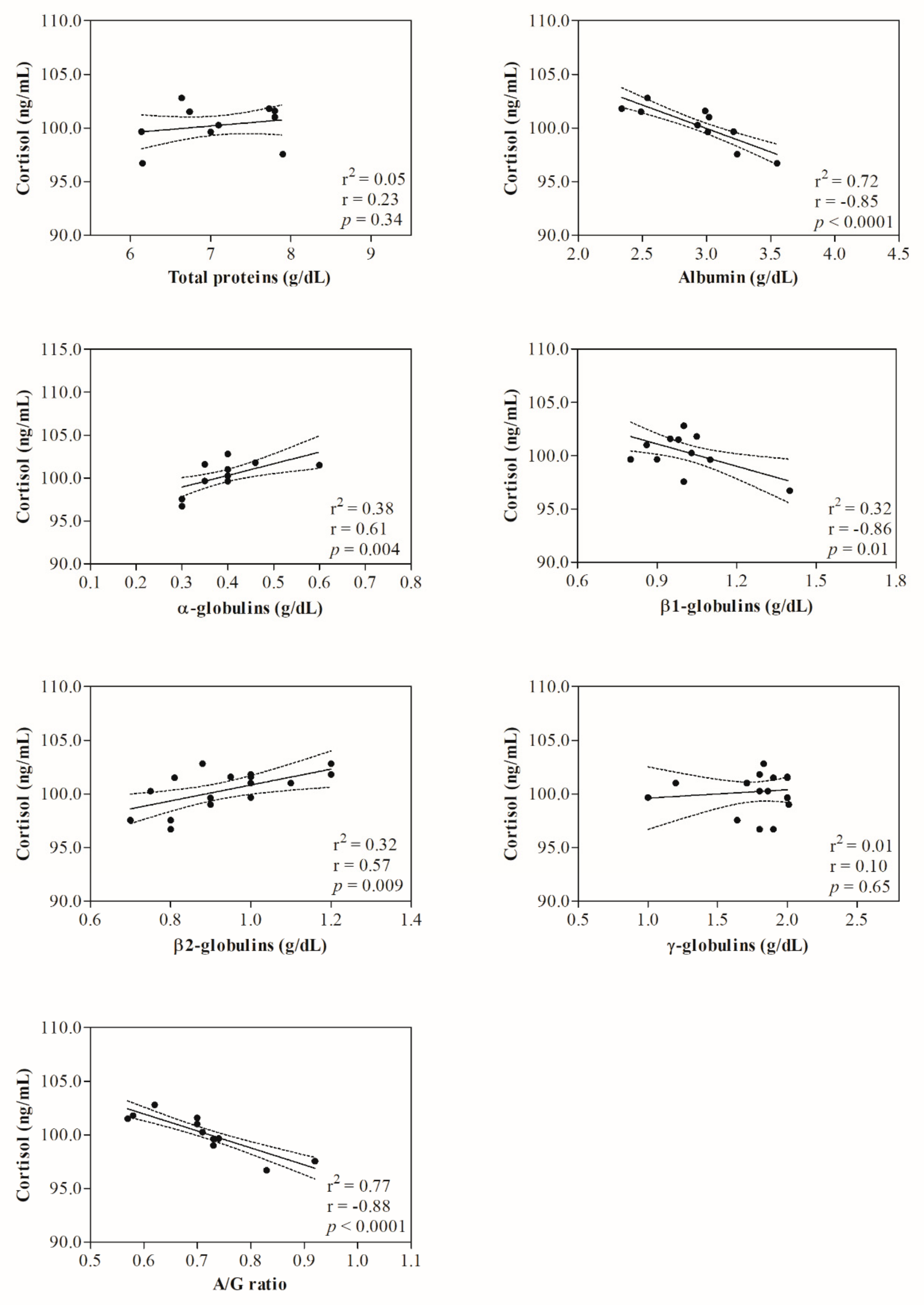
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Le Neindre, P.; Boivin, X.; Boissy, A. Handling of extensively kept animals. Appl. Anim. Behav. Sci. 1996, 49, 73–81. [Google Scholar] [CrossRef]
- Yardimci, M.; Sahin, E.H.; Cetingul, I.S.; Bayram, I.; Aslan, R.; Sengor, E. Stress responses to comparative handling procedures in sheep. Animal 2013, 7, 143–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dýrmundsson, O.R. Shearing time of sheep with special reference to conditions in northern Europe: A review. Bùvìsindi Icel. Agric. Sci. 1991, 5, 39–46. [Google Scholar]
- Piccione, G.; Casella, S.; Alberghina, D.; Zumbo, A.; Pennisi, P. Impact of shearing on body weight and serum total proteins in ewes. Span. J. Agric. Res. 2010, 8, 342–346. [Google Scholar] [CrossRef] [Green Version]
- Sanger, M.E.; Doyle, R.E.; Hinch, G.N.; Lee, C. Sheep exhibit a positive judgement bias and stress-induced hyperthermia following shearing. Appl. Anim. Behav. Sci. 2011, 131, 94–103. [Google Scholar] [CrossRef]
- Etim, N.N.; Evans, E.I.; Offiong, E.E.A.; Williams, M.E. Stress and the neuroendocrine system: Implications for animal well-being. Am. J. Biol. Life Sci. 2013, 1, 20–26. [Google Scholar]
- Kumar, B.; Manuja, A.; Aich, P. Stress and its impact on farm animals. Front. Biosci. Elite 2012, 4, 1759–1767. [Google Scholar] [CrossRef]
- Blokhuis, H.J.; Hopster, H.; Geverink, N.A.; Korte, S.M.; van Reenen, C.G. Studies of stress in farm animals. Comp. Haematol. Int. 1998, 8, 94–101. [Google Scholar] [CrossRef]
- Etim, N.N.; Williams, M.E.; Akpabio, U.; Offiong, E.E.A. Haematological Parameters and Factors Affecting Their Values. Agric. Sci. 2014, 2, 37–47. [Google Scholar] [CrossRef] [Green Version]
- Wingfield, J.C.; Kitaysky, A.S. Endocrine responses to unpredictable environmental events: Stress or anti-stress hormones? Integr. Comp. Biol. 2002, 42, 600–609. [Google Scholar] [CrossRef]
- Lay, D.C.; Friend, T.H.; Bowers, C.L.; Grissom, K.K.; Jenkins, O.C. A comparative physiological and behavioral study of freeze and hot-iron branding using dairy cows. J. Anim. Sci. 1992, 70, 1121–1130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Connell, T.X.; Horita, T.J.; Kasravi, B. Understanding and interpreting serum protein electrophoresis. Am. Fam. Physician 2005, 71, 105–112. [Google Scholar] [PubMed]
- Reczyńska, D.; Zalewska, M.; Czopowicz, M.; Kaba, J.; Zwierzchowski, L.; Bagnicka, E. Acute phase protein levels as an auxiliary tool in diagnosing viral diseases in ruminants-A Review. Viruses 2018, 10, 502. [Google Scholar] [CrossRef] [Green Version]
- Greguła-Kania, M.; Kosior-Korzecka, U.; Patkowski, K.; Juszczuk-Kubiak, E.; Plewik, M.; Gruszecki, T.M. Acute-phase proteins, cortisol and haematological parameters in ewes during the periparturient period. Reprod. Domest. Anim. 2020, 55, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Azim, W.; Azim, S.; Ahmed, K.; Shafi, H.; Rafi, T.; Luqman, M. Diagnostic significance of serum protein electrophoresis. Biomedica 2004, 20, 40–44. [Google Scholar]
- Ceciliani, F.; Ceron, J.J.; Eckersall, P.D.; Sauerwein, H. Acute phase proteins in ruminants. J. Proteom. 2012, 75, 4207–4231. [Google Scholar] [CrossRef] [PubMed]
- Bertoni, G.; Trevisi, E.; Han, X.; Bionaz, M. Effects of inflammatory conditions on liver activity in puerperium period and consequences for performance in dairy cows. J. Dairy Sci. 2008, 91, 3300–3310. [Google Scholar] [CrossRef] [Green Version]
- Piccione, G.; Caola, G.; Refinetti, R. Effect of shearing on the core temperature of three breeds of Mediterranean sheep. Small Rum. Res. 2002, 46, 211–215. [Google Scholar] [CrossRef]
- Piccione, G.; Caola, G. Influence of shearing on the circadian rhythm of body temperature in the sheep. J. Vet. Med. A 2003, 50, 235–240. [Google Scholar] [CrossRef]
- Piccione, G.; Casella, S.; Fazio, F.; Pennisi, P. Effect of shearing on some haematochemical parameters in ewes. Czech J. Anim. Sci. 2008, 53, 106–111. [Google Scholar] [CrossRef] [Green Version]
- Piccione, G.; Fazio, F.; Casella, S.; Pennisi, P.; Caola, G. Influence of shearing on oxidative stress and some physiological parameters in ewes. Anim. Sci. J. 2011, 82, 481–485. [Google Scholar] [CrossRef] [PubMed]
- Hargreaves, A.L.; Huston, G.D. The stress response in sheep during routine handling procedures. Appl. Anim. Behav. Sci. 1990, 26, 83–90. [Google Scholar] [CrossRef]
- Pennisi, P.; Costa, A.; Biondi, L.; Avondo, M.; Piccione, G. Influence of the fleece on thermal homeostasis and on body condition in Comisana ewe lambs. Anim. Res. 2004, 53, 13–19. [Google Scholar] [CrossRef] [Green Version]
- Aleksiev, Y. The effect of shearing on the behaviour of some physiological responses in lactating Pleven blackhead ewes. Bulg. J. Agric. Sci. 2009, 15, 446–452. [Google Scholar]
- Beatty, D.T.; Barnes, A.; Fleming, P.A.; Taylor, E.; Maloney, S.K. The effect of fleece on core and rumen temperature in sheep. J. Therm. Biol. 2008, 33, 437–443. [Google Scholar] [CrossRef]
- Casella, S.; Giudice, E.; Passantino, A.; Zumbo, A.; Di Pietro, S.; Piccione, G. Shearing induces secondary biomarkers responses of thermal stress in sheep. Anim. Sci. Pap. Rep. 2016, 34, 73–80. [Google Scholar]
- Hefnawy, A.; Helal, M.; Sabek, A.; Shousha, S. Clinical, behavioral and biochemical alterations due to shearing stress in Ossimi sheep. J. Vet. Med. Sci. 2018, 80, 1281–1286. [Google Scholar] [CrossRef] [Green Version]
- Piccione, G.; Lutri, L.; Casella, S.; Ferrantelli, V.; Pennisi, P. Effect of shearing and environmental conditions on physiological mechanisms in ewes. J. Environ. Biol. 2008, 29, 877–880. [Google Scholar]
- Kaneko, J.J.; Harvey, J.W.; Bruss, M.L. Clinical Biochemistry of Domestic Animals, 5th ed.; Academic Press Inc.: San Diego, CA, USA, 1997. [Google Scholar]
- Nagyová, V.; Arfuso, F.; Rizzo, M.; Nagy, O.; Piccione, G. Stability of ovine serum protein fractions under different storage conditions. J. Vet. Diagn. Investig. 2017, 29, 312–315. [Google Scholar] [CrossRef] [Green Version]
- Jern, C.; Wadenvik, H.; Mark, H.; Hallgren, J.; Jern, S. Haematological changes during acute mental stress. Br. J. Haematol. 1989, 71, 153–156. [Google Scholar] [CrossRef]
- Dede, S.; Altug, N.; Deger, Y.; Ozdal, N.; Ceylan, E. Serum biochemical profile and protein fractions in cattle with Theileriosis. Rev. Méd. Vét. 2014, 165, 137–143. [Google Scholar]
- Carcangiu, V.; Vacca, G.M.; Parmeggiani, A.; Mura, M.C.; Pazzola, M.; Dettori, M.L.; Bini, P.P. The effect of shearing procedures on blood levels of growth hormone, cortisol and other stress haematochemical parameters in Sarda sheep. Animal 2008, 2, 606–612. [Google Scholar] [CrossRef] [Green Version]
- Thompson, W.L.; Abeles, F.B.; Beall, F.A.; Dinterman, R.E.; Wannemacher, R.W., Jr. Influence of the adrenal glucocorticoids on the stimulation of synthesis of hepatic ribonucleic acid and plasma acute-phase globulins by leucocytic endogenous mediator. Biochem. J. 1976, 156, 25–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Gool, J.; Boers, W.; Sala, M.; Ladiges, N.C. Glucocorticoids and catecholamines as mediators of acute-phase proteins, especially rat alpha-macrofoetoprotein. Biochem. J. 1984, 220, 125–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Hematological Parameters | Experimental Period | ||
|---|---|---|---|
| TPRE | TPOST5 | TPOST60 | |
| WBCs (×103/µL) | 7.82 ± 1.58 | 7.48 ± 1.45 | 7.83 ± 1.61 |
| RBCs (×106/µL) | 10.71 ± 0.78 | 10.50 ± 0.62 | 10.47 ± 0.81 |
| Hct (%) | 36.55 ± 4.72 | 35.81 ± 4.92 | 34.41 ± 3.98 |
| Hb (g/dL) | 9.78 ± 0.91 | 9.68 ± 1.05 | 9.59 ± 0.97 |
| MCV (fL) | 35.60 ± 5.14 | 35.62 ± 5.16 | 34.50 ± 5.20 |
| MCH (pg) | 9.51 ± 0.87 | 9.60 ± 0.90 | 9.57 ± 0.86 |
| MCHC (%) | 26.94 ± 0.40 | 27.19 ± 0.83 | 28.03 ± 1.78 |
| PLTs (×103/µL) | 604.85 ± 168.48 | 636.08 ± 119.30 | 575.45 ± 101.08 |
| Serum Parameters | Experimental Period | ||
|---|---|---|---|
| TPRE | TPOST5 | TPOST60 | |
| Cortisol (ng/mL) | 94.99 ± 4.14 | 100.3 ± 1.93 a | 106.5 ± 2.98 a,b |
| Total proteins (d/dL) | 7.05 ± 0.80 | 7.65 ± 0.78 | 7.10 ± 0.68 |
| Albumin (g/dL) | 3.30 ± 0.28 | 3.71 ± 0.68 | 2.93 ± 0.38 b |
| α-globulins (g/dL) | 0.26 ± 0.06 | 0.28 ± 0.05 | 0.40 ± 0.08 a,b |
| β1-globulins (g/dL) | 0.90 ± 0.11 | 0.93 ± 0.10 | 1.03 ± 0.14 |
| β2-globulins (g/dL) | 0.58 ± 0.06 | 0.68 ± 0.08 | 0.88 ± 0.10 a,b |
| γ-globulins (g/dL) | 2.01 ± 0.33 | 2.05 ± 0.31 | 1.85 ± 0.13 |
| A/G ratio | 0.89 ± 0.10 | 0.96 ± 0.23 | 0.71 ± 0.11 b |
| WBCs (×103/µL) | RBCs (×106/µL) | Hct (%) | Hb (g/dL) | MCV (fL) | MCH (pg) | MCHC (%) | PLTs (×103/µL) | ||
|---|---|---|---|---|---|---|---|---|---|
| Cortisol (ng/mL) | TPRE | r = 0.24 | r = −0.99 | r = −0.02 | r = −0.20 | r = −0.10 | r = −0.30 | r = −0.13 | r = −0.20 |
| p = 0.31 | p = 0.70 | p = 0.95 | p = 0.40 | p = 0.67 | p = 0.20 | p = 0.59 | p = 0.40 | ||
| TPOST5 | r = −0.44 | r = −0.37 | r = 0.18 | r = 0.10 | r = 0.31 | r = 0.43 | r = 0.12 | r = −0.29 | |
| p = 0.06 | p = 0.11 | p = 0.46 | p = 0.68 | p = 0.18 | p = 0.06 | p = 0.61 | p = 0.21 | ||
| TPOST60 | r = −0.24 | r = 0.27 | r = −0.44 | r = −0.15 | r = −0.16 | r = 0.13 | r = 0.06 | r = −0.26 | |
| p = 0.30 | p = 0.26 | p = 0.06 | p = 0.54 | p = 0.50 | p = 0.61 | p = 0.81 | p = 0.27 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arfuso, F.; Fazio, F.; Chikhi, L.; Aymond, G.; Piccione, G.; Giannetto, C. Acute Stress Response of Sheep to Shearing Procedures: Dynamic Change of Cortisol Concentration and Protein Electrophoretic Pattern. Animals 2022, 12, 862. https://doi.org/10.3390/ani12070862
Arfuso F, Fazio F, Chikhi L, Aymond G, Piccione G, Giannetto C. Acute Stress Response of Sheep to Shearing Procedures: Dynamic Change of Cortisol Concentration and Protein Electrophoretic Pattern. Animals. 2022; 12(7):862. https://doi.org/10.3390/ani12070862
Chicago/Turabian StyleArfuso, Francesca, Francesco Fazio, Lucas Chikhi, Guillaume Aymond, Giuseppe Piccione, and Claudia Giannetto. 2022. "Acute Stress Response of Sheep to Shearing Procedures: Dynamic Change of Cortisol Concentration and Protein Electrophoretic Pattern" Animals 12, no. 7: 862. https://doi.org/10.3390/ani12070862
APA StyleArfuso, F., Fazio, F., Chikhi, L., Aymond, G., Piccione, G., & Giannetto, C. (2022). Acute Stress Response of Sheep to Shearing Procedures: Dynamic Change of Cortisol Concentration and Protein Electrophoretic Pattern. Animals, 12(7), 862. https://doi.org/10.3390/ani12070862







