Comparison of Flavonoid O-Glycoside, C-Glycoside and Their Aglycones on Antioxidant Capacity and Metabolism during In Vitro Digestion and In Vivo
Abstract
:1. Introduction
2. Materials and Methods
2.1. Selection of Flavonoid O-Glycoside, C-Glycoside, and Their Aglycones
2.2. Chemicals and Reagents
2.3. In Vitro Digestion
2.4. Antioxidant Activity Assays during In Vitro Digestion
2.4.1. DPPH Radical Scavenging Activity (DPPH Assay)
2.4.2. ABTS Radical Scavenging Activity (ABTS Assay)
2.4.3. Ferric Ion Reducing Antioxidant Power (FRAP Assay)
2.4.4. Oxygen Radical Absorbance Capacity (ORAC Assay)
2.5. HPLC–DAD Analysis
2.6. Animal Experiment
2.7. Total Antioxidant Capacity Assay (T-AOC) In Vivo
2.8. Q Exactive Analysis
2.9. Statistical Analysis
3. Results and Discussion
3.1. Antioxidant Activity of Flavonoids In Vitro Digestion
3.1.1. DPPH Assay of Flavonoids
3.1.2. ABTS Assay of Flavonoids
3.1.3. FRAP Assay of Flavonoids
3.1.4. ORAC Assay of Flavonoids
3.2. HPLC Analysis of Flavonoids during In Vitro Digestion
3.3. Analysis of Total Antioxidant Capacity and Metabolism of Flavonoids in Rat Plasma and Urine
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chiurchiù, V.; Maccarrone, M. Chronic inflammatory disorders and their redox control: From molecular mechanisms to therapeutic opportunities. Antioxid. Redox Signal. 2011, 15, 2605–2641. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Gan, R.; Li, H.; Yang, M.; McClements, D.J.; Gao, R.; Sun, Q. Absorption, metabolism, and bioactivity of vitexin: Recent advances in understanding the efficacy of an important nutraceutical. Crit. Rev. Food. Sci. Nutr. 2021, 61, 1049–1064. [Google Scholar] [CrossRef]
- Van Hung, P. Phenolic Compounds of Cereals and Their Antioxidant Capacity. Crit. Rev. Food. Sci. Nutr. 2016, 56, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Fraga, C.G.; Croft, K.D.; Kennedy, D.O.; Tomás-Barberán, F.A. The effects of polyphenols and other bioactives on human health. Food Funct. 2019, 10, 514–528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zorzi, M.; Gai, F.; Medana, C.; Aigotti, R.; Morello, S.; Peiretti, P.G. Bioactive Compounds and Antioxidant Capacity of Small Berries. Foods 2020, 9, 623. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Wang, Y.; Wang, J.; Li, P.; Ma, F. Structure-antioxidant capacity relationship of dihydrochalcone compounds in Malus. Food Chem. 2019, 275, 354–360. [Google Scholar] [CrossRef]
- Wen, L.; Zhao, Y.; Jiang, Y. Identification of a flavonoid C-glycoside as potent antioxidant. Free Radic. Biol. Med. 2017, 110, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Berhow, M.A.; Black, M.; Jeffery, E.H. A comparison of the absorption and metabolism of the major quercetin in brassica, quercetin-3-O-sophoroside, to that of quercetin aglycone, in rats. Food Chem. 2020, 311, 125880. [Google Scholar] [CrossRef]
- Ye, H.; Shaw, I.C. Food flavonoid ligand structure/estrogen receptor-α affinity relationships—Toxicity or food functionality? Food Chem. Toxicol. 2019, 129, 328–336. [Google Scholar] [CrossRef]
- Li, S.S.; Wu, J.; Chen, L.G.; Du, H.; Xu, Y.J.; Wang, L.J.; Zhang, H.J.; Zheng, X.C.; Wang, L.S. Biogenesis of C-glycosyl flavones and profiling of flavonoid glycosides in lotus (Nelumbo nucifera). PLoS ONE 2014, 9, e108860. [Google Scholar] [CrossRef]
- Plaza, M.; Pozzo, T.; Liu, J.; Gulshan Ara, K.Z.; Turner, C.; Nordberg Karlsson, E. Substituent effects on in vitro antioxidizing properties, stability, and solubility in flavonoids. J. Agric. Food Chem. 2014, 62, 3321–3333. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Capanoglu, E.; Jassbi, A.R.; Miron, A. Advance on the Flavonoid C-glycosides and Health Benefits. Crit. Rev. Food. Sci. Nutr. 2016, 56, S29–S45. [Google Scholar] [CrossRef] [PubMed]
- Courts, F.L.; Williamson, G. The Occurrence, Fate and Biological Activities of C-glycosyl Flavonoids in the Human Diet. Crit. Rev. Food. Sci. Nutr. 2015, 55, 1352–1367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, J.S.; Islam, M.N.; Ali, M.Y.; Kim, Y.M.; Park, H.J.; Sohn, H.S.; Jung, H.A. The effects of C-glycosylation of luteolin on its antioxidant, anti-Alzheimer’s disease, anti-diabetic, and anti-inflammatory activities. Arch. Pharm. Res. 2014, 37, 1354–1363. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Li, B.; Qiao, M.; Li, J.; Xu, H.; Zhang, L.; Zhang, X. Advances on the in vivo and in vitro glycosylations of flavonoids. Appl. Microbiol. Biotechnol. 2020, 104, 6587–6600. [Google Scholar] [CrossRef]
- Waki, T.; Nakanishi, I.; Matsumoto, K.; Kitajima, J.; Chikuma, T.; Kobayashi, S. Key role of chemical hardness to compare 2,2-diphenyl-1-picrylhydrazyl radical scavenging power of flavone and flavonol O-glycoside and C-glycoside derivatives. Chem. Pharm. Bull. (Tokyo) 2012, 60, 37–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olejnik, A.; Rychlik, J.; Kidoń, M.; Czapski, J.; Kowalska, K.; Juzwa, W.; Olkowicz, M.; Dembczyński, R.; Moyer, M.P. Antioxidant effects of gastrointestinal digested purple carrot extract on the human cells of colonic mucosa. Food Chem. 2016, 190, 1069–1077. [Google Scholar] [CrossRef] [PubMed]
- Czubinski, J.; Wroblewska, K.; Czyzniejewsk, M.; Górnaś, P.; Kachlicki, P.; Siger, A. Bioaccessibility of defatted lupin seed phenolic compounds in a standardized static in vitro digestion system. Food Res. Int. 2019, 116, 1126–1134. [Google Scholar] [CrossRef]
- Liang, S.; Xu, Z.; Ruan, Y.; Niu, T.; Guo, W.; Jiang, W.; Hou, J. Isoquercitrin Attenuates Renal Ischemia/Reperfusion Injury Through Antioxidation, Anti-inflammation, and Antiapoptosis in Mice. Transplant. Proc. 2020, 52, 1014–1019. [Google Scholar] [CrossRef]
- An, F.; Yang, G.; Tian, J.; Wang, S. Antioxidant effects of the orientin and vitexin in Trollius chinensis Bunge in D-galactose-aged mice. Neural Regen. Res. 2012, 7, 2565–2575. [Google Scholar]
- Tremmel, M.; Kiermaier, J.; Heilmann, J. In Vitro Metabolism of Six C-Glycosidic Flavonoids from Passiflora incarnata L. Int. J. Mol. Sci. 2021, 22, 6566. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.L.; Fan, M.X.; Wu, J.L.; Li, N.; Guo, M.Q. Antioxidant and anti-inflammatory properties of flavonoids from lotus plumule. Food Chem. 2019, 277, 706–712. [Google Scholar] [CrossRef] [PubMed]
- Minekus, M.; Alminger, M.; Alvito, P.; Balance, S.; Bohn, T.; Bourlieu, C.; Carrière, F. A standardised static in vitro digestion method suitable for food—An international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brodkorb, A.; Egger, L.; Alminger, M. INFOGEST static in vitro simulation of gastrointestinal food digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Yang, Y.; Gao, J.; Feng, J.; Shang, Y.; Wei, Z. Phenolics and antioxidant activity of bamboo leaves soup as affected by in vitro digestion. Food Chem. Toxicol. 2020, 135, 110941. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Song, J.; Zhang, M.; Wang, H.; Zhang, Y.; Suo, H. Comparison of In Vitro and In Vivo Antioxidant Activities of Six Flavonoids with Similar Structures. Antioxidants 2020, 9, 732. [Google Scholar] [CrossRef] [PubMed]
- Jakubec, P.; Bancirova, M.; Halouzka, V.; Lojek, A.; Ciz, M.; Denev, P.; Cibicek, N.; Vacek, J.; Vostalova, J.; Ulrichova, J.; et al. Electrochemical sensing of total antioxidant capacity and polyphenol content in wine samples using amperometry online-coupled with microdialysis. J. Agric. Food Chem. 2012, 60, 7836–7843. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Lee, S.J.; Yim, D.G.; Hur, S.J. Changes in the Content and Bioavailability of Onion Quercetin and Grape Resveratrol During In Vitro Human Digestion. Foods 2020, 9, 694. [Google Scholar] [CrossRef] [PubMed]
- Pavez-Guajardo, C.; Ferreira, S.R.S.; Mazzutt, S.; Guerra-Valle, M.E.; Sáez-Trautmann, G.; Moreno, J. Influence of In Vitro Digestion on Antioxidant Activity of Enriched Apple Snacks with Grape Juice. Foods 2020, 9, 1681. [Google Scholar] [CrossRef]
- Liu, G.; Ying, D.; Guo, B.; Cheng, L.J.; May, B.; Bird, T.; Sanguansri, L.; Cao, Y.; Augustin, M. Extrusion of apple pomace increases antioxidant activity upon in vitro digestion. Food Funct. 2019, 10, 951–963. [Google Scholar] [CrossRef] [PubMed]
- Bouayed, J.; Hoffmann, L.; Bohn, T. Total phenolics, flavonoids, anthocyanins and antioxidant activity following simulated gastro-intestinal digestion and dialysis of apple varieties: Bioaccessibility and potential uptake. Food Chem. 2011, 128, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Lucas-González, R.; Ángel Pérez-Álvarez, J.; Moscaritolo, S.; Fernández-López, J.; Sacchetti, G.; Viuda-Martos, M. Evaluation of polyphenol bioaccessibility and kinetic of starch digestion of spaghetti with persimmon (Dyospyros kaki) flours coproducts during in vitro gastrointestinal digestion. Food Chem. 2021, 338, 128142. [Google Scholar] [CrossRef] [PubMed]
- Dudonné, S.; Vitrac, X.; Coutière, P.; Woillez, M.; Mérillon, J.M. Comparative study of antioxidant properties and total phenolic content of 30 plant extracts of industrial interest using DPPH, ABTS, FRAP, SOD, and ORAC assays. J. Agric. Food Chem. 2009, 57, 1768–1774. [Google Scholar] [CrossRef] [PubMed]
- Lucas-González, R.; Viuda-Martos, M.; Pérez Álvarez, J.A.; Fernández-López, J. Changes in bioaccessibility, polyphenol profile and antioxidant potential of flours obtained from persimmon fruit (Diospyros kaki) co-products during in vitro gastrointestinal digestion. Food Chem. 2018, 256, 252–258. [Google Scholar] [CrossRef]
- José Jara-Palacios, M.; Gonçalves, S.; Hernanz, D.; Heredia, F.J.; Romano, A. Effects of in vitro gastrointestinal digestion on phenolic compounds and antioxidant activity of different white winemaking byproducts extracts. Food Res. Int. 2018, 109, 433–439. [Google Scholar] [CrossRef]
- Huang, H.; Sun, Y.; Lou, S.; Li, H.; Ye, X. In vitro digestion combined with cellular assay to determine the antioxidant activity in Chinese bayberry (Myrica rubra Sieb. et Zucc.) fruits: A comparison with traditional methods. Food Chem. 2014, 146, 363–370. [Google Scholar] [CrossRef]
- Zhang, Q.; Yang, W.; Liu, J. Identification of Six Flavonoids as Novel Cellular Antioxidants and Their Structure-Activity Relationship. Oxidative Med. Cell. Longev. 2020, 2020, 4150897. [Google Scholar] [CrossRef]
- Kahle, K.; Kempf, M.; Schreier, P.; Scheppach, W.; Schrenk, D.; Kautenburger, T.; Hecker, D.; Huemmer, W.; Ackermann, M.; Richling, E. Intestinal transit and systemic metabolism of apple polyphenols. Eur. J. Nutr. 2011, 50, 507–522. [Google Scholar] [CrossRef]
- Gulcin, İ. Antioxidants and antioxidant methods: An updated overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef] [Green Version]
- Xue, Y.; Liu, Y.; Xie, Y.; Cong, C.; Wang, G.; An, L.; Teng, Y.; Chen, M.; Zhang, L. Antioxidant activity and mechanism of dihydrochalcone C-glycosides: Effects of C-glycosylation and hydroxyl groups. Phytochemistry 2020, 179, 112393. [Google Scholar] [CrossRef]
- Pellegrini, M.; Lucas-Gonzalez, R.; Sayas-Barberá, E.; Fernández-López, J.; Pérez-Álvarez, J.A.; Viuda-Martos, M. Bioaccessibility of Phenolic Compounds and Antioxidant Capacity of Chia (Salvia hispanica L.) Seeds. Plant Foods Hum. Nutr. 2018, 73, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Xiang, D.; Wang, C.G.; Wang, W.Q.; Shi, C.Y.; Xiong, W.; Wang, M.D.; Fang, J.G. Gastrointestinal stability of dihydromyricetin, myricetin, and myricitrin: An in vitro investigation. Int. J. Food Sci. Nutr. 2017, 68, 704–711. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Tsao, R.; Wang, X.; Jiang, L.; Sun, Y.; Xiong, H. Phenolics of Yellow Pea (Pisum sativum L.) Hulls, Their Plasma and Urinary Metabolites, Organ Distribution, and In Vivo Antioxidant Activities. J. Agric. Food Chem. 2021, 69, 5013–5025. [Google Scholar] [CrossRef] [PubMed]
- Valentová, K.; Vrba, J.; Bancířová, M.; Ulrichová, J.; Křen, V. Isoquercitrin: Pharmacology, toxicology, and metabolism. Food Chem. Toxicol. 2014, 68, 267–282. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ouyang, L.; Mai, X.; Wang, H.; Liu, S.; Zeng, H.; Chen, T.; Li, J. Use of UHPLC-QTOF-MS/MS with combination of in silico approach for distributions and metabolites profile of flavonoids after oral administration of Niuhuang Shangqing tablets in rats. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2019, 1114–1115, 55–70. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Tie, X.; Bao, B.; Wu, X.; Zhang, Y. Metabolism of flavone C-glycosides and p-coumaric acid from antioxidant of bamboo leaves (AOB) in rats. Br. J. Nutr. 2007, 97, 484–494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ninfali, P.; Antonini, E.; Frati, A.; Scarpa, E.S. C-Glycosyl Flavonoids from Beta vulgaris Cicla and Betalains from Beta vulgaris rubra: Antioxidant, Anticancer and Antiinflammatory Activities-A Review. Phytother. Res. 2017, 31, 871–884. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J. Dietary flavonoid aglycones and their glycosides: Which show better biological significance? Crit. Rev. Food Sci. Nutr. 2017, 57, 1874–1905. [Google Scholar] [CrossRef]
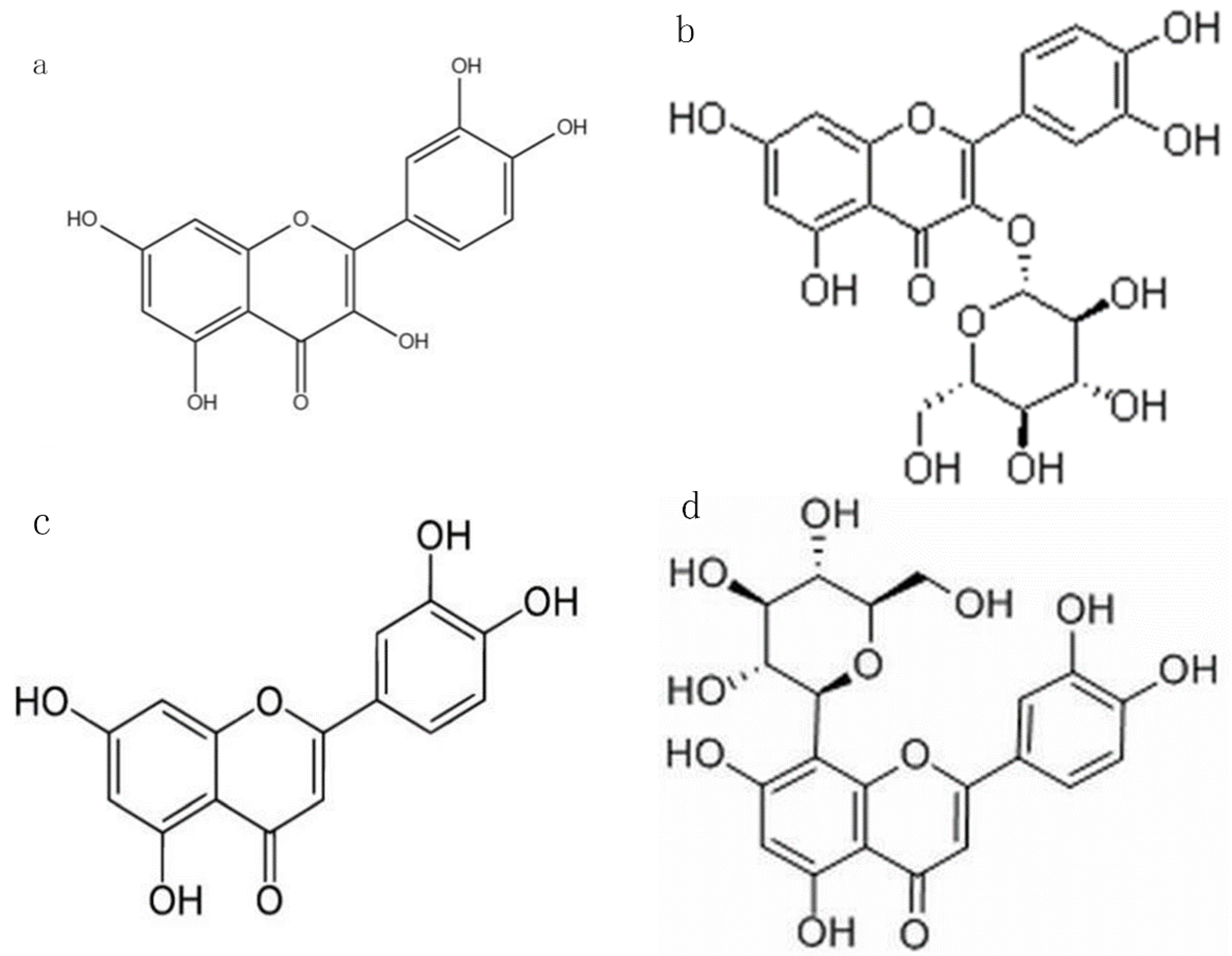
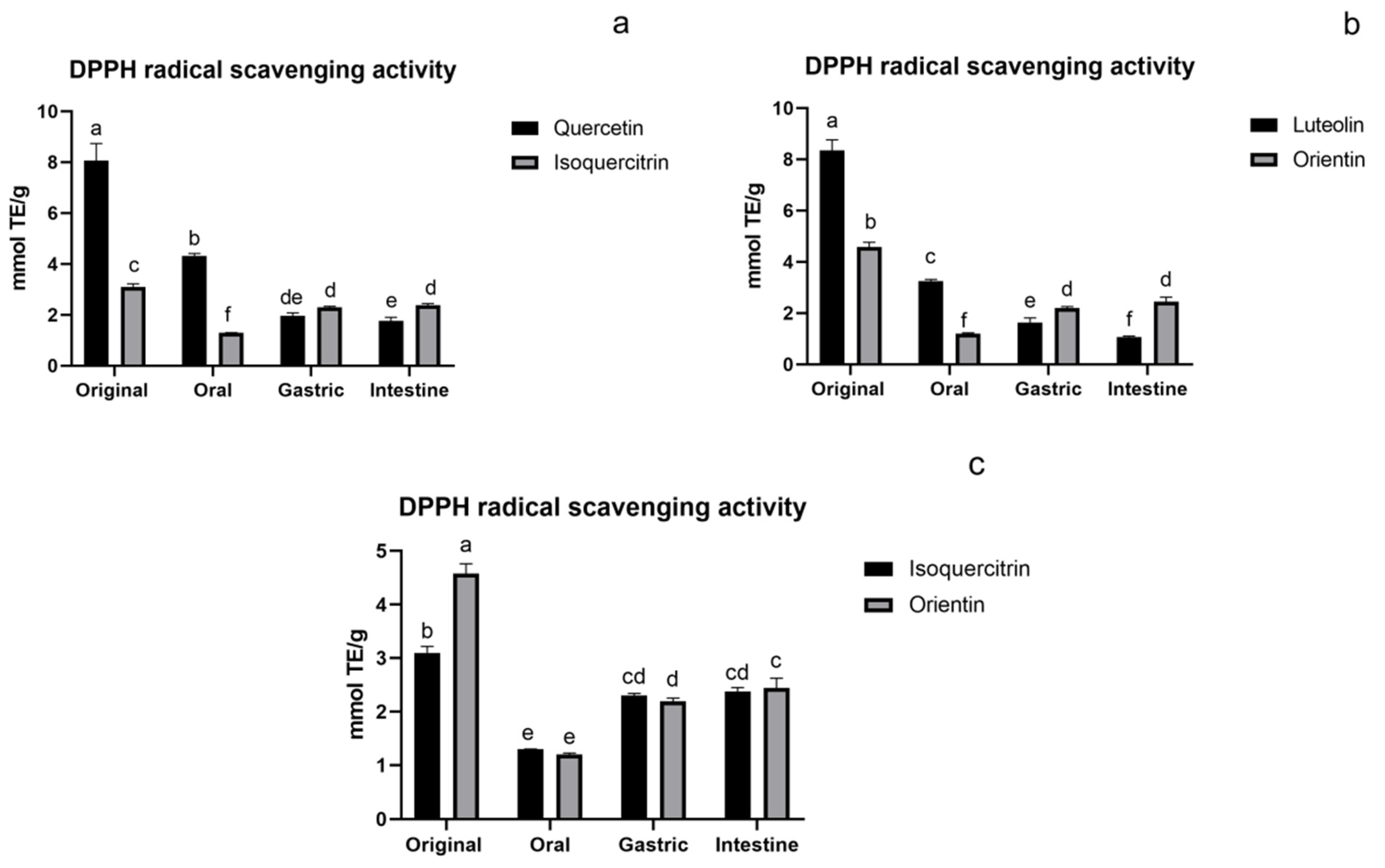
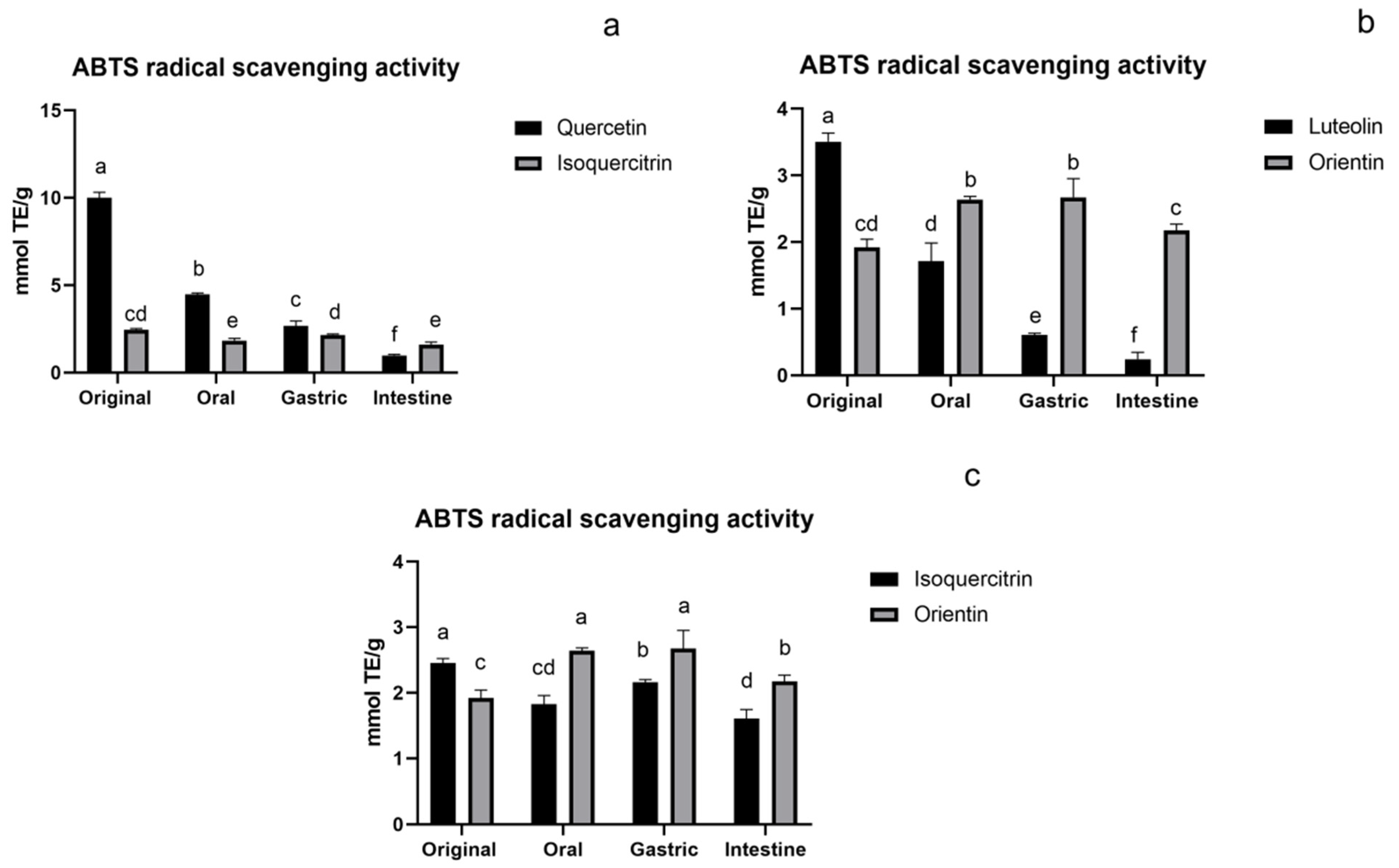
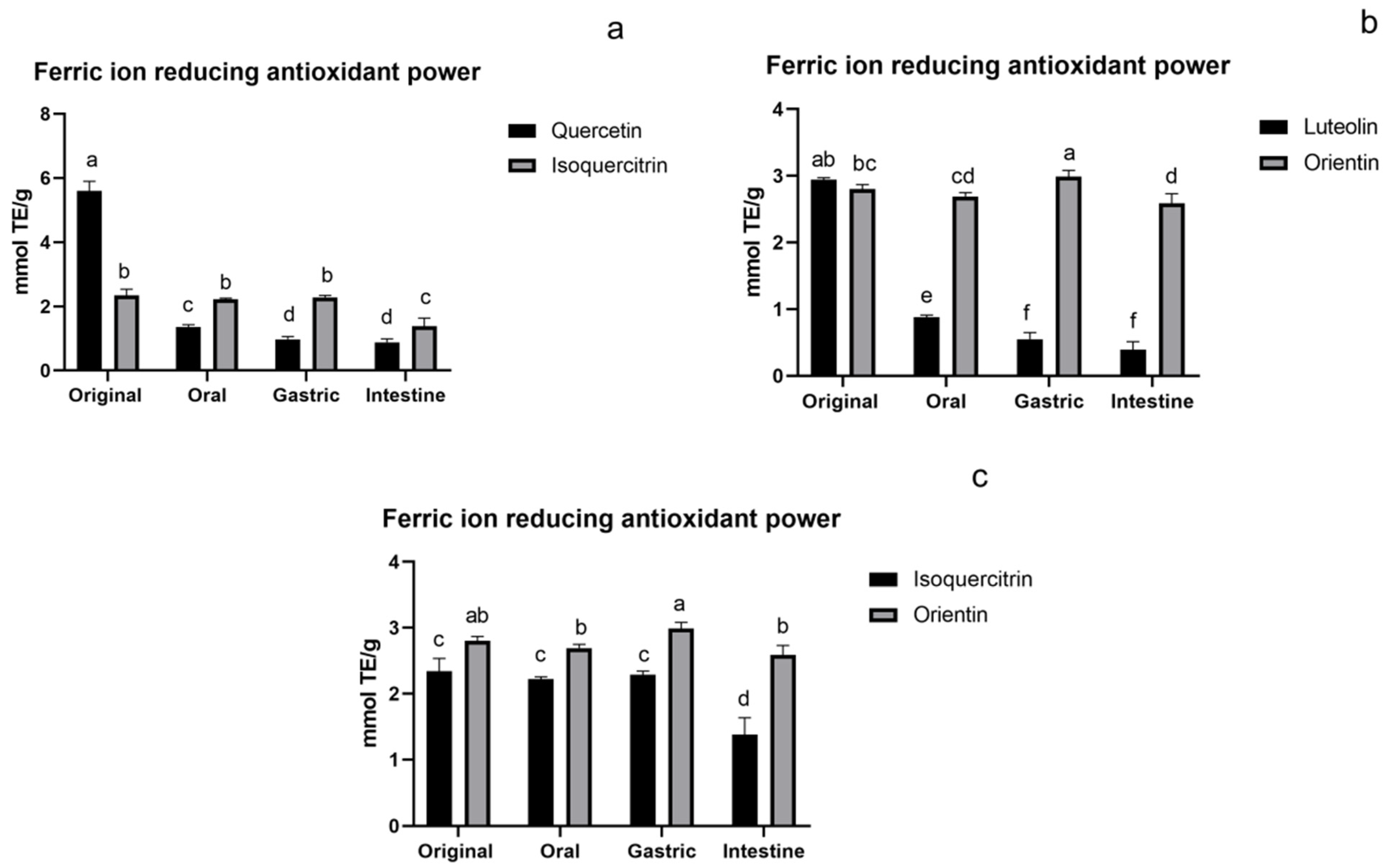

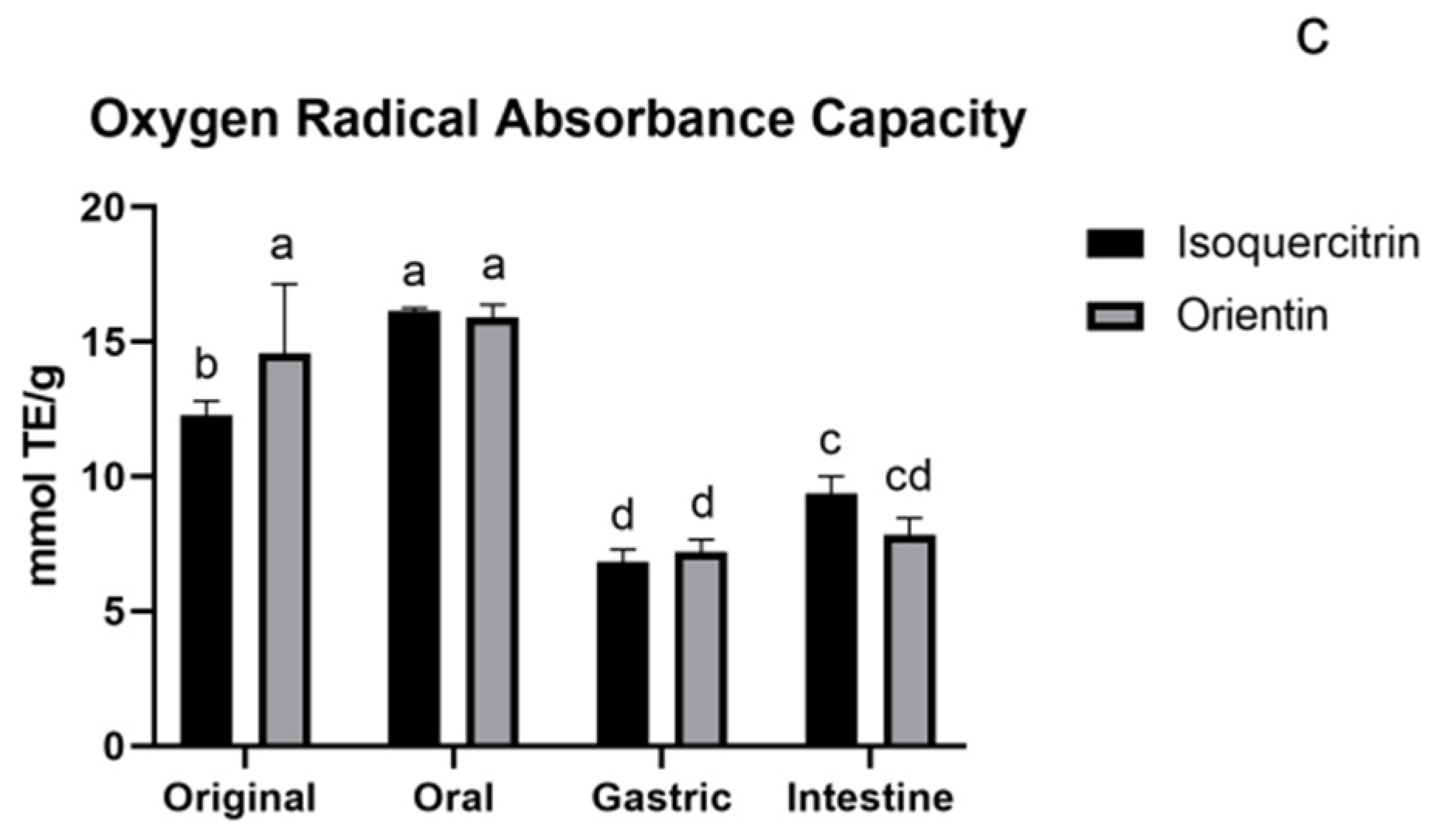
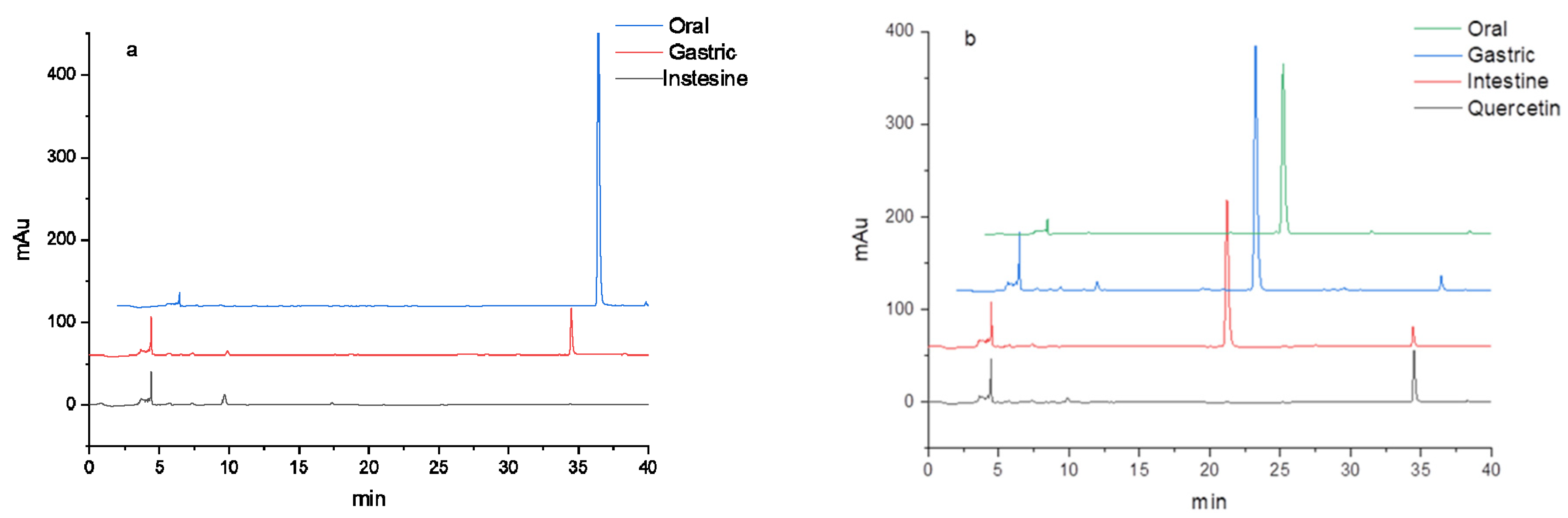

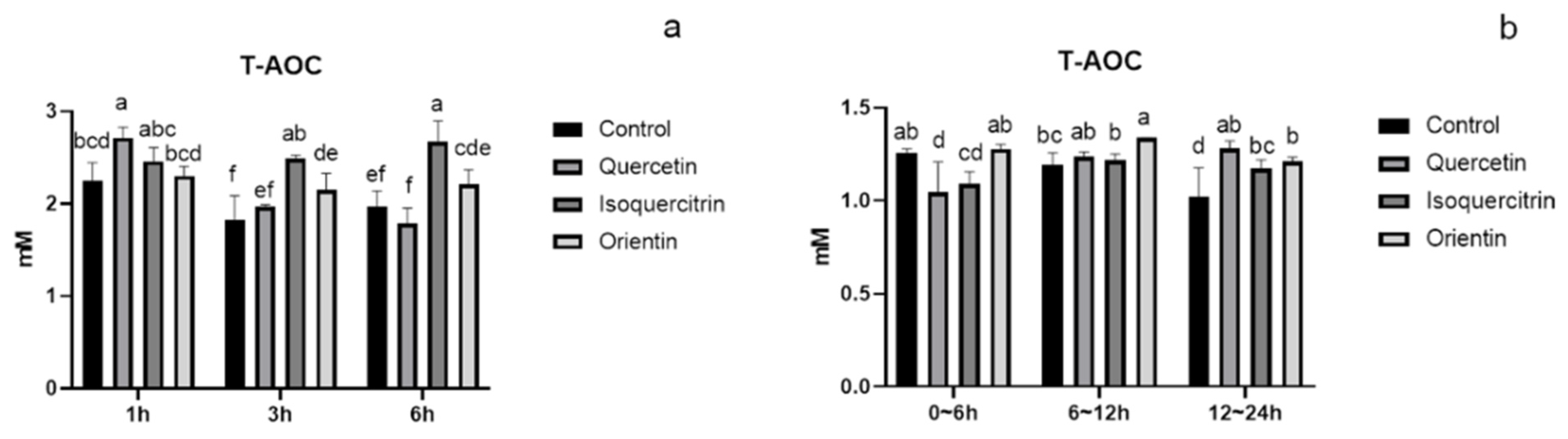
| Compounds | Stage (Content mg/g) | |||
|---|---|---|---|---|
| Original | Oral | Gastric | Intestine | |
| Quercetin | 1.00 a | 0.45 ± 0.12 b | 0.04 ± 0.01 c | ND |
| Isoquercitrin | 1.00 a | 0.65 ± 0.13 bc | 0.73 ± 0.08 b | 0.54 ± 0.08 c |
| Luteolin | 1.00 a | 0.84 ± 0.11 b | 0.13 ± 0.02 c | ND |
| Orientin | 1.00 a | 0.67 ± 0.01 b | 0.68 ± 0.10 b | 0.74 ± 0.03 b |
| NO | tR/Min | [M − H]− /[M + H]+ | PPM | Fragment Ions (m/z) | Mode | Formula | Transformations | Location | |
|---|---|---|---|---|---|---|---|---|---|
| P | U | ||||||||
| 1 | 1.247 | 377.053 | 4.21 | 301.038 | Neg | C17H14O10 | Hydration, Oxidat- ion, Acetylation | − | + |
| 2 | 5.419 | 477.067 | −1.83 | 301.035 151.002 | Neg | C21H18O13 | Glucuronide Conjugation | − | + |
| 3 | 5.552 | 447.091 | −2.92 | 153.017 | Pos | C21H18O11 | Dehydration, Gluc- oside Conjugation | − | + |
| 4 | 5.644 | 477.067 | −1.45 | 301.035 151.002 | Neg | C21H18O13 | Glucuronide Conjugation | − | + |
| 5 | 5.712 | 447.091 | −2.79 | 153.017 | Pos | C21H18O11 | Dehydration, Gluc- oside Conjugation | − | + |
| 6 | 9.047 | 297.047 | −1.83 | 151.057 | Neg | C16H10O6 | Dehydration, Methylation | − | + |
| 7 | 11.850 | 395.243 | −2.64 | 329.230 | Neg | C16H12O10 S | Methylation, Sulfation | − | + |
| 8 | 16.501 | 315.181 | −1.65 | 300.030 | Neg | C16H12O7 | Methylation | − | + |
| 9 | 22.344 | 311.020 | 3.10 | 151.110 | Neg | C16H8O7 | Didesaturation, Methylation | + | + |
| 10 | 24.500 | 329.233 | −2.12 | 301.200 151.040 | Neg | C16H10O8 | Desaturation, Oxid- ation, Methylation | + | + |
| 11 | 24.850 | 463.289 | −1.98 | 151.280 | Neg | C21H20O12 | Glucoside Conjugation | + | − |
| NO | tR/Min | [M − H]− /[M + H]+ | PPM | Fragment Ions (m/z) | Mode | Formula | Transformations | Location | |
|---|---|---|---|---|---|---|---|---|---|
| P | U | ||||||||
| 1 | 0.923 | 191.027 | −4.53 | 151.005 | Neg | C6H8O7 | Desaturation, Oxidation | + | − |
| 2 | 1.107 | 335.042 | 2.94 | 301.081 151.039 | Neg | C15H12O9 | Hydration, Oxidation | − | + |
| 3 | 1.118 | 491.082 | −1.71 | 315.051 300.027 | Neg | C22H20O13 | Desaturation, Oxida- tion, Methylation | − | + |
| 4 | 1.127 | 191.027 | −4.53 | 151.075 | Neg | C6H8O7 | Desaturation, Oxidation | + | − |
| 5 | 1.135 | 477.075 | −1.09 | 301.035 151.002 | Neg | C21H18O13 | Glucuronide Conjugation | − | + |
| 6 | 1.185 | 477.067 | −1.64 | 301.035 151.002 | Neg | C21H18O13 | Desaturation, Oxidation | − | + |
| 7 | 1.263 | 491.082 | −2.06 | 315.051 300.027 | Neg | C22H20O13 | Desaturation, Methylation | − | + |
| 8 | 1.269 | 491.090 | −1.96 | 315.051 300.027 | Neg | C22H20O13 | Desaturation, Oxida- tion, Methylation | − | + |
| 9 | 4.406 | 653.099 | −0.57 | 301.035 151.002 | Neg | C27H26O19 | Desaturation, Gluc- uronide Conjugation | + | + |
| 10 | 4.474 | 655.113 | −1.26 | 303.049 | Pos | C27H26O19 | Desaturation, Gluc- uronide Conjugation | + | + |
| 11 | 5.987 | 491.083 | −0.71 | 315.051 300.027 | Neg | C22H20O13 | Dehydration, Methylation | − | + |
| 12 | 6.283 | 491.090 | −1.32 | 315.051 300.027 | Neg | C22H20O13 | Desaturation, Oxida- tion, Methylation | − | + |
| 13 | 7.810 | 329.233 | −0.52 | 301.200 151.040 | Neg | C16H10O8 | Desaturation, Oxid- ation, Methylation | − | + |
| 14 | 8.908 | 317.078 | −1.26 | 153.018 | Pos | C16H12O7 | Methylation | − | + |
| 15 | 11.800 | 395.201 | 0.38 | 380.080 | Neg | C16H12O10 S | Methylation, Sulfation | − | + |
| 16 | 14.530 | 315.058 | −1.16 | 300.027 151.002 | Neg | C16H12O7 | Methylation | − | + |
| 17 | 15.593 | 557.022 | −3.15 | 301.036 | Neg | C21H18O16 S | Desaturation, Sulfation | + | + |
| 18 | 15.604 | 557.023 | −3.15 | 301.059 | Neg | C21H18O16 S | Desaturation, Oxida- tion, Sulfation | + | + |
| 19 | 22.620 | 301.165 | −3.94 | 151.004 | Neg | C15H10O7 | Deglycosylation | + | + |
| 20 | 32.814 | 315.058 | −2.81 | 300.027 151.003 | Neg | C16H12O7 | Methylation | − | + |
| NO | tR/Min | [M − H]− /[M + H]+ | PPM | Fragment Ions (m/z) | Mode | Formula | Transformations | Location | |
|---|---|---|---|---|---|---|---|---|---|
| P | U | ||||||||
| 1 | 6.700 | 447.093 | −0.67 | 357.135 | Neg | C21H20O11 | − | + | |
| 2 | 12.510 | 447.093 | −0.99 | 357.240 | Neg | C21H20O11 | − | + | |
| 3 | 19.310 | 445.272 | −0.74 | 297.250 | Neg | C21H18O11 | Dehydration | − | + |
| 4 | 27.445 | 465.102 | −4.52 | 357.736 | Neg | C21H22O12 | Hydration | + | − |
| 5 | 27.445 | 465.101 | −4.52 | 357.736 | Neg | C21H22O12 | Reduction | + | − |
| 6 | 27.529 | 465.101 | −4.06 | 357.736 | Neg | C21H22O12 | Reduction | + | − |
| 7 | 27.529 | 465.102 | −4.06 | 357.736 | Neg | C21H22O12 | Hydration | + | − |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, L.; Deng, Z.; Zhang, J.; Dong, H.; Wang, W.; Xing, B.; Liu, X. Comparison of Flavonoid O-Glycoside, C-Glycoside and Their Aglycones on Antioxidant Capacity and Metabolism during In Vitro Digestion and In Vivo. Foods 2022, 11, 882. https://doi.org/10.3390/foods11060882
Xie L, Deng Z, Zhang J, Dong H, Wang W, Xing B, Liu X. Comparison of Flavonoid O-Glycoside, C-Glycoside and Their Aglycones on Antioxidant Capacity and Metabolism during In Vitro Digestion and In Vivo. Foods. 2022; 11(6):882. https://doi.org/10.3390/foods11060882
Chicago/Turabian StyleXie, Liangqin, Zeyuan Deng, Jie Zhang, Huanhuan Dong, Wei Wang, Banghuai Xing, and Xiaoru Liu. 2022. "Comparison of Flavonoid O-Glycoside, C-Glycoside and Their Aglycones on Antioxidant Capacity and Metabolism during In Vitro Digestion and In Vivo" Foods 11, no. 6: 882. https://doi.org/10.3390/foods11060882
APA StyleXie, L., Deng, Z., Zhang, J., Dong, H., Wang, W., Xing, B., & Liu, X. (2022). Comparison of Flavonoid O-Glycoside, C-Glycoside and Their Aglycones on Antioxidant Capacity and Metabolism during In Vitro Digestion and In Vivo. Foods, 11(6), 882. https://doi.org/10.3390/foods11060882






