Exploring the Roles of Dietary Herbal Essential Oils in Aquaculture: A Review
Abstract
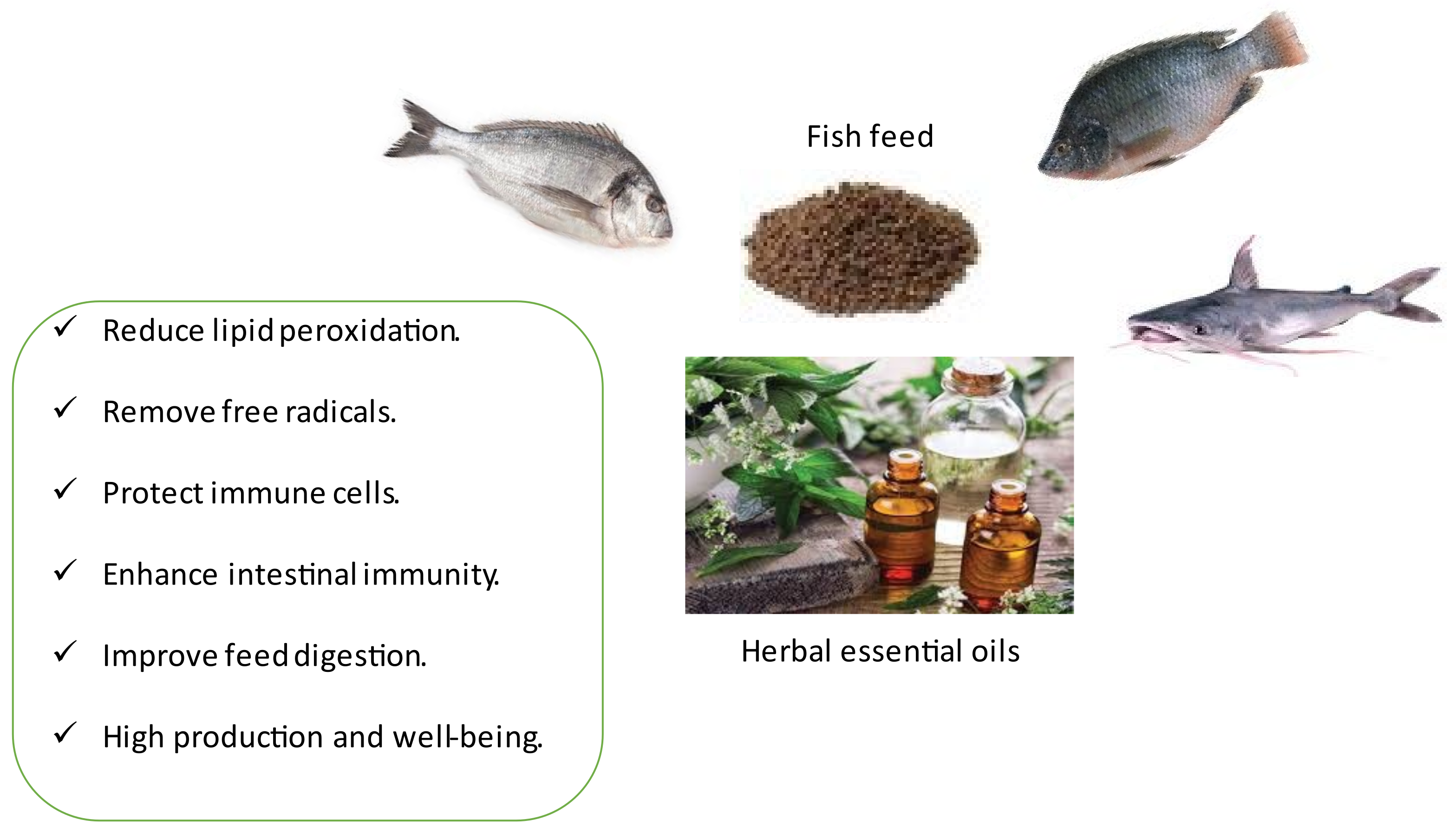
Simple Summary
Abstract
1. Introduction
2. Natural Sources of Essential Oils
2.1. Menthol
2.2. Linalool
2.3. Myrcene
2.4. Eucalyptol
2.5. Globulol (Ledol)
2.6. Spathulenol
2.7. Guaiol (Champacol)
2.8. Caryophyllene Oxide
2.9. Thymol
2.10. Carvacrol (CVC)
2.11. Terpinen-4-ol
2.12. Dehydrofukinone
3. Effects of Essential Oils on Growth and Gut Bacterial Communities
4. Essential Oils as Natural Antioxidants
5. Essential Oils as Immunostimulants
6. Concluding Remarks and Future Outlook
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tacon, A.G.J. Trends in global aquaculture and aquafeed production: 2000–2017. Rev. Fish. Sci. Aquac. 2020, 28, 43–56. [Google Scholar] [CrossRef]
- Dawood, M.A.O.; Noreldin, A.E.; Sewilam, H. Long term salinity disrupts the hepatic function, intestinal health, and gills antioxidative status in nile tilapia stressed with hypoxia. Ecotoxicol. Environ. Saf. 2021, 220, 112412. [Google Scholar] [CrossRef] [PubMed]
- Dawood, M.A.O. Nutritional immunity of fish intestines: Important insights for sustainable aquaculture. Rev. Aquac. 2021, 13, 642–663. [Google Scholar] [CrossRef]
- Garlock, T.; Asche, F.; Anderson, J.; Bjørndal, T.; Kumar, G.; Lorenzen, K.; Ropicki, A.; Smith, M.D.; Tveterås, R. A global blue revolution: Aquaculture growth across regions, species, and countries. Rev. Fish. Sci. Aquac. 2020, 28, 107–116. [Google Scholar] [CrossRef]
- Dawood, M.A.O.; Koshio, S.; Esteban, M.Á. Beneficial roles of feed additives as immunostimulants in aquaculture: A review. Rev. Aquac. 2018, 10, 950–974. [Google Scholar] [CrossRef]
- Yukgehnaish, K.; Kumar, P.; Sivachandran, P.; Marimuthu, K.; Arshad, A.; Paray, B.A.; Arockiaraj, J. Gut microbiota metagenomics in aquaculture: Factors influencing gut microbiome and its physiological role in fish. Rev. Aquac. 2020, 12, 1903–1927. [Google Scholar] [CrossRef]
- Mohammadi, G.; Hafezieh, M.; Karimi, A.A.; Azra, M.N.; Van Doan, H.; Tapingkae, W.; Abdelrahman, H.A.; Dawood, M.A.O. The synergistic effects of plant polysaccharide and Pediococcus acidilactici as a synbiotic additive on growth, antioxidant status, immune response, and resistance of Nile tilapia (Oreochromis niloticus) against Aeromonas hydrophila. Fish Shellfish Immunol. 2022, 120, 304–313. [Google Scholar] [CrossRef]
- Shourbela, R.M.; El-Hawarry, W.N.; Elfadadny, M.R.; Dawood, M.A.O. Oregano essential oil enhanced the growth performance, immunity, and antioxidative status of Nile tilapia (Oreochromis niloticus) reared under intensive systems. Aquaculture 2021, 542, 736868. [Google Scholar] [CrossRef]
- Elumalai, P.; Kurian, A.; Lakshmi, S.; Faggio, C.; Esteban, M.A.; Ringø, E. Herbal immunomodulators in aquaculture. Rev. Fish. Sci. Aquac. 2020, 29, 33–57. [Google Scholar] [CrossRef]
- Vaseeharan, B.; Thaya, R. Medicinal plant derivatives as immunostimulants: An alternative to chemotherapeutics and antibiotics in aquaculture. Aquac. Int. 2014, 22, 1079–1091. [Google Scholar] [CrossRef]
- Aydın, B.; Barbas, L.A.L. Sedative and anesthetic properties of essential oils and their active compounds in fish: A review. Aquaculture 2020, 520, 734999. [Google Scholar] [CrossRef]
- Zeng, Z.; Zhang, S.; Wang, H.; Piao, X. Essential oil and aromatic plants as feed additives in non-ruminant nutrition: A review. J. Anim. Sci. Biotechnol. 2015, 6, 7. [Google Scholar] [CrossRef] [PubMed]
- Abdelkhalek, N.K.; Risha, E.; El-Adl, M.A.; Salama, M.F.; Dawood, M.A.O. Antibacterial and antioxidant activity of clove oil against Streptococcus iniae infection in Nile tilapia (Oreochromis niloticus) and its effect on hepatic hepcidin expression. Fish Shellfish Immunol. 2020, 104, 478–488. [Google Scholar] [CrossRef] [PubMed]
- Alagawany, M.; Farag, M.R.; Salah, A.S.; Mahmoud, M.A. The role of oregano herb and its derivatives as immunomodulators in fish. Rev. Aquac. 2020, 12, 2481–2492. [Google Scholar] [CrossRef]
- Ghafarifarsani, H.; Kachuei, R.; Imani, A. Dietary supplementation of garden thyme essential oil ameliorated the deteriorative effects of aflatoxin B1 on growth performance and intestinal inflammatory status of rainbow trout (Oncorhynchus mykiss). Aquaculture 2021, 531, 735928. [Google Scholar] [CrossRef]
- Dawood, M.A.O.; El Basuini, M.F.; Zaineldin, A.I.; Yilmaz, S.; Hasan, M.T.; Ahmadifar, E.; El Asely, A.M.; Abdel-Latif, H.M.R.; Alagawany, M.; Abu-Elala, N.M.; et al. Antiparasitic and antibacterial functionality of essential oils: An alternative approach for sustainable aquaculture. Pathogens 2021, 10, 185. [Google Scholar] [CrossRef] [PubMed]
- Acar, U.; Kesbiç, O.S.; Yilmaz, S.; Gültepe, N.; Türker, A. Evaluation of the effects of essential oil extracted from sweet orange peel (Citrus sinensis) on growth rate of tilapia (Oreochromis mossambicus) and possible disease resistance against Streptococcus iniae. Aquaculture 2015, 437, 282–286. [Google Scholar] [CrossRef]
- Baba, E.; Acar, Ü.; Öntaş, C.; Kesbiç, O.S.; Yılmaz, S. Evaluation of citrus limon peels essential oil on growth performance, immune response of Mozambique tilapia Oreochromis mossambicus challenged with Edwardsiella tarda. Aquaculture 2016, 465, 13–18. [Google Scholar] [CrossRef]
- Ngugi, C.C.; Oyoo-Okoth, E.; Muchiri, M. Effects of dietary levels of essential oil (eo) extract from bitter lemon (Citrus limon) fruit peels on growth, biochemical, haemato-immunological parameters and disease resistance in juvenile Labeo victorianus fingerlings challenged with Aeromonas hydrophila. Aquac. Res. 2017, 48, 2253–2265. [Google Scholar]
- Abdel-Latif, H.M.R.; Abdel-Tawwab, M.; Khafaga, A.F.; Dawood, M.A.O. Dietary origanum essential oil improved antioxidative status, immune-related genes, and resistance of common carp (Cyprinus carpio L.) to Aeromonas hydrophila infection. Fish Shellfish Immunol. 2020, 104, 1–7. [Google Scholar] [CrossRef]
- Zheng, Z.L.; Tan, J.Y.W.; Liu, H.Y.; Zhou, X.H.; Xiang, X.; Wang, K.Y. Evaluation of oregano essential oil (Origanum heracleoticum L.) on growth, antioxidant effect and resistance against Aeromonas hydrophila in channel catfish (Ictalurus punctatus). Aquaculture 2009, 292, 214–218. [Google Scholar] [CrossRef]
- Diler, O.; Gormez, O.; Diler, I.; Metin, S. Effect of oregano (Origanum onites L.) essential oil on growth, lysozyme and antioxidant activity and resistance against Lactococcus garvieae in rainbow trout, Oncorhynchus mykiss (walbaum). Aquac. Nutr. 2017, 23, 844–851. [Google Scholar] [CrossRef]
- Anastasiou, T.I.; Mandalakis, M.; Krigas, N.; Vézignol, T.; Lazari, D.; Katharios, P.; Dailianis, T.; Antonopoulou, E. Comparative evaluation of essential oils from medicinal-aromatic plants of greece: Chemical composition, antioxidant cpacity and antimicrobial activity against bacterial fish pathogens. Molecules 2020, 25, 148. [Google Scholar] [CrossRef] [PubMed]
- Ahmadifar, E.; Yousefi, M.; Karimi, M.; Fadaei Raieni, R.; Dadar, M.; Yilmaz, S.; Dawood, M.A.O.; Abdel-Latif, H.M.R. Benefits of dietary polyphenols and polyphenol-rich additives to aquatic animal health: An overview. Rev. Fish. Sci. Aquac. 2021, 29, 478–511. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef] [PubMed]
- Carson, C.F.; Hammer, K.A. Chemistry and Bioactivity of Essential Oils; John Wiley & Sons, Ltd.: Chichester, UK, 2010; pp. 203–238. [Google Scholar]
- Hüsnü, K.; Baśer, C.; Demirci, F. Chemistry of Essential Oils; Springer: Berlin/Heidelberg, Germany, 2007; pp. 43–86. [Google Scholar]
- Bilia, A.R.; Guccione, C.; Isacchi, B.; Righeschi, C.; Firenzuoli, F.; Bergonzi, M.C. Essential oils loaded in nanosystems: A developing strategy for a successful therapeutic approach. Evid.-Based Complementary Altern. Med. 2014, 2014, 651593. [Google Scholar] [CrossRef]
- De FreitasSouza, C.; Baldissera, M.D.; Baldisserotto, B.; Heinzmann, B.M.; Martos-Sitcha, J.A.; Mancera, J.M. Essential oils as stress-reducing agents for fish aquaculture: A review. Front. Physiol. 2019, 10, 785. [Google Scholar]
- Góra, J.; Lis, A.; Kula, J.; Staniszewska, M.; Wołoszyn, A. Chemical composition variability of essential oils in the ontogenesis of some plants. Flavour Fragr. J. 2002, 17, 445–451. [Google Scholar] [CrossRef]
- Azmir, J.; Zaidul, I.S.M.; Rahman, M.M.; Sharif, K.M.; Mohamed, A.; Sahena, F.; Jahurul, M.H.A.; Ghafoor, K.; Norulaini, N.A.N.; Omar, A.K.M. Techniques for extraction of bioactive compounds from plant materials: A review. J. Food Eng. 2013, 117, 426–436. [Google Scholar] [CrossRef]
- Tongnuanchan, P.; Benjakul, S. Essential oils: Extraction, bioactivities, and their uses for food preservation. J. Food Sci. 2014, 79, R1231–R1249. [Google Scholar] [CrossRef]
- Manuale, D.L.; Betti, C.; Marchi, A.J.; Yori, J.C.; Romeo, E. Synthesis of liquid menthol by hydrogenation of dementholized peppermint oil over ni catalysts. Quim. Nova 2010, 33, 1231–1234. [Google Scholar] [CrossRef]
- Patel, T.; Ishiuji, Y.; Yosipovitch, G. Menthol: A refreshing look at this ancient compound. J. Am. Acad. Dermatol. 2007, 57, 873–878. [Google Scholar] [CrossRef] [PubMed]
- Kamatou, G.P.P.; Vermaak, I.; Viljoen, A.M.; Lawrence, B.M. Menthol: A simple monoterpene with remarkable biological properties. Phytochemistry 2013, 96, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Hoseini, S.M.; Taheri Mirghaed, A.; Yousefi, M. Application of herbal anaesthetics in aquaculture. Rev. Aquac. 2019, 11, 550–564. [Google Scholar] [CrossRef]
- Kalemba, D.; Synowiec, A. Agrobiological interactions of essential oils of two menthol mints: Mentha piperita and Mentha arvensis. Molecules 2020, 25, 59. [Google Scholar] [CrossRef] [PubMed]
- Tisserand, R.; Young, R. Essential Oil Safety: A Guide for Health Care Professionals, 2nd ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2014; pp. 1–780. [Google Scholar]
- Etzold, B.; Jess, A.; Nobis, M. Epimerisation of menthol stereoisomers: Kinetic studies of the heterogeneously catalysed menthol production. Catal. Today 2009, 140, 30–36. [Google Scholar] [CrossRef]
- Elsharif, S.A.; Banerjee, A.; Buettner, A. Structure-odor relationships of linalool, linalyl acetate and their corresponding oxygenated derivatives. Front. Chem. 2015, 3, 57. [Google Scholar] [CrossRef] [PubMed]
- Stashenko, E.E.; Martínez, J.R. Sampling flower scent for chromatographic analysis. J. Sep. Sci. 2008, 31, 2022–2031. [Google Scholar] [CrossRef] [PubMed]
- Behr, A.; Johnen, L. Myrcene as a natural base chemical in sustainable chemistry: A critical review. ChemSusChem 2009, 2, 1072–1095. [Google Scholar] [CrossRef] [PubMed]
- Giese, M.W.; Lewis, M.A.; Giese, L.; Smith, K.M. Method for the analysis of cannabinoids and terpenes in cannabis. J. AOAC Int. 2015, 98, 1503–1522. [Google Scholar] [CrossRef]
- Hazekamp, A.; Tejkalová, K.; Papadimitriou, S. Cannabis: From cultivar to chemovar ii—A metabolomics approach to cannabis classification. Cannabis Cannabinoid Res. 2016, 1, 202–215. [Google Scholar] [CrossRef]
- Russo, E.B.; Marcu, J. Chapter three-cannabis pharmacology: The usual suspects and a few promising leads. In Advances in Pharmacology; Kendall, D., Alexander, S.P.H., Eds.; Academic Press: Cambridge, MA, USA, 2017; Volume 80, pp. 67–134. [Google Scholar]
- Aprotosoaie, A.C.; Luca, V.S.; Trifan, A.; Miron, A. Antigenotoxic Potential of Some Dietary Non-Phenolic Phytochemicals; Elsevier: Amsterdam, The Netherlands, 2018; Volume 60, pp. 223–297. [Google Scholar]
- Flamini, G. Chapter 13-natural herbicides as a safer and more environmentally friendly approach to weed control: A review of the literature since 2000. In Studies in Natural Products Chemistry; Attaur, R., Ed.; Elsevier: Amsterdam, The Netherlands, 2012; Volume 38, pp. 353–396. [Google Scholar]
- Barbosa, L.; Filomeno, C.; Teixeira, R. Chemical variability and biological activities of Eucalyptus spp. Assential oils. Molecules 2016, 21, 1671. [Google Scholar] [CrossRef] [PubMed]
- Barnes, J. Herbal Medicines, 3rd ed.; Barnes, J., Anderson, L., Phillipson, D., Eds.; Pharmaceutical Press: London, UK, 2007. [Google Scholar]
- Tan, M.; Zhou, L.; Huang, Y.; Wang, Y.; Hao, X.; Wang, J. Antimicrobial activity of globulol isolated from the fruits of Eucalyptus globulus labill. Nat. Prod. Res. 2008, 22, 569–575. [Google Scholar] [CrossRef] [PubMed]
- Morais, S.M.; Cossolosso, D.S.; Silva, A.A.S.; de Moraes Filho, M.O.; Teixeira, M.J.; Campello, C.C.; Bonilla, O.H.; de Paula, V.F.; Vila-Nova, N.S. Essential oils from croton species: Chemical composition, in vitro and in silico antileishmanial evaluation, antioxidant and cytotoxicity activities. J. Braz. Chem. Soc. 2019, 30, 2404–2412. [Google Scholar] [CrossRef]
- Mendes, S.; Nunes, D.; Marques, M.; Tardivo, R.; Cechinel Filho, V.; Siminonatto, E.; Wisniewski, A., Jr. Essential oil of baccharis semiserrata, a source of spathulenol. Publ. UEPG-Cienc. Exatas E Da Terra Agrar. E Eng. 2008, 14, 241–245. [Google Scholar] [CrossRef]
- Apel, M.A.; Lima, M.E.L.; Sobral, M.; Young, M.C.M.; Cordeiro, I.; Schapoval, E.E.S.; Henriques, A.T.; Moreno, P.R.H. Anti-inflammatory activity of essential oil from leaves of Myrciaria tenella and Calycorectes sellowianus. Pharm. Biol. 2010, 48, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Kula, J.; Majda, T.; Stoyanova, A.; Georgiev, E. Chemical composition of Origanum vulgare L. Essential oil from Bulgaria. J. Essent. Oil-Bear. Plants 2007, 10, 215–220. [Google Scholar] [CrossRef]
- Hillig, K.W. A chemotaxonomic analysis of terpenoid variation in cannabis. Biochem. Syst. Ecol. 2004, 32, 875–891. [Google Scholar] [CrossRef]
- Kamal, B.S.; Kamal, F.; Lantela, D.E. Cannabis and the anxiety of fragmentation—A systems approach for finding an anxiolytic cannabis chemotype. Front. Neurosci. 2018, 12, 730. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Wu, J.; Luo, Y.; Huang, N.; Zhen, N.; Zhou, Y.; Sun, F.; Li, Z.; Pan, Q.; Li, Y. (−)-guaiol regulates rad51 stability via autophagy to induce cell apoptosis in non-small cell lung cancer. Oncotarget 2016, 7, 62585–62597. [Google Scholar] [CrossRef] [PubMed]
- Farag, R.S.; Shalaby, A.S.; El-Baroty, G.A.; Ibrahim, N.A.; Ali, M.A.; Hassan, E.M. Chemical and biological evaluation of the essential oils of different melaleuca species. Phytother. Res. 2004, 18, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Clarke, S. Families of Compounds that Occur in Essential Oils; Elsevier: Amsterdam, The Netherlands, 2008; pp. 41–77. [Google Scholar]
- Jafri, H.; Ansari, F.A.; Ahmad, I. Prospects of Essential Oils in Controlling Pathogenic Biofilm; Elsevier: Amsterdam, The Netherlands, 2018; pp. 203–236. [Google Scholar]
- Can Baser, K. Biological and pharmacological activities of carvacrol and carvacrol bearing essential oils. Curr. Pharm. Des. 2008, 14, 3106–3119. [Google Scholar] [CrossRef] [PubMed]
- Noma, Y.; Asakawa, Y. Biotransformation of Monoterpenoids; Elsevier Ltd.: Amsterdam, The Netherlands, 2010; Volume 3, pp. 669–801. [Google Scholar]
- Benelli, G.; Canale, A.; Flamini, G.; Cioni, P.L.; Demi, F.; Ceccarini, L.; Macchia, M.; Conti, B. Biotoxicity of Melaleuca alternifolia (myrtaceae) essential oil against the mediterranean fruit fly, Ceratitis capitata (diptera: Tephritidae), and its parasitoid Psyttalia concolor (hymenoptera: Braconidae). Ind. Crops Prod. 2013, 50, 596–603. [Google Scholar] [CrossRef]
- Gómez-Rincón, C.; Langa, E.; Murillo, P.; Valero, M.S.; Berzosa, C.; López, V. Activity of tea tree (Melaleuca alternifolia) essential oil against l3 larvae of Anisakis simplex. BioMed Res. Int. 2014, 2014, 549510. [Google Scholar] [CrossRef]
- Shapira, S.; Pleban, S.; Kazanov, D.; Tirosh, P.; Arber, N. Terpinen-4-ol: A novel and promising therapeutic agent for human gastrointestinal cancers. PLoS ONE 2016, 11, e0156540. [Google Scholar] [CrossRef]
- Yu, D.; Wang, J.; Shao, X.; Xu, F.; Wang, H. Antifungal modes of action of tea tree oil and its two characteristic components against (Botrytis cinerea). J. Appl. Microbiol. 2015, 119, 1253–1262. [Google Scholar] [CrossRef]
- Hart, P.H.; Brand, C.; Carson, C.F.; Riley, T.V.; Prager, R.H.; Finlay-Jones, J.J. Terpinen-4-ol, the main component of the essential oil of Melaleuca alternifolia (tea tree oil), suppresses inflammatory mediator production by activated human monocytes. Inflamm. Res. 2000, 49, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Barbas, L.A.L.; Maltez, L.C.; Stringhetta, G.R.; Garcia, L.d.O.; Monserrat, J.M.; da Silva, D.T.; Heinzmann, B.M.; Sampaio, L.A. Properties of two plant extractives as anaesthetics and antioxidants for juvenile tambaqui Colossoma macropomum. Aquaculture 2017, 469, 79–87. [Google Scholar] [CrossRef]
- Garlet, Q.I.; Pires, L.d.C.; Milanesi, L.H.; Marafiga, J.R.; Baldisserotto, B.; Mello, C.F.; Heinzmann, B.M. (+)-dehydrofukinone modulates membrane potential and delays seizure onset by gabaa receptor-mediated mechanism in mice. Toxicol. Appl. Pharmacol. 2017, 332, 52–63. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, M.; Jamshidi, A.; Raeisi, M.; Azizzadeh, M. The antibacterial andaantioxidant effects of clove (Syzygium aromaticum) and lemon verbena (Aloysia citriodora) essential oils. J. Hum. Environ. Health Promot. 2019, 5, 86–93. [Google Scholar]
- Galvez, C.E.; Jimenez, C.M.; Gomez, A.d.l.A.; Lizarraga, E.F.; Sampietro, D.A. Chemical composition and antifungal activity of essential oils from Senecio nutans, Senecio viridis, Tagetes terniflora and Aloysia gratissima against toxigenic Aspergillus and Fusarium species. Nat. Prod. Res. 2020, 34, 1442–1445. [Google Scholar] [CrossRef]
- Verni, M.C.; Garay, J.A.; Mendoza, L.; Bardón, A.; Borkosky, S.; Arena, M.E.; Cartagena, E. Lipophilic 9,10-dehydrofukinone action on pathogenic and non-pathogenic bacterial biofilms. Why is this main volatile metabolite in senecio? Chem. Biodivers. 2020, 17, e1900507. [Google Scholar] [CrossRef] [PubMed]
- Citarasu, T. Herbal biomedicines: A new opportunity for aquaculture industry. Aquac. Int. 2010, 18, 403–414. [Google Scholar] [CrossRef]
- El Basuini, M.F.; Teiba, I.I.; Zaki, M.A.A.; Alabssawy, A.N.; El-Hais, A.M.; Gabr, A.A.; Dawood, M.A.O.; Zaineldin, A.I.; Mzengereza, K.; Shadrack, R.S.; et al. Assessing the effectiveness of COQ10 dietary supplementation on growth performance, digestive enzymes, blood health, immune response, and oxidative-related genes expression of Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol. 2020, 98, 420–428. [Google Scholar] [CrossRef] [PubMed]
- Sutili, F.J.; Gatlin, D.M.; Heinzmann, B.M.; Baldisserotto, B. Plant essential oils as fish diet additives: Benefits on fish health and stability in feed. Rev. Aquac. 2018, 10, 716–726. [Google Scholar] [CrossRef]
- de Oliveira Hashimoto, G.S.; Neto, F.M.; Ruiz, M.L.; Acchile, M.; Chagas, E.C.; Chaves, F.C.M.; Martins, M.L. Essential oils of lippia sidoides and Mentha piperita against monogenean parasites and their influence on the hematology of Nile tilapia. Aquaculture 2016, 450, 182–186. [Google Scholar] [CrossRef]
- Harikrishnan, R.; Balasundaram, C.; Heo, M.-S. Impact of plant products on innate and adaptive immune system of cultured finfish and shellfish. Aquaculture 2011, 317, 1–15. [Google Scholar] [CrossRef]
- Penino, N.C.; Santos, G.D.O.; Rodrigues, M.F.; Bastos, H.B.D.A.; Winter, G.H.Z.; Bustamante-Filho, I.C.; Pimentel, A.M.; Gregory, R.M.; Mattos, R.C. Effect of intramuscular injection of butafosfan and cobalamin on the quality of fresh and cooled stallion semen. Semin. Cienc. Agrar. 2015, 36, 2603–2610. [Google Scholar] [CrossRef][Green Version]
- Reverter, M.; Bontemps, N.; Lecchini, D.; Banaigs, B.; Sasal, P. Use of plant extracts in fish aquaculture as an alternative to chemotherapy: Current status and future perspectives. Aquaculture 2014, 433, 50–61. [Google Scholar] [CrossRef]
- Saccol, E.M.H.; Uczay, J.; Pês, T.S.; Finamor, I.A.; Ourique, G.M.; Riffel, A.P.K.; Schmidt, D.; Caron, B.O.; Heinzmann, B.M.; Llesuy, S.F.; et al. Addition of Lippia alba (mill) n. E. Brown essential oil to the diet of the silver catfish: An analysis of growth, metabolic and blood parameters and the antioxidant response. Aquaculture 2013, 416–417, 244–254. [Google Scholar] [CrossRef]
- Sutili, F.J.; de Lima Silva, L.; Gressler, L.T.; Gressler, L.T.; Battisti, E.K.; Heinzmann, B.M.; de Vargas, A.C.; Baldisserotto, B. Plant essential oils against Aeromonas hydrophila: In vitro activity and their use in experimentally infected fish. J. Appl. Microbiol. 2015, 119, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Domadia, P.; Swarup, S.; Bhunia, A.; Sivaraman, J.; Dasgupta, D. Inhibition of bacterial cell division protein ftsz by cinnamaldehyde. Biochem. Pharmacol. 2007, 74, 831–840. [Google Scholar] [CrossRef] [PubMed]
- Nazzaro, F.; Fratianni, F.; De Martino, L.; Coppola, R.; De Feo, V. Effect of essential oils on pathogenic bacteria. Pharmaceuticals 2013, 6, 1451–1474. [Google Scholar] [CrossRef]
- Silva, N.C.C.; Fernandes Júnior, A. Biological properties of medicinal plants: A review of their antimicrobial activity. J. Venom. Anim. Toxins Incl. Trop. Dis. 2010, 16, 402–413. [Google Scholar] [CrossRef]
- Togashi, N.; Inoue, Y.; Hamashima, H.; Takano, A. Effects of two terpene alcohols on the antibacterial activity and the mode of action of farnesol against Staphylococcus aureus. Molecules 2008, 13, 3069–3076. [Google Scholar] [CrossRef]
- Laparra, J.M.; Sanz, Y. Interactions of gut microbiota with functional food components and nutraceuticals. Pharmacol. Res. 2010, 61, 219–225. [Google Scholar] [CrossRef]
- Gómez, G.D.; Balcázar, J.L. A review on the interactions between gut microbiota and innate immunity of fish: Table 1. FEMS Immunol. Med. Microbiol. 2008, 52, 145–154. [Google Scholar] [CrossRef]
- Pérez, T.; Balcázar, J.L.; Ruiz-Zarzuela, I.; Halaihel, N.; Vendrell, D.; de Blas, I.; Múzquiz, J.L. Host–microbiota interactions within the fish intestinal ecosystem. Mucosal Immunol. 2010, 3, 355–360. [Google Scholar] [CrossRef]
- Teiba, I.; Okunishi, S.; Yoshikawa, T.; Ikenaga, M.; Fouad El Basuini, M.; Mae S Santander-De Leon, S.; Maeda, H. Use of purple non-sulfur photosynthetic bacteria (Rhodobacter sphaeroides) in promoting ciliated protozoa growth. Biocontrol Sci. 2020, 25, 81–89. [Google Scholar] [CrossRef]
- Teiba, I.; Yoshikawa, T.; Okunishi, S.; Ikenaga, M.; Basuini, M.E.; Maeda, H. Diversity of the photosynthetic bacterial communities in highly eutrophicated Yamagawa bay sediments. Biocontrol Sci. 2020, 25, 25–33. [Google Scholar] [CrossRef]
- Ellis, A.E. Innate host defense mechanisms of fish against viruses and bacteria. Dev. Comp. Immunol. 2001, 25, 827–839. [Google Scholar] [CrossRef]
- Si, W.; Gong, J.; Tsao, R.; Zhou, T.; Yu, H.; Poppe, C.; Johnson, R.; Du, Z. Antimicrobial activity of essential oils and structurally related synthetic food additives towards selected pathogenic and beneficial gut bacteria. J. Appl. Microbiol. 2006, 100, 296–305. [Google Scholar] [CrossRef] [PubMed]
- Thapa, D.; Losa, R.; Zweifel, B.; John Wallace, R. Sensitivity of pathogenic and commensal bacteria from the human colon to essential oils. Microbiology 2012, 158, 2870–2877. [Google Scholar] [CrossRef] [PubMed]
- Bento, M.H.L.; Ouwehand, A.C.; Tiihonen, K.; Lahtinen, S.; Nurminen, P.; Saarinen, M.T.; Schulze, H.; Mygind, T.; Fischer, J. Essential oils and their use in animal feeds for monogastric animals—Effects on feed quality, gut microbiota, growth performance and food safety: A review. Vet. Med. 2013, 58, 449–458. [Google Scholar] [CrossRef]
- Chakraborty, S.B.; Horn, P.; Hancz, C. Application of Phytochemicals as Growth-Promoters and Endocrine Modulators in Fish Culture; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2014; Volume 6, pp. 1–19. [Google Scholar]
- Franz, C.; Baser, K.H.C.; Windisch, W. Essential oils and aromatic plants in animal feeding–A European perspective. A review. Flavour Fragr. J. 2010, 25, 327–340. [Google Scholar] [CrossRef]
- Sutili, F.J.; Velasquez, A.; Pinheiro, C.G.; Heinzmann, B.M.; Gatlin, D.M.; Baldisserotto, B. Evaluation of ocimum americanum essential oil as an additive in red drum (Sciaenops ocellatus) diets. Fish Shellfish Immunol. 2016, 56, 155–161. [Google Scholar] [CrossRef]
- Navarrete, P.; Toledo, I.; Mardones, P.; Opazo, R.; Espejo, R.; Romero, J. Effect of Thymus vulgaris essential oil on intestinal bacterial microbiota of rainbow trout, Oncorhynchus mykiss (walbaum) and bacterial isolates. Aquac. Res. 2010, 41, e667–e678. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, X.W.; Liu, L.L.; Cao, Y.C.; Zhu, H. Dietary oregano essential oil improved the immune response, activity of digestive enzymes, and intestinal microbiota of the koi carp, Cyprinus carpio. Aquaculture 2020, 518, 734781. [Google Scholar] [CrossRef]
- Al-Sagheer, A.A.; Mahmoud, H.K.; Reda, F.M.; Mahgoub, S.A.; Ayyat, M.S. Supplementation of diets for Oreochromis niloticus with essential oil extracts from lemongrass (Cymbopogon citratus) and geranium (Pelargonium graveolens) and effects on growth, intestinal microbiota, antioxidant and immune activities. Aquac. Nutr. 2018, 24, 1006–1014. [Google Scholar] [CrossRef]
- Giannenas, I.; Triantafillou, E.; Stavrakakis, S.; Margaroni, M.; Mavridis, S.; Steiner, T.; Karagouni, E. Assessment of dietary supplementation with carvacrol or thymol containing feed additives on performance, intestinal microbiota and antioxidant status of rainbow trout (Oncorhynchus mykiss). Aquaculture 2012, 350–353, 26–32. [Google Scholar] [CrossRef]
- Ran, C.; Hu, J.; Liu, W.; Liu, Z.; He, S.; Dan, B.C.T.; Diem, N.N.; Ooi, E.L.; Zhou, Z. Thymol and carvacrol affect hybrid tilapia through the combination of direct stimulation and an intestinal microbiota-mediated effect: Insights from a germ-free zebrafish model. J. Nutr. 2016, 146, 1132–1140. [Google Scholar] [CrossRef] [PubMed]
- Hernández, F.; Madrid, J.; García, V.; Orengo, J.; Megías, M.D. Influence of two plant extracts on broilers performance, digestibility, and digestive organ size. Poult. Sci. 2004, 83, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Michiels, J.; Missotten, J.; van Hoorick, A.; Ovyn, A.; Fremaut, D.; de Smet, S.; Dierick, N. Effects of dose and formulation of carvacrol and thymol on bacteria and some functional traits of the gut in piglets after weaning. Arch. Anim. Nutr. 2010, 64, 136–154. [Google Scholar] [CrossRef] [PubMed]
- Venketramalingam, K.; Christopher, J.G.; Citarasu, T. Zingiber officinalis an herbal appetizer in the tiger shrimp Penaeus monodon (fabricius) larviculture. Aquac. Nutr. 2007, 13, 439–443. [Google Scholar] [CrossRef]
- Adeshina, I.; Jenyo-Oni, A.; Emikpe, B.O.; Ajani, E.K.; Abdel-Tawwab, M. Stimulatory effect of dietary clove, Eugenia caryophyllata, bud extract on growth performance, nutrient utilization, antioxidant capacity, and tolerance of African catfish, Clarias gariepinus (b.), to Aeromonas hydrophila infection. J. World Aquac. Soc. 2019, 50, 390–405. [Google Scholar] [CrossRef]
- Huerta-Aguirre, G.; Paredes-Ramos, K.M.; Becerra-Amezcua, M.P.; Hernández-Calderas, I.; Matadamas-Guzman, M.; Guzmán-García, X. Histopathological Analysis of the Intestine from Mugil cephalus on Environment Reference Sites; Springer International Publishing: Berlin/Heidelberg, Germany, 2019; pp. 319–328. [Google Scholar]
- Abdel-Latif, H.M.R.; Abdel-Tawwab, M.; Khafaga, A.F.; Dawood, M.A.O. Dietary oregano essential oil improved the growth performance via enhancing the intestinal morphometry and hepato-renal functions of common carp (Cyprinus carpio L.) fingerlings. Aquaculture 2020, 526, 735432. [Google Scholar] [CrossRef]
- Abd El-Naby, A.S.; Al-Sagheer, A.A.; Negm, S.S.; Naiel, M.A.E. Dietary combination of chitosan nanoparticle and thymol affects feed utilization, digestive enzymes, antioxidant status, and intestinal morphology of Oreochromis niloticus. Aquaculture 2020, 515, 734577. [Google Scholar] [CrossRef]
- Ferreira, P.M.F.; Caldas, D.W.; Salaro, A.L.; Sartori, S.S.R.; Oliveira, J.M.; Cardoso, A.J.S.; Zuanon, J.A.S. Intestinal and liver morphometry of the yellow tail tetra (Astyanax altiparanae) fed with oregano oil. An. Da Acad. Bras. De Cienc. 2016, 88, 911–922. [Google Scholar] [CrossRef]
- de Oliveira, S.T.L.; Soares, R.A.N.; de Negreiros Sousa, S.M.; Fernandes, A.W.C.; Gouveia, G.V.; da Costa, M.M. Natural products as functional food ingredients for Nile tilapia challenged with Aeromonas hydrophila. Aquac. Int. 2020, 28, 913–926. [Google Scholar] [CrossRef]
- Rattanachaikunsopon, P.; Phumkhachorn, P. Assessment of synergistic efficacy of carvacrol and cymene against Edwardsiella tarda in vitro and in tilapia (Oreochromis niloticus). Afr. J. Microbiol. Res. 2010, 4, 420–425. [Google Scholar]
- Amer, S.A.; Metwally, A.E.; Ahmed, S.A.A. The influence of dietary supplementation of cinnamaldehyde and thymol on the growth performance, immunity and antioxidant status of monosex Nile tilapia fingerlings (Oreochromis niloticus). Egypt. J. Aquat. Res. 2018, 44, 251–256. [Google Scholar] [CrossRef]
- Aanyu, M.; Betancor, M.B.; Monroig, O. Effects of dietary limonene and thymol on the growth and nutritional physiology of Nile tilapia (Oreochromis niloticus). Aquaculture 2018, 488, 217–226. [Google Scholar] [CrossRef]
- Brum, A.; Pereira, S.A.; Owatari, M.S.; Chagas, E.C.; Chaves, F.C.M.; Mouriño, J.L.P.; Martins, M.L. Effect of dietary essential oils of clove basil and ginger on Nile tilapia (Oreochromis niloticus) following challenge with Streptococcus agalactiae. Aquaculture 2017, 468, 235–243. [Google Scholar] [CrossRef]
- Abo-State, H.A.; El-Monairy, M.M.; Hammouda, Y.A.; Elgendy, M.Y. Effect of a phytogenic feed additive on the growth performance and susceptibility of Oreochromis niloticus to Aeromonas hydrophila. J. Fish. Aquat. Sci. 2017, 12, 141–148. [Google Scholar] [CrossRef][Green Version]
- Hassaan, M.S.; Soltan, M.A. Evaluation of essential oil of fennel and garlic separately or combined with Bacillus licheniformis on the growth, feeding behaviour, hemato-biochemical indices of Oreochromis niloticus (L.) fry. J. Aquac. Res. Dev. 2016, 7, 422–429. [Google Scholar] [CrossRef]
- Zeppenfeld, C.C.; Hernández, D.R.; Santinón, J.J.; Heinzmann, B.M.; da Cunha, M.A.; Schmidt, D.; Baldisserotto, B. Essential oil of Aloysia triphylla as feed additive promotes growth of silver catfish (Rhamdia quelen). Aquac. Nutr. 2016, 22, 933–940. [Google Scholar] [CrossRef]
- Midhun, S.J.; Arun, D.; Edatt, L.; Sruthi, M.V.; Thushara, V.V.; Oommen, O.V.; Sameer Kumar, V.B.; Divya, L. Modulation of digestive enzymes, gh, igf-1 and igf-2 genes in the teleost, tilapia (Oreochromis mossambicus) by dietary curcumin. Aquac. Int. 2016, 24, 1277–1286. [Google Scholar] [CrossRef]
- Sönmez, A.Y.; Bilen, S.; Alak, G.; Hisar, O.; Yanık, T.; Biswas, G. Growth performance and antioxidant enzyme activities in rainbow trout (Oncorhynchus mykiss) juveniles fed diets supplemented with sage, mint and thyme oils. Fish Physiol. Biochem. 2015, 41, 165–175. [Google Scholar] [CrossRef]
- Ahmadifar, E.; Razeghi Mansour, M.; Keramat Amirkolaie, A.; Fadaii Rayeni, M. Growth efficiency, survival and haematological changes in great sturgeon (Huso huso linnaeus, 1758) juveniles fed diets supplemented with different levels of thymol–carvacrol. Anim. Feed Sci. Technol. 2014, 198, 304–308. [Google Scholar] [CrossRef]
- Ferreira, P.d.M.F.; Nascimento, L.d.S.; Dias, D.C.; Moreira, D.M.d.V.; Salaro, A.L.; de Freitas, M.B.D.; Carneiro, A.P.S.; Zuanon, J.A.S. Essential oregano oil as a growth promoter for the yellowtail tetra, Astyanax altiparanae. J. World Aquac. Soc. 2014, 45, 28–34. [Google Scholar] [CrossRef]
- Ahmadifar, E.; Falahatkar, B.; Akrami, R. Effects of dietary thymol-carvacrol on growth performance, hematological parameters and tissue composition of juvenile rainbow trout, Oncorhynchus mykiss. J. Appl. Ichthyol. 2011, 27, 1057–1060. [Google Scholar] [CrossRef]
- Chishti, S.; Kaloo, Z.A.; Sultan, P. Medicinal importance of genus origanum: A review. J. Pharmacogn. Phytother. Acad. J. 2013, 5, 170–177. [Google Scholar]
- Gonçalves, R.A.; Serradeiro, R.; Machado, M.; Costas, B.; Hunger, C.; Dias, J. Interactive effects of dietary fishmeal level and plant essential oils supplementation on European sea bass, Dicentrarchus labrax: Growth performance, nutrient utilization, and immunological response. J. World Aquac. Soc. 2019, 50, 1078–1092. [Google Scholar] [CrossRef]
- Morel, Y.; Barouki, R. Repression of gene expression by oxidative stress. Biochem. J. 1999, 342, 481–496. [Google Scholar] [CrossRef] [PubMed]
- Ray, P.D.; Huang, B.-W.; Tsuji, Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell. Signal. 2012, 24, 981–990. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Gutteridge, J.M. Free Radicals in Biology and Medicine; Oxford University Press: Oxford, MS, USA, 2015. [Google Scholar]
- Biller, J.D.; Takahashi, L.S. Oxidative stress and fish immune system: Phagocytosis and leukocyte respiratory burst activity. An. Da Acad. Bras. De Cienc. 2018, 90, 3403–3414. [Google Scholar] [CrossRef] [PubMed]
- Dawood, M.A.O.; Koshio, S. Vitamin C supplementation to optimize growth, health and stress resistance in aquatic animals. Rev. Aquac. 2018, 10, 334–350. [Google Scholar] [CrossRef]
- Birnie-Gauvin, K.; Costantini, D.; Cooke, S.J.; Willmore, W.G. A comparative and evolutionary approach to oxidative stress in fish: A review. Fish Fish. 2017, 18, 928–942. [Google Scholar] [CrossRef]
- Zaki, M.A.A.; Alabssawy, A.N.; Nour, A.E.A.M.; El Basuini, M.F.; Dawood, M.A.O.; Alkahtani, S.; Abdel-Daim, M.M. The impact of stocking density and dietary carbon sources on the growth, oxidative status and stress markers of Nile tilapia (Oreochromis niloticus) reared under biofloc conditions. Aquac. Rep. 2020, 16, 100282. [Google Scholar] [CrossRef]
- Tu, W.Y.; Pohl, S.; Summpunn, P.; Hering, S.; Kerstan, S.; Harwood, C.R. Comparative analysis of the responses of related pathogenic and environmental bacteria to oxidative stress. Microbiology 2012, 158, 636–647. [Google Scholar] [CrossRef]
- Brewer, M.S. Natural antioxidants: Sources, compounds, mechanisms of action, and potential applications. Compr. Rev. Food Sci. Food Saf. 2011, 10, 221–247. [Google Scholar] [CrossRef]
- Embuscado, M.E. Spices and herbs: Natural sources of antioxidants—A mini review. J. Funct. Foods 2015, 18, 811–819. [Google Scholar] [CrossRef]
- Su, L.; Yin, J.J.; Charles, D.; Zhou, K.; Moore, J.; Yu, L. Total phenolic contents, chelating capacities, and radical-scavenging properties of black peppercorn, nutmeg, rosehip, cinnamon and oregano leaf. Food Chem. 2007, 100, 990–997. [Google Scholar] [CrossRef]
- Abdel-Latif, H.M.R.; Khalil, R.H. Evaluation of two phytobiotics, Spirulina platensis and Origanum vulgare extract on growth, serum antioxidant activities and resistance of Nile tilapia (Oreochromis niloticus) to pathogenic Vibrio alginolyticus. Int. J. Fish. Aquat. Stud. 2014, 1, 250–255. [Google Scholar]
- El-Hawarry, W.N.; Mohamed, R.A.; Ibrahim, S.A. Collaborating effects of rearing density and oregano oil supplementation on growth, behavioral and stress response of Nile tilapia (Oreochromis niloticus). Egypt. J. Aquat. Res. 2018, 44, 173–178. [Google Scholar] [CrossRef]
- Peterson, B.C.; Bosworth, B.G.; Li, M.H.; Beltran, R.; Santos, G.A. Assessment of a phytogenic feed additive (Digestarom PEP MGE) on growth performance, processing yield, fillet composition, and survival of channel catfish. J. World Aquac. Soc. 2014, 45, 206–212. [Google Scholar] [CrossRef]
- Zeppenfeld, C.C.; Saccol, E.M.H.; Pês, T.S.; Salbego, J.; Koakoski, G.; dos Santos, A.C.; Heinzmann, B.M.; da Cunha, M.A.; Barcellos, L.J.G.; Pavanato, M.A.; et al. Aloysia triphylla essential oil as food additive for Rhamdia quelen-stress and antioxidant parameters. Aquac. Nutr. 2017, 23, 1362–1367. [Google Scholar] [CrossRef]
- Hsieh, T.J.; Wang, J.C.; Hu, C.Y.; Li, C.T.; Kuo, C.M.; Hsieh, S.L. Effects of rutin from Toona sinensis on the immune and physiological responses of white shrimp (Litopenaeus vannamei) under Vibrio alginolyticus challenge. Fish Shellfish Immunol. 2008, 25, 581–588. [Google Scholar] [CrossRef]
- de Freitas Souza, C.; Baldissera, M.D.; Bianchini, A.E.; da Silva, E.G.; Mourão, R.H.V.; da Silva, L.V.F.; Schmidt, D.; Heinzmann, B.M.; Baldisserotto, B. Citral and linalool chemotypes of Lippia alba essential oil as anesthetics for fish: A detailed physiological analysis of side effects during anesthetic recovery in silver catfish (Rhamdia quelen). Fish Physiol. Biochem. 2018, 44, 21–34. [Google Scholar] [CrossRef]
- Saccol, E.M.H.; Londero, É.P.; Bressan, C.A.; Salbego, J.; Gressler, L.T.; Silva, L.V.F.; Mourão, R.H.V.; Oliveira, R.B.; Llesuy, S.F.; Baldisserotto, B.; et al. Oxidative and biochemical responses in brycon amazonicus anesthetized and sedated with Myrcia sylvatica (g. Mey.) dc. And Curcuma longa L. Essential oils. Vet. Anaesth. Analg. 2017, 44, 555–566. [Google Scholar] [CrossRef]
- Gressler, L.T.; Riffel, A.P.K.; Parodi, T.V.; Saccol, E.M.H.; Koakoski, G.; da Costa, S.T.; Pavanato, M.A.; Heinzmann, B.M.; Caron, B.; Schmidt, D.; et al. Silver catfish Rhamdia quelen immersion anaesthesia with essential oil of Aloysia triphylla (l’hérit) britton or tricaine methanesulfonate: Effect on stress response and antioxidant status. Aquac. Res. 2014, 45, 1061–1072. [Google Scholar] [CrossRef]
- Baldissera, M.D.; Souza, C.F.; Júnior, G.B.; de Vargas, A.C.; Boligon, A.A.; de Campos, M.M.A.; Stefani, L.M.; Baldisserotto, B. Melaleuca alternifolia essential oil enhances the non-specific immune system and prevents oxidative damage in Rhamdia quelen experimentally infected by Aeromonas hydrophila: Effects on cholinergic and purinergic systems in liver tissue. Fish Shellfish Immunol. 2017, 61, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ali, B.; Al-Wabel, N.A.; Shams, S.; Ahamad, A.; Khan, S.A.; Anwar, F. Essential oils used in aromatherapy: A systemic review. Asian Pac. J. Trop. Biomed. 2015, 5, 601–611. [Google Scholar] [CrossRef]
- Huang, C.-F.; Lin, S.-S.; Liao, P.-H.; Young, S.-C.; Yang, C.-C. The immunopharmaceutical effects and mechanisms of herb medicine. Cell. Mol. Immunol. 2008, 5, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Peterfalvi, A.; Miko, E.; Nagy, T.; Reger, B.; Simon, D.; Miseta, A.; Czéh, B.; Szereday, L. Much more than a pleasant scent: A review on essential oils supporting the immune system. Molecules 2019, 24, 4530. [Google Scholar] [CrossRef] [PubMed]
- Manion, C.R.; Widder, R.M. Essentials of essential oils. Am. J. Health-Syst. Pharm. 2017, 74, e153–e162. [Google Scholar] [CrossRef] [PubMed]
- Bousbia, N.; Abert Vian, M.; Ferhat, M.A.; Petitcolas, E.; Meklati, B.Y.; Chemat, F. Comparison of two isolation methods for essential oil from rosemary leaves: Hydrodistillation and microwave hydrodiffusion and gravity. Food Chem. 2009, 114, 355–362. [Google Scholar] [CrossRef]
- Elyemni, M.; Louaste, B.; Nechad, I.; Elkamli, T.; Bouia, A.; Taleb, M.; Chaouch, M.; Eloutassi, N. Extraction of essential oils of Rosmarinus officinalis L. By two different methods: Hydrodistillation and microwave assisted hydrodistillation. Sci. World J. 2019, 2019, 3659432. [Google Scholar] [CrossRef]
- Kumar, D.; Arya, V.; Kaur, R.; Bhat, Z.A.; Gupta, V.K.; Kumar, V. A review of immunomodulators in the Indian traditional health care system. J. Microbiol. Immunol. Infect. 2012, 45, 165–184. [Google Scholar] [CrossRef]
- SaiRam, M.; Sharma, S.K.; Ilavazhagan, G.; Kumar, D.; Selvamurthy, W. Immunomodulatory effects of nim-76, a volatile fraction from neem oil. J. Ethnopharmacol. 1997, 55, 133–139. [Google Scholar] [CrossRef]
- Dalmo, R.A.; Ingebrigtsen, K.; Bogwald, J. Non-specific defence mechanisms in fish, with particular reference to the reticuloendothelial system (res). J. Fish Dis. 1997, 20, 241–273. [Google Scholar] [CrossRef]
- Cecchini, S.; Terova, G.; Caricato, G.; Saroglia, M. Lysozyme activity in embryos and larvae of sea bass (Dicentrarchus labrax L.), spawned by broodstocks fed with vitamin C enriched diets. Bull. Eur. Assoc. Fish Pathol. 2000, 20, 120–124. [Google Scholar]
- Kawakami, H.; Yamashita, H.; Sakai, M. Comparative sensitivity of yellowtail Seriola quinqueradiata and goldstriped amberjack S. aureovittata to photobacterium damsela subsp. Piscicida. J. World Aquac. Soc. 2000, 31, 213–217. [Google Scholar] [CrossRef]
- Dossou, S.; Koshio, S.; Ishikawa, M.; Yokoyama, S.; Dawood, M.A.O.; El Basuini, M.F.; Olivier, A.; Zaineldin, A.I. Growth performance, blood health, antioxidant status and immune response in red sea bream (Pagrus major) fed Aspergillus oryzae fermented rapeseed meal (RM-KOJI). Fish Shellfish Immunol. 2018, 75, 253–262. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, M.W.; de Brito, S.T.; de A Prado, S.; de Oliveira, G.C.; De Paula, C.A.; de Melo, C.D.; Ribeiro, A.P.P. Cinnamon (Cinnamomum sp.) inclusion in diets for Nile tilapia submitted to acute hypoxic stress. Fish Shellfish Immunol. 2016, 54, 551–555. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, E.; Ergün, S.; Yilmaz, S. Influence of carvacrol on the growth performance, hematological, non-specific immune and serum biochemistry parameters in rainbow trout (Oncorhynchus mykiss). Food Nutr. Sci. 2015, 6, 523–531. [Google Scholar] [CrossRef]
- Volpatti, D.; Chiara, B.; Francesca, T.; Marco, G. Growth parameters, innate immune response and resistance to Listonella Vibrio anguillarum of Dicentrarchus labrax fed carvacrol supplemented diets. Aquac. Res. 2013, 45, 31–44. [Google Scholar] [CrossRef]
- Sheikhzadeh, N.; Soltani, M.; Ebrahimzadeh-Mousavi, H.A.; Shahbazian, N.; Norouzi, M. Effects of Zataria multiflora and Eucalyptus globolus essential oils on haematological parameters and respiratory burst activity in Cyprinus carpio. Iran. J. Fish. Sci. 2011, 10, 316–323. [Google Scholar]
- Rattanachaikunsopon, P.; Phumkhachorn, P. Potential of cinnamon (Cinnamomum verum) oil to control streptococcus iniae infection in tilapia (Oreochromis niloticus). Fish. Sci. 2010, 76, 287–293. [Google Scholar] [CrossRef]
- Soltani, M.; Sheikhzadeh, N.; Ebrahimzadeh-Mousavi, H.; Zargar, A. Effects of Zataria multiflora essential oil on innate immune responses of common carp (Cyprinus carpio). J. Fish. Aquat. Sci. 2010, 5, 191–199. [Google Scholar] [CrossRef]
- Azambuja, C.R.; Mattiazzi, J.; Riffel, A.P.K.; Finamor, I.A.; Garcia, L.d.O.; Heldwein, C.G.; Heinzmann, B.M.; Baldisserotto, B.; Pavanato, M.A.; Llesuy, S.F. Effect of the essential oil of Lippia alba on oxidative stress parameters in silver catfish (Rhamdia quelen) subjected to transport. Aquaculture 2011, 319, 156–161. [Google Scholar] [CrossRef]
- Awad, E.; Austin, D.; Lyndon, A.R. Effect of black cumin seed oil (Nigella sativa) and nettle extract (quercetin) on enhancement of immunity in rainbow trout, Oncorhynchus mykiss (walbaum). Aquaculture 2013, 388–391, 193–197. [Google Scholar] [CrossRef]
- Sheikhzadeh, N.; Soltani, M.; Mousavi, H.E.; Khosravi, A.; Bagheri, H.; Fathi, E.; Zargar, A. Effects of Eucalyptus globules labill essential oil on some immunological variables of common carp (Cyprinus carpio). J. Vet. Res. 2009, 64, Pe47–Pe54. [Google Scholar]
- Valladão, G.M.R.; Gallani, S.U.; Pala, G.; Jesus, R.B.; Kotzent, S.; Costa, J.C.; Silva, T.F.A.; Pilarski, F. Practical diets with essential oils of plants activate the complement system and alter the intestinal morphology of Nile tilapia. Aquac. Res. 2017, 48, 5640–5649. [Google Scholar] [CrossRef]
- Sönmez, A.Y.; Bilen, S.; Albayrak, M.; Yılmaz, S.; Biswas, G.; Hisar, O.; Yanık, T. Effects of dietary supplementation of herbal oils containing 1, 8-cineole, carvacrol or pulegone on growth performance, survival, fatty acid composition, and liver and kidney histology of rainbow trout (Oncorhynchus mykiss) fingerlings. Turk. J. Fish. Aquat. Sci. 2015, 15, 813–819. [Google Scholar] [CrossRef]
- Rafieepour, A.; Hajirezaee, S.; Rahimi, R. Dietary oregano extract (Origanum vulgare L.) enhances the antioxidant defence in rainbow trout, Oncorhynchus mykiss against toxicity induced by organophosphorus pesticide, diazinon. Toxin Rev. 2019, 39, 397–407. [Google Scholar] [CrossRef]
- De Moraes França Ferreira, P.; da Silva Nascimento, L.; Coelho Dias, D.; da Veiga Moreira, D.M.; Lúcia Salaro, A.; Duca de Freitas, M.B.; Souza Carneiro, A.P.; Sampaio Zuanon, J.A. Essential oregano oil as a growth promoter for the yellowtail tetra, Astyanax altiparanae. J. World Aquac. Soc. 2014, 45, 28–34. [Google Scholar] [CrossRef]
- Dawood, M.A.O.; El-Salam Metwally, A.; Elkomy, A.H.; Gewaily, M.S.; Abdo, S.E.; Abdel-Razek, M.A.S.; Soliman, A.A.; Amer, A.A.; Abdel-Razik, N.I.; Abdel-Latif, H.M.R.; et al. The impact of menthol essential oil against inflammation, immunosuppression, and histopathological alterations induced by chlorpyrifos in Nile tilapia. Fish Shellfish Immunol. 2020, 102, 316–325. [Google Scholar] [CrossRef]
| Aquatic Species | Essential Oil | Dose and Duration | Influence | Reference |
|---|---|---|---|---|
| Common carp (Cyprinus carpio) | Zataria multiflora | 30–120 ppm/kg diet for 22 days |
| [163] |
| Silver catfish (Rhamdia quelen) | Lippia alba | 10 μL/L for 7 h |
| [164] |
| Rainbow trout (Oncorhynchus mykiss) | Black cumin seed oil | 1, 2, and 3 for 14 days |
| [165] |
| Rainbow trout (Oncorhynchus mykiss) | Carvacrol and thymol | 1 g/kg for 8 weeks |
| [101] |
| Silver catfish (Rhamdia quelen) | Lippia alba | 0.25, 0.5, 1.0, or 2.0 mL/kg diet for 60 days |
| [80] |
| Red drum (Sciaenops ocellatus) | Lime basil | 0, 0.25, 0.5, 1.0, and 2.0 g/kg diet for 7 weeks |
| [97] |
| Nile tilapia (Oreochromis niloticus) | Limonene and thymol | 0, 200, 400, and 600 mg/kg for 63 days |
| [114] |
| Common carp (Cyprinus carpio) | Blue gum | 30, 60, and 120 µL/L or mg/kg feed for 8 days |
| [166] |
| Nile tilapia (Oreochromis niloticus) | Pepper rosemary and peppermint | 20–40 mg/L (3 baths for 10 min each) |
| [76] |
| Rainbow trout (Oncorhynchus mykiss) | Carvacrol | 0, 1, 3, or 5 g/kg for 60 days |
| [159] |
| Common carp (Cyprinus carpio L.) | Oregano | 0, 5, 10, 15, and 20 g/kg diet for 2 months |
| [108] |
| Nile tilapia (Oreochromis niloticus) | Peppermint and tea tree | 100 and 250 mg/kg for 60 days |
| [167] |
| Rainbow trout (Oncorhynchus mykiss) | 1,8-cineole, carvacrol or pulegone | 0.5, 1, and 1.5% for 60 days |
| [168] |
| Rainbow trout (Oncorhynchus mykiss) | Oregano | 6 and 10 g/kg diet |
| [169] |
| Yellowtail Tetra (Astyanax altiparanae) | Oregano | 0.0, 0.5, 1.0, 1.5, 2, and 2.5 g/kg for 90 days |
| [170] |
| Nile tilapia (Oreochromis niloticus) | Oregano | 0.0, 1.0, and 2 mL/kg for 10 weeks |
| [138] |
| Nile tilapia (Oreochromis niloticus) | Cinnamaldehyde and thymol | 1 and 2 mL/kg diet for 75 days |
| [113] |
| Great sturgeon (Huso huso Linnaeus, 1758) | Thymol–carvacrol | 0, 1.0, 2.0, and 3.0 g/kg for 60 days |
| [121] |
| Nile tilapia (Oreochromis niloticus) | Menthol | 0.25% for 30 days |
| [171] |
 ): no significant change; WBCs: white blood cells; LPO: lipoperoxidation; CAT: catalase; SOD: superoxide dismutase; GST: glutathione-S-transferase; IgM: immunoglobulin; MDA: malondialdehyde; IGF-I: insulin growth factor I; MUC: mucin-like protein; PEPT1: oligo-peptide transporter I; LPL: lipoprotein lipase; ALP: alkaline phosphatase; RBC: red blood cells; TAC: total antioxidant capacity; LHD: lactate dehydrogenase; ALT: alanine aminotransferase; AST: aspartate aminotransferase; GR: glutathione reductase; NO: nitric oxide.
): no significant change; WBCs: white blood cells; LPO: lipoperoxidation; CAT: catalase; SOD: superoxide dismutase; GST: glutathione-S-transferase; IgM: immunoglobulin; MDA: malondialdehyde; IGF-I: insulin growth factor I; MUC: mucin-like protein; PEPT1: oligo-peptide transporter I; LPL: lipoprotein lipase; ALP: alkaline phosphatase; RBC: red blood cells; TAC: total antioxidant capacity; LHD: lactate dehydrogenase; ALT: alanine aminotransferase; AST: aspartate aminotransferase; GR: glutathione reductase; NO: nitric oxide.Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dawood, M.A.O.; El Basuini, M.F.; Yilmaz, S.; Abdel-Latif, H.M.R.; Alagawany, M.; Kari, Z.A.; Abdul Razab, M.K.A.; Hamid, N.K.A.; Moonmanee, T.; Van Doan, H. Exploring the Roles of Dietary Herbal Essential Oils in Aquaculture: A Review. Animals 2022, 12, 823. https://doi.org/10.3390/ani12070823
Dawood MAO, El Basuini MF, Yilmaz S, Abdel-Latif HMR, Alagawany M, Kari ZA, Abdul Razab MKA, Hamid NKA, Moonmanee T, Van Doan H. Exploring the Roles of Dietary Herbal Essential Oils in Aquaculture: A Review. Animals. 2022; 12(7):823. https://doi.org/10.3390/ani12070823
Chicago/Turabian StyleDawood, Mahmoud A. O., Mohammed F. El Basuini, Sevdan Yilmaz, Hany M. R. Abdel-Latif, Mahmoud Alagawany, Zulhisyam Abdul Kari, Mohammad Khairul Azhar Abdul Razab, Noor Khalidah Abdul Hamid, Tossapol Moonmanee, and Hien Van Doan. 2022. "Exploring the Roles of Dietary Herbal Essential Oils in Aquaculture: A Review" Animals 12, no. 7: 823. https://doi.org/10.3390/ani12070823
APA StyleDawood, M. A. O., El Basuini, M. F., Yilmaz, S., Abdel-Latif, H. M. R., Alagawany, M., Kari, Z. A., Abdul Razab, M. K. A., Hamid, N. K. A., Moonmanee, T., & Van Doan, H. (2022). Exploring the Roles of Dietary Herbal Essential Oils in Aquaculture: A Review. Animals, 12(7), 823. https://doi.org/10.3390/ani12070823











