Kaempferol Regresses Carcinogenesis through a Molecular Cross Talk Involved in Proliferation, Apoptosis and Inflammation on Human Cervical Cancer Cells, HeLa
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Maintenance and Preparation of Kaempferol Solutions
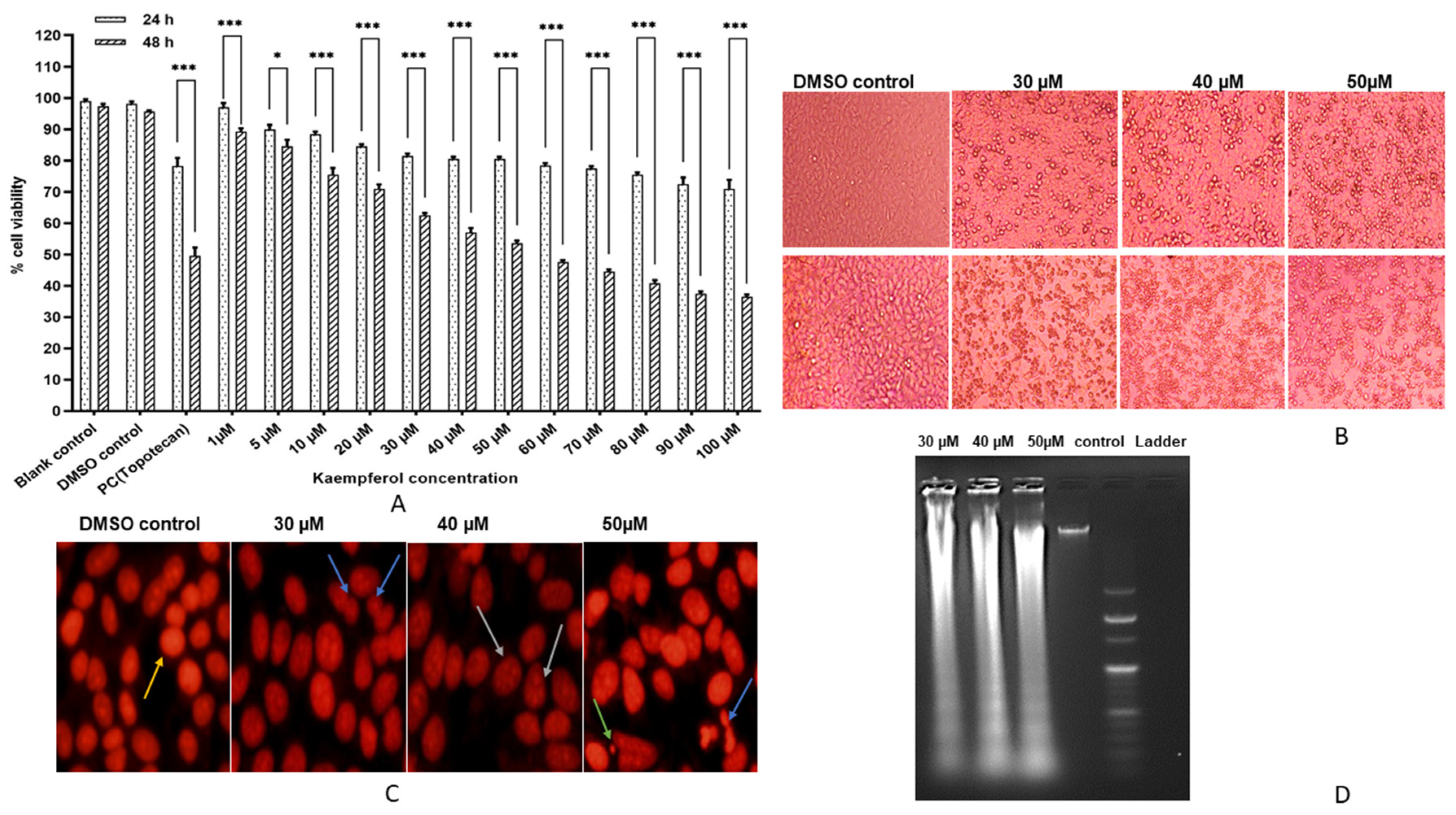
2.2. Cell Viability Assay
2.3. Analysis of Nuclear Morphology of Apoptotic Cells Using Propidium Iodide
2.4. DNA Ladder Assay
2.5. Cell Cycle Analysis
2.6. Quantitation of Cell Apoptosis
2.7. Detection of Mitochondrial Membrane Potential (∆ψm)
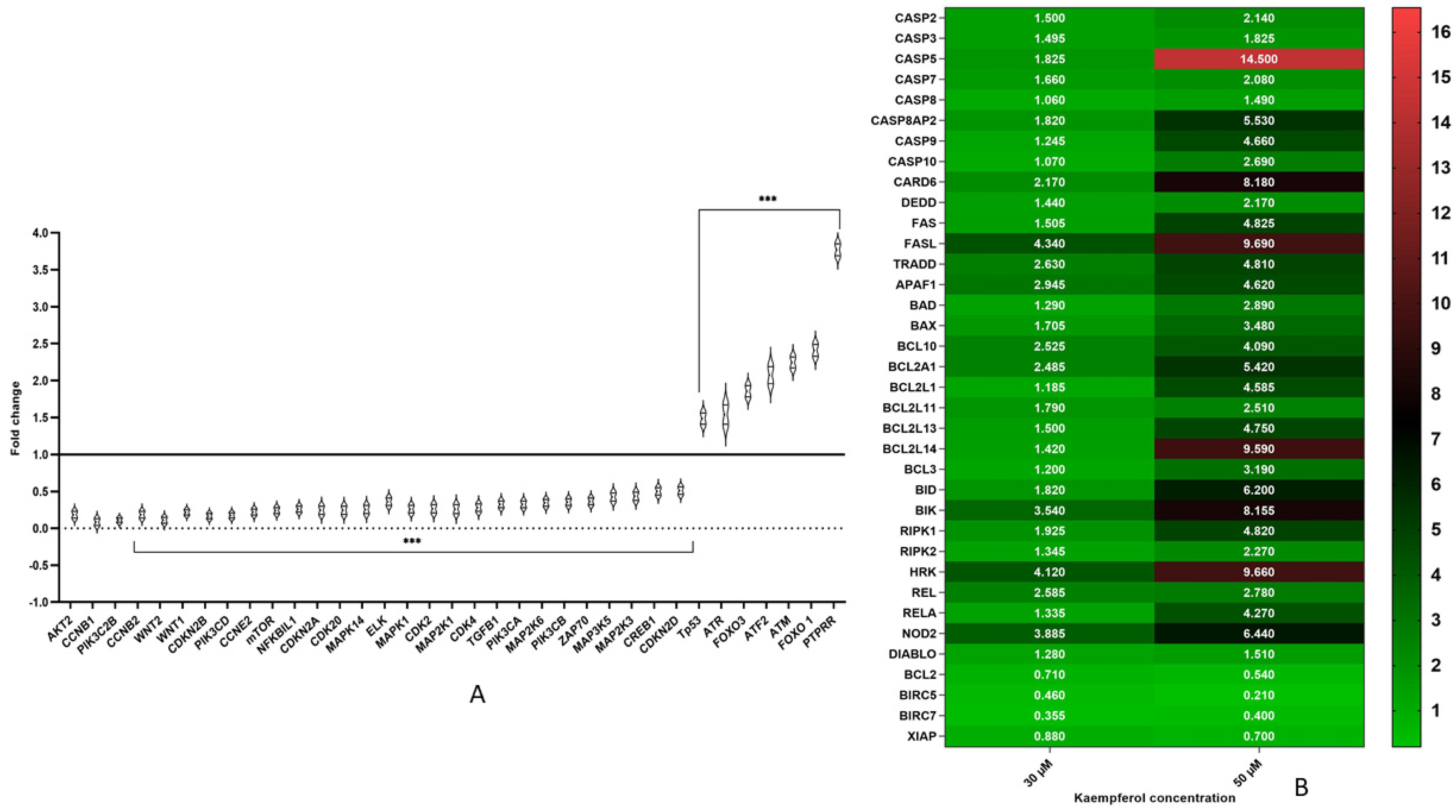
2.8. Gene Expression by TaqMan Apoptosis Array
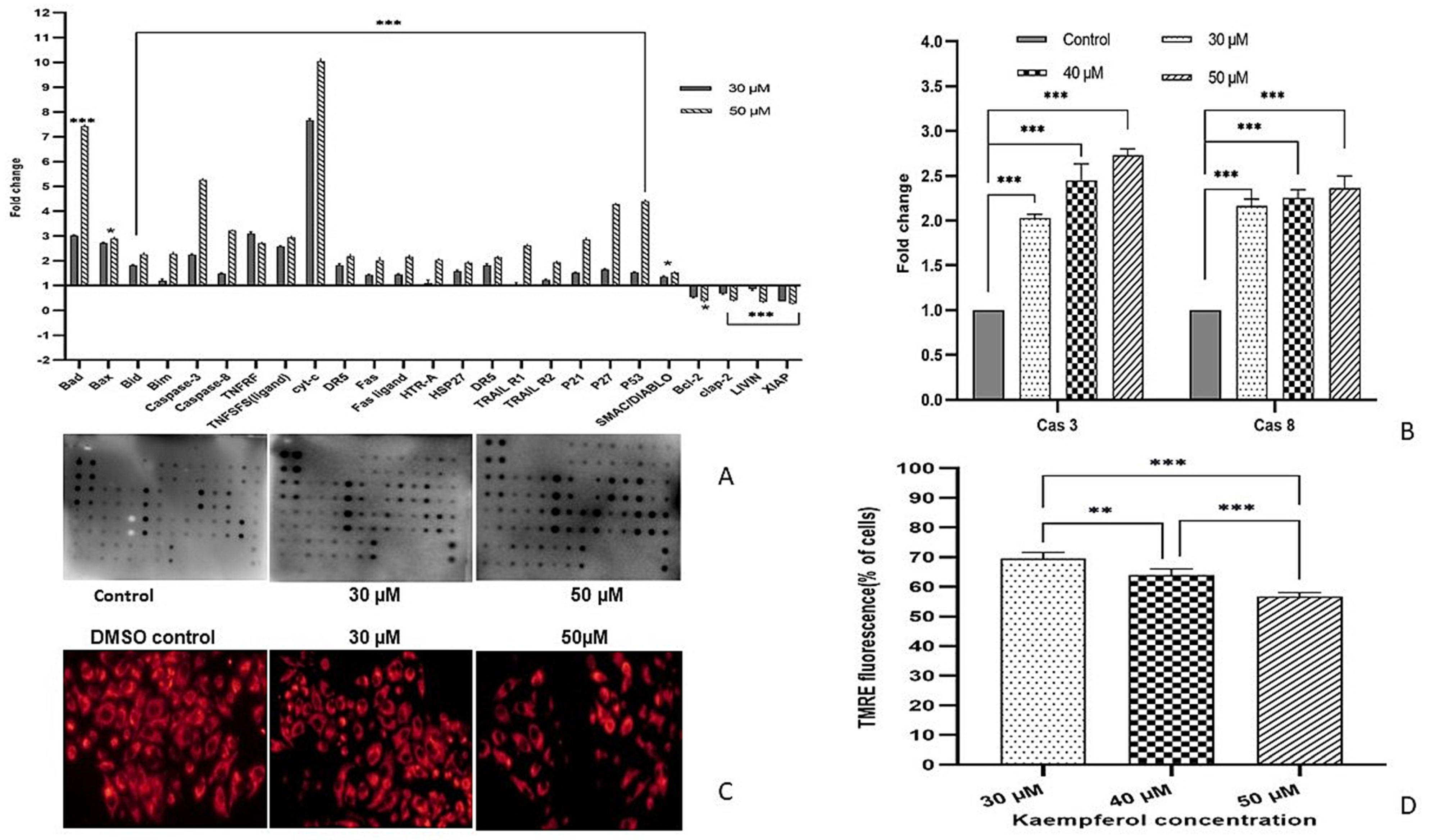
2.9. Quantitation of Apoptosis Related Protein
2.10. Detection of Caspase Multiplex Activity
2.11. Quantitation of GSH Activity
2.12. Quantitation of Inflammatory Cytokines Expression through Antibody Array
2.13. Analysis of Phosphorylated Proteins Expression Pertaining to Various Signalling Pathways
2.14. Statistical Analysis
3. Results
3.1. Kaempferol Inhibits HeLa Cell Proliferation
3.2. Kaempferol Mediates Nuclear Aberrations and DNA Fragmentation
3.3. Kaempferol Induces G2/M Arrest in HeLa Cells
3.4. Kaempferol Increases Early Apoptosis in HeLa Cells
3.5. Kaempferol Modulates Expression of Various Genes Involved in Cell Cycle Regulation and Signalling Pathways
3.6. Kaempferol Mediates Apoptosis Via Both Extrinsic and Intrinsic Pathways
3.7. Modulation of Pro-Survival and Anti-Survival Proteins by Kaempferol
3.8. Kaempferol Induces Apoptosis by Activating Caspase-3 and Caspase-8
3.9. Kaempferol Reduces ∆ψm and Fluorescence Intensity
3.10. Kaempferol Ameliorates Inflammatory Response by Altering Pro-Inflammatory and Anti-Inflammatory Cytokines/Proteins
3.11. Kaempferol Upregulates GSH Activity
3.12. Kaempferol Suppresses Cell Growth, Survival and Inflammation by Regulating Aberrant MAPK, PI3K/AKT/mTOR, NF-kB and JAK-STAT Pathways
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Sudhakar, A. History of cancer, ancient and modern treatment methods. J. Cancer Sci. Ther. 2009, 1, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehta, R.G.; Murillo, G.; Naithani, R.; Peng, X. Cancer chemoprevention by natural products: How far have we come? Pharm. Res. 2010, 27, 950–961. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.P.; Dhanalakshmi, S.; Agarwal, R. Phytochemicals as cell cycle modulators a less toxic approach in halting human cancers. Cell Cycle 2002, 1, 155–160. [Google Scholar] [CrossRef] [Green Version]
- Imran, M.; Shah, Z.A.; Imran, A.; Umair, M.; Peters, D.G.; Mubarak, M.S. Chemo—preventive and therapeutic effect of the dietary flavonoid kaempferol: A comprehensive review. Phytother. Res. 2019, 33, 263–275. [Google Scholar] [CrossRef]
- Raina, R.; Afroze, N.; Sundaram, M.K.; Haque, S.; Bajbouj, K.; Hamad, M.; Hussain, A. Chrysin inhibits propagation of HeLa cells by attenuating cell survival and inducing apoptotic pathways. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 2206–2220. [Google Scholar]
- Kedhari Sundaram, M.; Raina, R.; Afroze, N.; Bajbouj, K.; Hamad, M.; Haque, S.; Hussain, A. Quercetin modulates signaling pathways and induces apoptosis in cervical cancer cells. Biosci. Rep. 2019, 39, BSR20190720. [Google Scholar] [CrossRef] [Green Version]
- You, Y.; Wang, R.; Shao, N.; Zhi, F.; Yang, Y. luteolin suppresses tumor proliferation through inducing apoptosis and autophagy via MaPK activation in glioma. Onco Targets Ther. 2019, 12, 2383. [Google Scholar] [CrossRef] [Green Version]
- Raj, L.; Ide, T.; Gurkar, A.U.; Foley, M.; Schenone, M.; Li, X.; Tolliday, N.J.; Golub, T.R.; Carr, S.A.; Shamji, A.F.; et al. Selective killing of cancer cells by a small molecule targeting the stress response to, R.O.S. Nature 2011, 475, 231–234. [Google Scholar] [CrossRef]
- Bostan, M.; Mihaila, M.; Hotnog, C.; Bleotu, G.A.C.; Anton, G.; Roman, V.; Brasoveanu, L.I. Modulation of Apoptosis in Colon Cancer Cells by Bioactive Compounds. Color Cancer Pathog. Treat. 2016, 1174–1446. [Google Scholar]
- Matthews, H.K.; Bertoli, C.; de Bruin, R.A.M. Cell cycle control in cancer. Nat. Rev. Mol. Cell Biol. 2021, 23, 1–15. [Google Scholar] [CrossRef]
- Elmore, S. Apoptosis: A Review of Programmed Cell Death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Xue, L. Kaempferol Suppresses Proliferation and Induces Cell Cycle Arrest, Apoptosis, and DNA Damage in Breast Cancer Cells. Oncol. Res. 2020, 27, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Tu, L.Y.; Bai, H.H.; Cai, J.Y.; Deng, S.P. The Mechanism of Kaempferol Induced Apoptosis and Inhibited Proliferation in Human Cervical Cancer SiHa Cell: From Macro to Nano. Scanning 2016, 38, 644–653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karunagaran, D.; Rashmi, R.; Kumar, T.R. Induction of apoptosis by curcumin and its implications for cancer therapy. Curr. Cancer Drug Targets 2005, 5, 117–129. [Google Scholar] [CrossRef]
- Huang, C.-Y.; Han, Z.; Li, X.; Xie, H.-H.; Zhu, S.-S. Mechanism of EGCG promoting apoptosis of MCF-7 cell line in human breast cancer. Oncol. Lett. 2017, 14, 3623–3627. [Google Scholar] [CrossRef] [Green Version]
- Ahn, W.S.; Huh, S.W.; Bae, S.-M.; Lee, I.P.; Lee, J.M.; Namkoong, S.E.; Kim, C.K.; Sin, J.-I. A major constituent of green tea, EGCG, inhibits the growth of a human cervical cancer cell line, CaSki cells, through apoptosis, G1 arrest, and regulation of gene expression. DNA Cell Biol. 2003, 22, 217–224. [Google Scholar] [CrossRef]
- Lin, H.; Tang, H.; Davis, F.B.; Davis, P.J. Resveratrol and apoptosis. Ann. N. Y. Acad. Sci. 2011, 1215, 79–88. [Google Scholar] [CrossRef]
- Kadioglu, O.; Nass, J.; Saeed, M.E.M.; Schuler, B.; Efferth, T. Kaempferol is an anti-inflammatory compound with activity towards NF-κB pathway proteins. Anticancer Res. 2015, 35, 2645–2650. [Google Scholar]
- Alam, W.; Khan, H.; Shah, M.A.; Cauli, O.; Saso, L. Kaempferol as a dietary anti-inflammatory agent: Current therapeutic standing. Molecules 2020, 25, 4073. [Google Scholar] [CrossRef]
- Meeran, S.M.; Katiyar, S.K. Cell cycle control as a basis for cancer chemoprevention through dietary agents. Front. Biosci. J. Virtual Libr. 2008, 13, 2191. [Google Scholar] [CrossRef] [Green Version]
- Pal, H.C.; Sharma, S.; Elmets, C.A.; Athar, M.; Afaq, F. Fisetin inhibits growth, induces G 2/M arrest and apoptosis of human epidermoid carcinoma A 431 cells: Role of mitochondrial membrane potential disruption and consequent caspases activation. Exp. Dermatol. 2013, 22, 470–475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kasha, E.; Moradzadeh, M.; Mohamadkhani, A.; Erfanian, S. ScienceDirect Kaempferol increases apoptosis in human cervical cancer HeLa cells via PI3K/AKT and telomerase pathways. Biomed. Pharmacother. 2017, 89, 573–577. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.; Choi, K.; Hwang, K. Kaempferol, a phytoestrogen, suppressed triclosan-induced epithelial-mesenchymal transition and metastatic-related behaviors of MCF-7 breast cancer cells. Environ. Toxicol Pharmacol. 2017, 49, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Fang, X.; Ge, L.; Cao, F.; Zhao, L.; Wang, Z.; Xiao, W. Antitumor, antioxidant and anti-inflammatory activities of kaempferol and its corresponding glycosides and the enzymatic preparation of kaempferol. PLoS ONE 2018, 13, e0197563. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Z.; Ye, G.; Huang, B. Kaempferol alleviates the interleukin-1β-induced inflammation in rat osteoarthritis chondrocytes via suppression of NF-κB. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2017, 23, 3925. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liao, W.; Chen, L.; Ma, X.; Jiao, R.; Li, X.; Wang, Y. Protective effects of kaempferol against reactive oxygen species-induced hemolysis and its antiproliferative activity on human cancer cells. Eur. J. Med. Chem. 2016, 114, 24–32. [Google Scholar] [CrossRef]
- Rajendran, P.; Rengarajan, T.; Nandakumar, N.; Palaniswami, R.; Nishigaki, Y.; Nishigaki, I. Kaempferol, a potential cytostatic and cure for inflammatory disorders. Eur. J. Med. Chem. 2014, 86, 103–112. [Google Scholar] [CrossRef]
- Yeon, M.J.; Lee, M.H.; Kim, D.H.; Yang, J.Y.; Woo, H.J.; Kwon, H.J.; Moon, C.; Kim, S.-H.; Kim, J.-B. Anti-inflammatory effects of Kaempferol on Helicobacter pylori-induced inflammation. Biosci. Biotechnol. Biochem. 2019, 83, 166–173. [Google Scholar] [CrossRef]
- He, M.; Xia, L.; Li, J. Potential Mechanisms of Plant-Derived Natural Products in the Treatment of Cervical Cancer. Biomolecules 2021, 11, 1539. [Google Scholar] [CrossRef]
- Basnet, P.; Skalko-Basnet, N. Curcumin: An anti-inflammatory molecule from a curry spice on the path to cancer treatment. Molecules 2011, 16, 4567–4598. [Google Scholar] [CrossRef] [Green Version]
- García-Lafuente, A.; Guillamón, E.; Villares, A.; Rostagno, M.A.; Martínez, J.A. Flavonoids as anti-inflammatory agents: Implications in cancer and cardiovascular disease. Inflamm. Res. 2009, 58, 537–552. [Google Scholar] [CrossRef] [PubMed]
- Kim, R.; Emi, M.; Tanabe, K. Cancer cell immune escape and tumor progression by exploitation of anti-inflammatory and pro-inflammatory responses. Cancer Biol. Ther. 2005, 4, 924–933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, W.; Tsai, S.; Peng, S.; Lin, M.; Chiang, J. Kaempferol induces autophagy through AMPK and AKT signaling molecules and causes G2/M arrest via downregulation of CDK1/cyclin B in SK-HEP-1 human hepatic cancer cells. Int. J. Oncol. 2013, 42, 2069–2077. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Choi, K. Anti-cancer Effect and Underlying Mechanism (s) of Kaempferol, a Phytoestrogen, on the Regulation of Apoptosis in Diverse Cancer Cell Models. Toxicol. Res. 2013, 29, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Raina, R.; Pramodh, S.; Rais, N.; Haque, S.; Shafarin, J.; Bajbouj, K.; Hamad, M.; Hussain, A. Luteolin inhibits proliferation, triggers apoptosis and modulates Akt/mTOR and MAP kinase pathways in HeLa cells. Oncol. Lett. 2021, 21, 1. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Cheng, Y.; Qu, W.; Sun, Y.; Wang, Z.; Wang, H.; Tian, B. Fisetin, a dietary flavonoid, induces cell cycle arrest and apoptosis through activation of p53 and inhibition of NF-kappa B pathways in bladder cancer cells. Basic Clin. Pharmacol. Toxicol. 2011, 108, 84–93. [Google Scholar] [CrossRef]
- Dang, Q.; Song, W.; Xu, D.; Ma, Y.; Li, F.; Zeng, J.; Zhu, G.; Wang, X.; Chang, L.S.; He, D.; et al. Kaempferol suppresses bladder cancer tumor growth by inhibiting cell proliferation and inducing apoptosis. Mol. Carcinog. 2015, 54, 831–840. [Google Scholar] [CrossRef]
- Yang, S.; Si, L.; Jia, Y.; Jian, W.; Yu, Q.; Wang, M.; Lin, R. Kaempferol exerts anti-proliferative effects on human ovarian cancer cells by inducing apoptosis, G0/G1 cell cycle arrest and modulation of MEK/ERK and STAT3 pathways. J. Buon. 2019, 24, 975–981. [Google Scholar]
- Shiloh, Y. ATM and ATR: Networking cellular responses to DNA damage. Curr. Opin. Genet. Dev. 2001, 11, 71–77. [Google Scholar] [CrossRef]
- Wang, X.; Zeng, L.; Wang, J.; Chau, J.F.L.; Lai, K.P.; Jia, D.; Poonepalli, A.; Hande, M.P.; Liu, H.; He, G.; et al. A positive role for c-Abl in Atm and Atr activation in DNA damage response. Cell Death Differ. 2011, 18, 5–15. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Xu, Z.-P.; Huang, Y.; Hamrick, H.E.; Duerksen-Hughes, P.J.; Yu, Y.-N. ATM and ATR: Sensing DNA damage. World J. Gastroenterol. 2004, 10, 155. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Cho, H.J.; Yu, R.; Lee, K.W.; Chun, H.S. Mechanisms Underlying Apoptosis-Inducing Effects of Kaempferol in HT-29 Human Colon Cancer Cells. Int. J. Mol. Sci. 2014, 15, 2722–2737. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.J.; Han, J.; Park, Y. Kaempferol Induces Cell Cycle Arrest in HT-29 Human Colon Cancer Cells. J. Cancer Prev. 2013, 18, 257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, A.Y.; Charlie, Y. A review of the dietary flavonoid, kaempferol on human health and cancer chemoprevention. Food Chem. 2013, 138, 2099–2107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Sun, W.; Sun, X.; Wang, Y.; Zhou, M. Kaempferol ameliorates Cisplatin induced nephrotoxicity by modulating oxidative stress, inflammation and apoptosis via ERK and NF-κB pathways. AMB Express 2020, 10, 58. [Google Scholar] [CrossRef]
- Moradzadeh, M.; Tabarraei, A.; Sadeghnia, H.R.; Ghorbani, A.; Mohamadkhani, A.; Erfanian, S.; Sahebkar, A. Kaempferol increases apoptosis in human acute promyelocytic leukemia cells and inhibits multidrug resistance genes. J. Cell Biochem. 2018, 119, 2288–2297. [Google Scholar] [CrossRef]
- Yoshida, T.; Konishi, M.; Horinaka, M.; Yasuda, T.; Goda, A.E.; Taniguchi, H.; Yano, K.; Wakada, M.; Sakai, T. Kaempferol sensitizes colon cancer cells to TRAIL-induced apoptosis. Biochem. Biophys. Res. Commun. 2008, 375, 129–133. [Google Scholar] [CrossRef]
- Isemura, M.; Saeki, K.; Kimura, T.; Hayakawa, S.; Minami, T.; Sazuka, M. Tea catechins and related polyphenols as anti-cancer agents. Biofactors 2000, 13, 81–85. [Google Scholar] [CrossRef]
- Oz, H.S.; Ebersole, J.L. Green tea polyphenols mediated apoptosis in intestinal epithelial cells by a FADD-dependent pathway. J. Cancer Ther. 2010, 1, 105. [Google Scholar] [CrossRef] [Green Version]
- Zahra, K.; Patel, S.; Dey, T.; Pandey, U.; Mishra, S.P. A study of oxidative stress in cervical cancer-an institutional study. Biochem. Biophys. Rep. 2021, 25, 100881. [Google Scholar] [CrossRef]
- Ehren, J.L.; Maher, P. Concurrent regulation of the transcription factors Nrf2 and ATF4 mediates the enhancement of glutathione levels by the flavonoid fisetin. Biochem. Pharmacol. 2013, 85, 1816–1826. [Google Scholar] [CrossRef] [PubMed]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative stress and antioxidant defense. World Allergy Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naidu, M.S.K.; Suryakar, A.N.; Swami, S.C.; Katkam, R.V.; Kumbar, K.M. Oxidative stress and antioxidant status in cervical cancer patients. Indian J. Clin. Biochem. 2007, 22, 140–144. [Google Scholar] [CrossRef] [Green Version]
- Tavsan, Z.; Kayali, H.A. Flavonoids showed anticancer effects on the ovarian cancer cells: Involvement of reactive oxygen species, apoptosis, cell cycle and invasion. Biomed. Pharmacother. 2019, 116, 109004. [Google Scholar] [CrossRef] [PubMed]
- Althunibat, O.Y.; Al Hroob, A.M.; Abukhalil, M.H.; Germoush, M.O.; Bin-Jumah, M.; Mahmoud, A.M. Fisetin ameliorates oxidative stress, inflammation and apoptosis in diabetic cardiomyopathy. Life Sci. 2019, 221, 83–92. [Google Scholar] [CrossRef]
- Rodgers, E.H.; Grant, M.H. The effect of the flavonoids, quercetin, myricetin and epicatechin on the growth and enzyme activities of MCF7 human breast cancer cells. Chem. Biol. Interact. 1998, 116, 213–228. [Google Scholar] [CrossRef]
- Moskaug, J.Ø.; Carlsen, H.; Myhrstad, M.C.W.; Blomhoff, R. Polyphenols and glutathione synthesis regulation. Am. J. Clin. Nutr. 2005, 81, 277S–283S. [Google Scholar] [CrossRef]
- Giovannini, C.; Filesi, C.; D’Archivio, M.; Scazzocchio, B.; Santangelo, C.; Masella, R. Polyphenols and endogenous antioxidant defences: Effects on glutathione and glutathione related enzymes. Ann. Ist. Super Sanita 2006, 42, 336–347. [Google Scholar]
- Song, H.; Bao, J.; Wei, Y.; Chen, Y.; Mao, X.; Li, J.; Yang, Z.; Xue, Y. Kaempferol inhibits gastric cancer tumor growth: An in vitro and in vivo study. Oncol. Rep. 2015, 33, 868–874. [Google Scholar] [CrossRef]
- Hung, T.-W.; Chen, P.-N.; Wu, H.-C.; Wu, S.-W.; Tsai, P.-Y.; Hsieh, Y.-S.; Chang, H.-R. Kaempferol inhibits the invasion and migration of renal cancer cells through the downregulation of AKT and FAK pathways. Int. J. Med. Sci. 2017, 14, 984. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Kim, J.H. Kaempferol inhibits pancreatic cancer cell growth and migration through the blockade of EGFR-related pathway in vitro. PLoS ONE 2016, 11, e0155264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palacz-Wrobel, M.; Borkowska, P.; Paul-Samojedny, M.; Kowalczyk, M.; Fila-Danilow, A.; Suchanek-Raif, R.; Kowalski, J. Effect of apigenin, kaempferol and resveratrol on the gene expression and protein secretion of tumor necrosis factor alpha (TNF-α) and interleukin-10 (IL-10) in RAW-264.7 macrophages. Biomed. Pharmacother. 2017, 93, 1205–1212. [Google Scholar] [CrossRef] [PubMed]
- Maurya, A.; Singh, A.K.; Yadav, H.; Mishra, G.; Awasthi, R.; Mishra, S.K.; Chaudhary, S.K. Role of Phytoconstituents in Targeting Cytokines for Managing Pathophysiology of Lung Diseases. In Targeting Cellular Signalling Pathways in Lung Diseases; Springer: Berlin/Heidelberg, Germany, 2021; pp. 783–803. [Google Scholar]
- Ghosh, S.; Baltimore, D. Activation in vitro of NF-κB″ by phosphorylation of its inhibitor IκB″. Nature 1990, 344, 678–682. [Google Scholar] [CrossRef]
- Atkinson, G.P.; Nozell, S.E.; Benveniste ET, N. NF-κB and STAT3 signaling in glioma: Targets for future therapies. Expert Rev. Neurother. 2010, 10, 575–586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brooks, A.J.; Putoczki, T. JAK-STAT Signalling Pathway in Cancer. Cancers 2020, 12, 1971. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Hoya, A.; Soto-Cruz, I. Role of the JAK/STAT pathway in cervical cancer: Its relationship with HPV E6/E7 oncoproteins. Cells 2020, 9, 2297. [Google Scholar] [CrossRef]
- Choi, E.J.; Ahn, W.S. Kaempferol induced the apoptosis via cell cycle arrest in human breast cancer MDA-MB-453 cells. Nutr. Res. Pract. 2008, 2, 322–325. [Google Scholar] [CrossRef] [Green Version]
- Hayakawa, J.; Ohmichi, M.; Kurachi, H.; Kanda, Y.; Hisamoto, K.; Nishio, Y.; Adachi, K.; Tasaka, K.; Kanzaki, T.; Murata, Y. Inhibition of BAD phosphorylation either at serine 112 via extracellular signal-regulated protein kinase cascade or at serine 136 via Akt cascade sensitizes human ovarian cancer cells to cisplatin. Cancer Res. 2000, 60, 5988–5994. [Google Scholar]
- Scheid, M.P.; Schubert, K.M.; Duronio, V. Regulation of Bad phosphorylation and association with Bcl-xL by the MAPK/Erk kinase. J. Biol. Chem. 1999, 274, 31108–31113. [Google Scholar] [CrossRef] [Green Version]
- Fang, X.; Yu, S.; Eder, A.; Mao, M.; Bast, R.C.; Boyd, D.; Mills, G.B. Regulation of BAD phosphorylation at serine 112 by the Ras-mitogen-activated protein kinase pathway. Oncogene 1999, 48, 6635–6640. [Google Scholar] [CrossRef] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Afroze, N.; Pramodh, S.; Almutary, A.G.; Rizvi, T.A.; Rais, N.; Raina, R.; Faiyazuddin, M.; Alnuqaydan, A.M.; Hussain, A. Kaempferol Regresses Carcinogenesis through a Molecular Cross Talk Involved in Proliferation, Apoptosis and Inflammation on Human Cervical Cancer Cells, HeLa. Appl. Sci. 2022, 12, 3155. https://doi.org/10.3390/app12063155
Afroze N, Pramodh S, Almutary AG, Rizvi TA, Rais N, Raina R, Faiyazuddin M, Alnuqaydan AM, Hussain A. Kaempferol Regresses Carcinogenesis through a Molecular Cross Talk Involved in Proliferation, Apoptosis and Inflammation on Human Cervical Cancer Cells, HeLa. Applied Sciences. 2022; 12(6):3155. https://doi.org/10.3390/app12063155
Chicago/Turabian StyleAfroze, Nazia, Sreepoorna Pramodh, Abdulmajeed G. Almutary, Tahir A. Rizvi, Naushad Rais, Ritu Raina, Md. Faiyazuddin, Abdullah M. Alnuqaydan, and Arif Hussain. 2022. "Kaempferol Regresses Carcinogenesis through a Molecular Cross Talk Involved in Proliferation, Apoptosis and Inflammation on Human Cervical Cancer Cells, HeLa" Applied Sciences 12, no. 6: 3155. https://doi.org/10.3390/app12063155





