Prophylactic Addition of Glucose Suppresses Cyanobacterial Abundance in Lake Water
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Collection of Harsha Lake Samples
2.3. Incubation of Treated Water Samples
2.4. Analysis of Microcystin Using Enzyme-Linked Immunosorbent Assay (ELISA)
2.5. DNA Extraction and High-Throughput Sequencing
2.6. Amplicon Processing
2.7. Data Analysis
3. Results
3.1. Microcystin Concentrations after Glucose Treatments
3.2. Changes in Microbial Community Structure Based on 16S rRNA Gene Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Disclaimer
References
- Carmichael, W.W. Health effects of toxin-producing cyanobacteria: The CyanoHABS. Hum. Ecol. Risk Assess. Int. J. 2001, 7, 1393–1407. [Google Scholar] [CrossRef]
- Amorim, C.A.; Moura, A.D.N. Ecological impacts of freshwater algal blooms on water quality, plankton biodiversity, structure, and ecosystem functioning. Sci. Total Environ. 2021, 758, 143605. [Google Scholar] [CrossRef]
- Olson, N.E.; Cooke, M.E.; Shi, J.H.; Birbeck, J.A.; Westrick, J.A.; Ault, A.P. Harmful algal bloom toxins in aerosol generated from inland lake water. Environ. Sci. Technol. 2020, 54, 4769–4780. [Google Scholar] [CrossRef]
- Asgodom, M.E.; Liu, D.; Fu, H.; Xie, H.; Kong, J. Effect of the near-infrared activated photocatalyst Cu(2)(OH)PO(4) nanoparticles on the growth of harmful algal blooms causing Microcystis aeruginosa. Environ. Sci. Pollut. Res. Int. 2021, 28, 20762–20771. [Google Scholar] [CrossRef]
- Buley, R.P.; Adams, C.; Belfiore, A.P.; Fernandez-Figueroa, E.G.; Gladfelter, M.F.; Garner, B.; Wilson, A.E. Field evaluation of seven products to control cyanobacterial blooms in aquaculture. Environ. Sci. Pollut. Res. Int. 2021, 128, 29971–29983. [Google Scholar] [CrossRef]
- Kinley-Baird, C.; Calomeni, A.; Berthold, D.E.; Lefler, F.W.; Barbosa, M.; Rodgers, J.H.; Laughinghouse, H.D. Laboratory-scale evaluation of algaecide effectiveness for control of microcystin-producing cyanobacteria from Lake Okeechobee, Florida (USA). Ecotoxicol. Environ. Saf. 2020, 207, 11233. [Google Scholar] [CrossRef]
- Chen, C.; Wang, Y.; Chen, K.; Shi, X.; Yang, G. Using hydrogen peroxide to control cyanobacterial blooms: A mesocosm study focused on the effects of algal density in Lake Chaohu, China. Environ. Pollut. 2021, 272, 115923. [Google Scholar] [CrossRef]
- Mucci, N.; Dugheri, S.; Bonari, A.; Farioli, A.; Rapisarda, V.; Garzaro, G.; Cappelli, G.; Arcangeli, G. Health risk assessment related to hydrogen peroxide presence in the workplace atmosphere—Analytical methods evaluation for an innovative monitoring protocol. Int. J. Occup. Med. Environ. Health 2020, 33, 137–150. [Google Scholar] [CrossRef]
- VandKhanghah, M.M.; Hedayati, A.; Nazeri, S.; Mohammadi Azarm, H.; Ghorbani, R. Biomagnification of copper along the aquatic food chain (Artemia franciscana, Danio rerio, and Astronotus ocellatus). Biol. Trace Elem. Res. 2021, 200, 1854–1860. [Google Scholar] [CrossRef]
- Zhu, X.; Dao, G.; Tao, Y.; Zhan, X.; Hu, H. A review on control of harmful algal blooms by plant-derived allelochemicals. J. Hazard. Mater. 2021, 401, 123403. [Google Scholar] [CrossRef]
- Zerrifi, S.E.A.; Mugani, R.; Redouane, E.M.; El Khalloufi, F.; Campos, A.; Vasconcelos, V.; Oudra, B. Harmful Cyanobacterial Blooms (HCBs): Innovative green bioremediation process based on anti-cyanobacteria bioactive natural products. Arch. Microbiol. 2021, 203, 31–44. [Google Scholar] [CrossRef]
- Zeng, Y.; Wang, J.; Yang, C.; Ding, M.; Hamilton, P.B.; Zhang, X.; Yang, C.; Zhnag, L.; Dai, X. A Streptomyces globisporus strain kills Microcystis aeruginosa via cell-to-cell contact. Sci. Total Environ. 2021, 769, 144489. [Google Scholar] [CrossRef]
- He, L.; Lin, Z.; Wang, Y.; He, X.; Zhou, J.; Guan, M.; Zhou, J. Facilitating harmful algae removal in fresh water via joint effects of multi-species algicidal bacteria. J. Hazard. Mater. 2021, 403, 123662. [Google Scholar] [CrossRef]
- Li, X.; Xia, Z.; Wang, B.; Lai, L.; Wang, J.; Jiang, L.; Li, T.; Wu, J.; Wang, L. Malformin C, an algicidal peptide from marine fungus Aspergillus species. Ecotoxicology 2021, 30, 996–1003. [Google Scholar] [CrossRef]
- Mohamed, Z.A.; Hashem, M.; Alamri, S.; Campos, A.; Vasconcelos, V. Fungal biodegradation and removal of cyanobacteria and microcystins: Potential applications and research needs. Environ. Sci. Pollut. Res. Int. 2021, 28, 37041–37050. [Google Scholar] [CrossRef]
- Kurtz, T.; Zeng, T.; Rosario-Ortiz, F.L. Photodegradation of cyanotoxins in surface waters. Water Res. 2021, 192, 116804. [Google Scholar] [CrossRef]
- Pal, M.; Yesankar, P.J.; Dwivedi, A.; Qureshi, A. Biotic control of harmful algal blooms (HABS): A brief review. J. Environ. Manag. 2020, 268, 110687. [Google Scholar] [CrossRef]
- Carmichael, W.W. Cyanobacteria secondary metabolites—The cyanotoxins. J. Appl. Bacteriol. 1992, 72, 445–459. [Google Scholar] [CrossRef]
- Wiegand, C.; Pflugmacher, S. Ecotoxicological effects of selected cyanobacterial secondary metabolites: A short review. Toxicol. Appl. Pharmacol. 2005, 203, 201–218. [Google Scholar] [CrossRef]
- Zurawell, R.W.; Chen, H.; Burke, J.M.; Prepas, E.E. Hepatotoxic cyanobacteria: A review of the biological importance of microcystins in freshwater environments. J. Toxicol. Environ. Health Part B 2005, 8, 1–37. [Google Scholar] [CrossRef]
- Chen, K.; Allen, J.; Lu, J. Community structures of phytoplankton with emphasis on toxic cyanobacteria in an Ohio inland lake during bloom season. J. Water Resour. Prot. 2017, 9, 1–29. [Google Scholar] [CrossRef] [Green Version]
- Lu, J.; Zhu, B.; Struewing, I.; Xu, N.; Duan, S. Nitrogen-phosphorus-associated metabolic activities during the development of a cyanobacterial bloom revealed by metatranscriptomics. Sci. Rep. 2019, 9, 2480. [Google Scholar] [CrossRef] [Green Version]
- Zhu, B.; Cao, H.; Li, G.; Du, W.; Xu, G.; Domingo, J.S.; Gu, H.; Xu, N.; Duan, S.; Lu, J. Biodiversity and dynamics of cyanobacterial communities during blooms in temperate lake (Harsha Lake, Ohio, USA). Harmful Algae 2019, 181, 9–18. [Google Scholar] [CrossRef]
- Kozich, J.J.; Westcott, S.L.; Baxter, N.T.; Highlander, S.K.; Schlossa, P.D. Development of a dual-index sequencing strategy and curation pipeline for analyzing amplicon sequence data on the MiSeq Illumina sequencing platform. Appl. Environ. Microbiol. 2013, 79, 5112–5120. [Google Scholar] [CrossRef] [Green Version]
- Bagely, M.; Pilgrim, E.; Knapp, M.; Yoder, C.; Domingo, J.S.; Banerji, A. High- throughput environmental DNA analysis informs a biological assessment of an urban stream. Ecol. Indic. 2019, 104, 378–389. [Google Scholar] [CrossRef]
- Neilan, B.A.; Jacobs, D.; Del Dot, T.; Blackall, L.L.; Hawkins, P.R.; Cox, P.T.; Goodman, A.E. rRNA sequences and evolutionary relationships among toxic and nontoxic cyanobacteria of the genus Microcystis. Int. J. Syst. Bacteriol. 1997, 47, 693–697. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [Green Version]
- Bokulich, N.A.; Kaehler, B.D.; Rideout, J.R.; Dillon, M.; Bolyen, E.; Knight, R.; Huttley, G.A.; Gregory Caporaso, J. Optimizing taxonomic classification of marker-gene amplicon sequences with QIIME 2’s q2-feature-classifier plugin. Microbiome 2018, 6, 90. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web- based tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef]
- Glöckner, F.O.; Yilmaz, P.; Quast, C.; Gerken, J.; Beccati, A.; Ciuprina, A.; Bruns, G.; Yarza, P.; Peplies, J.; Westram, R.; et al. 25 years of serving the community with ribosomal RNA gene reference databases and tools. J. Biotechnol. 2017, 261, 169–176. [Google Scholar] [CrossRef]
- Yilmaz, P.; Parfrey, L.W.; Yarza, P.; Gerken, J.; Pruesse, E.; Quast, C.; Schweer, T.; Peplies, J.; Ludwig, W.; Glöckner, F.O. The SILVA and “All-species Living Tree Project (LTP)” taxonomic frameworks. Nucleic Acids Res. 2014, 42, D643–D648. [Google Scholar] [CrossRef] [Green Version]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 14 December 2021).
- McMurdie, P.J.; Holmes, S. Phyloseq: An R package for reproducible interactive analysis and graphics of microbiome census data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef] [Green Version]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Stevens, M.H.H. Vegan: Community Ecology Package; R Package Version 2.5–7. 2018. Available online: https://CRAN.R-project.org/package=vegan (accessed on 14 December 2021).
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; ISBN 978-3-319-24277-4. Available online: https://ggplot2.tidyverse.org (accessed on 4 January 2022).
- Clarke, K.R. Non-parametric multivariate analyses of changes in community structure. Austral. J. Ecol. 1993, 18, 117–143. [Google Scholar] [CrossRef]
- Suttle, C.A.; Fuhrman, J.A.; Capone, D.G. Rapid ammonium cycling and concentration- dependent partitioning of ammonium and phosphate: Implications for carbon transfer in planktonic communities. Limnol. Oceanogr. 1990, 35, 424–433. [Google Scholar] [CrossRef]
- Jansson, M. Uptake, exchange, and excretion of orthophosphate in phosphate-starved Scenedesmus quadricauda and Pseudomonas K7. Limnol. Oceanogr. 1993, 38, 1162–1178. [Google Scholar] [CrossRef]
- Deng, W.; Wang, S.; Wan, X.; Zheng, Z.; Jiao, N.; Kao, S.J.; Moore, J.K.; Zhang, Y. Potential competition between marine heterotrophic prokaryotes and autotrophic picoplankton for nitrogen substrates. Limnol. Oceanogr. 2021, 66, 3338–3355. [Google Scholar] [CrossRef]
- Ho, L.; Hoefel, D.; Palazot, S.; Sawade, E.; Newcombe, G.; Saint, C.P.; Brookes, J.D. Investigations into the biodegradation of microcystin-LR in wastewaters. J. Hazard. Mater. 2010, 180, 628–633. [Google Scholar] [CrossRef]
- Ma, G.; Pei, H.; Hu, W.; Xu, X.; Ma, C.; Li, X. The removal of cyanobacteria and their metabolites through anoxic biodegradation in drinking water sludge. Bioresour. Technol. 2014, 165, 191–198. [Google Scholar] [CrossRef]
- Ma, G.; Pei, H.; Hu, W.; Xu, X.; Ma, C.; Pei, R. Effects of glucose on microcystin-LR removal and the bacterial community composition through anoxic biodegradation in drinking water sludge. Environ. Technol. 2016, 371, 64–73. [Google Scholar] [CrossRef]
- Li, H.; Baber, M.; Lu, J.; Goel, R. Microbial community successions and their dynamic functions during harmful cyanobacterial blooms in a freshwater lake. Water Res. 2020, 185, 116292. [Google Scholar] [CrossRef]
- Wang, K.; Mou, X.; Cao, H.; Struewing, I.; Allen, J.; Lu, J. Co-occurring microorganisms regulate the succession of cyanobacterial harmful algal blooms. Environ. Pollut. 2021, 288, 117682. [Google Scholar] [CrossRef]
- Lu, J.; Struewing, I.; Wymer, L.; Tettenhorst, D.R.; Shoemaker, J.; Allen, J. Use of qPCR and RT-qPCR for monitoring variations of microcystin producers and as an early warning system to predict toxin production in an Ohio inland lake. Water Res. 2020, 170, 115262. [Google Scholar] [CrossRef]

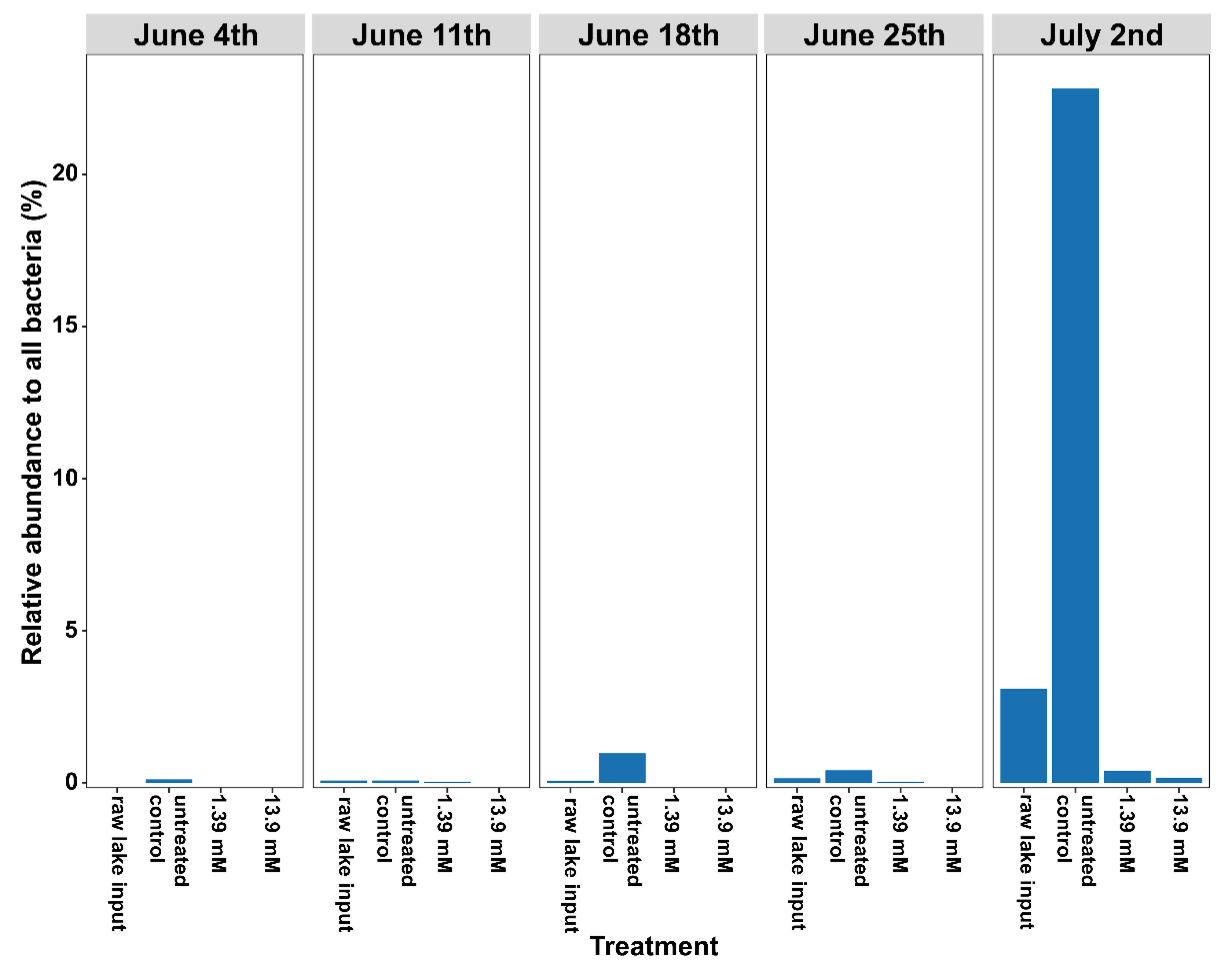
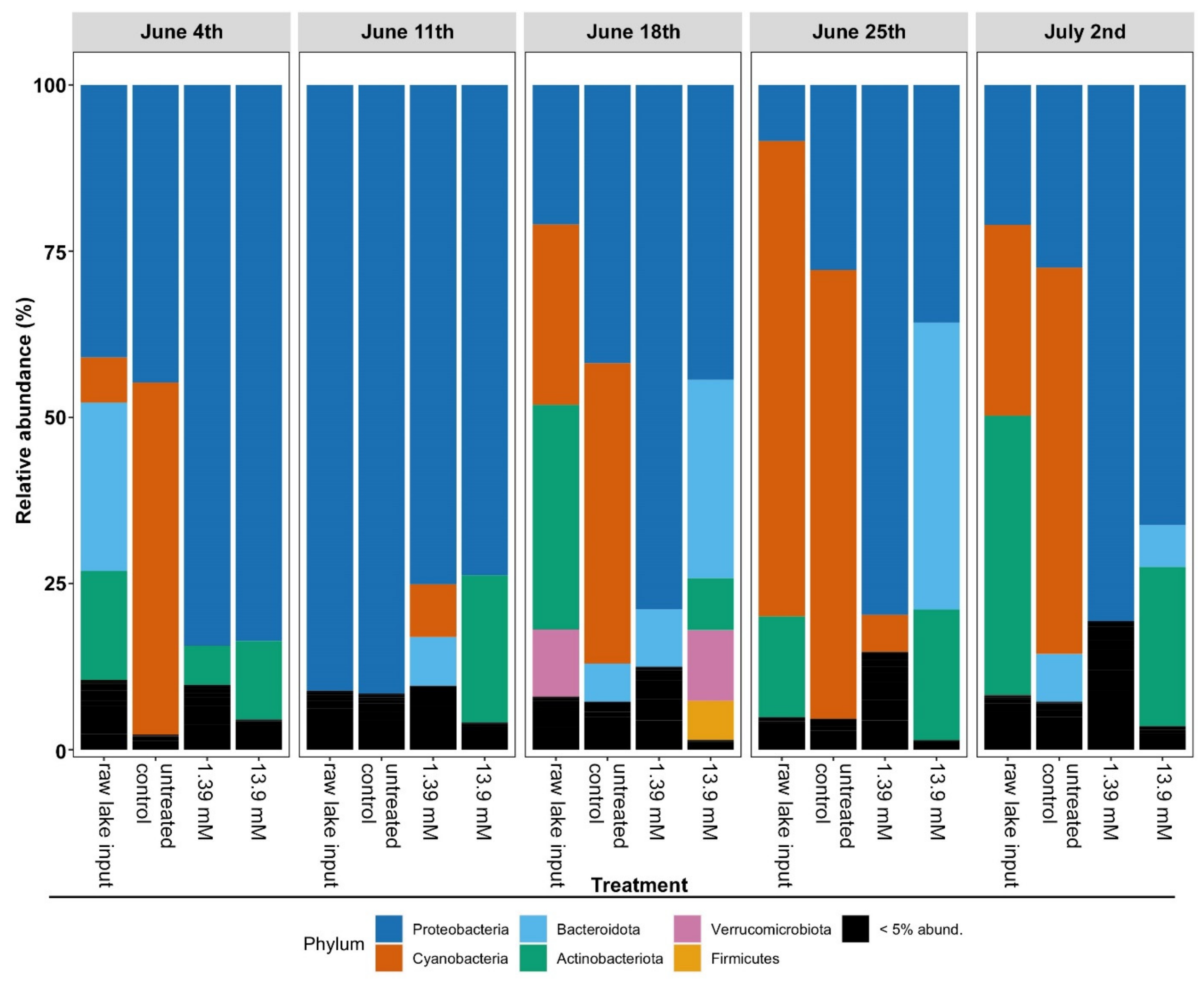
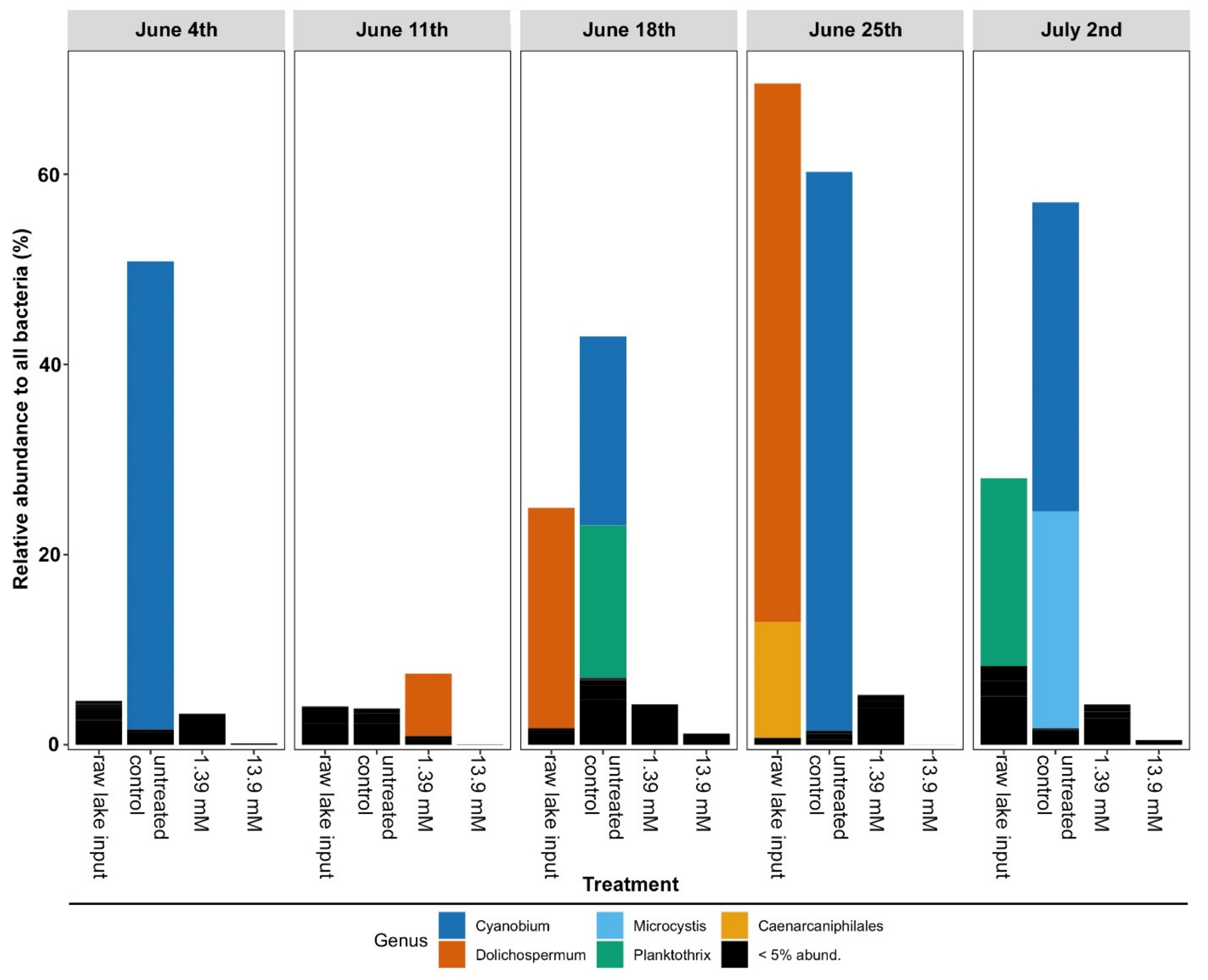

| Glucose Concentrations | ||||
|---|---|---|---|---|
| 0 mM | 1.39 mM | 13.9 mM | ||
| Sample Week | Gene Target | Copy Number ±SD/mL | Copy Number ±SD/mL | Copy Number ±SD/mL |
| 6/4 | McyEmic | 1.6 ± 0.4 × 103 | 0 | 0 |
| 16SMic | 1.1 ± ND × 103 | 0 | 0 | |
| 6/11 | McyEmic | 2.4 ± 0.8 × 103 | 0 | 0 |
| 16SMic | 0.5 ± 0.1 × 103 | 42 ± 4 | 0 | |
| 6/18 | McyEmic | 5.7 ± 2.0 × 103 | 0 | 0 |
| 16SMic | 6.2 ± 0.4 × 103 | 0 | 0 | |
| 6/25 | McyEmic | 1.3 ± 0.4 × 103 | 30 ± 16 | 0 |
| 16SMic | 3.0 ± 0.7 × 103 | 89 ± 15 | 0 | |
| 7/2 | McyEmic | 1.6 ± ND × 105 | 2.9 ± 2.2 × 103 | 5.9 ± 4.9 × 103 |
| 16SMic | 3.6 ± ND × 105 | 0.9 ± 0.1 × 103 | 0.8 ± ND × 103 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vesper, S.; Sienkiewicz, N.; Struewing, I.; Linz, D.; Lu, J. Prophylactic Addition of Glucose Suppresses Cyanobacterial Abundance in Lake Water. Life 2022, 12, 385. https://doi.org/10.3390/life12030385
Vesper S, Sienkiewicz N, Struewing I, Linz D, Lu J. Prophylactic Addition of Glucose Suppresses Cyanobacterial Abundance in Lake Water. Life. 2022; 12(3):385. https://doi.org/10.3390/life12030385
Chicago/Turabian StyleVesper, Stephen, Nathan Sienkiewicz, Ian Struewing, David Linz, and Jingrang Lu. 2022. "Prophylactic Addition of Glucose Suppresses Cyanobacterial Abundance in Lake Water" Life 12, no. 3: 385. https://doi.org/10.3390/life12030385
APA StyleVesper, S., Sienkiewicz, N., Struewing, I., Linz, D., & Lu, J. (2022). Prophylactic Addition of Glucose Suppresses Cyanobacterial Abundance in Lake Water. Life, 12(3), 385. https://doi.org/10.3390/life12030385





