Relationships of Nutritional Factors and Agrochemical Exposure with Parkinson’s Disease in the Province of Brescia, Italy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
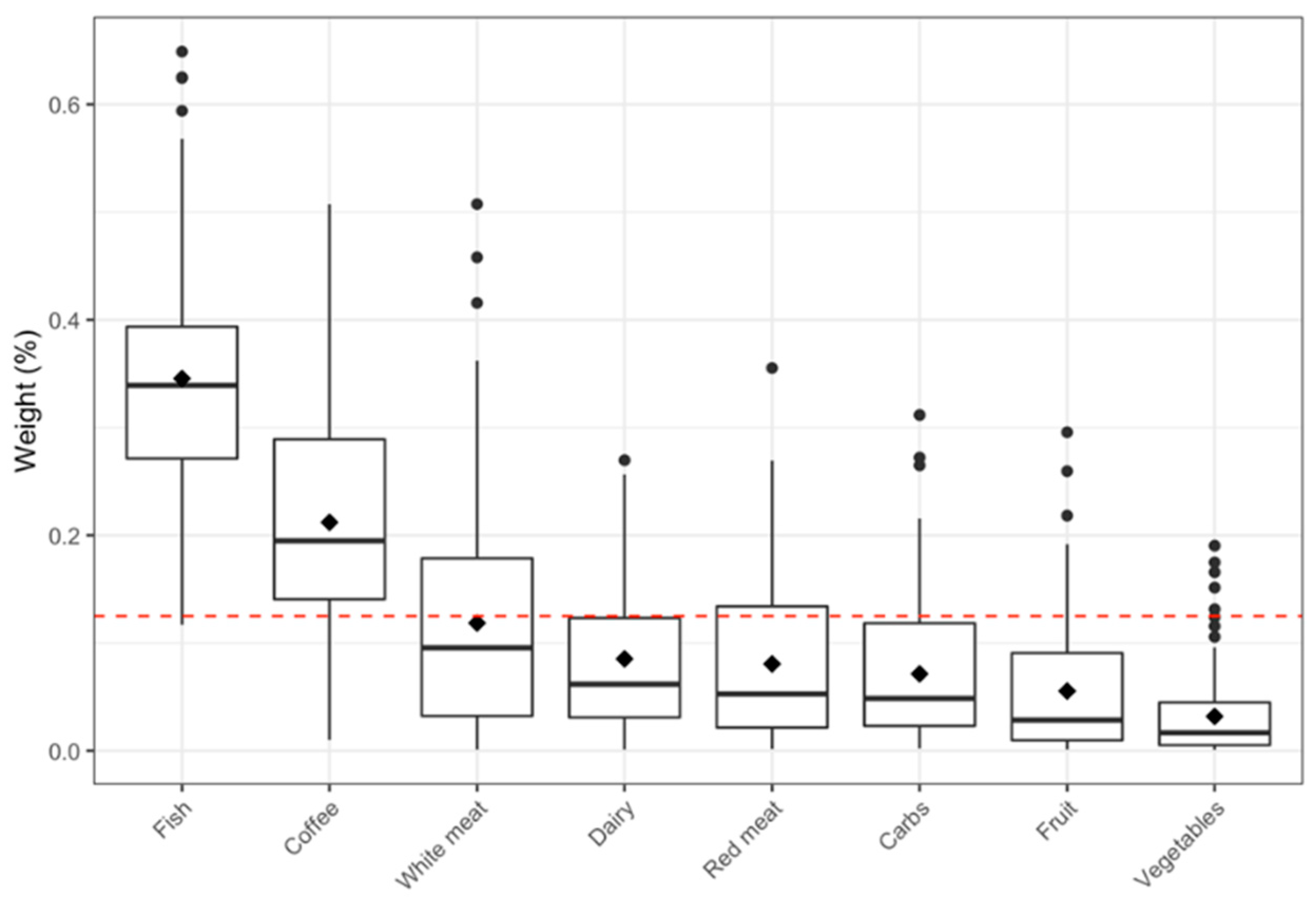
2.2. Nutritional Exposure
2.3. Exposures to Agricultural Chemical and Metals
2.4. Genotyping
2.5. Covariates
2.6. Statistical Analysis
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Lesage, S.; Brice, A. Parkinson’s disease: From monogenic forms to genetic susceptibility factors. Hum. Mol. Genet. 2009, 18, R48–R59. [Google Scholar] [CrossRef] [PubMed]
- De Lau, L.M.L.; Breteler, M.M.B. Epidemiology of Parkinson’s disease. Lancet Neurol. 2006, 5, 525–535. [Google Scholar] [CrossRef]
- DeMaagd, G.; Philip, A. Parkinson’s Disease and Its Management: Part 1: Disease Entity, Risk Factors, Pathophysiology, Clinical Presentation, and Diagnosis. Pharm. Ther. 2015, 40, 504–532. [Google Scholar]
- Lill, C.M. Genetics of Parkinson’s disease. Mol. Cell. Probes 2016, 30, 386–396. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Shu, L.; Sun, Q.; Pan, H.; Guo, J.; Tang, B. A Comprehensive Analysis of the Association Between SNCA Polymorphisms and the Risk of Parkinson’s Disease. Front. Mol. Neurosci. 2018, 11, 391. [Google Scholar] [CrossRef] [Green Version]
- Polymeropoulos, M.H.; Lavedan, C.; Leroy, E.; Ide, S.E.; Dehejia, A.; Dutra, A.; Pike, B.; Root, H.; Rubenstein, J.; Boyer, R.; et al. Mutation in the α-Synuclein Gene Identified in Families with Parkinson’s Disease. Science 1997, 276, 2045–2047. [Google Scholar] [CrossRef] [Green Version]
- Lucchini, R.G.; Guazzetti, S.; Renzetti, S.; Broberg, K.; Caci, M.; Covolo, L.; Crippa, P.; Gelatti, U.; Hashim, D.; Oppini, M.; et al. Metal Exposure and SNCA rs356219 Polymorphism Associated with Parkinson Disease and Parkinsonism. Front. Neurol. 2020, 11, 1650. [Google Scholar] [CrossRef]
- Chin-Chan, M.; Navarro-Yepes, J.; Quintanilla-Vega, B. Environmental pollutants as risk factors for neurodegenerative disorders: Alzheimer and Parkinson diseases. Front. Cell. Neurosci. 2015, 9, 124. [Google Scholar] [CrossRef] [Green Version]
- Ball, N.; Teo, W.-P.; Chandra, S.; Chapman, J. Parkinson’s Disease and the Environment. Front. Neurol. 2019, 10, 218. [Google Scholar] [CrossRef] [Green Version]
- Ascherio, A.; Chen, H.; Weisskopf, M.G.; O’Reilly, E.; ScD, M.L.M.; Calle, E.E.; Schwarzschild, M.A.; Thun, M.J. Pesticide exposure and risk for Parkinson’s disease. Ann. Neurol. 2006, 60, 197–203. [Google Scholar] [CrossRef]
- Lucchini, R.G.; Albini, E.; Benedetti, L.; Borghesi, S.; Coccaglio, R.; Malara, E.C.; Parrinello, G.; Garattini, S.; Resola, S.; Alessio, L. High prevalence of parkinsonian disorders associated to manganese exposure in the vicinities of ferroalloy industries. Am. J. Ind. Med. 2007, 50, 788–800. [Google Scholar] [CrossRef]
- Butler, L.; Gennings, C.; Peli, M.; Borgese, L.; Placidi, D.; Zimmerman, N.; Hsu, H.-H.L.; Coull, B.A.; Wright, R.; Smith, D.R.; et al. Assessing the contributions of metals in environmental media to exposure biomarkers in a region of ferroalloy industry. J. Expo. Sci. Environ. Epidemiol. 2018, 29, 674–687. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Zhang, Y.; Liu, L.; Shi, N.; Yan, H. Pesticide exposure and risk of Parkinson’s disease: Dose-response meta-analysis of observational studies. Regul. Toxicol. Pharmacol. 2018, 96, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, H.; Abushouk, A.I.; Gabr, M.; Negida, A.; Abdel-Daim, M.M. Parkinson’s disease and pesticides: A meta-analysis of disease connection and genetic alterations. Biomed. Pharmacother. 2017, 90, 638–649. [Google Scholar] [CrossRef] [PubMed]
- Pezzoli, G.; Cereda, E. Exposure to pesticides or solvents and risk of Parkinson disease. Neurology 2013, 80, 2035–2041. [Google Scholar] [CrossRef] [PubMed]
- Mezzaroba, L.; Alfieri, D.F.; Simão, A.N.C.; Reiche, E.M.V. The role of zinc, copper, manganese and iron in neurodegenerative diseases. Neurotoxicology 2019, 74, 230–241. [Google Scholar] [CrossRef] [PubMed]
- Ascherio, A.; Schwarzschild, M.A. The epidemiology of Parkinson’s disease: Risk factors and prevention. Lancet Neurol. 2016, 15, 1257–1272. [Google Scholar] [CrossRef]
- Agnihotri, A.; Aruoma, O.I. Alzheimer’s Disease and Parkinson’s Disease: A Nutritional Toxicology Perspective of the Impact of Oxidative Stress, Mitochondrial Dysfunction, Nutrigenomics and Environmental Chemicals. J. Am. Coll. Nutr. 2019, 39, 16–27. [Google Scholar] [CrossRef] [Green Version]
- Ajibawo-Aganbi, U.; Saleem, S.; Khan, S.Z.A.; Veliginti, S.; Bastidas, M.V.P.; Lungba, R.M.; Cancarevic, I. Can Nutritional Adequacy Help Evade Neurodegeneration in Older Age? A Review. Cureus 2020, 12, e10921. [Google Scholar] [CrossRef]
- Seidl, S.E.; Santiago, J.A.; Bilyk, H.; Potashkin, J.A. The emerging role of nutrition in Parkinson’s disease. Front. Aging Neurosci. 2014, 6, 36. [Google Scholar] [CrossRef] [Green Version]
- Ross, G.W.; Abbott, R.D.; Petrovitch, H.; Morens, D.M.; Grandinetti, A.; Tung, K.-H.; Tanner, C.M.; Masaki, K.H.; Blanchette, P.L.; Curb, J.D.; et al. Association of Coffee and Caffeine Intake with the Risk of Parkinson Disease. JAMA 2000, 283, 2674–2679. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Chen, H.; Fung, T.T.; Logroscino, G.; Schwarzschild, M.A.; Hu, F.B.; Ascherio, A. Prospective study of dietary pattern and risk of Parkinson disease. Am. J. Clin. Nutr. 2007, 86, 1486–1494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbaresko, J.; Lellmann, A.W.; Schmidt, A.; Lehmann, A.; Amini, A.M.; Egert, S.; Schlesinger, S.; Nöthlings, U. Dietary Factors and Neurodegenerative Disorders: An Umbrella Review of Meta-Analyses of Prospective Studies. Adv. Nutr. Int. Rev. J. 2020, 11, 1161–1173. [Google Scholar] [CrossRef] [PubMed]
- Torti, M.; Fossati, C.; Casali, M.; De Pandis, M.F.; Grassini, P.; Radicati, F.G.; Stirpe, P.; Vacca, L.; Iavicoli, I.; Leso, V.; et al. Effect of family history, occupation and diet on the risk of Parkinson disease: A case-control study. PLoS ONE 2020, 15, e0243612. [Google Scholar] [CrossRef]
- Logroscino, G.; Marder, K.; Cote, L.; Tang, M.-X.; Shea, S.; Mayeux, R. Dietary lipids and antioxidants in Parkinson’s disease: A population-based, case-control study. Ann. Neurol. 1996, 39, 89–94. [Google Scholar] [CrossRef]
- Fall, P.-A.; Fredrikson, M.; Axelson, O. Nutritional and occupational factors influencing the risk of Parkinson’s disease: A case-control study in southeastern Sweden. Mov. Disord. 1999, 14, 28–37. [Google Scholar] [CrossRef]
- Anderson, C.; Checkoway, H.; Franklin, G.M.; Beresford, S.; Smith-Weller, T.; Swanson, P.D. Dietary factors in Parkinson’s disease: The role of food groups and specific foods. Mov. Disord. 1999, 14, 21–27. [Google Scholar] [CrossRef]
- Marras, C.; Canning, C.G.; Goldman, S. Environment, lifestyle, and Parkinson’s disease: Implications for prevention in the next decade. Mov. Disord. 2019, 34, 801–811. [Google Scholar] [CrossRef]
- Shrestha, S.; Parks, C.G.; Umbach, D.M.; Richards-Barber, M.; Hofmann, J.N.; Chen, H.; Blair, A.; Freeman, L.E.B.; Sandler, D.P. Pesticide use and incident Parkinson’s disease in a cohort of farmers and their spouses. Environ. Res. 2020, 191, 110186. [Google Scholar] [CrossRef]
- Hugh-Jones, M.E.; Peele, R.H.; Wilson, V.L. Parkinson’s Disease in Louisiana, 1999–2012: Based on Hospital Primary Discharge Diagnoses, Incidence, and Risk in Relation to Local Agricultural Crops, Pesticides, and Aquifer Recharge. Int. J. Environ. Res. Public Health 2020, 17, 1584. [Google Scholar] [CrossRef] [Green Version]
- Lucchini, R.G.; Martin, C.J.; Doney, B.C. From Manganism to Manganese-Induced Parkinsonism: A Conceptual Model Based on the Evolution of Exposure. Neuromol. Med. 2009, 11, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Zoni, S.; Bonetti, G.; Lucchini, R. Olfactory functions at the intersection between environmental exposure to manganese and Parkinsonism. J. Trace Elem. Med. Biol. 2012, 26, 179–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferri, R.; Hashim, D.; Smith, D.R.; Guazzetti, S.; Donna, F.; Ferretti, E.; Curatolo, M.; Moneta, C.; Beone, G.M.; Lucchini, R.G. Metal contamination of home garden soils and cultivated vegetables in the province of Brescia, Italy: Implications for human exposure. Sci. Total Environ. 2015, 518–519, 507–517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borgese, L.; Federici, S.; Zacco, A.; Gianoncelli, A.; Rizzo, L.; Smith, D.R.; Donna, F.; Lucchini, R.; Depero, L.E.; Bontempi, E. Metal fractionation in soils and assessment of environmental contamination in Vallecamonica, Italy. Environ. Sci. Pollut. Res. 2013, 20, 5067–5075. [Google Scholar] [CrossRef] [Green Version]
- Wahlberg, K.E.; Guazzetti, S.; Pineda, D.; Larsson, S.C.; Fedrighi, C.; Cagna, G.; Zoni, S.; Placidi, D.; Wright, R.O.; Smith, D.R.; et al. Polymorphisms in Manganese Transporters SLC30A10 and SLC39A8 Are Associated With Children’s Neurodevelopment by Influencing Manganese Homeostasis. Front. Genet. 2018, 9, 664. [Google Scholar] [CrossRef] [Green Version]
- Rentschler, G.; Covolo, L.; Haddad, A.A.; Lucchini, R.G.; Zoni, S.; Broberg, K. ATP13A2 (PARK9) polymorphisms influence the neurotoxic effects of manganese. Neurotoxicology 2012, 33, 697–702. [Google Scholar] [CrossRef] [Green Version]
- Wahlberg, K.; Kippler, M.; Alhamdow, A.; Rahman, S.M.; Smith, D.R.; Vahter, M.; Lucchini, R.G.; Broberg, K. Common Polymorphisms in the Solute Carrier SLC30A10 are Associated with Blood Manganese and Neurological Function. Toxicol. Sci. 2015, 149, 473–483. [Google Scholar] [CrossRef] [Green Version]
- Williams, D.R.; Litvan, I. Parkinsonian Syndromes. Contin. Lifelong Learn. Neurol. 2013, 19, 1189–1212. [Google Scholar] [CrossRef]
- McKeith, I.; Galasko, D.; Kosaka, K.; Perry, E.; Dickson, D.; Hansen, L.; Salmon, D.; Lowe, J.; Mirra, S.; Byrne, E.; et al. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB). Neurology 1996, 47, 1113–1124. [Google Scholar] [CrossRef]
- Litvan, I.; Agid, Y.; Calne, D.; Campbell, G.; Dubois, B.; Duvoisin, R.C.; Goetz, C.G.; Golbe, L.I.; Grafman, J.; Growdon, J.H.; et al. Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome). Neurology 1996, 47, 1–9. [Google Scholar] [CrossRef]
- Armstrong, M.J.; Litvan, I.; Lang, A.E.; Bak, T.H.; Bhatia, K.P.; Borroni, B.; Boxer, A.L.; Dickson, D.W.; Grossman, M.; Hallett, M.; et al. Criteria for the diagnosis of corticobasal degeneration. Neurology 2013, 80, 496–503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gilman, S.; Wenning, G.; Low, P.A.; Brooks, D.; Mathias, C.J.; Trojanowski, J.Q.; Wood, N.; Colosimo, C.; Durr, A.; Fowler, C.J.; et al. Second consensus statement on the diagnosis of multiple system atrophy. Neurology 2008, 71, 670–676. [Google Scholar] [CrossRef] [PubMed]
- Lucchini, R.G.; Guazzetti, S.; Zoni, S.; Donna, F.; Peter, S.; Zacco, A.; Salmistraro, M.; Bontempi, E.; Zimmerman, N.J.; Smith, D.R. Tremor, olfactory and motor changes in Italian adolescents exposed to historical ferro-manganese emission. Neurotoxicology 2012, 33, 687–696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carrico, C.; Gennings, C.; Wheeler, D.C.; Factor-Litvak, P. Characterization of Weighted Quantile Sum Regression for Highly Correlated Data in a Risk Analysis Setting. J. Agric. Biol. Environ. Stat. 2014, 20, 100–120. [Google Scholar] [CrossRef] [PubMed]
- Tanner, E.M.; Bornehag, C.-G.; Gennings, C. Repeated holdout validation for weighted quantile sum regression. MethodsX 2019, 6, 2855–2860. [Google Scholar] [CrossRef] [PubMed]
- Peli, M.; Bostick, B.C.; Barontini, S.; Lucchini, R.G.; Ranzi, R. Profiles and species of Mn, Fe and trace metals in soils near a ferromanganese plant in Bagnolo Mella (Brescia, IT). Sci. Total Environ. 2020, 755, 143123. [Google Scholar] [CrossRef] [PubMed]
- Pavilonis, B.T.; Lioy, P.J.; Guazzetti, S.; Bostick, B.C.; Donna, F.; Peli, M.; Zimmerman, N.J.; Bertrand, P.; Lucas, E.; Smith, D.R.; et al. Manganese concentrations in soil and settled dust in an area with historic ferroalloy production. J. Expo. Sci. Environ. Epidemiol. 2014, 25, 443–450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferri, R.; Donna, F.; Smith, D.R.; Guazzetti, S.; Zacco, A.; Rizzo, L.; Bontempi, E.; Zimmerman, N.J.; Lucchini, R.G. Heavy Metals in Soil and Salad in the Proximity of Historical Ferroalloy Emission. J. Environ. Prot. 2012, 03, 374–385. [Google Scholar] [CrossRef] [Green Version]
- Sääksjärvi, K.; Knekt, P.; Lundqvist, A.; Männistö, S.; Heliövaara, M.; Rissanen, H.; Järvinen, R. A cohort study on diet and the risk of Parkinson’s disease: The role of food groups and diet quality. Br. J. Nutr. 2012, 109, 329–337. [Google Scholar] [CrossRef] [Green Version]
- De Lau, L.M.L.; Bornebroek, M.; Witteman, J.C.M.; Hofman, A.; Koudstaal, P.J.; Breteler, M.M.B. Dietary fatty acids and the risk of Parkinson disease: The Rotterdam Study. Neurology 2005, 64, 2040–2045. [Google Scholar] [CrossRef]
- Hughes, K.C.; Gao, X.; Kim, I.Y.; Wang, M.; Weisskopf, M.G.; Schwarzschild, M.A.; Ascherio, A. Intake of dairy foods and risk of Parkinson disease. Neurology 2017, 89, 46–52. [Google Scholar] [CrossRef] [PubMed]
| No PD (N = 389) | PD (N = 347) | Total (N = 736) | p-Value | |
|---|---|---|---|---|
| Age | <0.001 | |||
| Mean (SD) | 69.5 (9.7) | 71.9 (9.6) | 70.6 (9.7) | |
| Sex | 0.754 | |||
| Female | 158 (40.6%) | 137 (39.5%) | 295 (40.1%) | |
| Male | 231 (59.4%) | 210 (60.5%) | 441 (59.9%) | |
| SES | 0.792 | |||
| Low | 254 (65.3%) | 232 (66.9%) | 486 (66.0%) | |
| Medium | 83 (21.3%) | 67 (19.3%) | 150 (20.4%) | |
| High | 52 (13.4%) | 48 (13.8%) | 100 (13.6%) | |
| Parent history of PD | <0.001 | |||
| No | 381 (97.9%) | 322 (92.8%) | 703 (95.5%) | |
| Yes | 8 (2.1%) | 25 (7.2%) | 33 (4.5%) | |
| Agricultural Chemical exposure | 0.001 | |||
| No | 342 (87.9%) | 275 (79.3%) | 617 (83.8%) | |
| Yes | 47 (12.1%) | 72 (20.7%) | 119 (16.2%) | |
| Head injury | 0.490 | |||
| No | 340 (87.4%) | 309 (89.0%) | 649 (88.2%) | |
| Yes | 49 (12.6%) | 38 (11.0%) | 87 (11.8%) | |
| Ever smoke | 0.153 | |||
| No | 214 (55.0%) | 209 (60.2%) | 423 (57.5%) | |
| Yes | 175 (45.0%) | 138 (39.8%) | 313 (42.5%) | |
| Province of Birth | 0.011 | |||
| Brescia | 329 (84.6%) | 315 (90.8%) | 644 (87.5%) | |
| Other | 60 (15.4%) | 32 (9.2%) | 92 (12.5%) | |
| Metal exposure | 0.006 | |||
| No | 354 (93.7%) | 301 (87.8%) | 655 (90.8%) | |
| Yes | 24 (6.3%) | 42 (12.2%) | 66 (9.2%) | |
| SNCA rs356219 | 0.004 | |||
| TT | 167 (43.9%) | 113 (34.1%) | 280 (39.4%) | |
| TC | 167 (43.9%) | 153 (46.2%) | 320 (45.0%) | |
| CC | 46 (12.1%) | 65 (19.6%) | 111 (15.6%) | |
| Coffee intake | 0.067 | |||
| Low | 152 (39.1%) | 164 (47.3%) | 316 (42.9%) | |
| Medium | 191 (49.1%) | 143 (41.2%) | 334 (45.4%) | |
| High | 46 (11.8%) | 40 (11.5%) | 86 (11.7%) | |
| Vegetable intake | 0.041 | |||
| Low | 114 (29.3%) | 102 (29.4%) | 216 (29.3%) | |
| Medium-Low | 106 (27.2%) | 82 (23.6%) | 188 (25.5%) | |
| Medium-High | 129 (33.2%) | 103 (29.7%) | 232 (31.5%) | |
| High | 40 (10.3%) | 60 (17.3%) | 100 (13.6%) | |
| Fruit intake | 0.139 | |||
| Low | 339 (87.1%) | 289 (83.3%) | 628 (85.3%) | |
| High | 50 (12.9%) | 58 (16.7%) | 108 (14.7%) | |
| Fish intake | 0.002 | |||
| Low | 80 (20.6%) | 99 (28.5%) | 179 (24.3%) | |
| Medium-Low | 94 (24.2%) | 102 (29.4%) | 196 (26.6%) | |
| Medium-High | 106 (27.2%) | 81 (23.3%) | 187 (25.4%) | |
| High | 109 (28.0%) | 65 (18.7%) | 174 (23.6%) | |
| Red meat intake | 0.336 | |||
| Low | 151 (38.8%) | 120 (34.6%) | 271 (36.8%) | |
| Medium | 208 (53.5%) | 192 (55.3%) | 400 (54.3%) | |
| High | 30 (7.7%) | 35 (10.1%) | 65 (8.8%) | |
| White meat intake | 0.367 | |||
| Low | 349 (89.7%) | 304 (87.6%) | 653 (88.7%) | |
| High | 40 (10.3%) | 43 (12.4%) | 83 (11.3%) | |
| Carbs intake | 0.521 | |||
| Low | 121 (31.1%) | 106 (30.5%) | 227 (30.8%) | |
| Medium | 201 (51.7%) | 170 (49.0%) | 371 (50.4%) | |
| High | 67 (17.2%) | 71 (20.5%) | 138 (18.8%) | |
| Dairy intake | 0.264 | |||
| Low | 114 (29.3%) | 83 (23.9%) | 197 (26.8%) | |
| Medium-Low | 104 (26.7%) | 91 (26.2%) | 195 (26.5%) | |
| Medium-High | 116 (29.8%) | 124 (35.7%) | 240 (32.6%) | |
| High | 55 (14.1%) | 49 (14.1%) | 104 (14.1%) |
| Model 1 | Model 2 a | |||||
|---|---|---|---|---|---|---|
| Predictors | OR | 95%CI | p-Value | OR | 95%CI | p-Value |
| Age | 1.03 | 1.01–1.05 | <0.001 | 1.03 | 1.01–1.05 | <0.001 |
| Male | 0.98 | 0.69–1.40 | 0.929 | 0.99 | 0.69–1.41 | 0.947 |
| SES Medium vs. Low | 1.12 | 0.74–1.71 | 0.586 | 1.12 | 0.74–1.71 | 0.596 |
| SES High vs. Low | 1.14 | 0.70–1.86 | 0.603 | 1.11 | 0.68–1.81 | 0.674 |
| Parental PD history | 3.58 | 1.58–8.94 | 0.004 | 3.64 | 1.59–9.16 | 0.003 |
| Head Injury | 0.88 | 0.52–1.46 | 0.611 | 0.88 | 0.53–1.47 | 0.63 |
| Ever smoked | 0.81 | 0.57–1.15 | 0.234 | 0.81 | 0.57–1.14 | 0.228 |
| SNCA rs356219 (TC vs. TT) | 1.32 | 0.93–1.87 | 0.125 | 1.32 | 0.93–1.87 | 0.124 |
| SNCA rs356219 (CC vs. TT) | 2.1 | 1.30–3.43 | 0.003 | 2.09 | 1.29–3.41 | 0.003 |
| Agricultural chemical exposure | 1.98 | 1.28–3.10 | 0.003 | 1.95 | 1.25–3.05 | 0.003 |
| Metal exposure (Yes vs. No) | 2.34 | 1.31–4.27 | 0.004 | 2.33 | 1.30–4.25 | 0.005 |
| Coffee | 1 | 0.99–1.00 | 0.109 | 1 | 0.99–1.00 | 0.105 |
| Fish | 0.98 | 0.96–1.00 | 0.06 | 0.98 | 0.96–1.00 | 0.066 |
| Fruit | 1.27 | 1.02–1.59 | 0.036 | 0.94 | 0.58–1.49 | 0.807 |
| Vegetables | 1.01 | 1.00–1.02 | 0.018 | 1.32 | 0.81–2.16 | 0.268 |
| White meat | 1.01 | 0.98–1.04 | 0.432 | 1.01 | 0.98–1.04 | 0.432 |
| Red meat | 1.01 | 0.99–1.04 | 0.315 | 1.01 | 0.99–1.04 | 0.291 |
| Dairy | 1 | 0.99–1.01 | 0.849 | 1 | 0.99–1.01 | 0.838 |
| Carbs | 1 | 1.00–1.01 | 0.586 | 1 | 1.00–1.01 | 0.579 |
| Born in Brescia (BS) | 1.73 | 1.05–2.90 | 0.035 | 1.69 | 1.02–2.84 | 0.043 |
| (Fruit and BS) vs. Other | 1.31 | 0.81–2.21 | 0.286 | |||
| (Vegetables and BS) vs. Other | 0.93 | 0.55–1.55 | 0.773 | |||
| Model P a | Model N a | |||
|---|---|---|---|---|
| OR | 95%CI | OR | 95%CI | |
| WQS index for diet mixture b | 1.305 | (0.88, 1.936) | 0.721 | (0.525, 0.991) |
| Age | 1.034 | (1.019, 1.049) | 1.031 | (1.016, 1.046) |
| Males vs. Females | 0.98 | (0.764, 1.258) | 0.979 | (0.757, 1.265) |
| SES Medium vs. Low | 1.135 | (0.774, 1.663) | 1.138 | (0.776, 1.669) |
| SES High vs. Low | 1.13 | (0.766, 1.668) | 1.182 | (0.795, 1.758) |
| Parent PD history | 4.165 | (2.136, 8.12) | 4.145 | (2.096, 8.196) |
| Head Injury | 0.883 | (0.58, 1.344) | 0.881 | (0.578, 1.343) |
| Ever smoked | 0.767 | (0.565, 1.041) | 0.759 | (0.559, 1.031) |
| Agricultural chemical exposure | 1.843 | (1.173, 2.897) | 2.113 | (1.413, 3.159) |
| Metal exposure | 2.501 | (1.607, 3.893) | 2.504 | (1.610, 3.893) |
| Born in Brescia vs. others | 1.843 | (1.173, 2.897) | 1.825 | (1.169, 2.848) |
| SNCA rs356219 | ||||
| TC vs. TT | 1.396 | (1.036, 1.88) | 1.393 | (1.037, 1.871) |
| CC vs. TT | 2.138 | (1.417, 3.225) | 2.17 | (1.434, 3.284) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belingheri, M.; Chiu, Y.-H.M.; Renzetti, S.; Bhasin, D.; Wen, C.; Placidi, D.; Oppini, M.; Covolo, L.; Padovani, A.; Lucchini, R.G. Relationships of Nutritional Factors and Agrochemical Exposure with Parkinson’s Disease in the Province of Brescia, Italy. Int. J. Environ. Res. Public Health 2022, 19, 3309. https://doi.org/10.3390/ijerph19063309
Belingheri M, Chiu Y-HM, Renzetti S, Bhasin D, Wen C, Placidi D, Oppini M, Covolo L, Padovani A, Lucchini RG. Relationships of Nutritional Factors and Agrochemical Exposure with Parkinson’s Disease in the Province of Brescia, Italy. International Journal of Environmental Research and Public Health. 2022; 19(6):3309. https://doi.org/10.3390/ijerph19063309
Chicago/Turabian StyleBelingheri, Michael, Yueh-Hsiu Mathilda Chiu, Stefano Renzetti, Deepika Bhasin, Chi Wen, Donatella Placidi, Manuela Oppini, Loredana Covolo, Alessandro Padovani, and Roberto G. Lucchini. 2022. "Relationships of Nutritional Factors and Agrochemical Exposure with Parkinson’s Disease in the Province of Brescia, Italy" International Journal of Environmental Research and Public Health 19, no. 6: 3309. https://doi.org/10.3390/ijerph19063309
APA StyleBelingheri, M., Chiu, Y.-H. M., Renzetti, S., Bhasin, D., Wen, C., Placidi, D., Oppini, M., Covolo, L., Padovani, A., & Lucchini, R. G. (2022). Relationships of Nutritional Factors and Agrochemical Exposure with Parkinson’s Disease in the Province of Brescia, Italy. International Journal of Environmental Research and Public Health, 19(6), 3309. https://doi.org/10.3390/ijerph19063309







