The Management of Urinary Tract Infections during the COVID-19 Pandemic: What Do We Need to Know?
Abstract
:1. Background and Aims
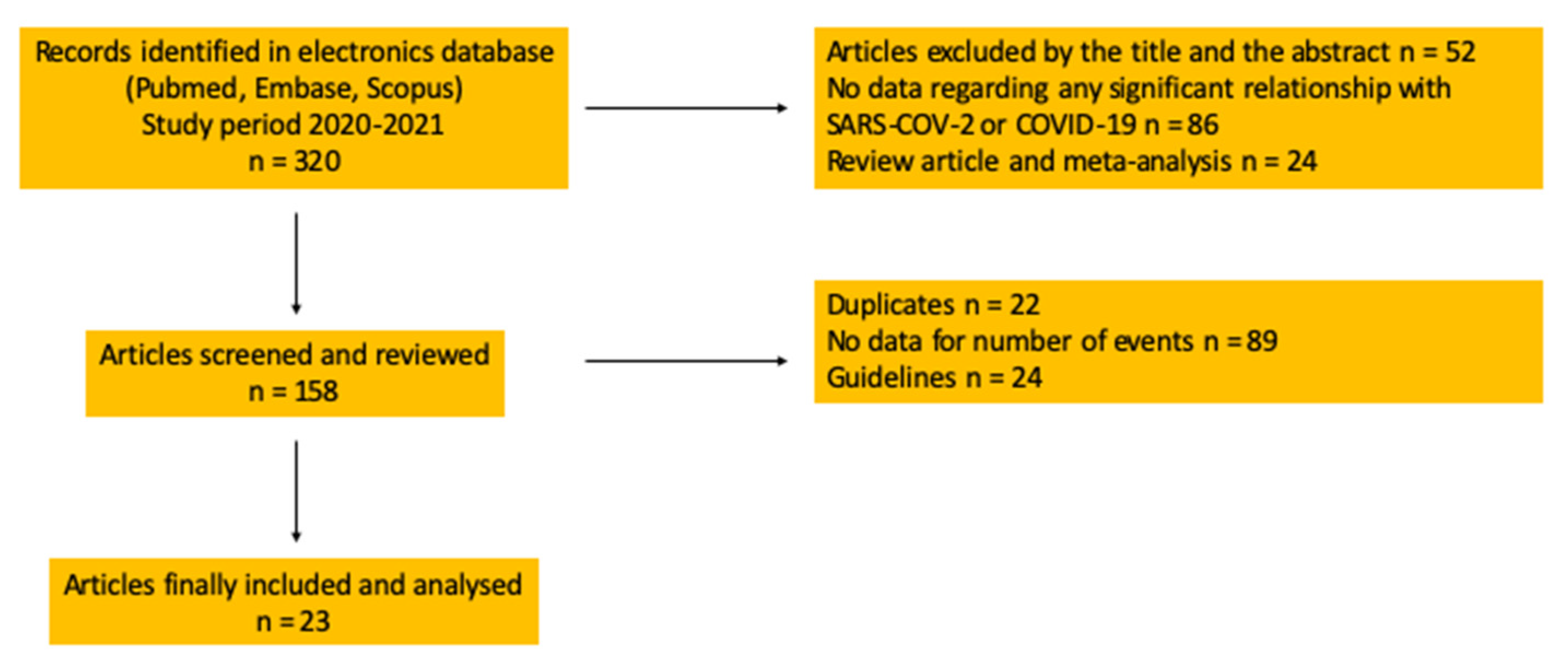
2. Materials and Methods
Search of Evidence
3. Evidence and Recommendations
3.1. Evidence
3.1.1. The Prevalence of Bacterial Co-Infections in COVID-19
3.1.2. The Prevalence of UTI Co-Infections in COVID-19
3.1.3. Antibiotic Prescriptions and Antimicrobial Stewardship Considerations in COVID-19
3.2. Recommendations
3.2.1. The COVID-19 Pandemic: An Excellent Reminder of Antimicrobial Stewardship Principles
3.2.2. Think Twice before Prescribing Antimicrobials to COVID-19 Positive Patients!
3.2.3. Asymptomatic Bacteriuria Is Not a Risk Factor for Future Complications in COVID-19 Patients
3.2.4. Antimicrobial Prophylaxis before Urological Procedures and Surgery in COVID-19 Positive Patients
3.2.5. Urosepsis and COVID-19
3.3. Limitations
4. Conclusions and Final Remarks
- Avoid using antibiotics in COVID-19 patients without any sign and/or symptoms related to bacterial infections.
- The presence of fever in the absence of symptoms related to UTIs is not an indication for the use of antibiotics.
- In case of symptoms related to UTIs, empirical antimicrobial treatment in accordance with international guidelines is the most appropriate practice.
- Asymptomatic bacteriuria is not a risk factor in patients affected by COVID-19 infection. The management of asymptomatic bacteriuria should also be in line with international guidelines, but elderly patients with bacteriuria and delirium require meticulous evaluation and continuous attention to a less harmful approach than antibiotic treatment.
- The care of COVID-19 patients is more difficult than for patients in a standard hospital setting. Isolation procedures might increase the use of urinary catheters and cause higher prevalence of catheter associated UTI and hospital acquired UTIs. The indications for catheterization in patients affected by COVID-19 requires careful considerations.
- Patients affected by COVID-19 have the same risk as non-COVID-19 patients to develop infectious complications after urological procedures. There is no evidence to deviate from international guidelines on antimicrobial prophylaxis before urological surgical procedures.
- Before prescription of antibiotic therapy, physicians must consider all possible collateral damages caused by antibiotics.
- It is urgently required to change current practice of preemptive broad-spectrum antibiotic prescription in COVID-19 patients. We must pay more attention to available evidence and the principles of antimicrobial stewardship!
- Please don’t forget to consider the patient’s quality of life in association with antimicrobial stewardship in everyday clinical practice! [32].
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Coronavirus Pandemic (COVID-19)—The Data. Available online: https://ourworldindata.org/coronavirus-data (accessed on 19 November 2021).
- WHO Coronavirus (COVID-19). Available online: https://COVID19.who.int (accessed on 19 November 2021).
- Barycka, K.; Szarpak, L.; Filipiak, K.J.; Jaguszewski, M.; Smereka, J.; Ladny, J.R.; Turan, O. Comparative effectiveness of N95 respirators and surgical/face masks in preventing airborne infections in the era of SARS-CoV2 pandemic: A meta-analysis of randomized trials. PLoS ONE 2020, 15, e0242901. [Google Scholar] [CrossRef] [PubMed]
- Estrada, A.D.B.; Parra, J.C.; Carracedo, E.F.; Míguez, A.M.; Martínez, A.R.; Rubio, E.M.; Rubio-Rivas, M.; Agudo, P.; Fernández, F.A.; Perez, V.E.; et al. Inadequate use of antibiotics in the COVID-19 era: Effectiveness of antibiotic therapy. BMC Infect. Dis. 2021, 21, 1144. [Google Scholar]
- International Severe Acute Respiratory and Emerging Infection Consortium. COVID-19 Report. 2020. Available online: https://media.tghn.org/medialibrary/2020/04/ISARIC_Data_Platform_COVID-19_Report_8APR20.pdf (accessed on 12 December 2021).
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann. Intern. Med. 2009, 151, 264–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Köves, B.; Cai, T.; Veeratterapillay, R.; Pickard, R.; Seisen, T.; Lam, T.B.; Yuan, Y.; Bruyere, F.; Wagenlehner, F.; Bartoletti, R.; et al. Benefits and Harms of Treatment of Asymptomatic Bacteriuria: A Systematic Review and Meta-analysis by the European Association of Urology Urological Infection Guidelines Panel. Eur. Urol. 2017, 72, 865–868. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, B.; Li, Q.; Wen, L.; Zhang, R. Clinical Features of 69 Cases with Coronavirus Disease 2019 in Wuhan, China. Clin. Infect. Dis. 2020, 71, 769–777. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, C.; Chen, X.; Cai, Y.; Xia, J.; Zhou, X.; Xu, S.; Huang, H.; Zhang, L.; Zhou, X.; Du, C.; et al. Risk Factors Associated with Acute Respiratory Distress Syndrome and Death in Patients with Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern. Med. 2020, 180, 934–943. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wan, S.; Xiang, Y.; Fang, W.; Zheng, Y.; Li, B.; Hu, Y.; Lang, C.; Huang, D.; Sun, Q.; Xiong, Y.; et al. Clinical features and treatment of COVID-19 patients in northeast Chongqing. J. Med. Virol. 2020, 92, 797–806. [Google Scholar] [CrossRef]
- Lansbury, L.; Lim, B.; Baskaran, V.; Lim, W.S. Co-infections in people with COVID-19: A systematic review and meta-analysis. J. Infect. 2020, 81, 266–275. [Google Scholar] [CrossRef]
- Rawson, T.M.; Moore, L.S.P.; Zhu, N.; Ranganathan, N.; Skolimowska, K.; Gilchrist, M.; Satta, G.; Cooke, G.; Holmes, A. Bacterial and Fungal Coinfection in Individuals with Coronavirus: A Rapid Review to Support COVID-19 Antimicrobial Prescribing. Clin. Infect. Dis. 2020, 71, 2459–2468. [Google Scholar] [CrossRef]
- Bardi, T.; Pintado, V.; Gomez-Rojo, M.; Escudero-Sanchez, R.; Azzam Lopez, A.; Diez-Remesal, Y.; Martinez Castro, N.; Ruiz-Garbajosa, P.; Pestaña, D. Nosocomial infections associated to COVID-19 in the intensive care unit: Clinical characteristics and outcome. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 495–502. [Google Scholar] [CrossRef]
- Karaba, S.M.; Jones, G.; Helsel, T.; Smith, L.L.; Avery, R.; Dzintars, K.; Salinas, A.B.; Keller, S.C.; Townsend, J.L.; Klein, E.; et al. Prevalence of Co-infection at the Time of Hospital Admission in COVID-19 Patients, A Multicenter Study. Open Forum Infect. Dis. 2020, 8, ofaa578. [Google Scholar] [CrossRef] [PubMed]
- Van Laethem, J.; Wuyts, S.C.M.; Pierreux, J.; Seyler, L.; Verschelden, G.; Depondt, T.; Meuwissen, A.; Lacor, P.; Piérard, D.; Allard, S.D. Presumed Urinary Tract Infection in Patients Admitted with COVID-19: Are We Treating Too Much? Antibiotics 2021, 10, 1493. [Google Scholar] [CrossRef] [PubMed]
- Langford, B.J.; So, M.; Raybardhan, S.; Leung, V.; Westwood, D.; MacFadden, D.R. Bacterial co-infection and secondary infection in patients with COVID-19: A living rapid review and meta-analysis. Clin. Microbiol. Infect. 2020, 26, 1622–1629. [Google Scholar] [CrossRef] [PubMed]
- Rawson, T.M.; Moore, L.S.P.; Castro-Sanchez, E.; Charani, E.; Davies, F.; Satta, G. COVID-19 and the potential long-term impact on antimicrobial resistance. J. Antimicrob. Chemother. 2020, 75, 1681–1684. [Google Scholar] [CrossRef]
- Lai, J.; Ma, S.; Wang, Y.; Cai, Z.; Hu, J.; Wei, N. Factors associated with mental health outcomes among health care workers exposed to coronavirus disease 2019. JAMA Netw. Open 2020, 3, e203976. [Google Scholar] [CrossRef]
- Huttner, B.D.; Catho, G.; Pano-Pardo, J.R.; Pulcini, C.; Schouten, J. COVID-19: Don’t neglect antimicrobial stewardship principles! Clin. Microbiol. Infect. 2020, 26, 808–810. [Google Scholar] [CrossRef]
- Borek, A.J.; Maitland, K.; McLeod, M.; Campbell, A.; Hayhoe, B.; Butler, C.C.; Morrell, L.; Roope, L.S.J.; Holmes, A.; Walker, A.S.; et al. Impact of the COVID-19 Pandemic on Community Antibiotic Prescribing and Stewardship: A Qualitative Interview Study with General Practitioners in England. Antibiotics 2021, 10, 1531. [Google Scholar] [CrossRef]
- Han, J.; Gatheral, T.; Williams, C. Procalcitonin for patient stratification and identification of bacterial co-infection in COVID-19. Clin. Med. 2020, 20, e47. [Google Scholar] [CrossRef]
- Müller, B.; Becker, K.L.; Schächinger, H.; Rickenbacher, P.R.; Huber, P.R.; Zimmerli, W.; Ritz, R. Calcitonin precursors are reliable markers of sepsis in a medical intensive care unit. Crit. Care Med. 2000, 28, 977–983. [Google Scholar] [CrossRef]
- Geehan Suleyman, M.D.; Rita Kassab, D.O.; Smitha Gudipati, M.D.; Ramesh Mayur, M.D.; Indira Brar, M.D. 779. COVID-19 Pandemic and Catheter-associated Urinary Tract Infection Trends. Open Forum Infect. Dis. 2021, 8, S486–S487. [Google Scholar] [CrossRef]
- van de Pol, A.C.; Boeijen, J.A.; Venekamp, R.P.; Platteel, T.; Damoiseaux, R.A.M.J.; Kortekaas, M.F.; van der Velden, A.W. Impact of the COVID-19 Pandemic on Antibiotic Prescribing for Common Infections in The Netherlands: A Primary Care-Based Observational Cohort Study. Antibiotics 2021, 10, 196. [Google Scholar] [CrossRef] [PubMed]
- Reyes, R.; Bono, G.; Finucane, T.E. So-called Urinary Tract Infection in the Era of COVID-19. J. Am. Geriatr. Soc. 2020, 68, 1927–1928. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, M.; Brymer, C.; Elsayed, S. Treatment of asymptomatic UTI in older delirious medical in-patients: A prospective cohort study. Arch. Gerontol. Geriatr. 2017, 72, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Antonello, V.S.; Dallé, J.; Antonello, I.C.F.; Benzano, D.; Ramos, M.C. Surgical Site Infection after Cesarean Delivery in Times of COVID-19. Rev. Bras. Ginecol. Obstet. 2021, 43, 374–376. [Google Scholar] [CrossRef] [PubMed]
- Jue, S.; Alameddine, M. COVID-19 Coagulopathy: Considerations for Urologists. J. Urol. 2020, 204, 640–641. [Google Scholar] [CrossRef]
- Manne, B.K.; Denorme, F.; Middleton, E.A.; Portier, I.; Rowley, J.W.; Stubben, C.; Petrey, A.C.; Tolley, N.D.; Guo, L.; Cody, M.; et al. Platelet gene expression and function in COVID-19 patients. Blood 2020, 136, 1317–1329. [Google Scholar] [CrossRef]
- Cai, T.; Tandogdu, Z.; Wagenlehner, F.M.E.; Bjerklund Johansen, T.E. Re: COVID-19 Coagulopathy: Considerations for Urologists. J. Urol. 2020, 204, 848–849. [Google Scholar] [CrossRef]
- Bramer, W.M.; Rethlefsen, M.L.; Kleijnen, J.; Franco, O.H.; Bramer, W.M.; Rethlefsen, M.L.; Kleijnen, J.; Franco, O.H. Optimal database combinations for literature searches in systematic reviews: A prospective exploratory study. Syst. Rev. 2017, 6, 245. [Google Scholar] [CrossRef]
- Cai, T.; Verze, P.; Bjerklund Johansen, T.E. The Quality of Life Definition: Where Are We Going? Uro 2021, 1, 14–22. [Google Scholar] [CrossRef]
| Author | Year | Setting | Type of Study | Aim of Study | Sample Size | Median Age (IQR) | Gender | Most Important Findings |
|---|---|---|---|---|---|---|---|---|
| Wang Z et al. [8] | 2020 | Hospital (China) | Retrospective | Clinical features of COVID19 infection | 69 | 42 (35–62) | Male 46% Female 54% | COVID-19 symptoms: Fever (87%), cough (55%), fatigue (42%), Sp02 < 90% (20%) |
| Wu C et al. [9] | 2020 | Hospital (China) | Retrospective | Risk factors associated with ARDS and death | 201 | 51 (43–60) | Male 63.7% Female 36.3% | Older age is associated with greater risk of ARDS (41.8%) and death (52.4%) |
| Wan S et al. [10] | 2020 | Hospital (China, North-east)-Chongqing | Retrospective | Clinical features of COVID-19 infection | 135 | 47 (36–55) | Male: 53.3% Female: 46.7% | COVID-19 symptoms: Fever (88.9%), cough (76.5%), fatigue (32.5%) |
| Lansbury L et al. [11] | 2020 | Hospital (China, Europe, USA) | Retrospective | Clinical features of COVID-19 infection | 2590 (22 studies included) | 47 (36–55) | Male 53.3% Female 46.7% | Bacterial co-infections: 7% Common pathogens: Mycoplasma pneumonia, Pseudomonas aeruginosa, Haemophilus influenzae Routine use of antibiotics should be avoided |
| Rawson TM et al. [12] | 2020 | China, USA, Saudi Arabia, Taiwan, South-Korea, Canada, Hong-Kong | Systematic review | Evaluation of patients with concomitant bacterial and fungal co-infection in patients with COVID 19 | 2010 patients (18 studies included) -806 COVID-19 -811 No COVID-19 | - | - | COVID-19 positive: 8% No COVID-19: 11% 72% of the overall cohort received wide-spectrum antimicrobial therapy |
| Bardi T et al. [13] | 2021 | Single-centre; Case-control study (Spain, Europe) | Retrospective | Evaluation of ICU-acquired infections in COVID-19 patients | 140 | 61 (57–67) | Male 77% Female 33% | HAUTI Incidence: 47%; Primary (31%); catheter-related (25%); pneumonia (23%); tracheobronchitis (10%); UTI (8%) with 60% of risk of septic shock |
| Karaba SM et al. [14] | 2020 | Hospitals, Multicentric (USA) | Retrospective | Incidence of bacterial respiratory and non-respiratory co-infections | 1016 | 62 (48–74) | Male 54% Female 46% | Bacterial respiratory: 1.2%; Bacterial pneumonia: 1.1% UTI: 3% |
| Van Laethem J et al. [15] | 2021 | Hospital, single center (Belgium) | Retrospective | Quantitative/Qualitative evaluation of UTI in COVID-19 ward | 622 | 63 | Male 58% Female 42% | UTI Incidence 13% 12% of UTI subgroup under antibiotic therapy 61% overdiagnosis rate (unnecessary therapy) |
| Langford BJ et al. [16] | 2020 | Hospitals, Multicentric (China, USA, Europe) | Systematic review and meta-analysis | Prevalence of bacterial co-infection (admission) and secondary infection (during hospitalization) in COVID-19 wards | 3338 24 included studies | 2–71 | Male: 54.2% Female: 45.8% | Low incidence of bacterial co-infection in COVID-19 wards (6.9%) but 71.9% of patients received antimicrobial therapy |
| Rawson TM et al. [17] | 2020 | Editorial comment (Point of debate) | Comment | Potential impacts of healthcare system adaption during COVID-19 pandemic on antimicrobial resistance | - | - | - | Reinforce antimicrobial stewardship independently of COVID-19 pandemic |
| Lai J et al. [18] | 2020 | Hospitals, multicentric (China) | Cross-sectional survey | Mental evaluation of health-care workers | 1257 60.8% Nurses 39.2% Physicians | 26–40 | Male: 23.3% Female: 76.7% | High psychological burden. Depression, anxiety, distress more pronounced in nurse staff during the pandemic |
| Huttner BD et al. [19] | 2020 | Editorial comment | - | Antimicrobial stewardship principles in the COVID-19 pandemic era | - | - | - | Unmet needs: -National recommendations on antimicrobial stewardship in COVID-19 patients -Establish incidence of co-infections and super-infections -Assess relationship between antibiotic-resistance and COVID-19 infection |
| Borek AJ et al. [20] | 2021 | Survey report–Audio Structured-recorded Questionnaires (UK) | - | GPs experience and perception of COVID-19 pandemic | 18 | - | - | -Increased use of telemedicine in COVID-19 pandemic -No impact on antibiotic prescriptions -Increased workload due to COVID-19 pandemic |
| Han J et al. [21] | 2020 | Letter to the editor | - | The role of procalcitonin for identifying bacterial co-infection in COVID-19 patients | - | - | - | Procalcitonin may represent a negative prognostic factor (clinical deterioration) in patients admitted to COVID-19 wards |
| Muller et al. [22] | 2000 | Prospective multicentric study (Europe, USA) | Role of calcitonin precursors in predicting septic shock | 101 ICU patients | 59 (23–86) | Male: 60% Female: 40% | Calcitonin precursors are sensitive markers of sepsis compared to serum C-reactive and interleukins | |
| Suleyman G et al. [23] | 2021 | Hospitals, USA (Abstract) | Retrospective–cross sectional study (USA) (Abstract) | Impact of COVID-19 on catheter-associated urinary tract infections (CAUTIs) | 877 | - | - | COVID-19 pandemic did not significantly change CAUTI incidence |
| Van de Pol AC [24] | 2021 | JGPN (Julius General Practitioners’ Network) registry 2019–2020 | Care-based observational cohort study | Evaluation of number of infectious disease episodes, complications, and antibiotic prescription rates | 2019: 27.263 2020: 37.604 (consultations) | - | 2019: Respiratory (21%); Urinary (54%); GI (3%); Skin (31%)–2020 Respiratory (13%); Urinary (57%); GI (4%); Skin 34% | Decrease in number of infectious diseases treated with antibiotics due to lockdown restrictions |
| Reyes R et al. [25] | 2020 | Editorial comment | Editorial comment | The impact of urinary tract infection in the era of COVID-19 | - | - | - | Careful clinical observation before antimicrobial treatment |
| Dasgupta M et al. [26] | 2017 | Hospital, Canada | Prospective cohort study | Determination of asymptomatic UTI treatment rate in older medically ill delirious patients | 343 | 85.3 (range not reported) | Male: 29% Female: 71% | 27% treated for a UTI. Treatment of UTI associated with poor functional recovery and consequently of questionable utility |
| Antonello VS et al. [27] | 2021 | Hospital, Brazil, Maternity Unit | Retrospective analysis | Consumption of personal protective equipment and products (PPEP) and frequency of surgical site infection among non-COVID 19 patients | Not reported | Not described | Not described | Increased safety of healthcare workers associated with reduction of SSI |
| Jue S et al. [28] | 2020 | Editorial comment | Editorial comment | Thromboprophylaxis guidelines in COVID-19 patients | - | - | - | COVID-19 caused a hypercoagulability state with increased risk of DIC (Disseminated intravascular coagulation) |
| Manne BK et al. [29] | 2020 | Hospital, Single center (USA) | Retrospective series | Pathway analysis of COVID-19 infection on platelets | 58 (Healthy donor s = 17) (non-ICU COVID-19 = 24) ICU COVID-19 (=17) | 49.9 | Male: 53% Female: 47% | COVID-19 increases platelet hyperreactivity |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, T.; Tascini, C.; Novelli, A.; Anceschi, U.; Bonkat, G.; Wagenlehner, F.; Bjerklund Johansen, T.E. The Management of Urinary Tract Infections during the COVID-19 Pandemic: What Do We Need to Know? Uro 2022, 2, 55-64. https://doi.org/10.3390/uro2010008
Cai T, Tascini C, Novelli A, Anceschi U, Bonkat G, Wagenlehner F, Bjerklund Johansen TE. The Management of Urinary Tract Infections during the COVID-19 Pandemic: What Do We Need to Know? Uro. 2022; 2(1):55-64. https://doi.org/10.3390/uro2010008
Chicago/Turabian StyleCai, Tommaso, Carlo Tascini, Andrea Novelli, Umberto Anceschi, Gernot Bonkat, Florian Wagenlehner, and Truls E. Bjerklund Johansen. 2022. "The Management of Urinary Tract Infections during the COVID-19 Pandemic: What Do We Need to Know?" Uro 2, no. 1: 55-64. https://doi.org/10.3390/uro2010008
APA StyleCai, T., Tascini, C., Novelli, A., Anceschi, U., Bonkat, G., Wagenlehner, F., & Bjerklund Johansen, T. E. (2022). The Management of Urinary Tract Infections during the COVID-19 Pandemic: What Do We Need to Know? Uro, 2(1), 55-64. https://doi.org/10.3390/uro2010008









