Effect of Microcystin-LR, Nodularin, Anatoxin-a, β-N-Methylamino-L-Alanine and Domoic Acid on Antioxidant Properties of Glutathione
Abstract
:1. Introduction
2. Materials and Methods
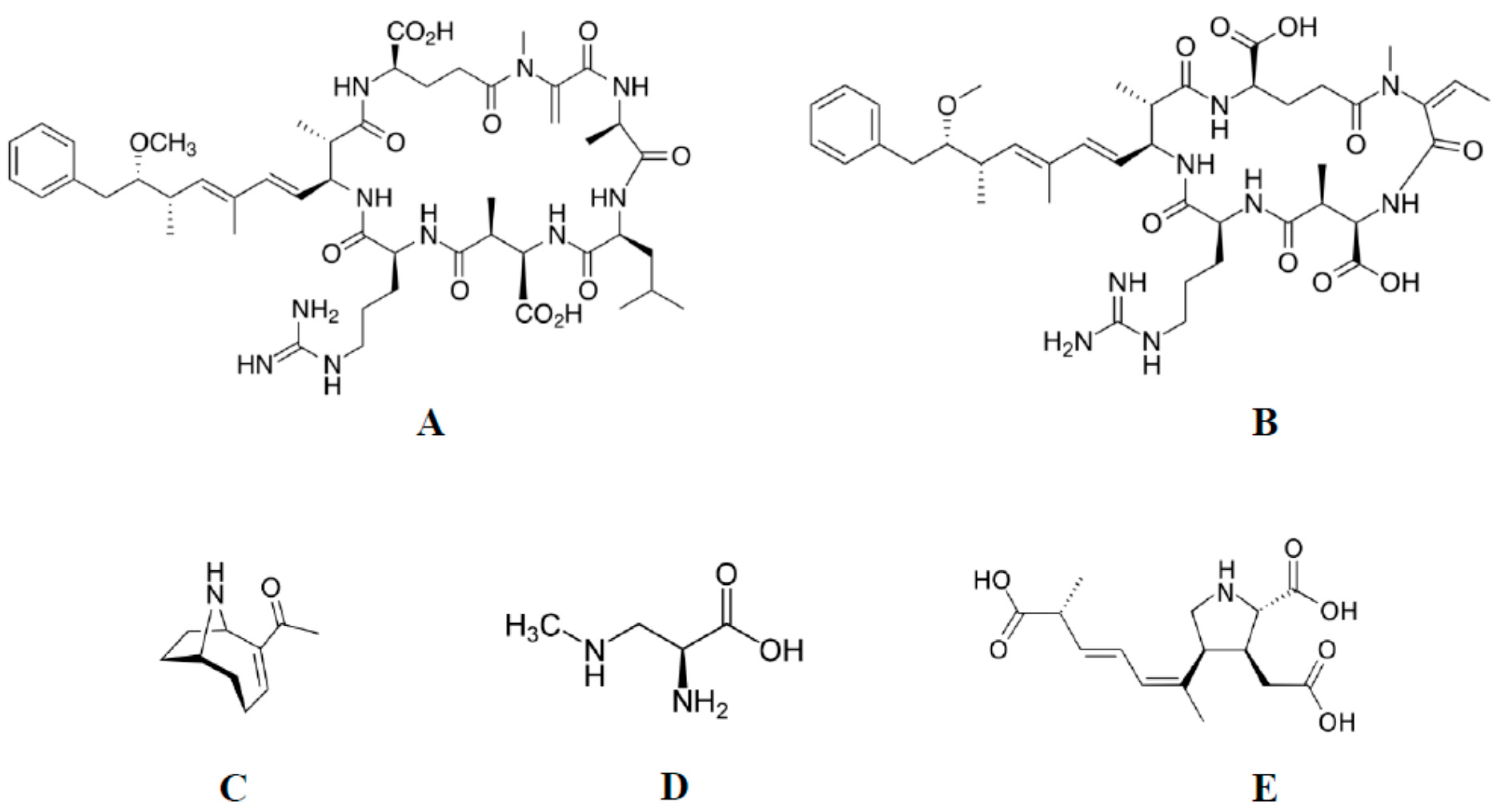
2.1. Source of Toxins
2.2. Cyanobacterial Growth Conditions
2.3. MC-LR and ANTX-a Extraction and Purification
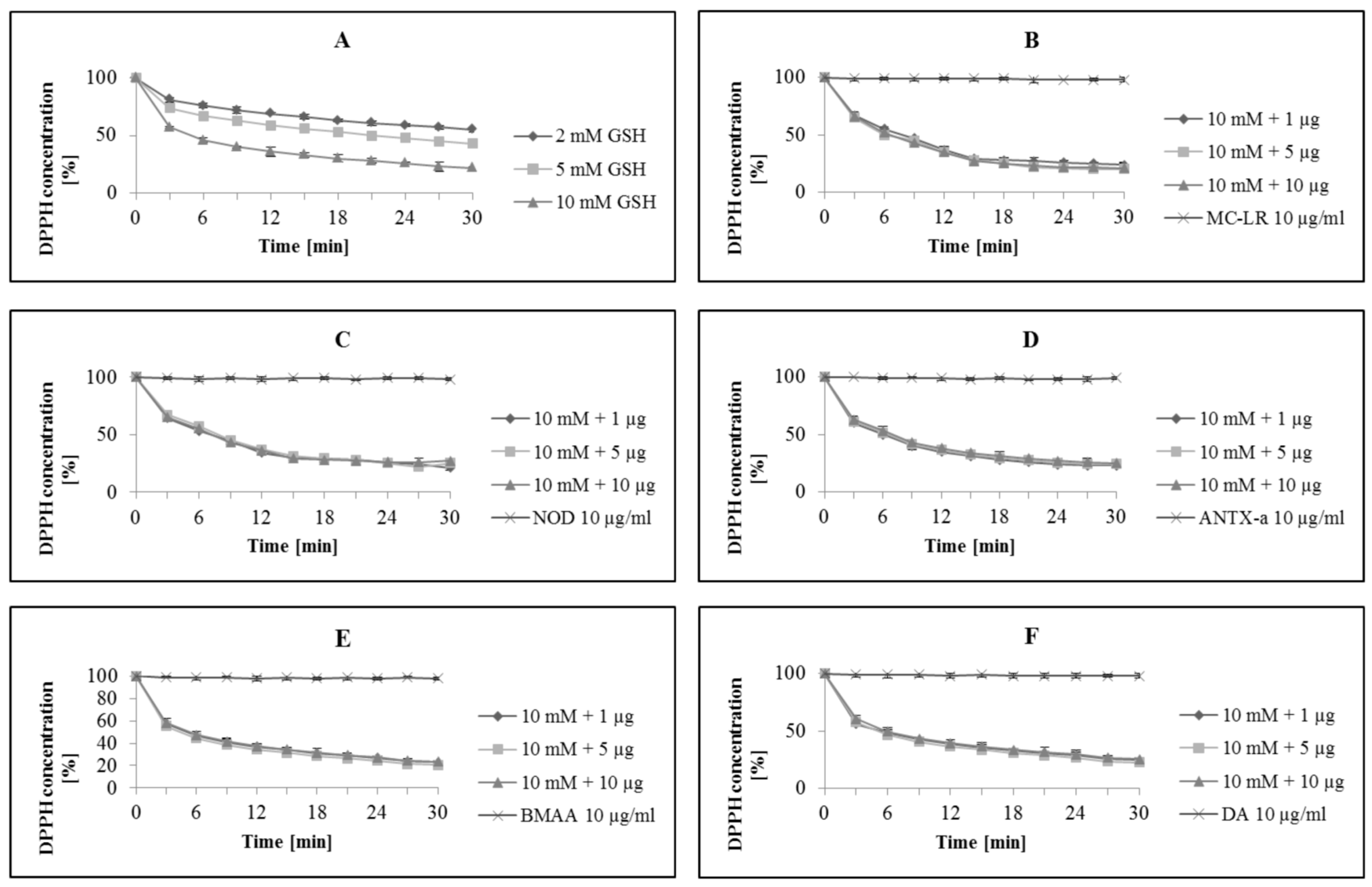
2.4. Determination of In Vitro Antioxidant Properties of GSH under the Influence of Cyanotoxins and DA
2.5. Statistical Analysis
2.6. Chemicals
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Rzymski, P.; Poniedziałek, B. Dermatotoxins synthesized by blue-green algae (Cyanobacteria). Postepy Dermatol. I Alergol. 1995, 29, 47–50. [Google Scholar]
- Adamski, M.; Chrapusta, E.; Bober, B.; Kamiński, A.; Białczyk, J. Cylindrospermopsin: Cyanobacterial secondary metabolite. Biological aspects and potential risk for human health and life. Oceanol. Hydrobiol. Stud. 2014, 43, 442–449. [Google Scholar] [CrossRef]
- Huisman, J.; Codd, G.A.; Paerl, H.W.; Ibelings, B.W.; Verspagen, J.M.H.; Visser, P.M. Cyanobacterial blooms. Nat. Rev. Microbiol. 2018, 16, 471–483. [Google Scholar] [CrossRef]
- Svirčev, Z.; Lalić, D.; Bojadžija Savić, G.; Tokodi, N.; Drobac Backović, D.; Chen, L.; Meriluoto, J.; Codd, G.A. Global geographical and historical overview of cyanotoxin distribution and cyanobacterial poisonings. Arch. Toxicol. 2019, 93, 2429–2481. [Google Scholar] [CrossRef] [PubMed]
- Adamski, M.; Zimolag, E.; Kaminski, A.; Drukała, J.; Bialczyk, J. Effects of cylindrospermopsin, its decomposition products, and anatoxin-a on human keratinocytes. Sci. Total Environ. 2020, 765, 142670. [Google Scholar] [CrossRef] [PubMed]
- Pearson, L.; Mihali, T.; Moffitt, M.; Kellmann, R.; Neilan, B. On the chemistry, toxicology and genetics of the cyanobacterial toxins, microcystin, nodularin, saxitoxin and cylindrospermopsin. Mar. Drugs 2010, 8, 1650–1680. [Google Scholar] [CrossRef] [Green Version]
- Runnegar, M.; Berndt, N.; Kaplowitz, N. Microcystin uptake and inhibition of protein phosphatases: Effects of chemoprotectants and self-inhibition in relation to known hepatic transporters. Toxicol. Appl. Pharmacol. 1995, 134, 264–272. [Google Scholar] [CrossRef]
- Lone, Y.; Koiri, R.K.; Bhide, M. An overview of the toxic effect of potential human carcinogen Microcystin-LR on testis. Toxicol. Rep. 2015, 2, 289–296. [Google Scholar] [CrossRef] [Green Version]
- Runnegar, M.; Shouming, K.; Berndt, N. Protein phosphatase inhibition and in vivo hepatotoxicity of microcystins. Am. J. Physiol. 1993, 265, 224–230. [Google Scholar] [CrossRef]
- Welten, R.D.; Meneely, J.P.; Elliott, C.T. A Comparative Review of the Effect of Microcystin-LR on the Proteome. Expo. Health 2020, 12, 111–129. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.; Chen, L.; Liu, W.; Qiao, Q.; Wu, K.; Wen, J.; Huang, C.; Tang, R.; Zhang, X. Involvement of oxidative stress and cytoskeletal disruption in microcystin-induced apoptosis in CIK cells. Aquat. Toxicol. 2015, 165, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Shan, Z.; Xu, W.; Wang, X.; Zhou, J.; Kong, D.; Xu, J. Microcystin-LR induced reactive oxygen species mediate cytoskeletal disruption and apoptosis of hepatocytes in Cyprinus carpio L. PLoS ONE 2013, 8, e84768. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Falconer, I.R.; Yeung, D.S.K. Cytoskeletal changes in hepatocytes induced by Microcystis toxins and their relation to hyperphosphorylation of cell proteins. Chem. -Biol. Interact. 1992, 81, 181–196. [Google Scholar] [CrossRef]
- Massey, I.Y.; Yang, F. A mini review on microcystins and bacterial degradation. Toxins 2020, 12, 268. [Google Scholar] [CrossRef] [Green Version]
- Svirčev, Z.; Lujić, J.; Marinović, Z.; Drobac, D.; Tokodi, N.; Stojiljković, B.; Meriluoto, J. Toxicopathology induced by microcystins and nodularin: A histopathological review. J. Environ. Sci. Health Part C Environ. Carcinog. Ecotoxicol. Rev. 2015, 33, 125–167. [Google Scholar] [CrossRef]
- Osswald, J.; Rellán, S.; Gago, A.; Vasconcelos, V. Toxicology and detection methods of the alkaloid neurotoxin produced by cyanobacteria, anatoxin-a. Environ. Int. 2007, 33, 1070–1089. [Google Scholar] [CrossRef]
- Proctor, E.A.; Mowrey, D.D.; Dokholyan, N.V. β-Methylamino-L-alanine substitution of serine in SOD1 suggests a direct role in ALS etiology. PLoS Comput. Biol. 2019, 15, e1007225. [Google Scholar] [CrossRef] [Green Version]
- van Onselen, R.; Downing, T.G. BMAA-protein interactions: A possible new mechanism of toxicity. Toxicon 2020, 143, 74–80. [Google Scholar] [CrossRef]
- Banack, S.A.; Caller, T.A.; Stommel, E.W. The cyanobacteria derived toxin beta-N-methylamino-L-alanine and amyotrophic lateral sclerosis. Toxins 2010, 2, 2837–2850. [Google Scholar] [CrossRef]
- Holtcamp, W. The emerging science of BMAA: Do cyanobacteria contribute to neurodegenerative disease? Environ. Health Perspect. 2012, 120, 110–116. [Google Scholar] [CrossRef] [Green Version]
- Chiu, A.S.; Gehringer, M.M.; Welch, J.H.; Neilan, B.A. Does α-amino-β-methylaminopropionic acid (BMAA) play a role in neurodegeneration? Int. J. Environ. Res. Public Health 2011, 8, 3728–3746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.Q.; Rush, T.; Zapata, J.; Lobner, D. β-N-methylamino-l-alanine induces oxidative stress and glutamate release through action on system Xc-. Exp. Neurol. 2009, 217, 429–433. [Google Scholar] [CrossRef] [PubMed]
- Lobner, D. Mechanisms of β-N-methylamino-L-alanine induced neurotoxicity. Amyotroph. Lateral Scler. 2009, 10 (Suppl. 2), 56–60. [Google Scholar] [CrossRef] [PubMed]
- Zabaglo, K.; Chrapusta, E.; Bober, B.; Kaminski, A.; Adamski, M.; Bialczyk, J. Environmental roles and biological activity of domoic acid: A review. Algal Res. 2016, 13, 94–101. [Google Scholar] [CrossRef]
- Scott, N.; Hatlelid, K.M.; MacKenzie, N.E.; Carter, D.E. Reactions of Arsenic (III) and Arsenic (V) Species with Glutathione. Chem. Res. Toxicol. 1993, 6, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Umansky, C.; Morellato, A.; Scheidegger, M.; Rieckher, M.; Martinefski, M.R.; Fernandez, G.A.; Kolesnikova, K.; Vesting, A.J.; Karakasilioti, I.; Reingruber, H.; et al. Endogenous formaldehyde scavenges cellular glutathione resulting in cytotoxic redox disruption. bioRxiv 2020. [Google Scholar] [CrossRef]
- Adamski, M.; Kaminski, A. Impact of cylindrospermopsin and its decomposition products on antioxidant properties of glutathione. Algal Res. 2021, 56, 102305. [Google Scholar] [CrossRef]
- Stanier, R.Y.; Deruelles, J.; Rippka, R.; Herdman, M.; Waterbury, J.B. Generic Assignments, Strain Histories and Properties of Pure Cultures of Cyanobacteria. Microbiology 1979, 111, 1–61. [Google Scholar] [CrossRef] [Green Version]
- Meriluoto, J.; Codd, G.A. Toxic: Cyanobacterial Monitoring and Cyanotoxin Analysis; Åbo Akademi University Press: Åbo, Finland, 2005. [Google Scholar]
- Adamski, M.; Zmudzki, P.; Chrapusta, E.; Bober, B.; Kaminski, A.; Zabaglo, K.; Latkowska, E.; Bialczyk, J. Effect of pH and temperature on the stability of cylindrospermopsin. Characterization of decomposition products. Algal Res. 2016, 15, 129–134. [Google Scholar] [CrossRef]
- Adamski, M.; Zmudzki, P.; Chrapusta, E.; Kaminski, A.; Bober, B.; Zabaglo, K.; Bialczyk, J. Characterization of cylindrospermopsin decomposition products formed under irradiation conditions. Algal Res. 2016, 18, 1–6. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. Lebensm. Wiss. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Forman, H.J.; Zhang, H.; Rinna, A. Glutathione: Overview of its protective roles, measurement, and biosynthesis. Mol. Asp. Med. 2009, 30, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Gaucher, C.; Boudier, A.; Bonetti, J.; Clarot, I.; Leroy, P.; Parent, M. Glutathione: Antioxidant properties dedicated to nanotechnologies. Antioxidants 2018, 7, 62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lushchak, V.I. Glutathione Homeostasis and Functions: Potential Targets for Medical Interventions. J. Amino Acids 2012, 2012, 736837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giordano, G.; White, C.C.; Mohar, I.; Kavanagh, T.J.; Costa, L.G. Glutathione levels modulate domoic acid-induced apoptosis in mouse cerebellar granule cells. Toxicol. Sci. 2007, 100, 433–444. [Google Scholar] [CrossRef]
- Hinojosa, M.G.; Prieto, A.I.; Gutiérrez-Praena, D.; Moreno, F.J.; Cameán, A.M.; Jos, A. In vitro assessment of the combination of cylindrospermopsin and the organophosphate chlorpyrifos on the human neuroblastoma SH-SY5Y cell line. Ecotoxicol. Environ. Saf. 2020, 191, 110222. [Google Scholar] [CrossRef]
- Norris, R.L.G.; Seawright, A.A.; Shaw, G.R.; Senogles, P.; Eaglesham, G.K.; Smith, M.J.; Chiswell, R.K.; Moore, M.R. Hepatic xenobiotic metabolism of cylindrospermopsin in vivo in the mouse. Toxicon 2002, 40, 471–476. [Google Scholar] [CrossRef]
- Won, E.; Kim, D.; Yoo, J.; In, S.; Shin, K.; Lee, Y. Oxidative stress responses in brackish water flea exposed to microcystin-LR and algal bloom waters from Nakdong River, Republic of Korea. Mar. Pollut. Bull. 2021, 162, 111868. [Google Scholar] [CrossRef]
- Puerto, M.; Pichardo, S.; Jos, Á.; Prieto, A.I.; Sevilla, E.; Frías, J.E.; Cameán, A.M. Differential oxidative stress responses to pure Microcystin-LR and Microcystin-containing and non-containing cyanobacterial crude extracts on Caco-2 cells. Toxicon 2010, 55, 514–522. [Google Scholar] [CrossRef]
- Sicińska, P.; Bukowska, B.; Michałowicz, J.; Duda, W. Damage of cell membrane and antioxidative system in human erythrocytes incubated with microcystin-LR in vitro. Toxicon 2006, 47, 387–397. [Google Scholar] [CrossRef]
- Qiu, T.; Xie, P.; Ke, Z.; Li, L.; Guo, L. In situ studies on physiological and biochemical responses of four fishes with different trophic levels to toxic cyanobacterial blooms in a large Chinese lake. Toxicon 2007, 50, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Martins, N.D.; Yunes, J.S.; Monteiro, D.A.; Rantin, F.T.; Kalinin, A.L. Microcystin-LR leads to oxidative damage and alterations in antioxidant defense system in liver and gills of Brycon amazonicus (SPIX & AGASSIZ 2017, 1829). Toxicon 2017, 139, 109–116. [Google Scholar] [CrossRef]
- Min, B.H.; Ravikumar, Y.; Lee, D.H.; Choi, K.S.; Kim, B.M.; Rhee, J.S. Age-dependent antioxidant responses to the bioconcentration of microcystin-LR in the mysid crustacean, Neomysis awatschensis. Environ. Pollut. 2005, 232, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Takenaka, S. Covalent glutathione conjugation to cyanobacterial hepatotoxin microcystin LR by F344 rat cytosolic and microsomal glutathione S-transferases. Environ. Toxicol. Pharmacol. 2001, 9, 135–139. [Google Scholar] [CrossRef]
- Kondo, F.; Ikai, Y.; Oka, H.; Okumura, M.; Ishikawa, N.; Harada, K.; Matsuura, K.; Murata, H.; Suzuki, M. Formation, Characterization, and Toxicity of the Glutathione and Cysteine Conjugates of Toxic Heptapeptide Microcystins. Chem. Res. Toxicol. 1992, 5, 591–596. [Google Scholar] [CrossRef]
- Gehringer, M.M.; Shephard, E.G.; Downing, T.G.; Wiegand, C.; Neilan, B.A. An investigation into the detoxification of microcystin-LR by the glutathione pathway in Balb/c mice. Int. J. Biochem. Cell Biol. 2004, 36, 931–941. [Google Scholar] [CrossRef] [PubMed]
- Zong, W.S.; Zhang, S.H.; Wang, Q.; Teng, Y.; Liu, Y.Z.; Du, Y.G. Evaluation of the Direct and Indirect Regulation Pathways of Glutathione Target to the Hepatotoxicity of Microcystin-LR. BioMed Res. Int. 2018, 2018, 5672637. [Google Scholar] [CrossRef]
- Beattie, K.A.; Ressler, J.; Wiegand, C.; Krause, E.; Codd, G.A.; Steinberg, C.E.; Pflugmacher, S. Comparative effects and metabolism of two microcystins and nodularin in the brine shrimp Artemia salina. Aquat. Toxicol. 2003, 62, 219–226. [Google Scholar] [CrossRef]
- Bouaïcha, N.; Maatouk, I. Microcystin-LR and nodularin induce intracellular glutathione alteration, reactive oxygen species production and lipid peroxidation in primary cultured rat hepatocytes. Toxicol. Lett. 2004, 148, 53–63. [Google Scholar] [CrossRef]
- Ha, M.H.; Contardo-Jara, V.; Pflugmacher, S. Uptake of the cyanobacterial neurotoxin, anatoxin-a, and alterations in oxidative stress in the submerged aquatic plant Ceratophyllum demersum. Ecotoxicol. Environ. Saf. 2014, 101, 205–212. [Google Scholar] [CrossRef]
- Ha, M.H.; Pflugmacher, S. Phytotoxic effects of the cyanobacterial neurotoxin anatoxin-a: Morphological, physiological and biochemical responses in aquatic macrophyte, Ceratophyllum demersum. Toxicon 2013, 70, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kaminski, A.; Chrapusta, E.; Adamski, M.; Bober, B.; Zabaglo, K.; Bialczyk, J. Determination of the time-dependent response of Lemna trisulca to the harmful impact of the cyanotoxin anatoxin-a. Algal Res. 2016, 16, 368–375. [Google Scholar] [CrossRef]
- Mitrovic, S.M.; Pflugmacher, S.; James, K.J.; Furey, A. Anatoxin-a elicits an increase in peroxidase and glutathione S-transferase activity in aquatic plants. Aquat. Toxicol. 2004, 68, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Delcourt, N.; Claudepierre, T.; Maignien, T.; Arnich, N.; Mattei, C. Cellular and molecular aspects of the β-N-Methylamino-l-alanine (BMAA) mode of action within the neurodegenerative pathway: Facts and controversy. Toxins 2018, 10, 6. [Google Scholar] [CrossRef] [Green Version]
- Lobner, D.; Piana PM, T.; Salous, A.K.; Peoples, R.W. β-N-methylamino-l-alanine enhances neurotoxicity through multiple mechanisms. Neurobiol. Dis. 2007, 25, 360–366. [Google Scholar] [CrossRef] [Green Version]
- Giordano, G.; White, C.C.; McConnachie, L.A.; Fernandez, C.; Kavanagh, T.J.; Costa, L.G. Neurotoxicity of domoic acid in cerebellar granule neurons in a genetic model of glutathione deficiency. Mol. Pharmacol. 2006, 70, 2116–2126. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adamski, M.; Kaminski, A. Effect of Microcystin-LR, Nodularin, Anatoxin-a, β-N-Methylamino-L-Alanine and Domoic Acid on Antioxidant Properties of Glutathione. Life 2022, 12, 227. https://doi.org/10.3390/life12020227
Adamski M, Kaminski A. Effect of Microcystin-LR, Nodularin, Anatoxin-a, β-N-Methylamino-L-Alanine and Domoic Acid on Antioxidant Properties of Glutathione. Life. 2022; 12(2):227. https://doi.org/10.3390/life12020227
Chicago/Turabian StyleAdamski, Michal, and Ariel Kaminski. 2022. "Effect of Microcystin-LR, Nodularin, Anatoxin-a, β-N-Methylamino-L-Alanine and Domoic Acid on Antioxidant Properties of Glutathione" Life 12, no. 2: 227. https://doi.org/10.3390/life12020227
APA StyleAdamski, M., & Kaminski, A. (2022). Effect of Microcystin-LR, Nodularin, Anatoxin-a, β-N-Methylamino-L-Alanine and Domoic Acid on Antioxidant Properties of Glutathione. Life, 12(2), 227. https://doi.org/10.3390/life12020227





