Micropropagation of Plum (Prunus domestica L.) in Bioreactors Using Photomixotrophic and Photoautotrophic Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Establishment of Shoot Multiplication Cultures
2.2. Culture System, Hormonal Supplementation and Frequency of Immersion
2.3. Growth Conditions and Sucrose Supplementation
2.3.1. Biochemical Quantifications
Soluble Monosaccharides
Total Soluble Sugars
Photosynthetic Pigments
Total Soluble Phenolic Compounds
Antioxidant Activity
2.4. Rooting and Acclimatization
2.5. Data Recording and Statistical Analysis
3. Results
3.1. Culture System and Hormonal Composition of the Media
3.2. Culture System and Number of Immersions
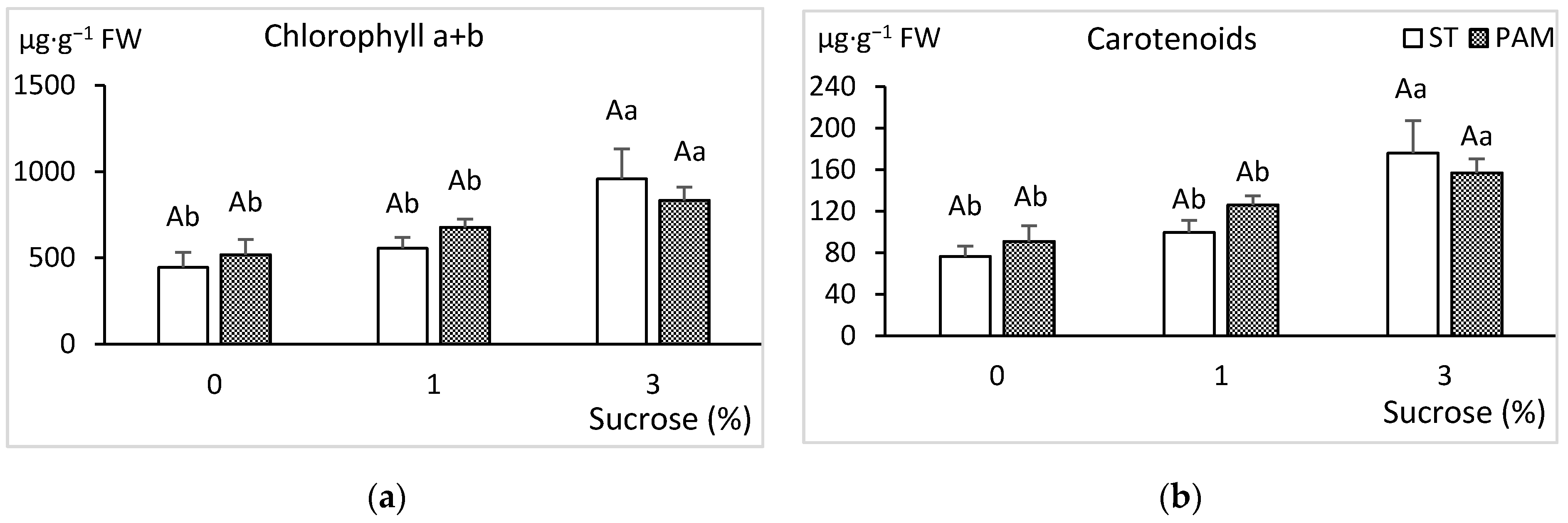
3.3. Growth Conditions and Sucrose Supplementation
3.4. Effect of the Culture System on Rooting and Acclimation
3.5. Effect of Sucrose Supplementation on Rooting and Acclimation
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sottile, F.; Caltagirone, C.; Giacalone, G.; Peano, C. Unlocking Plum Genetic Potential: Where Are We At ? Horticulturae 2022, 8, 128. [Google Scholar] [CrossRef]
- Zhebentyayeva, T.; Shankar, V.; Scorza, R.; Callahan, A.; Ravelonandro, M.; Castro, S.; DeJong, T.; Saski, C.A.; Dardick, C. Genetic characterization of worldwide Prunus domestica (plum) germplasm using sequence-based genotyping. Hortic. Res. 2019, 6, 12. [Google Scholar] [CrossRef] [Green Version]
- Miller, A.J.; Gross, B.L. From forest to field: Perennial fruit crop domestication. Am. J. Bot. 2011, 98, 1389–1414. [Google Scholar] [CrossRef] [Green Version]
- Goldschmidt, E.E. The Evolution of Fruit Tree Productivity: A Review. Econ. Bot. 2013, 67, 51–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaši, F.; Sehic, J.; Grahic, J.; Hjeltnes, S.H.; Ordidge, M.; Benedikova, D.; Blouin-Delmas, M.; Drogoudi, P.; Giovannini, D.; Höfer, M.; et al. Genetic assessment of the pomological classification of plum Prunus domestica L. accessions sampled across Europe. Genet. Resour. Crop Evol. 2020, 67, 1137–1161. [Google Scholar] [CrossRef]
- Lansari, A.; Kester, D.E.; Iezzoni, A.F. Inbreeding, coancestry, anf founding clones of almonds of California, Mediterranean shores, and Russia. J. Am. Soc. Hortic. Sci. 1994, 119, 1279–1285. [Google Scholar] [CrossRef] [Green Version]
- Maxted, N.; Kell, S.; Ford-Lloyd, B.; Dulloo, E.; Toledo, Á. Toward the systematic conservation of global crop wild relative diversity. Crop Sci. 2012, 52, 774–785. [Google Scholar] [CrossRef]
- Caballero, A.; García-Dorado, A. Allelic diversity and its implications for the rate of adaptation. Genetics 2013, 195, 1373–1384. [Google Scholar] [CrossRef] [Green Version]
- Marconi, G.; Ferradini, N.; Russi, L.; Concezzi, L.; Veronesi, F.; Albertini, E. Genetic characterization of the apple germplasm collection in central Italy: The value of local varieties. Front. Plant Sci. 2018, 9, 1460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aranzana, M.J.; Decroocq, V.; Dirlewanger, E.; Eduardo, I.; Gao, Z.S.; Gasic, K.; Iezzoni, A.; Jung, S.; Peace, C.; Prieto, H.; et al. Prunus genetics and applications after de novo genome sequencing: Achievements and prospects. Hortic. Res. 2019, 6, 58. [Google Scholar] [CrossRef] [Green Version]
- Urrestarazu, J.; Errea, P.; Miranda, C.; Santesteban, L.G.; Pina, A. Genetic diversity of Spanish Prunus domestica L. germplasm reveals a complex genetic structure underlying. PLoS ONE 2018, 13, e0195591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrás-Sexto, C.; O’Flanagan, P. Small-holdings and sustainable family farming in Galicia and Ireland. A comparative case study. Norois 2012, 224, 61–76. [Google Scholar] [CrossRef] [Green Version]
- Pereira-Lorenzo, S.; Urrestarazu, J.; Ramos-Cabrer, A.M.; Miranda, C.; Pina, A.; Dapena, E.; Moreno, M.A.; Errea, P.; Llamero, N.; Díaz-Hernández, M.B.; et al. Analysis of the genetic diversity and structure of the Spanish apple genetic resources suggests the existence of an Iberian genepool. Ann. Appl. Biol. 2017, 171, 424–440. [Google Scholar] [CrossRef]
- Goded, S.; Ekroos, J.; Domínguez, J.; Guitián, J.A.; Smith, H.G. Effects of organic farming on bird diversity in North-West Spain. Agric. Ecosyst. Environ. 2018, 257, 60–67. [Google Scholar] [CrossRef]
- FAO. Contributing to Food Security and Sustainability in a Changing World; FAO: Rome, Italy, 2011; ISBN 9789251067482. [Google Scholar]
- Miloševic, T.; Miloševic, N. Plum (Prunus spp.) Breeding. In Advances in Plant Breeding Strategies: Fruits; Al-Khayri, J.M., Jain, S.M., Johnson, D.V., Eds.; Springer: Berlin/Heidelberg, Germany, 2018; pp. 165–215. [Google Scholar]
- Dulloo, M.E.; Hunter, D.; Borelli, T. Ex situ and in situ conservation of agricultural biodiversity: Major advances and research needs. Not. Bot. Horti Agrobot. Cluj-Napoca 2010, 38, 123–135. [Google Scholar] [CrossRef]
- Postman, J.; Hummer, K.; Stover, E.; Krueger, R.; Forsline, P.; Grauke, L.J.; Zee, F.; Ayala-Silva, T.; Irish, B. Fruit and Nut Genebanks in the U.S. National Plant Germplasm System. HortScience 2006, 41, 1188–1194. [Google Scholar] [CrossRef] [Green Version]
- Pence, V.C. In Vitro Methods and the Challenge of Exceptional Species for Target 8 of the Global Strategy for Plant Conservation 1. Ann. Mo. Bot. Gard. 2013, 99, 214–220. [Google Scholar] [CrossRef]
- Druart, P.; Gruselle, R. Plum (Prunus domestica). In Biotechnology in Agriculture and Forestry, Vol 1: Trees I; Bajaj, Y., Ed.; Springer: Berlin/Heidelberg, Germany, 1986; pp. 130–154. ISBN 3-540-15581-3. [Google Scholar]
- Druart, P. In Vitro Culture and Micropropagation of Plum (Prunus spp.). In High-Tech and Micropropagation II Biotechnology in Agriculture and Forestry, Vol 18; Bajaj, Y.P.S., Ed.; Springer: Berlin/Heidelberg, Germany, 1992; pp. 279–303. [Google Scholar]
- Andreu, P.; Marín, J.A. In vitro culture establishment and multiplication of the Prunus rootstock ‘Adesoto 101′ (P. insititia L.) as affected by the type of propagation of the donor plant and by the culture medium composition. Sci. Hortic. 2005, 106, 258–267. [Google Scholar] [CrossRef] [Green Version]
- Ruzic, D.; Vujovic, T.; Cerovic, R. In vitro preservation of autochthonous plum genotypes. Bulg. J. Agric. Sci. 2012, 18, 55–62. [Google Scholar]
- Wolella, E.K. Surface sterilization and in vitro propagation of Prunus domestica L. cv. Stanley using axillary buds as explants. J. Biotech Res. 2017, 8, 18–26. [Google Scholar]
- Alburquerque, N.; Faize, L.; Burgos, L. Silencing of Agrobacterium tumefaciens oncogenes ipt and iaaM induces resistance to crown gall disease in plum but not in apricot. Pest Manag. Sci. 2017, 73, 2163–2173. [Google Scholar] [CrossRef] [PubMed]
- Vujović, T.; Jevremović, D.; Marjanović, T.; Glišić, I. In vitro propagation and medium-term conservation of autochthonous plum cultivar “Crvena Ranka”. Acta Agric. Serbica 2020, 25, 141–147. [Google Scholar] [CrossRef]
- Etienne, H.; Berthouly, M. Temporary immersion systems in plant micropropagation. Plant Cell Tissue Organ Cult. 2002, 69, 215–231. [Google Scholar] [CrossRef]
- Vidal, N.; Sánchez, C. Use of bioreactor systems in the propagation of forest trees. Eng. Life Sci. 2019, 19, 896–915. [Google Scholar] [CrossRef] [Green Version]
- Sanchez, M.C.; Vieitez, A.M. In vitro morphogenetic competence of basal sprouts and crown branches of mature chestnut. Tree Physiol. 1991, 8, 59–70. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A Revised Medium for Rapid Growth and Bio Assays with Tobacco Tissue Cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Cuenca Valera, B.; Aldrey Villar, A.; Blanco Beiro, B.; Vidal González, N. Use of a Continuous Immersion System (CIS) for micropropagation of chestnut in photoautotrophic and photomixotrophic conditions. In Woody Plant Production Integrating Genetic and Vegetative Propagation Technologies, Proceedings of the 3rd International Conference of the IUFRO Unit 2.09.02, Vitoria-Gasteiz, Spain, 8–12 September 2014; Park, Y.S., Bonga, J.M., Eds.; IUFRO: Vienna, Austria, 2015; pp. 112–116. [Google Scholar]
- Miller, G.L. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Lindsay, H. A colorimetric estimation of reducing sugars in potatoes with 3,5-dinitrosalicylic acid. Potato Res. 1973, 16, 176–179. [Google Scholar] [CrossRef]
- Yemm, E.W.; Willis, A.J. The Estimation of Carbohydrates in Plant Extracts by Anthrone. Biochem. J. 1954, 57, 508–514. [Google Scholar] [CrossRef] [Green Version]
- Wellburn, A.R. The Spectral Determination of Chlorophylls a and b, as well as Total Carotenoids, Using Various Solvents with Spectrophotometers of Different Resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Díaz, J.; Bernal, A.; Pomar, F.; Merino, F. Induction of shikimate dehydrogenase and peroxidase in pepper (Capsicum annuum L.) seedlings in response to copper stress and its relation to lignification. Plant Sci. 2001, 161, 179–188. [Google Scholar] [CrossRef]
- Singleton, V.; Rossi, J. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–168. [Google Scholar]
- Blois, M.S. Antioxidant Determinations by the Use of a Stable Free Radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Murch, S.J.; Liu, C.; Romero, R.M.; Saxena, P.K. In vitro Culture and Temporary Immersion Bioreactor Production of Crescentia cujete. Plant Cell Tissue Organ Cult. 2004, 78, 63–68. [Google Scholar] [CrossRef]
- McAlister, B.; Finnie, J.; Watt, M.; Blakeway, F. Use of the temporary immersion bioreactor system (RITA®) for production of commercial Eucalyptus clones in Mondi Forests (SA). Plant Cell Tissue Organ Cult. 2005, 81, 347–358. [Google Scholar] [CrossRef]
- Zhu, L.-H.; Li, X.-Y.; Welander, M. Optimisation of growing conditions for the apple rootstock M26 grown in RITA containers using temporary immersion principle. Plant Cell Tissue Organ Cult. 2005, 81, 313–318. [Google Scholar] [CrossRef]
- Quiala, E.; Cañal, M.J.; Meijón, M.; Rodríguez, R.; Chávez, M.; Valledor, L.; de Feria, M.; Barbón, R. Morphological and physiological responses of proliferating shoots of teak to temporary immersion and BA treatments. Plant Cell Tissue Organ Cult. 2012, 109, 223–234. [Google Scholar] [CrossRef] [Green Version]
- Aguilar, M.E.; Garita, K.; Kim, Y.W.; Kim, J.-A.; Moon, H.K. Simple Protocol for the Micropropagation of Teak (Tectona grandis Linn.) in Semi-Solid and Liquid Media in RITA Bioreactors and ex Vitro Rooting. Am. J. Plant Sci. 2019, 10, 1121–1141. [Google Scholar] [CrossRef] [Green Version]
- Akdemir, H.; Süzerer, V.; Onay, A.; Tilkat, E.; Ersali, Y.; Çiftçi, Y.O. Micropropagation of the pistachio and its rootstocks by temporary immersion system. Plant Cell Tissue Organ Cult. 2014, 117, 65–76. [Google Scholar] [CrossRef]
- Vidal, N.; Blanco, B.; Cuenca, B. A temporary immersion system for micropropagation of axillary shoots of hybrid chestnut. Plant Cell Tissue Organ Cult. 2015, 123, 229–243. [Google Scholar] [CrossRef]
- Latawa, J.; Shukla, M.R.; Saxena, P.K. An efficient temporary immersion system for micropropagation of hybrid hazelnut. Botany 2016, 94, 1–8. [Google Scholar] [CrossRef]
- Luna, C.V.; Gonzalez, A.M.; Mroginski, L.A.; Sansberro, P.A. Anatomical and histological features of Ilex paraguariensis leaves under different in vitro shoot culture systems. Plant Cell Tissue Organ Cult. 2017, 129, 457–467. [Google Scholar] [CrossRef]
- Regueira, M.; Rial, E.; Blanco, B.; Bogo, B.; Aldrey, A.; Correa, B.; Varas, E.; Sánchez, C.; Vidal, N. Micropropagation of axillary shoots of Salix viminalis using a temporary immersion system. Trees 2018, 32, 61–71. [Google Scholar] [CrossRef]
- Benelli, C.; De Carlo, A. In vitro multiplication and growth improvement of Olea europaea L. cv Canino with temporary immersion system (Plantform™). 3 Biotech 2018, 8, 317. [Google Scholar] [CrossRef]
- San José, M.C.; Blázquez, N.; Cernadas, M.J.; Janeiro, L.V.; Cuenca, B.; Sánchez, C.; Vidal, N. Temporary immersion systems to improve alder micropropagation. Plant Cell Tissue Organ Cult. 2020, 143, 265–275. [Google Scholar] [CrossRef]
- Lotfi, M.; Bayoudh, C.; Werbrouck, S.; Mars, M. Effects of meta–topolin derivatives and temporary immersion on hyperhydricity and in vitro shoot proliferation in Pyrus communis. Plant Cell Tissue Organ Cult. 2020, 143, 499–505. [Google Scholar] [CrossRef]
- Georgiev, V.; Schumann, A.; Pavlov, A.; Bley, T. Temporary immersion systems in plant biotechnology. Eng. Life Sci. 2014, 14, 607–621. [Google Scholar] [CrossRef]
- Xiao, Y.; Niu, G.; Kozai, T. Development and application of photoautotrophic micropropagation plant system. Plant Cell Tissue Organ Cult. 2011, 105, 149–158. [Google Scholar] [CrossRef]
- Watt, M.P. The status of temporary immersion system (TIS) technology for plant micropropagation. Afr. J. Biotechnol. 2012, 11, 14036–14043. [Google Scholar] [CrossRef]
- Kirdmanee, C.; Kitaya, Y.; Kozai, T. Effects of CO2 enrichment and supporting materialin vitro on photoautotrophic growth ofEucalyptus plantletsin vitro andex vitro. Vitr. Cell. Dev. Biol.-Plant 1995, 31, 144–149. [Google Scholar] [CrossRef]
- Zobayed, S.M.A.; Afreen, F.; Kozai, T. Physiology of Eucalyptus plantlets grown photoautotrophically in a scaled-up vessel. Vitr. Cell. Dev. Biol.-Plant 2001, 37, 807–813. [Google Scholar] [CrossRef]
- Zobayed, S. Mass Propagation of Eucalyptus camaldulensis in a Scaled-up Vessel Under In Vitro Photoautotrophic Condition. Ann. Bot. 2000, 85, 587–592. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, M.; Giang, D.T.T.; Murakami, A. Application of a novel disposable film culture system to photoautotrophic micropropagation of Eucalyptus uro-grandis (Urophylia × grandis). Vitr. Cell. Dev. Biol.-Plant 2005, 41, 173–180. [Google Scholar] [CrossRef]
- Fuljahn, S.; Tantau, H.-J. Process engineering as a means of regulating the microclimate in a photoautotrophic in vitro culture. Acta Hortic. 2009, 817, 143–150. [Google Scholar] [CrossRef]
- Arencibia, A.D.; Gómez, A.; Poblete, M.; Vergara, C. High-performance micropropagation of dendroenergetic poplar hybrids in photomixotrophic Temporary Immersion Bioreactors (TIBs). Ind. Crops Prod. 2017, 96, 102–109. [Google Scholar] [CrossRef]
- García-Ramírez, Y.; Barrera, G.P.; Freire-Seijo, M.; Barbón, R.; Concepción-Hernández, M.; Mendoza-Rodríguez, M.F.; Torres-García, S. Effect of sucrose on physiological and biochemical changes of proliferated shoots of Bambusa vulgaris Schrad. Ex Wendl in temporary immersion. Plant Cell Tissue Organ Cult. 2019, 137, 239–247. [Google Scholar] [CrossRef]
- Gago, D.; Vilavert, S.; Bernal, M.Á.; Sánchez, C.; Aldrey, A.; Vidal, N. The Effect of Sucrose Supplementation on the Micropropagation of Salix viminalis L. Shoots in Semisolid Medium and Temporary Immersion Bioreactors. Forests 2021, 12, 1408. [Google Scholar] [CrossRef]
- Greenboim-Wainberg, Y.; Maymon, I.; Borochov, R.; Alvarez, J.; Olszewski, N.; Ori, N.; Eshed, Y.; Weiss, D. Cross Talk between Gibberellin and Cytokinin: The Arabidopsis GA Response Inhibitor SPINDLY Plays a Positive Role in Cytokinin Signaling. Plant Cell 2005, 17, 92–102. [Google Scholar] [CrossRef] [Green Version]
- Silva, L.A.S.; Costa, A.D.O.; Batista, D.S.; Silva, M.L.D.; Costa Netto, A.P.D.; Rocha, D.I. Exogenous gibberellin and cytokinin in a novel system for in vitro germination and development of African iris (Dietes bicolor). Rev. Ceres 2020, 67, 402–409. [Google Scholar] [CrossRef]
- Deng, Z.; Chu, J.; Wang, Q.; Wang, L. Effect of different carbon sources on the accumulation of carbohydrate, nutrient absorption and the survival rate of Chinese Ash (Fraxinus mandshurica) explants in vitro. Afr. J. Agric. Res. 2012, 7, 3111–3119. [Google Scholar] [CrossRef]
- Kim, N.-Y.; Hwang, H.-D.; Kim, J.-H.; Kwon, B.-M.; Kim, D.; Park, S.-Y. Efficient production of virus-free apple plantlets using the temporary immersion bioreactor system. Hortic. Environ. Biotechnol. 2020, 61, 779–785. [Google Scholar] [CrossRef]
- Sha Valli Khan, P.S.; Kozai, T.; Nguyen, Q.T.; Kubota, C.; Dhawan, V. Growth and Water Relations of Paulownia fortunei Under Photomixotrophic and Photoautotrophic Conditions. Biol. Plant. 2003, 46, 161–166. [Google Scholar] [CrossRef]
- Mosaleeyanon, K.; Cha-um, S.; Kirdmanee, C. Enhanced growth and photosynthesis of rain tree (Samanea saman Merr.) plantlets in vitro under a CO2-enriched condition with decreased sucrose concentrations in the medium. Sci. Hortic. 2004, 103, 51–63. [Google Scholar] [CrossRef]
- Cha-um, S.; Chanseetis, C.; Chintakovid, W.; Pichakum, A.; Supaibulwatana, K. Promoting root induction and growth of in vitro macadamia (Macadamia tetraphylla L. ‘Keaau’) plantlets using CO2-enriched photoautotrophic conditions. Plant Cell Tissue Organ Cult. 2011, 106, 435–444. [Google Scholar] [CrossRef]
- Fortini, E.A.; Batista, D.S.; Mamedes-Rodrigues, T.C.; Felipe, S.H.S.; Correia, L.N.F.; Chagas, K.; Silva, P.O.; Rocha, D.I.; Otoni, W.C. Gas exchange rates and sucrose concentrations affect plant growth and production of flavonoids in Vernonia condensata grown in vitro. Plant Cell Tissue Organ Cult. 2021, 144, 593–605. [Google Scholar] [CrossRef]
- Hassankhah, A.; Vahdati, K.; Lotfi, M.; Mirmasoumi, M.; Preece, J.; Assareh, M.-H. Effects of Ventilation and Sucrose Concentrations on the Growth and Plantlet Anatomy of Micropropagated Persian Walnut Plants. Int. J. Hort. Sci. Technol. 2014, 1, 111–120. [Google Scholar]
- Mingozzi, M.; Montello, P.; Merkle, S. Adventitious shoot regeneration from leaf explants of eastern cottonwood (Populus deltoides) cultured under photoautotrophic conditions. Tree Physiol. 2009, 29, 333–343. [Google Scholar] [CrossRef]
- Saldanha, C.W.; Otoni, C.G.; Notini, M.M.; Kuki, K.N.; da Cruz, A.C.F.; Neto, A.R.; Dias, L.L.C.; Otoni, W.C. A CO2-enriched atmosphere improves in vitro growth of Brazilian ginseng [Pfaffia glomerata (Spreng.) Pedersen]. Vitr. Cell. Dev. Biol.-Plant 2013, 49, 433–444. [Google Scholar] [CrossRef]
- Ševčíková, H.; Lhotáková, Z.; Hamet, J.; Lipavská, H. Mixotrophic in vitro cultivations: The way to go astray in plant physiology. Physiol. Plant. 2019, 167, 365–377. [Google Scholar] [CrossRef]
- Badr, A.; Angers, P.; Desjardins, Y. Metabolic profiling of photoautotrophic and photomixotrophic potato plantlets (Solanum tuberosum) provides new insights into acclimatization. Plant Cell Tissue Organ Cult. 2011, 107, 13–24. [Google Scholar] [CrossRef]
- Lucchesini, M.; Monteforti, G.; Mensuali-Sodi, A.; Serra, G. Leaf ultrastructure, photosynthetic rate and growth of myrtle plantlets under different in vitro culture conditions. Biol. Plant. 2006, 50, 161–168. [Google Scholar] [CrossRef]
- Sáez, P.L.; Bravo, L.A.; Latsague, M.I.; Sánchez, M.E.; Ríos, D.G. Increased light intensity during in vitro culture improves water loss control and photosynthetic performance of Castanea sativa grown in ventilated vessels. Sci. Hortic. 2012, 138, 7–16. [Google Scholar] [CrossRef]
- Gaspar, T.; Franck, T.; Bisbis, B.; Kevers, C.; Jouve, L.; Hausman, J.F.; Dommes, J. Concepts in plant stress physiology. Application to plant tissue cultures. Plant Growth Regul. 2002, 37, 263–285. [Google Scholar] [CrossRef]
- Ahmad, P.; Jaleel, C.A.; Salem, M.A.; Nabi, G.; Sharma, S. Roles of enzymatic and nonenzymatic antioxidants in plants during abiotic stress. Crit. Rev. Biotechnol. 2010, 30, 161–175. [Google Scholar] [CrossRef]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive Oxygen Species, Oxidative Damage, and Antioxidative Defense Mechanism in Plants under Stressful Conditions. J. Bot. 2012, 2012, 217037. [Google Scholar] [CrossRef] [Green Version]
- Gharibi, S.; Tabatabaei, B.E.S.; Saeidi, G.; Goli, S.A.H. Effect of Drought Stress on Total Phenolic, Lipid Peroxidation, and Antioxidant Activity of Achillea Species. Appl. Biochem. Biotechnol. 2016, 178, 796–809. [Google Scholar] [CrossRef]
- García-Ramírez, Y.; Gonzáles, M.G.; Mendoza, E.Q.; Seijo, M.F.; Cárdenas, M.L.O.; Moreno-Bermúdez, L.J.; Ribalta, O.H. Effect of BA Treatments on Morphology and Physiology of Proliferated Shoots of Bambusa vulgaris Schrad. Ex Wendl in Temporary Immersion. Am. J. Plant Sci. 2014, 05, 205–211. [Google Scholar] [CrossRef] [Green Version]
- Malik, M.; Warchoł, M.; Pawłowska, B. Liquid culture systems affect morphological and biochemical parameters during Rosa canina plantlets in vitro production. Not. Bot. Horti Agrobot. Cluj-Napoca 2018, 46, 58–64. [Google Scholar] [CrossRef] [Green Version]
- Regni, L.; Del Buono, D.; Micheli, M.; Facchin, S.L.; Tolisano, C.; Proietti, P. Effects of Biogenic ZnO Nanoparticles on Growth, Physiological, Biochemical Traits and Antioxidants on Olive Tree In Vitro. Horticulturae 2022, 8, 161. [Google Scholar] [CrossRef]
- Newel, C.; Growns, D.; McComb, J. The influence of medium aeration on in vitro rooting of Australian plant microcuttings. Plant Cell Tissue Organ Cult. 2003, 75, 131–142. [Google Scholar] [CrossRef]
- Dutta Gupta, S.; Prasad, V.S.S. Matrix-Supported Liquid Culture Systems for Efficient Micropropagation of Floricultural Plants. In Floriculture, Ornamental and Plant Biotechnology; Teixeira da Silva, J., Ed.; Global Science Books: Kagawa, Japan, 2006; pp. 487–495. [Google Scholar]
- Afreen-Zobayed, F.; Zobayed, S.M.A.; Kubota, C.; Kozai, T.; Hasegawa, O. Supporting material affects the growth and development of in vitro sweet potato plantlets cultured photoautotrophically. Vitr. Cell. Dev. Biol.-Plant 1999, 35, 470–474. [Google Scholar] [CrossRef]
- Maner, L.; Merkle, S. Polymerized peat plugs improve American chestnut somatic embryo germination in vitro. J. Am. Chestnut Found. 2010, 24, 16. [Google Scholar]
- Cuenca, B.; Sánchez, C.; Aldrey, A.; Bogo, B.; Blanco, B.; Correa, B.; Vidal, N. Micropropagation of axillary shoots of hybrid chestnut (Castanea sativa × C. crenata) in liquid medium in a continuous immersion system. Plant Cell Tissue Organ Cult. 2017, 131, 307–320. [Google Scholar] [CrossRef]
- Kodym, A.; Leeb, C.J. Back to the roots: Protocol for the photoautotrophic micropropagation of medicinal Cannabis. Plant Cell Tissue Organ Cult. 2019, 138, 399–402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adelberg, J.; Naylor-Adelberg, J.; Miller, S.; Gasic, K.; Schnabel, G.; Bryson, P.; Saski, C.; Parris, S.; Reighard, G. In vitro co-culture system for Prunus spp. and Armillaria mellea in phenolic foam rooting matric. Vitr. Cell. Dev. Biol.-Plant 2021, 57, 387–397. [Google Scholar] [CrossRef]
- Gianní, S.; Sottile, F. In vitro storage of plum germplasm by slow growth. Hortic. Sci. 2016, 42, 61–69. [Google Scholar] [CrossRef] [Green Version]
- Godoy, S.; Tapia, E.; Seit, P.; Andrade, D.; Sánchez, E.; Andrade, P.; Almeida, A.M.; Prieto, H. Temporary immersion systems for the mass propagation of sweet cherry cultivars and cherry rootstocks: Development of a micropropagation procedure and effect of culture conditions on plant quality. Vitr. Cell. Dev. Biol.-Plant 2017, 53, 494–504. [Google Scholar] [CrossRef]
- Zare Khafri, A.; Solouki, M.; Zarghami, R.; Fakheri, B.; Mahdinezhad, N.; Naderpour, M. In vitro propagation of three Iranian apricot cultivars. Vitr. Cell. Dev. Biol.-Plant 2021, 57, 102–117. [Google Scholar] [CrossRef]
- Damiano, C.; Monticelli, S.; La Starza, S.R.; Gentile, A.; Frattarelli, A. Temperate fruit plant propagation through temporary immersion. Acta Hortic. 2003, 625, 193–200. [Google Scholar] [CrossRef]
- Cantabella, D.; Mendoza, C.R.; Teixidó, N.; Vilaró, F.; Torres, R.; Dolcet-Sanjuan, R. GreenTray® TIS bioreactor as an effective in vitro culture system for the micropropagation of Prunus spp. rootstocks and analysis of the plant-PGPMs interactions. Sci. Hortic. 2022, 291, 110622. [Google Scholar] [CrossRef]












| Culture System | NS | SL (mm) | LL (mm) | LW (mm) |
|---|---|---|---|---|
| Jars | 5.3 ± 0.27 b | 18.9 ± 0.68 b | 15.3 ± 0.63 c | 6.9 ± 0.30 b |
| RITA© (3 immersions/day) | 7.7 ± 0.59 a | 22.3 ± 0.70 a | 21.5 ± 0.51 b | 9.3 ± 0.26 a |
| RITA© (6 immersions/day) | 8.7 ± 0.61 a | 24.5 ± 0.83 a | 23.8 ± 0.69 a | 10.1 ± 0.41 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gago, D.; Sánchez, C.; Aldrey, A.; Christie, C.B.; Bernal, M.Á.; Vidal, N. Micropropagation of Plum (Prunus domestica L.) in Bioreactors Using Photomixotrophic and Photoautotrophic Conditions. Horticulturae 2022, 8, 286. https://doi.org/10.3390/horticulturae8040286
Gago D, Sánchez C, Aldrey A, Christie CB, Bernal MÁ, Vidal N. Micropropagation of Plum (Prunus domestica L.) in Bioreactors Using Photomixotrophic and Photoautotrophic Conditions. Horticulturae. 2022; 8(4):286. https://doi.org/10.3390/horticulturae8040286
Chicago/Turabian StyleGago, Diego, Conchi Sánchez, Anxela Aldrey, Colin Bruce Christie, María Ángeles Bernal, and Nieves Vidal. 2022. "Micropropagation of Plum (Prunus domestica L.) in Bioreactors Using Photomixotrophic and Photoautotrophic Conditions" Horticulturae 8, no. 4: 286. https://doi.org/10.3390/horticulturae8040286






