Membrane Hormone Receptors and Their Signaling Pathways as Targets for Endocrine Disruptors
Abstract
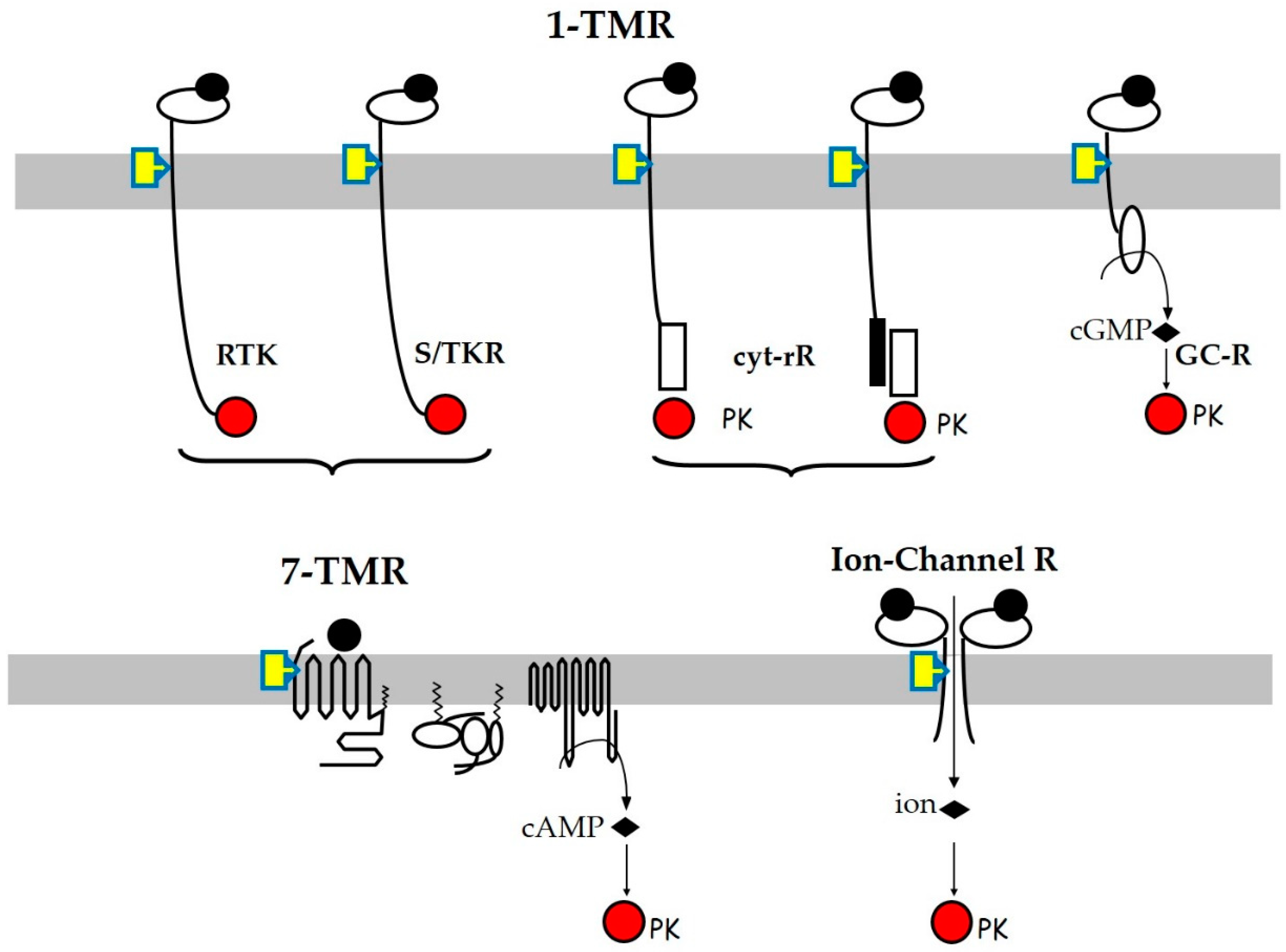
1. Introduction
2. Tyr-Kinase Receptors (RTKs)
3. Ser/Thr-Kinase Receptors (STKRs)
4. Cytokine and Related Receptors
5. Seven-Transmembrane Domain Receptors (7TM-R/GPCR)
6. Guanyl Cyclase Receptors
7. Ion-Channel Receptors
8. Non-Receptor Membrane Proteins
9. Discussion
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Heindel, J.J.; Blumberg, B.; Cave, M.; Machtinger, R.; Mantovani, A.; Mendez, M.A.; Nadal, A.; Palanza, P.; Panzica, G.; Sargis, R.; et al. Metabolism disrupting chemicals and metabolic disorders. Reprod. Toxicol. 2017, 68, 3–33. [Google Scholar] [CrossRef] [PubMed]
- Toporova, L.; Balaguer, P. Nuclear receptors are the major targets of endocrine disrupting chemicals. Mol. Cell. Endocrinol. 2020, 502, 110665. [Google Scholar] [CrossRef] [PubMed]
- Combarnous, Y.; Nguyen, T.M.D. Comparative Overview of the Mechanisms of Action of Hormones and Endocrine Disruptor Compounds. Toxics 2019, 7, 5. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wu, J.; Chen, Q.; Tan, H.; Huang, F.; Guo, J.; Zhang, X.; Yu, H.; Shi, W. Allosteric binding on nuclear receptors: Insights on screening of non-competitive endocrine-disrupting chemicals. Environ. Int. 2022, 159, 107009. [Google Scholar] [CrossRef] [PubMed]
- Lamb, J.C.T.; Boffetta, P.; Foster, W.G.; Goodman, J.E.; Hentz, K.L.; Rhomberg, L.R.; Staveley, J.; Swaen, G.; Van Der Kraak, G.; Williams, A.L. Comments on the opinions published by Bergman et al. (2015) on Critical Comments on the WHO-UNEP State of the Science of Endocrine Disrupting Chemicals (Lamb et al., 2014). Regul. Toxicol. Pharmacol. 2015, 73, 754–757. [Google Scholar] [CrossRef]
- Bergman, Å.; Becher, G.; Blumberg, B.; Bjerregaard, P.; Bornman, R.; Brandt, I.; Casey, S.C.; Frouin, H.; Giudice, L.C.; Heindel, J.J. Manufacturing doubt about endocrine disrupter science–A rebuttal of industry-sponsored critical comments on the UNEP/WHO report “State of the Science of Endocrine Disrupting Chemicals 2012”. Regul. Toxicol. Pharmacol. 2015, 73, 1007–1017. [Google Scholar] [CrossRef]
- Lamb, J.C.t.; Boffetta, P.; Foster, W.G.; Goodman, J.E.; Hentz, K.L.; Rhomberg, L.R.; Staveley, J.; Swaen, G.; Van Der Kraak, G.; Williams, A.L. Critical comments on the WHO-UNEP State of the Science of Endocrine Disrupting Chemicals—2012. Regul. Toxicol. Pharmacol. 2014, 69, 22–40. [Google Scholar] [CrossRef]
- La Merrill, M.A.; Vandenberg, L.N.; Smith, M.T.; Goodson, W.; Browne, P.; Patisaul, H.B.; Guyton, K.Z.; Kortenkamp, A.; Cogliano, V.J.; Woodruff, T.J.; et al. Consensus on the key characteristics of endocrine-disrupting chemicals as a basis for hazard identification. Nat. Rev. Endocrinol. 2020, 16, 45–57. [Google Scholar] [CrossRef]
- Motlagh, H.N.; Wrabl, J.O.; Li, J.; Hilser, V.J. The ensemble nature of allostery. Nature 2014, 508, 331–339. [Google Scholar] [CrossRef]
- Bhat, A.S.; Dustin Schaeffer, R.; Kinch, L.; Medvedev, K.E.; Grishin, N.V. Recent advances suggest increased influence of selective pressure in allostery. Curr. Opin. Struct. Biol. 2020, 62, 183–188. [Google Scholar] [CrossRef]
- Fasciani, I.; Petragnano, F.; Aloisi, G.; Marampon, F.; Carli, M.; Scarselli, M.; Maggio, R.; Rossi, M. Allosteric Modulators of G Protein-Coupled Dopamine and Serotonin Receptors: A New Class of Atypical Antipsychotics. Pharmaceuticals 2020, 13, 388. [Google Scholar] [CrossRef] [PubMed]
- Ni, D.; Wei, J.; He, X.; Rehman, A.U.; Li, X.; Qiu, Y.; Pu, J.; Lu, S.; Zhang, J. Discovery of cryptic allosteric sites using reversed allosteric communication by a combined computational and experimental strategy. Chem. Sci. 2021, 12, 464–476. [Google Scholar] [CrossRef] [PubMed]
- Bindslev, N. Allosteric transition: A comparison of two models. BMC Pharmacol. Toxicol. 2013, 14, 4. [Google Scholar] [CrossRef]
- Yang, C.-Y. Identification of Potential Small Molecule Allosteric Modulator Sites on IL-1R1 Ectodomain Using Accelerated Conformational Sampling Method. PLoS ONE 2015, 10, e0118671. [Google Scholar] [CrossRef] [PubMed]
- Damian, M.; Louet, M.; Gomes, A.A.S.; M’Kadmi, C.; Denoyelle, S.; Cantel, S.; Mary, S.; Bisch, P.M.; Fehrentz, J.-A.; Catoire, L.J.; et al. Allosteric modulation of ghrelin receptor signaling by lipids. Nat. Commun. 2021, 12, 3938. [Google Scholar] [CrossRef]
- Brannigan, G.; Henin, J.; Law, R.; Eckenhoff, R.; Klein, M.L. Embedded cholesterol in the nicotinic acetylcholine receptor. Proc. Natl. Acad. Sci. USA 2008, 105, 14418–14423. [Google Scholar] [CrossRef]
- Hardesty, J.E.; Al-Eryani, L.; Wahlang, B.; Falkner, K.C.; Shi, H.; Jin, J.; Vivace, B.J.; Ceresa, B.P.; Prough, R.A.; Cave, M.C. Epidermal Growth Factor Receptor Signaling Disruption by Endocrine and Metabolic Disrupting Chemicals. Toxicol. Sci. 2018, 162, 622–634. [Google Scholar] [CrossRef]
- Küblbeck, J.; Niskanen, J.; Honkakoski, P. Metabolism-Disrupting Chemicals and the Constitutive Androstane Receptor CAR. Cells 2020, 9, 2306. [Google Scholar] [CrossRef]
- Hinke, S.A.; Cieniewicz, A.M.; Kirchner, T.; D’Aquino, K.; Nanjunda, R.; Aligo, J.; Perkinson, R.; Cooper, P.; Boayke, K.; Chiu, M.L.; et al. Unique pharmacology of a novel allosteric agonist/sensitizer insulin receptor monoclonal antibody. Mol. Metab. 2018, 10, 87–99. [Google Scholar] [CrossRef]
- Yunn, N.-O.; Koh, A.; Han, S.; Lim, J.H.; Park, S.; Lee, J.; Kim, E.; Jang, S.K.; Berggren, P.-O.; Ryu, S.H. Agonistic aptamer to the insulin receptor leads to biased signaling and functional selectivity through allosteric modulation. Nucleic Acids Res. 2015, 43, 7688–7701. [Google Scholar] [CrossRef]
- De Smet, F.; Christopoulos, A.; Carmeliet, P. Allosteric targeting of receptor tyrosine kinases. Nat. Biotechnol. 2014, 32, 1113–1120. [Google Scholar] [CrossRef] [PubMed]
- Marsiglia, W.M.; Katigbak, J.; Zheng, S.; Mohammadi, M.; Zhang, Y.; Traaseth, N.J. A Conserved Allosteric Pathway in Tyrosine Kinase Regulation. Structure 2019, 27, 1308–1315.e1303. [Google Scholar] [CrossRef] [PubMed]
- Sargis, R.M.; Neel, B.A.; Brock, C.O.; Lin, Y.; Hickey, A.T.; Carlton, D.A.; Brady, M.J. The novel endocrine disruptor tolylfluanid impairs insulin signaling in primary rodent and human adipocytes through a reduction in insulin receptor substrate-1 levels. Biochim. Biophys. Acta 2012, 1822, 952–960. [Google Scholar] [CrossRef] [PubMed]
- Chaikuad, A.; Bullock, A.N. Structural Basis of Intracellular TGF-β Signaling: Receptors and Smads. Cold Spring Harb. Perspect. Biol. 2016, 8, a022111. [Google Scholar] [CrossRef]
- Cui, X.; Shang, S.; Lv, X.; Zhao, J.; Qi, Y.; Liu, Z. Perspectives of small molecule inhibitors of activin receptor-like kinase in anti-tumor treatment and stem cell differentiation (Review). Mol. Med. Rep. 2019, 19, 5053–5062. [Google Scholar] [CrossRef]
- Seth, B.; Yadav, A.; Agarwal, S.; Tiwari, S.K.; Chaturvedi, R.K. Inhibition of the transforming growth factor-beta/SMAD cascade mitigates the anti-neurogenic effects of the carbamate pesticide carbofuran. J. Biol. Chem. 2017, 292, 19423–19440. [Google Scholar] [CrossRef]
- Huang, T.; Ditzel, E.J.; Perrera, A.B.; Broka, D.M.; Camenisch, T.D. Arsenite Disrupts Zinc-Dependent TGFβ2-SMAD Activity During Murine Cardiac Progenitor Cell Differentiation. Toxicol. Sci. 2015, 148, 409–420. [Google Scholar] [CrossRef][Green Version]
- Rizk, S.S.; Kouadio, J.-L.K.; Szymborska, A.; Duguid, E.M.; Mukherjee, S.; Zheng, J.; Clevenger, C.V.; Kossiakoff, A.A. Engineering synthetic antibody binders for allosteric inhibition of prolactin receptor signaling. Cell Commun. Signal. 2015, 13, 1. [Google Scholar] [CrossRef]
- Schjenken, J.E.; Green, E.S.; Overduin, T.S.; Mah, C.Y.; Russell, D.L.; Robertson, S.A. Endocrine Disruptor Compounds-A Cause of Impaired Immune Tolerance Driving Inflammatory Disorders of Pregnancy? Front. Endocrinol. 2021, 12, 607539. [Google Scholar] [CrossRef]
- Kovats, S. Estrogen receptors regulate innate immune cells and signaling pathways. Cell. Immunol. 2015, 294, 63–69. [Google Scholar] [CrossRef]
- Schamel, W.W.A.; Alarcon, B.; Höfer, T.; Minguet, S. The Allostery Model of TCR Regulation. J. Immunol. 2017, 198, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-W.; Ha, S.K.; Kim, Y. Bisphenol A disrupts inflammatory responses via Nod-like receptor protein 3 pathway in macrophages. Appl. Biol. Chem. 2020, 63, 78. [Google Scholar] [CrossRef]
- Lo, C.H.; Huber, E.C.; Sachs, J.N. Conformational states of TNFR1 as a molecular switch for receptor function. Protein Sci. 2020, 29, 1401–1415. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, J.; Porter, J.; Kroeplien, B.; Norman, T.; Rapecki, S.; Davis, R.; McMillan, D.; Arakaki, T.; Burgin, A.; Fox Iii, D.; et al. Small molecules that inhibit TNF signalling by stabilising an asymmetric form of the trimer. Nat. Commun. 2019, 10, 5795. [Google Scholar] [CrossRef] [PubMed]
- Lightwood, D.J.; Munro, R.J.; Porter, J.; McMillan, D.; Carrington, B.; Turner, A.; Scott-Tucker, A.; Hickford, E.S.; Schmidt, A.; Fox, D.; et al. A conformation-selective monoclonal antibody against a small molecule-stabilised signalling-deficient form of TNF. Nat. Commun. 2021, 12, 583. [Google Scholar] [CrossRef] [PubMed]
- Olson, K.M.; Traynor, J.R.; Alt, A. Allosteric Modulator Leads Hiding in Plain Site: Developing Peptide and Peptidomimetics as GPCR Allosteric Modulators. Front. Chem. 2021, 9, 671483. [Google Scholar] [CrossRef] [PubMed]
- Suteau, V.; Rodien, P.; Munier, M. G-Protein Coupled Hormone Receptors of the Hypothalamic-Pituitary-Gonadal Axis are Targets of Endocrine Disrupting Chemicals; IntechOpen: London, UK, 2021. [Google Scholar] [CrossRef]
- Rossi, M.; Dimida, A.; Ferrarini, E.; Silvano, E.; De Marco, G.; Agretti, P.; Aloisi, G.; Simoncini, T.; Di Bari, L.; Tonacchera, M.; et al. Presence of a putative steroidal allosteric site on glycoprotein hormone receptors. Eur. J. Pharmacol. 2009, 623, 155–159. [Google Scholar] [CrossRef]
- Landomiel, F.; De Pascali, F.; Raynaud, P.; Jean-Alphonse, F.; Yvinec, R.; Pellissier, L.P.; Bozon, V.; Bruneau, G.; Crepieux, P.; Poupon, A.; et al. Biased Signaling and Allosteric Modulation at the FSHR. Front. Endocrinol. 2019, 10, 148. [Google Scholar] [CrossRef]
- Aathi, M.S.; Kumar, C.; Prabhudesai, K.S.; Shanmugarajan, D.; Idicula-Thomas, S. Mapping of FSHR agonists and antagonists binding sites to identify potential peptidomimetic modulators. Biochim. Biophys. Acta (BBA)—Biomembr. 2022, 1864, 183842. [Google Scholar] [CrossRef]
- Anderson, R.C.; Newton, C.L.; Millar, R.P. Small Molecule Follicle-Stimulating Hormone Receptor Agonists and Antagonists. Front. Endocrinol. 2018, 9, 757. [Google Scholar] [CrossRef]
- Perian, S.; Vanacker, J.M. GPER as a Receptor for Endocrine-Disrupting Chemicals (EDCs). Front. Endocrinol. 2020, 11, 545. [Google Scholar] [CrossRef] [PubMed]
- Perian, S.; Cerutti, C.; Forcet, C.; Tribollet, V.; Vanacker, J.M. A Cell-Based Method to Detect Agonist and Antagonist Activities of Endocrine-Disrupting Chemicals on GPER. Front. Endocrinol. 2020, 11, 547. [Google Scholar] [CrossRef] [PubMed]
- Bouskine, A.; Nebout, M.; Brucker-Davis, F.; Benahmed, M.; Fenichel, P. Low doses of bisphenol A promote human seminoma cell proliferation by activating PKA and PKG via a membrane G-protein-coupled estrogen receptor. Environ. Health Perspect. 2009, 117, 1053–1058. [Google Scholar] [CrossRef] [PubMed]
- Miglioli, A.; Balbi, T.; Besnardeau, L.; Dumollard, R.; Canesi, L. Bisphenol A interferes with first shell formation and development of the serotoninergic system in early larval stages of Mytilus galloprovincialis. Sci. Total Environ. 2021, 758, 144003. [Google Scholar] [CrossRef]
- Yao, J.; Wang, J.; Wu, L.; Lu, H.; Wang, Z.; Yu, P.; Xiao, H.; Gao, R.; Yu, J. Perinatal exposure to bisphenol A causes a disturbance of neurotransmitter metabolic pathways in female mouse offspring: A focus on the tryptophan and dopamine pathways. Chemosphere 2020, 254, 126715. [Google Scholar] [CrossRef]
- Gonkowski, S. Bisphenol A (BPA)-Induced Changes in the Number of Serotonin-Positive Cells in the Mucosal Layer of Porcine Small Intestine-the Preliminary Studies. Int. J. Mol. Sci. 2020, 21, 1079. [Google Scholar] [CrossRef]
- Nguyen, T.M.; Combarnous, Y.; Praud, C.; Duittoz, A.; Blesbois, E. Ca2+/Calmodulin-Dependent Protein Kinase Kinases (CaMKKs) Effects on AMP-Activated Protein Kinase (AMPK) Regulation of Chicken Sperm Functions. PLoS ONE 2016, 11, e0147559. [Google Scholar] [CrossRef]
- Xiao, B.; Sanders, M.J.; Carmena, D.; Bright, N.J.; Haire, L.F.; Underwood, E.; Patel, B.R.; Heath, R.B.; Walker, P.A.; Hallen, S.; et al. Structural basis of AMPK regulation by small molecule activators. Nat. Commun. 2013, 4, 3017. [Google Scholar] [CrossRef]
- Niemuth, N.J.; Klaper, R.D. Low-dose metformin exposure causes changes in expression of endocrine disruption-associated genes. Aquat. Toxicol. 2018, 195, 33–40. [Google Scholar] [CrossRef]
- Quesada, I.; Fuentes, E.; Viso-Leon, M.C.; Soria, B.; Ripoll, C.; Nadal, A. Low doses of the endocrine disruptor bisphenol-A and the native hormone 17beta-estradiol rapidly activate transcription factor CREB. FASEB J. 2002, 16, 1671–1673. [Google Scholar] [CrossRef]
- Oguro, A.; Sugitani, A.; Kobayashi, Y.; Sakuma, R.; Imaoka, S. Bisphenol A stabilizes Nrf2 via Ca2+ influx by direct activation of the IP3 receptor. J. Toxicol. Sci. 2021, 46, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kucka, M.; Pogrmic-Majkic, K.; Fa, S.; Stojilkovic, S.S.; Kovacevic, R. Atrazine acts as an endocrine disrupter by inhibiting cAMP-specific phosphodiesterase-4. Toxicol. Appl. Pharm. 2012, 265, 19–26. [Google Scholar] [CrossRef]
- Liu, C.; Li, Q.; Feng, X.; Zhu, J.; Li, Q. Deterioration of diabetic nephropathy via stimulating secretion of cytokines by atrial natriuretic peptide. BMC Endocr. Disord. 2021, 21, 204. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhao, Z.; Wang, J. Natriuretic peptide receptor A as a novel target for cancer. World J. Surg. Oncol. 2014, 12, 174. [Google Scholar] [CrossRef]
- Serafino, A.; Pierimarchi, P. Atrial natriuretic peptide: A magic bullet for cancer therapy targeting Wnt signaling and cellular pH regulators. Curr. Med. Chem. 2014, 21, 2401–2409. [Google Scholar] [CrossRef] [PubMed]
- Januzzi, J.L.; Mohebi, R. Obesity-Mediated Disruption of Natriuretic Peptide–Blood Pressure Rhythms. J. Am. Coll. Cardiol. 2021, 77, 2304–2306. [Google Scholar] [CrossRef]
- Changeux, J.-P. The nicotinic acetylcholine receptor: A typical ‘allosteric machine’. Philos. Trans. R. Soc. B Biol. Sci. 2018, 373, 20170174. [Google Scholar] [CrossRef]
- Taly, A.; Hénin, J.; Changeux, J.-P.; Cecchini, M. Allosteric regulation of pentameric ligand-gated ion channels: An emerging mechanistic perspective. Channels 2014, 8, 350–360. [Google Scholar] [CrossRef]
- Hilton, J.K.; Kim, M.; Van Horn, W.D. Structural and Evolutionary Insights Point to Allosteric Regulation of TRP Ion Channels. Acc. Chem. Res. 2019, 52, 1643–1652. [Google Scholar] [CrossRef]
- Deba, F.; Munoz, K.; Peredia, E.; Akk, G.; Hamouda, A.K. Assessing Potentiation of the (α4)3(β2)2 Nicotinic Acetylcholine Receptor by the Allosteric Agonist CMPI. J. Biol. Chem. 2021, 298, 101455. [Google Scholar] [CrossRef]
- Derouiche, S.; Mariot, P.; Warnier, M.; Vancauwenberghe, E.; Bidaux, G.; Gosset, P.; Mauroy, B.; Bonnal, J.L.; Slomianny, C.; Delcourt, P.; et al. Activation of TRPA1 Channel by Antibacterial Agent Triclosan Induces VEGF Secretion in Human Prostate Cancer Stromal Cells. Cancer Prev. Res. 2017, 10, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Kow, L.-M.; Pfaff, D.W. Rapid estrogen actions on ion channels: A survey in search for mechanisms. Steroids 2016, 111, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Scarano, W.R.; Pinho, C.F.; Pissinatti, L.; Gonçalves, B.F.; Mendes, L.O.; Campos, S.G.P. Cell junctions in the prostate: An overview about the effects of Endocrine Disrupting Chemicals (EDCS) in different experimental models. Reprod. Toxicol. 2018, 81, 147–154. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Combarnous, Y.; Nguyen, T.M.D. Membrane Hormone Receptors and Their Signaling Pathways as Targets for Endocrine Disruptors. J. Xenobiot. 2022, 12, 64-73. https://doi.org/10.3390/jox12020007
Combarnous Y, Nguyen TMD. Membrane Hormone Receptors and Their Signaling Pathways as Targets for Endocrine Disruptors. Journal of Xenobiotics. 2022; 12(2):64-73. https://doi.org/10.3390/jox12020007
Chicago/Turabian StyleCombarnous, Yves, and Thi Mong Diep Nguyen. 2022. "Membrane Hormone Receptors and Their Signaling Pathways as Targets for Endocrine Disruptors" Journal of Xenobiotics 12, no. 2: 64-73. https://doi.org/10.3390/jox12020007
APA StyleCombarnous, Y., & Nguyen, T. M. D. (2022). Membrane Hormone Receptors and Their Signaling Pathways as Targets for Endocrine Disruptors. Journal of Xenobiotics, 12(2), 64-73. https://doi.org/10.3390/jox12020007






