In Silico Screening for Pesticide Candidates against the Desert Locust Schistocerca gregaria
Abstract
:1. Introduction
- The structures of the three endogenous AKHs were determined by nuclear magnetic resonance techniques in dodecylphosphocholine micelles; a turn structure was demonstrated for each peptide.
- A 3D model of Schgr-AKHR was constructed; the human kappa opioid receptor was found to be the best template.
- The AKHs were individually docked to the Schgr-AKHR, and a dynamic simulation of the ligand–receptor complexes in a model membrane was performed; the results demonstrated that the three AKHs bind to a common region on the receptor, interact with similar residues of the receptor, and have comparable binding constants.
2. Materials and Methods
2.1. In Silico Screening
2.2. Insects
2.3. Biological Assay
2.4. Synthetic Peptide and Test Compound
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pener, M.P.; Simpson, S.J. Locust phase polyphenism: An update. Adv. Insect Physiol. 2009, 36, 1–286. [Google Scholar]
- Simpson, S.J.; McCaffery, A.R.; Hägele, B.F. A behavioural analysis of phase change in the desert locust. Biol. Rev. 1999, 74, 461–480. [Google Scholar] [CrossRef]
- Brader, L.; Djibo, H.; Faye, F.; Ghaout, S.; Lazar, M.; Luzietoso, P.; Babah, M.O. Towards a More Effective Response to Desert Locusts and Their Impacts on Food Security, Livelihoods and Poverty: Multilateral Evaluation of the 2003–05 Desert Locust Campaign; FAO: Rome, Italy, 2006; Available online: https://www.fao.org/ag/locusts/common/ecg/1913/en/DesertLocustEvalReportE.pdf (accessed on 28 January 2020).
- Gäde, G.; Goldsworthy, G.J. Insect peptide hormones: A selective review of their physiology and potential application for pest control. Pest. Manag. Sci. 2003, 59, 1063–1075. [Google Scholar] [CrossRef] [PubMed]
- Gäde, G. The explosion of structural information on insect neuropeptides. In Progress in the Chemistry of Organic Natural Products; Herz, W., Kirby, G.W., Moore, R.E., Steglich, W., Tamm, C.H., Eds.; Springer: New York, NY, USA, 1997; Volume 71, pp. 1–128. [Google Scholar]
- Marco, H.G.; Gäde, G. Adipokinetic hormone: A hormone for all seasons? In Advances in Invertebrate (Neuro)Endocrinology: A Collection of Reviews in the Post-Genomic Era; Saleuddin, S., Lange, A.B., Orchard, I., Eds.; Apple Academic Press: Cambridge, MA, USA, 2020; Volume 2, pp. 126–170. [Google Scholar]
- Gäde, G.; Hoffmann, K.-H.; Spring, J.H. Hormonal regulation in insects: Facts, gaps, and future directions. Physiol. Rev. 1997, 77, 963–1032. [Google Scholar] [CrossRef] [PubMed]
- Hansen, K.K.; Stafflinger, E.; Schneider, M.; Hauser, F.; Cazzamali, G.; Williamson, M.; Kollmann, M.; Schachtner, J.; Grimmelikhuijzen, C.J. Discovery of a novel insect neuropeptide signaling system closely related to the insect adipokinetic hormone and corazonin hormonal systems. J. Biol. Chem. 2010, 285, 10736–10747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roch, G.J.; Busby, E.R.; Sherwood, N.M. Evolution of GnRH: Diving deeper. Gen. Comp. Endocrinol. 2011, 171, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Gäde, G.; Šimek, P.; Marco, H.G. An invertebrate [hydroxyproline]-modified neuropeptide: Further evidence for a close evolutionary relationship between insect adipokinetic hormone and mammalian gonadotropin hormone family. Biochem. Biophys Res. Commun. 2011, 414, 592–597. [Google Scholar] [CrossRef]
- Gäde, G. Peptides of the adipokinetic hormone/red pigment-concentrating hormone family: A new take on biodiversity. Ann. N. Y. Acad. Sci. 2009, 1163, 125–136. [Google Scholar] [CrossRef]
- Gäde, G.; Šimek, P.; Marco, H.G. The adipokinetic peptides in Diptera: Structure, function, and evolutionary trends. Front. Endocrinol. 2020, 11, 153. [Google Scholar] [CrossRef]
- Marco, H.G.; Šimek, P.; Gäde, G. Unique members of the adipokinetic hormone family in butterflies and moths (Insecta, Lepidoptera). Front. Physiol. 2020, 11, 614552. [Google Scholar] [CrossRef]
- Stone, J.V.; Mordue, W.; Batley, K.E.; Morris, H.R. Structure of locust adipokinetic hormone, a neurohormone that regulates lipid utilisation during flight. Nature 1976, 263, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Siegert, K.; Morgan, P.; Mordue, W. Primary structures of locust adipokinetic hormones II. Biol. Chem. Hoppe-Seyler 1985, 366, 723–727. [Google Scholar] [CrossRef] [PubMed]
- Gäde, G.; Goldsworthy, G.J.; Schaffer, M.H.; Cook, J.C.; Rinehart, K.L., Jr. Sequence analyses of adipokinetic hormones II from corpora cardiaca of Schistocerca nitans, Schistocerca gregaria, and Locusta migratoria by fast atom bombardment mass spectrometry. Biochem. Biophys. Res. Commun. 1986, 134, 723–730. [Google Scholar] [CrossRef]
- Oudejans, R.C.H.M.; Kooiman, F.P.; Heerma, W.; Versluis, C.; Slotboom, A.J.; Beenakkers, A.M.T. Isolation and structure elucidation of a novel adipokinetic hormone (Lom-AKH-III) from the glandular lobes of the corpus cardiacum of the migratory locust, Locusta migratoria. Eur. J. Biochem. 1991, 195, 351–359. [Google Scholar] [CrossRef]
- Veenstra, J.A. The contribution of the genomes of a termite and a locust to our understanding of insect neuropeptides and neurohormones. Front Physiol. 2014, 5, 454. [Google Scholar] [CrossRef] [Green Version]
- Marchal, E.; Schellens, S.; Monjon, E.; Bruyninckx, E.; Marco, H.G.; Gäde, G.; Vanden Broeck, J.; Verlinden, H. Analysis of peptide ligand specificity of different insect adipokinetic hormone receptors. Int. J. Mol. Sci. 2018, 19, 542. [Google Scholar] [CrossRef] [Green Version]
- Kaufmann, C.; Merzendorfer, H.; Gäde, G. The adipokinetic hormone system in Culicinae (Diptera: Culicidae): Molecular identification and characterization of two adipokinetic hormone (AKH) precursors from Aedes aegypti and Culex pipiens and two putative AKH receptor variants from A. aegypti. Insect Biochem. Mol. Biol. 2009, 39, 770–781. [Google Scholar] [CrossRef]
- Staubli, F.; Jorgensen, T.J.D.; Cazzamali, G.; Williamson, M.; Lenz, C.; Sondergaard, L.; Roepstorff, P.; Grimmelikhuijzen, C.J. Molecular identification of the insect adipokinetic hormone receptors. Proc. Natl. Acad. Sci. USA 2002, 99, 3446–3451. [Google Scholar] [CrossRef] [Green Version]
- Park, Y.; Kim, Y.J.; Adams, M.E. Identification of G protein-coupled receptors for Drosophila PRXamide peptides, CCAP, corazonin, and AKH supports a theory of ligand-receptor coevolution. Proc. Natl. Acad. Sci. USA 2002, 99, 11423–11428. [Google Scholar] [CrossRef] [Green Version]
- Abdulganiyyu, I.A.; Kaczmarek, K.; Zabrocki, J.; Nachman, R.J.; Marchal, E.; Schellens, S.; Verlinden, H.; Vanden Broeck, J.V.; Marco, H.G.; Jackson, G.E. Conformational analysis of a cyclic AKH neuropeptide analog that elicits selective activity on locust versus honeybee receptor. Insect Biochem. Mol. Biol. 2020, 125, 103362. [Google Scholar] [CrossRef]
- Altstein, M.; Nässel, D.R. Neuropeptide signalling in insects. In Neuropeptide Systems as Targets for Parasite and Pest Control Advances in Experimental Medicine and Biology; Geary, T.G., Maule, A.G., Eds.; Springer: Boston, MA, USA, 2010. [Google Scholar]
- Audsley, N.; Down, R.E. G-protein coupled receptors as targets for next generation pesticides. Insect Biochem. Mol. Biol. 2015, 67, 27–37. [Google Scholar] [CrossRef]
- Verlinden, H.; Vleugels, R.; Zels, S.; Dillen, S.; Lenaerts, C.; Crabbé, K.; Spit, J.; Vanden Broeck, J. Receptors for neuronal or endocrine signalling molecules as potential targets for the control of insect pests. Adv. Insect Physiol. 2014, 46, 167–303. [Google Scholar]
- Jackson, G.E.; Pavadai, E.; Gäde, G.; Andersen, N.H. The adipokinetic hormones and their cognate receptor from the desert locust, Schistocerca gregaria: Solution structure of endogenous peptides and models of their binding to the receptor. Peer J. 2019, 7, e7514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schrödinger, LLC. Schrödinger Release 2021-4. Virtual Screening Workflow (VSW); Schrödinger, LLC: New York, NY, USA, 2021. [Google Scholar]
- Schrödinger, LLC. Schrödinger Release 2021-4. LigPrep; Schrödinger, LLC: New York, NY, USA, 2021. [Google Scholar]
- Shivakumar, D.; Williams, J.; Wu, Y.; Damm, W.; Shelley, J.; Sherman, W. Prediction of absolute solvation free energies using molecular dynamics free energy perturbation and the OPLS force field. J. Chem. Theory Comput. 2010, 6, 1509–1519. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, J.R.; Calkins, D.; Sullivan, A.P.; Shelley, J.C. Towards the comprehensive, rapid, and accurate prediction of the favorable tautomeric states of drug like molecules in aqueous solution. J. Comp.-Aid Mol. Des. 2010, 24, 591–604. [Google Scholar] [CrossRef] [PubMed]
- Shelley, J.C.; Cholleti, A.; Frye, L.L.; Greenwood, J.R.; Timlin, M.R.; Uchimaya, M. Epik: A software program for pKa prediction and protonation state generation for drug-like molecules. J. Comp.-Aid Mol. Des. 2007, 21, 681–691. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, C.A. Lead- and drug-like compounds: The rule-of-five revolution. Drug Discov. Today Technol. 2004, 1, 337–341. [Google Scholar] [CrossRef]
- Halgren, T.A.; Murphy, R.B.; Friesner, R.A.; Beard, H.S.; Frye, L.L.; Pollard, W.T.; Banks, J.L. Glide: A new approach for rapid, accurate docking and scoring. 2. Enrichment factors in database screening J. Med. Chem. 2004, 47, 1750–1759. [Google Scholar] [PubMed]
- Friesner, R.A.; Banks, J.L.; Murphy, R.B.; Halgren, T.A.; Klicic, J.J.; Mainz, D.T.; Repasky, M.P.; Knoll, E.H.; Shaw, D.E.; Shelley, M.; et al. Glide: A new approach for rapid, accurate docking and scoring. 1. Method and assessment of docking accuracy. J. Med. Chem. 2004, 47, 1739–1749. [Google Scholar] [CrossRef]
- Friesner, R.A.; Murphy, R.B.; Repasky, M.P.; Frye, L.L.; Greenwood, J.R.; Halgren, T.A.; Sanschagrin, P.C.; Mainz, D.T. Extra precision glide: Docking and scoring incorporating a model of hydrophobic enclosure for protein-ligand complexes. J. Med. Chem. 2006, 49, 6177–6196. [Google Scholar] [CrossRef] [Green Version]
- NCI. 2012 Open Database. Available online: https://cactus.nci.nih.gov/download/nci/ (accessed on 7 July 2021).
- Irwin, J.J.; Tang, K.G.; Young, J.; Dandarchuluun, C.; Wong, B.R.; Khurelbaatar, M.; Moroz, Y.S.; Mayfield, J.; Sayle, R.A. ZINC20—A Free Ultralarge-Scale Chemical Database for Ligand Discovery. J. Chem. Inform. Mod. 2020, 60, 6065–6073. [Google Scholar] [CrossRef] [PubMed]
- D.E. Shaw Research. Schrödinger Release 2021-4. Desmond Molecular Dynamics System; Maestro-Desmond Interoperability Tools; D.E. Shaw Research: New York, NY, USA; Schrödinger: New York, NY, USA, 2021. [Google Scholar]
- Bowers, K.J.; Chow, E.; Xu, H.; Dror, R.O.; Eastwood, M.P.; Gregersen, B.A.; Klepeis, J.L.; Kolossvary, I.; Moraes, M.A.; Sacerdoti, F.D.; et al. Scalable algorithms for molecular dynamics simulations on commodity clusters. In Proceedings of the ACM/IEEE Conference on Supercomputing (SC06), Tampa, FL, USA, 11–17 November 2006. [Google Scholar]
- Schrödinger, LLC. Schrödinger Release 2021-4. Maestro; Schrödinger, LLC: New York, NY, USA, 2021. [Google Scholar]
- Gäde, G. Further characteristics of adipokinetic and hyperglycaemic factor(s) of stick insects. J. Insect Physiol. 1980, 26, 351–360. [Google Scholar] [CrossRef]
- Holwerda, D.A.; van Doorn, J.; Beenakkers, A.M.T. Characterization of the adipokinetic and hyperglycaemic substances from the locust corpus cardiacum. Insect Biochem. 1977, 7, 151–157. [Google Scholar] [CrossRef]
- Lambert, D.G. Drugs and receptors. Contin. Educ. Anaesth. Crit. Care Pain 2004, 4, 181–184. [Google Scholar] [CrossRef]
- Thompson, S.J.; Hattotuwagama, C.K.; Holliday, J.D.; Flower, D.R. On the hydrophobicity of peptides: Comparing empirical predictions of peptide log P values. Bioinformation 2006, 1, 237–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gäde, G. Structure-function studies on hypertrehalosaemic and adipokinetic hormones: Activity of naturally occurring analogues and some N- and C-terminal modified analogues. Physiol. Entomol. 1990, 15, 299–316. [Google Scholar] [CrossRef]
- Venkatakrishnan, A.J.; Ma, A.K.; Fonseca, R.; Latorraca, N.R.; Kelly, B.; Betz, R.M.; Asawa, C.; Kobilka, B.K.; Dror, R.O. Diverse GPCRs exhibit conserved water networks for stabilization and activation. Proc. Natl. Acad. Sci. USA 2019, 116, 3288–3293. [Google Scholar] [CrossRef] [Green Version]
- Iyison, N.B.; Sinmaz, M.G.; Sahbaz, B.D.; Shahraki, A.; Aksoydan, B.; Durdagi, S. In silico characterization of adipokinetic hormone receptor and screening for pesticide candidates against stick insect, Carausius morosus. J. Mol. Graph. Mod. 2020, 101, 107720. [Google Scholar] [CrossRef]
- Miao, Y.; McCammon, J.A. GPCR graded activation and free energy landscapes. Proc. Natl. Acad. Sci. USA 2016, 113, 12162–12167. [Google Scholar] [CrossRef] [Green Version]
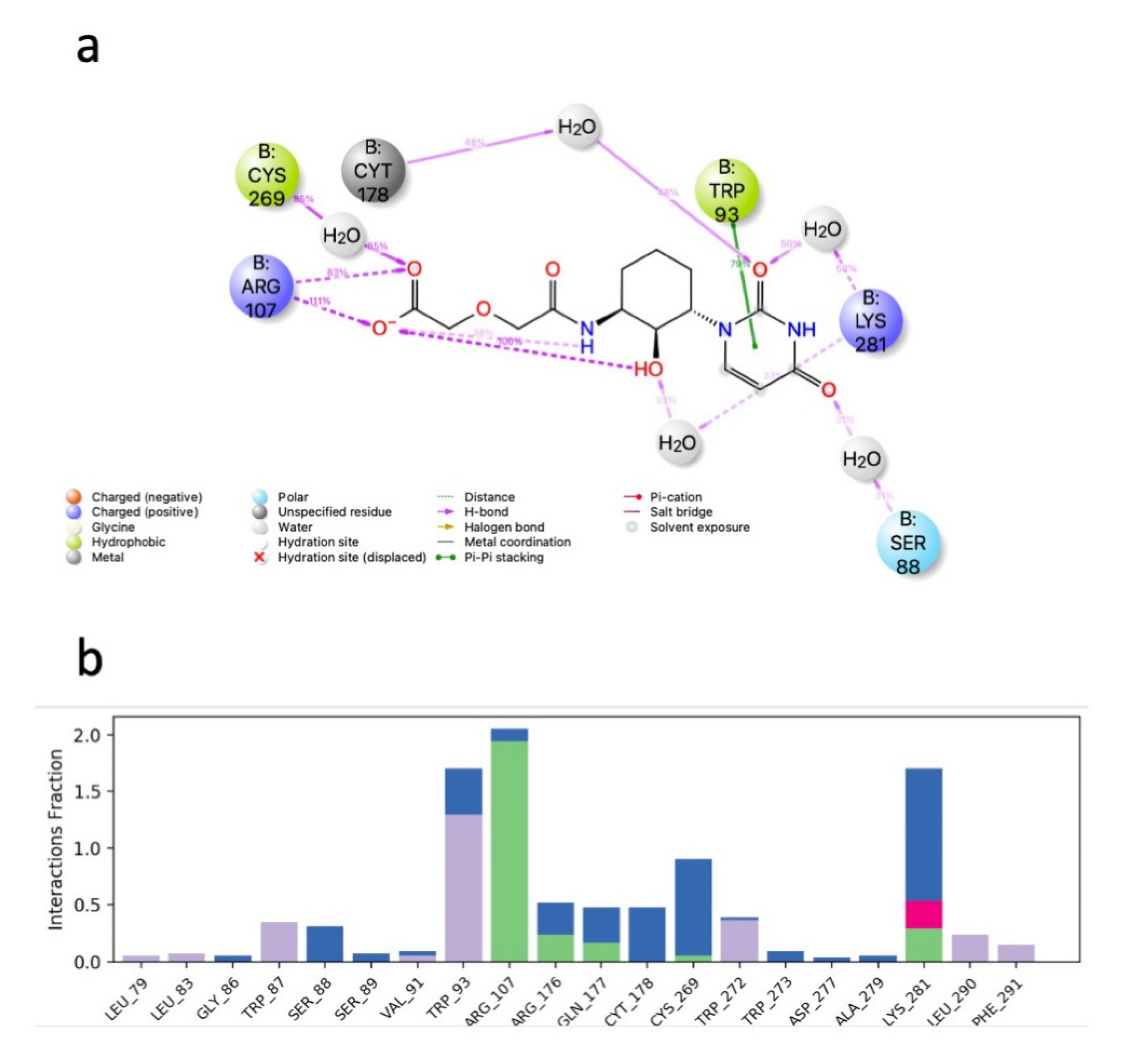
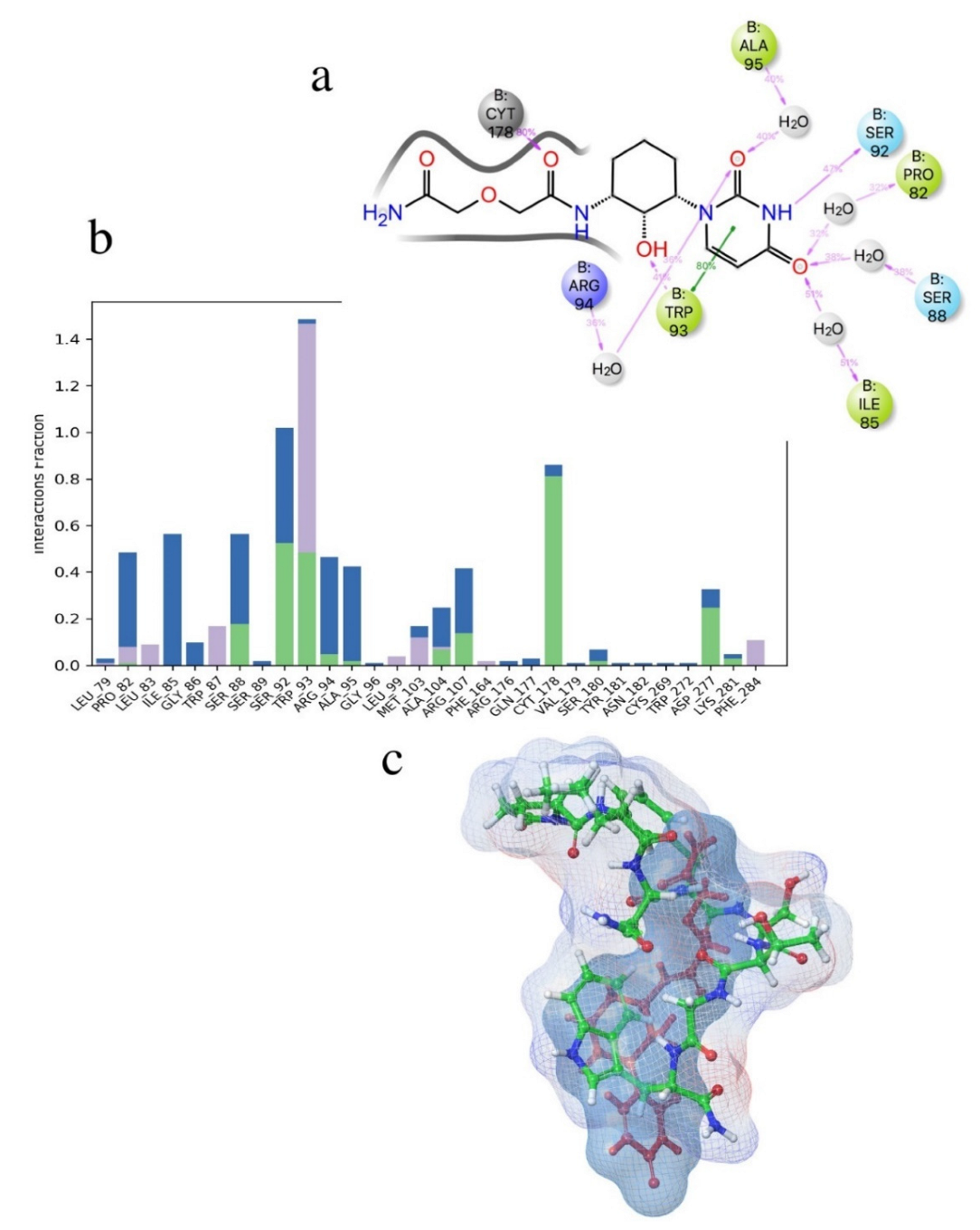

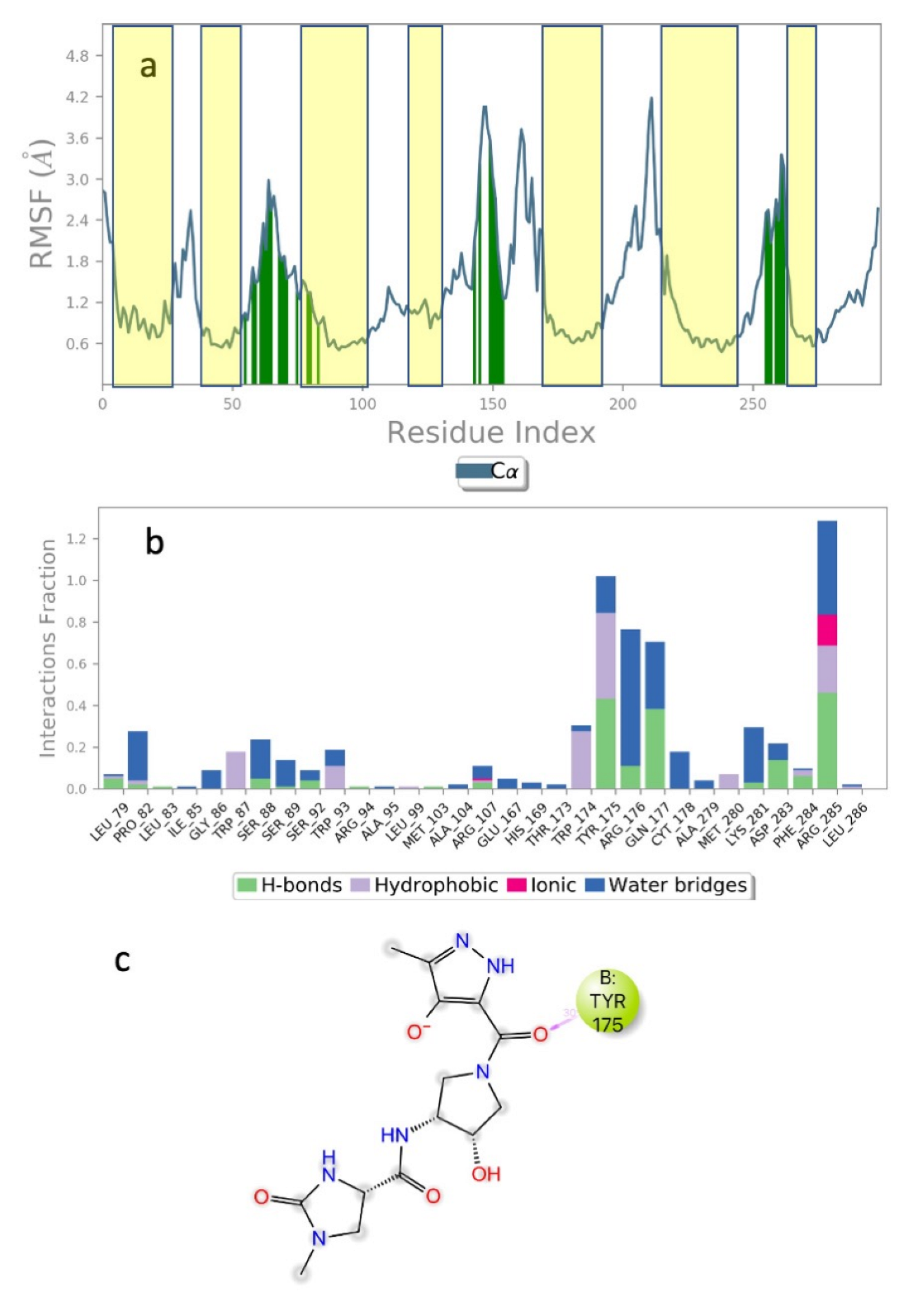
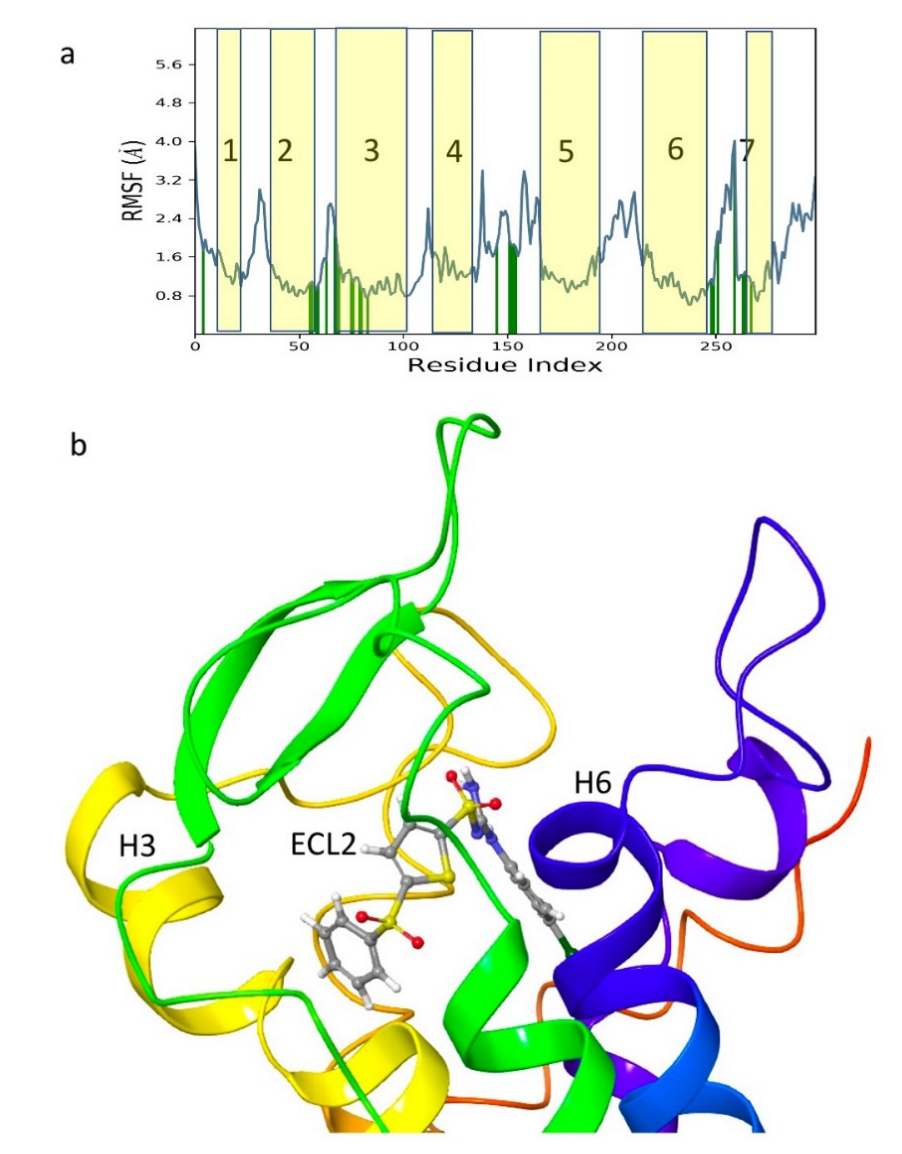
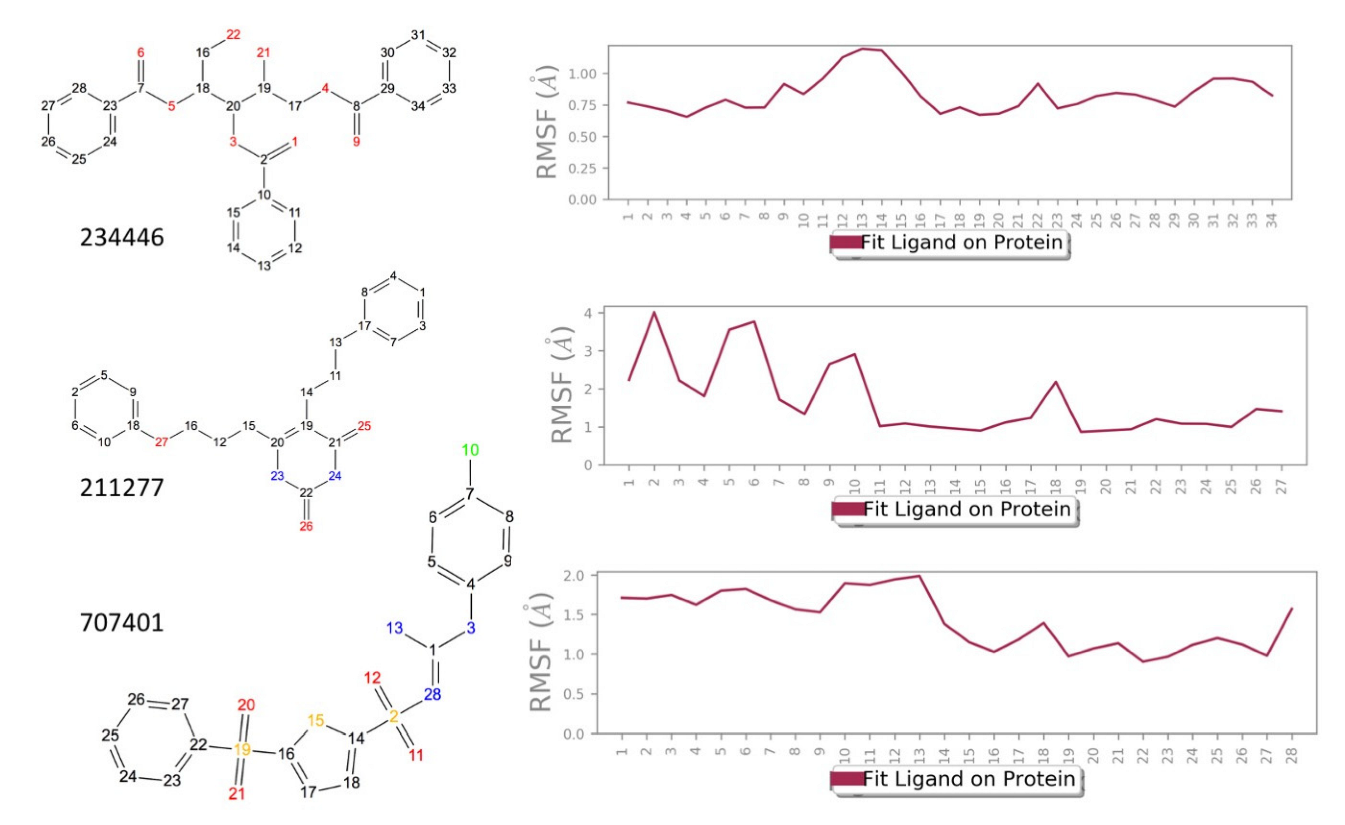
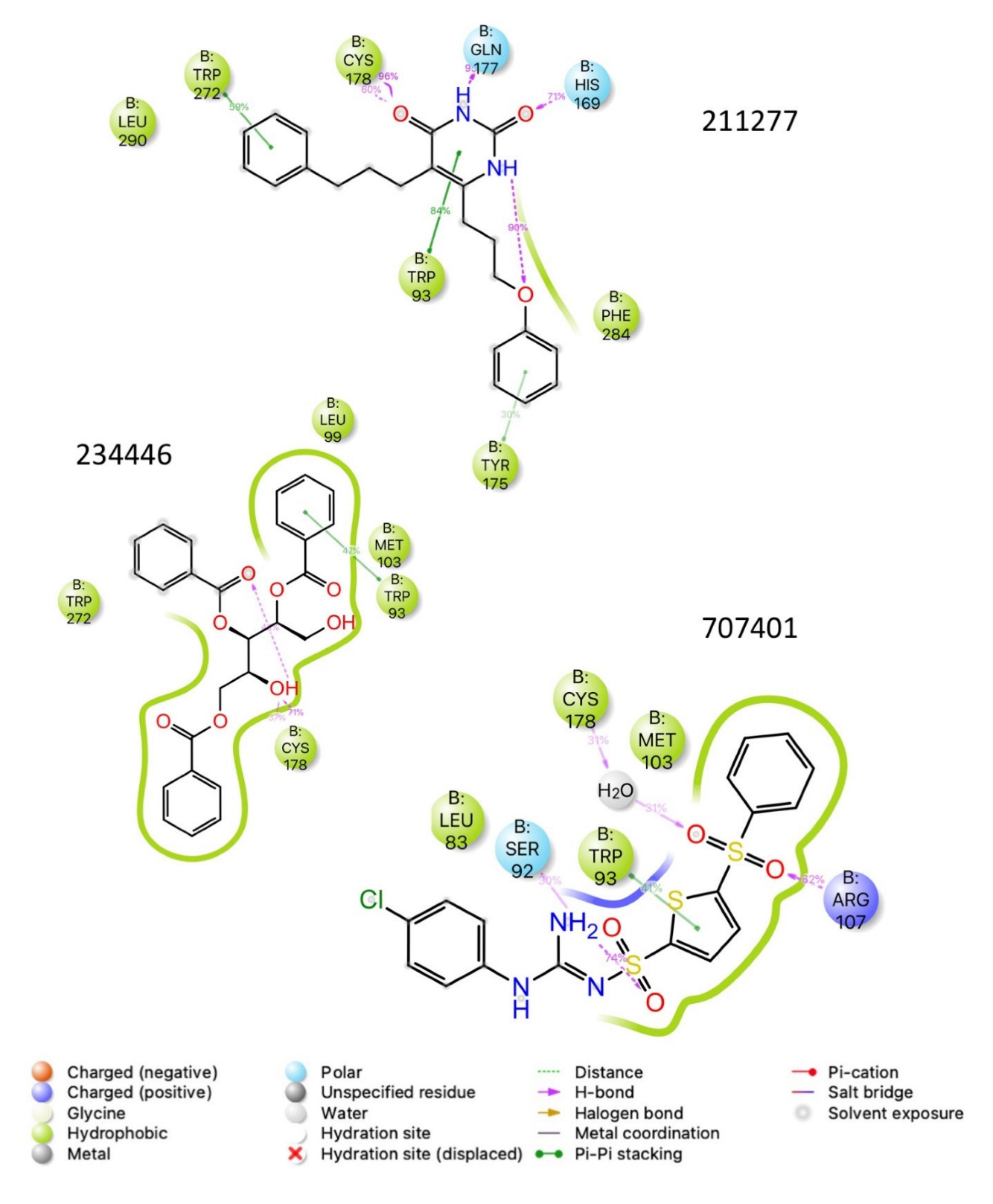
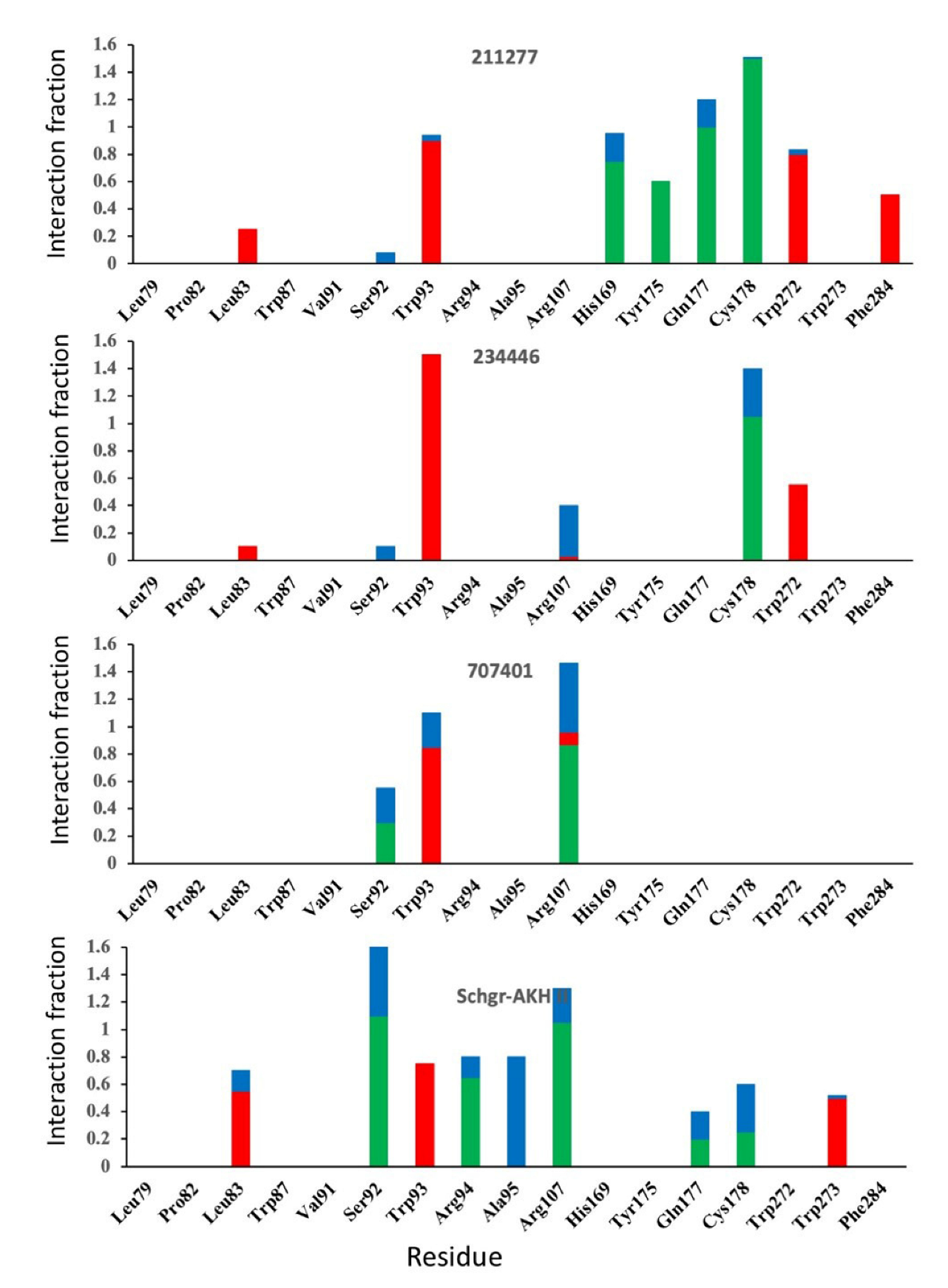
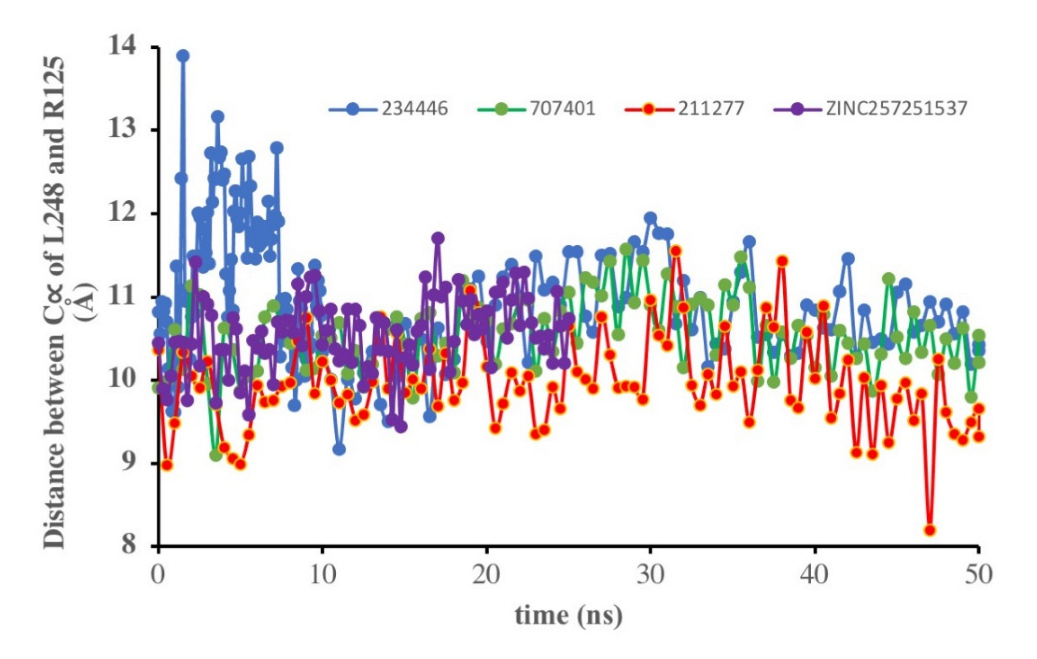
| Structure | Name | gScore | ΔGbind |
|---|---|---|---|
 | 234446 | −10.9 | −60.1 |
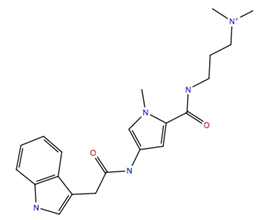 | 722562 | −10.8 | −52.5 |
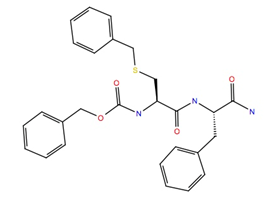 | 333451 | −10.8 | −58.5 |
 | 707401 | −10.7 | −72.1 |
 | 702438 | −10.7 | −36.2 |
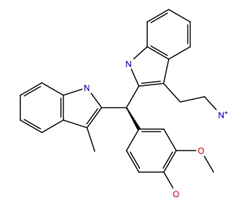 | 529379 | −10.7 | −45.4 |
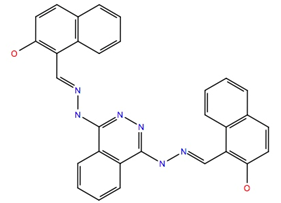 | 103663 | −10.7 | −60.7 |
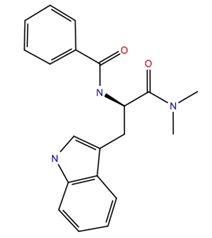 | 201298 | −10.4 | −60.3 |
 | 211277 | −10.3 | −72.3 |
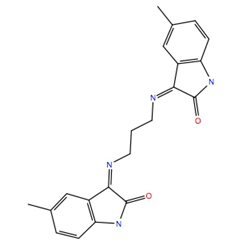 | 142427 | −10.7 | −53.9 |
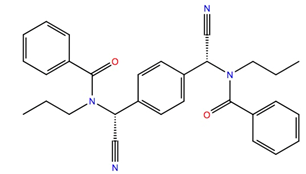 | 681749 | −10.2 | −48.0 |
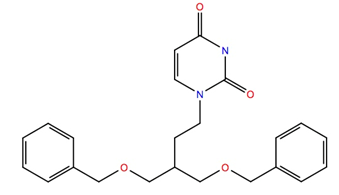 | 151836 | −10.1 | −67.8 |
 | 152001 | −10.2 | −56.3 |
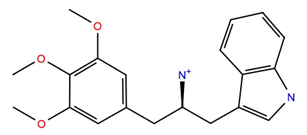 | 613572 | −10.1 | −53.1 |
| Ligand | ΔGbind | ΔGcoulomb | ΔGcovalent | ΔGH-bond | ΔGLipophilic | ΔGSolvGB | ΔGvdW |
|---|---|---|---|---|---|---|---|
| 211277 | −87(9) | −17(4) | 0.7(1) | −1.3(0.3) | −29(3) | 19(3) | −52(3) |
| 234446 | −80(4) | −16(2) | 3.2(1) | −0.8(0.2) | −26(1) | 22(2) | −61(3) |
| 707401 | −72(6) | 4(5) | 1.6(1) | −1(0.5) | −21(2) | 7(4) | −56(4) |
| Schgr-AKH II | −93(10) | −30(9) | −0.3(3) | −1.3(1) | −31(3) | 58(7) | −88(7) |
| Treatment | n | [Lipid]T0min (µg/µL) | [Lipid]T90min (µg/µL) | Difference (µg/µL) | p * |
|---|---|---|---|---|---|
| 1% DMSO | 6 | 8.51 ± 2.04 | 7.36 ± 3.03 | −1.14 ± 2.84 | NS |
| ZINC25725137 (500 pmol) | 5 | 8.87 ± 1.62 | 9.23 ± 2.15 | 0.35 ± 1.32 | NS |
| ZINC25725137 (1466 pmol) | 10 | 6.98 ± 1.15 | 7.74 ± 2.04 | 0.76 ± 1.48 | NS |
| ZINC25725137 (1466 pmol) challenged after 5 min with Schgr-AKH-II (10 pmol) | 15 | 7.79 ± 1.04 | 13.14 ± 2.63 | 5.35 ± 2.35 | ≤0.001 |
| Schgr-AKH-II (10 pmol) | 10 | 10.26 ± 4.45 | 20.69 ± 10.51 | 10.44 ± 7.23 | ≤0.001 |
| S. gregaria corpora cardiaca (0.1 gland pair equivalent) | 5 | 7.61 ± 1.35 | 21.62 ± 10.05 | 14.01 ± 8.93 | ≤0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jackson, G.E.; Gäde, G.; Marco, H.G. In Silico Screening for Pesticide Candidates against the Desert Locust Schistocerca gregaria. Life 2022, 12, 387. https://doi.org/10.3390/life12030387
Jackson GE, Gäde G, Marco HG. In Silico Screening for Pesticide Candidates against the Desert Locust Schistocerca gregaria. Life. 2022; 12(3):387. https://doi.org/10.3390/life12030387
Chicago/Turabian StyleJackson, Graham E., Gerd Gäde, and Heather G. Marco. 2022. "In Silico Screening for Pesticide Candidates against the Desert Locust Schistocerca gregaria" Life 12, no. 3: 387. https://doi.org/10.3390/life12030387
APA StyleJackson, G. E., Gäde, G., & Marco, H. G. (2022). In Silico Screening for Pesticide Candidates against the Desert Locust Schistocerca gregaria. Life, 12(3), 387. https://doi.org/10.3390/life12030387






