The Multifunctional Role of Herbal Products in the Management of Diabetes and Obesity: A Comprehensive Review
Abstract
:1. Introduction
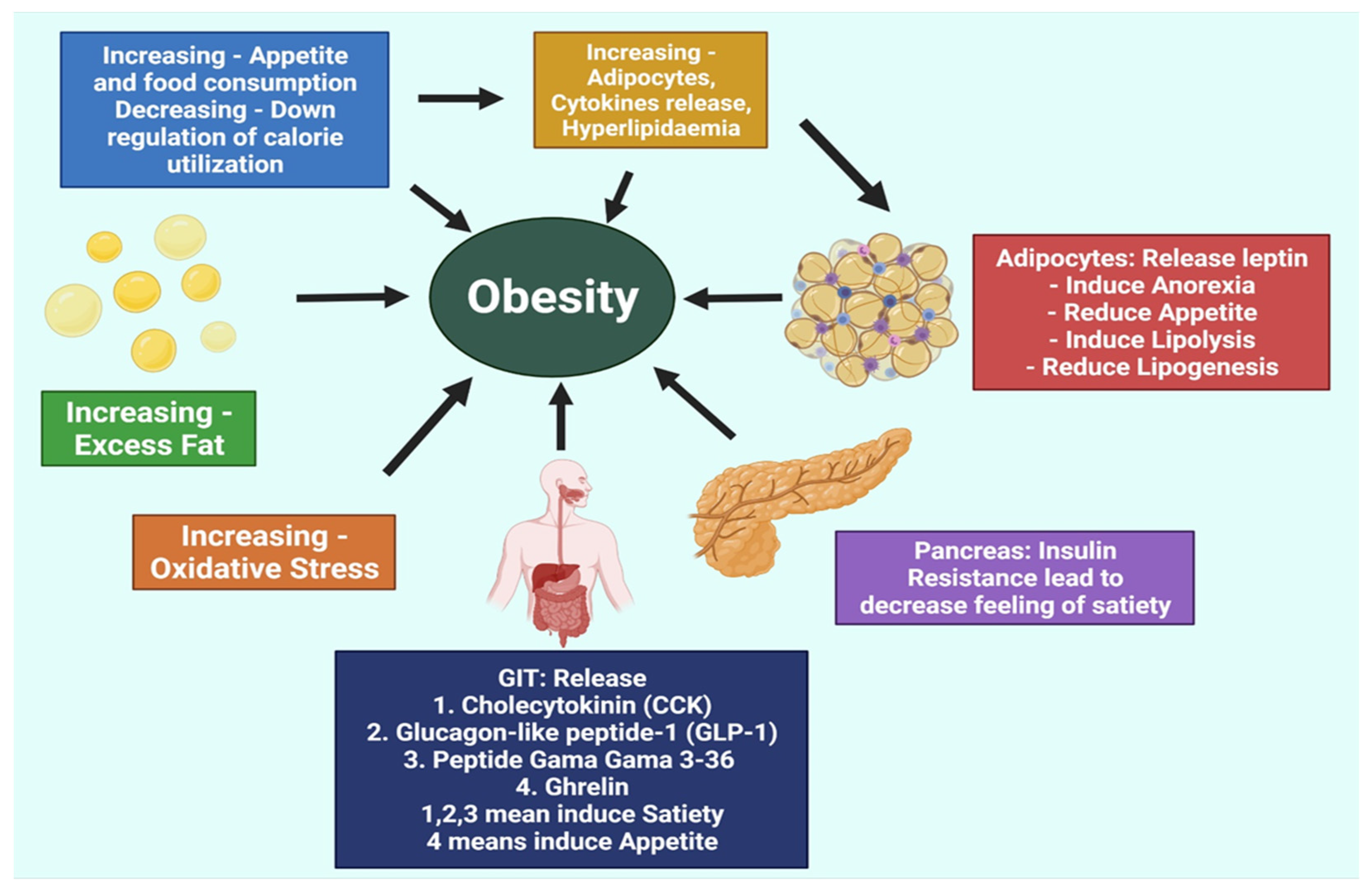
2. Pathogenesis of Obesity
3. Obesity and Diabetes
3.1. Obesity: Current Concerns and Treatments
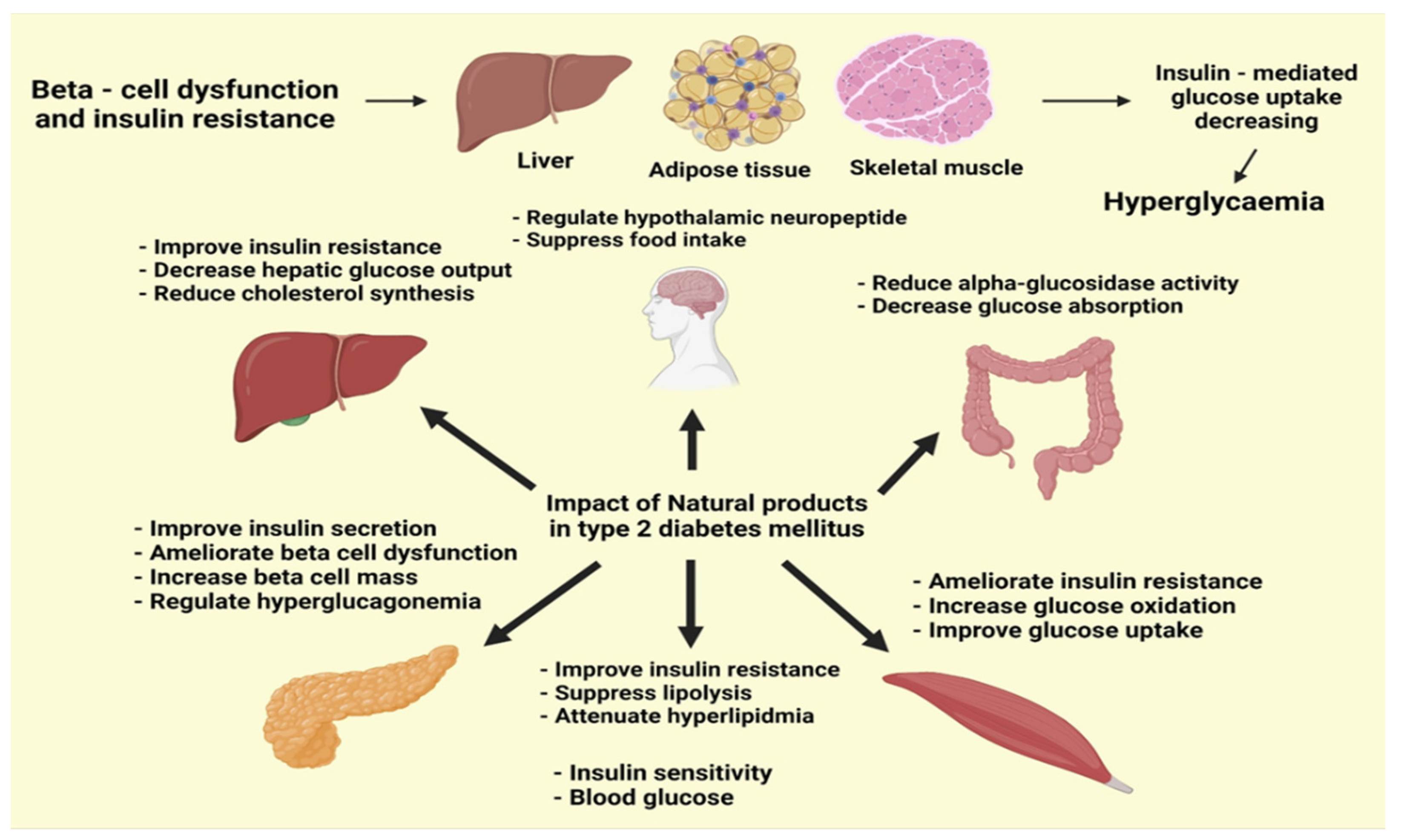
3.2. Diabetes: Current Concerns and Treatments
4. Relationship between Diabetes and Obesity
Genetic Factors Linking Obesity and Diabetes
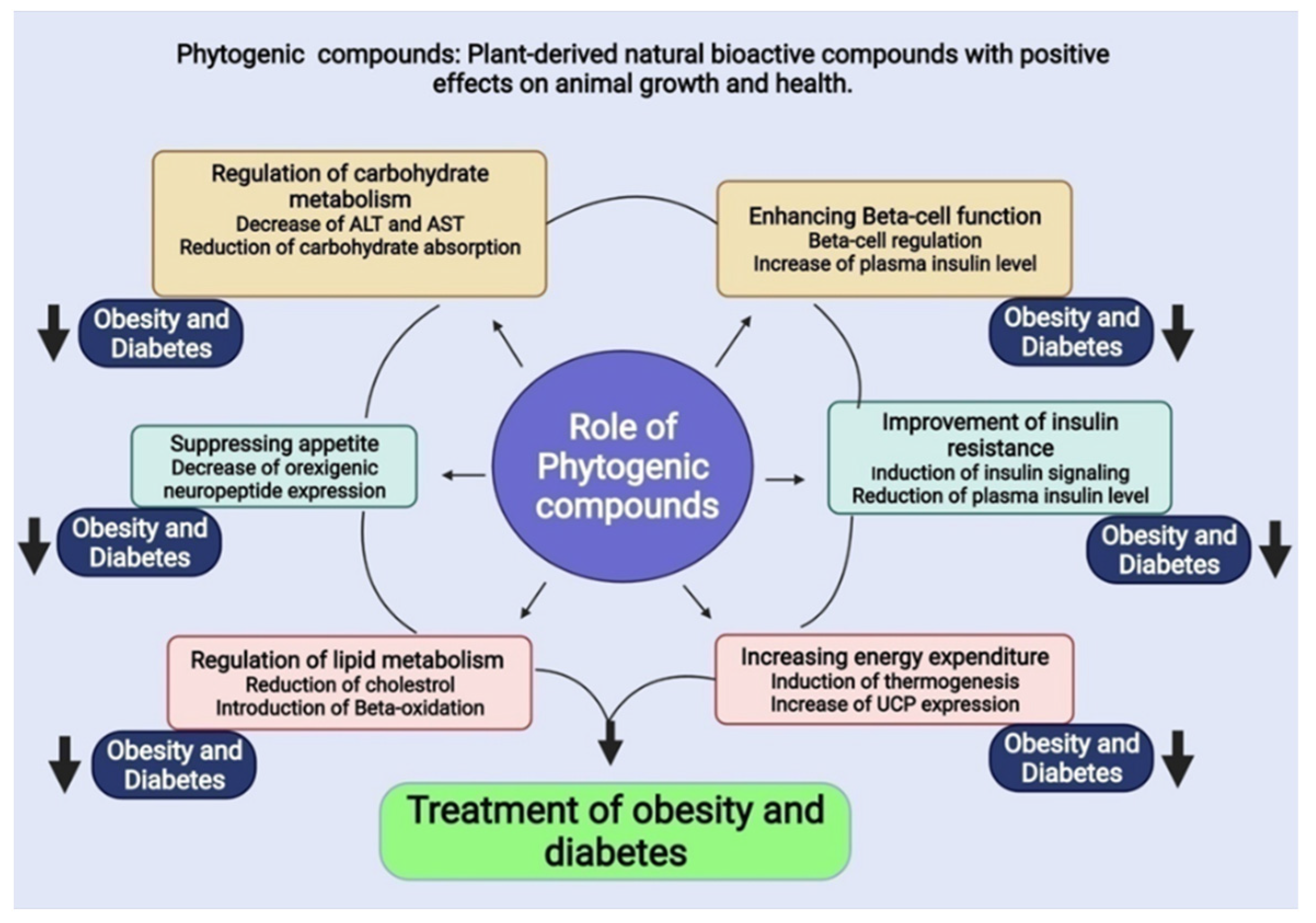
5. Phytogenic Compounds
5.1. Possible Therapeutic Compounds for Obesity
5.1.1. Compounds Suppress Food Intake
Panax quinquefolius (American Ginseng)
Panax ginseng (Asian Ginseng)
Hoodia gordonii (Hoodia)
Vaccinium spp. (Blueberry)
5.1.2. Compounds Stimulate Energy Expenditure
Nelumbo nucifera (Indian Lotus)
Capsicum annuum (Chili Pepper)
5.1.3. Compounds Regulate Lipid Metabolism
Camellia sinensis (Green Tea)
Vaccinium angustifolium (Wild Blueberry)
Cinnamomum spp. (Cinnamon)
5.1.4. Possible Therapeutic Compounds That Regulate Carbohydrate Metabolism
Camellia sinensis (Teas)
Glycine max Merr (Soybean)
5.2. Possible Therapeutic Compounds for Diabetes
5.2.1. Possible Therapeutic Compounds That Regulate Insulin Resistance
Vaccinium spp. (Blueberry)
Glycyrrhiza glabra (Liquorice)
Trigonella foenum-graecum (Fenugreek)
Cinnamomum spp. (Cinnamon)
Gymnema sylvestre
5.2.2. Possible Therapeutic Compounds Regulate β-Cell Function
Ervatamia microphylla (Kerr)
Anoectochilus roxburghii (Jewel Orchid)
Nymphaea stellata
5.2.3. Compounds with Multiple Antidiabetic Activities
Capsicum frutescens (Solanaceae)
Momordica charantia (Cucurbitaceae)
Vitis vinifera (Grape Vine)
5.3. Possible Therapeutic Compounds for Both Obesity and Diabetes
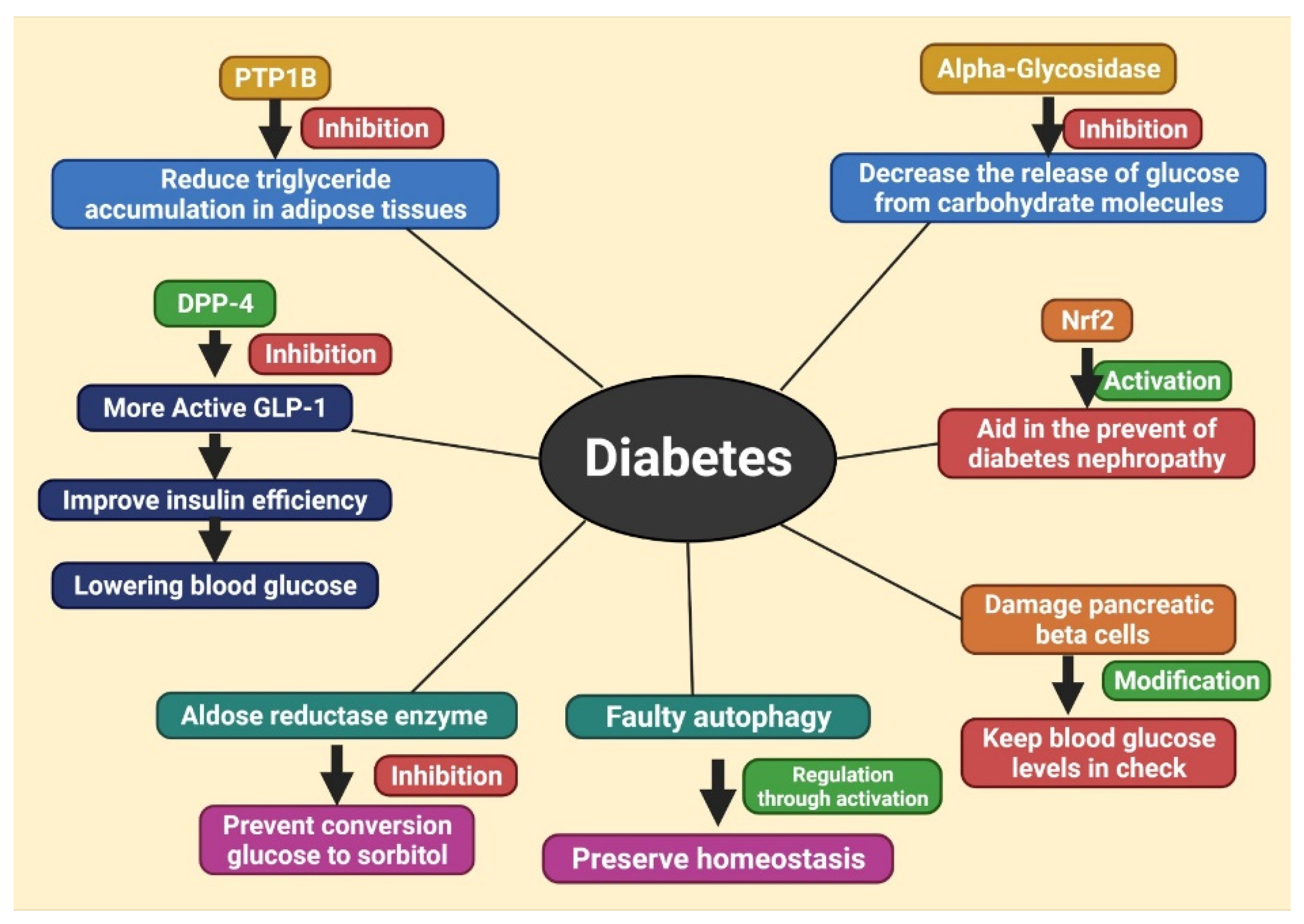
6. Different Therapeutic Targets of Diabetes, Treating with Herbal Products
6.1. Inhibition of DPP-4
6.2. Inhibition of Protein Tyrosine Phosphatase 1B (PTP1B)
6.3. Inhibition of α-Glycosidase
6.4. Activation of Nrf2
6.5. Modification of Pancreatic Beta Cells
6.6. Inhibition of Aldose Reductase Enzyme
6.7. Regulation of Autophagy
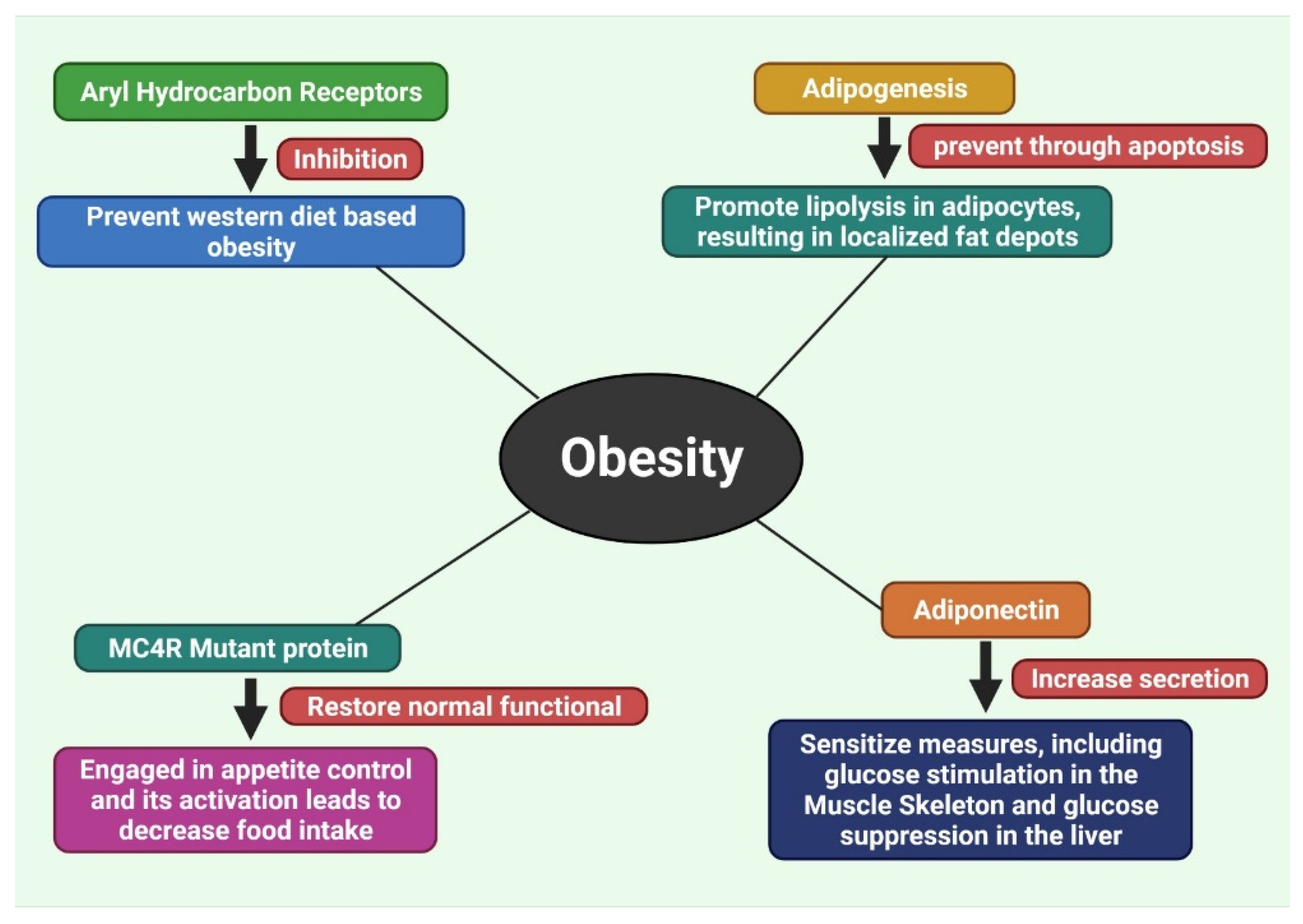
7. Different Therapeutic Targets of Obesity, Treating with Herbal Products
7.1. Inhibition of Aryl Hydrocarbon Receptors
7.2. Inhibition of Adipogenesis by Methylxanthine
7.3. Recover the Disruption of Melanocortin 4 Receptor (MC4R) Protein
7.4. Increase the Secretion of Adiponectin
8. Conclusions and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, Q.; Chen, L.; Hu, L.; Guo, Y.; Shen, X. Small molecules from natural sources, targeting signaling pathways in diabetes. Biochim. Biophys. Acta-Gene Regul. Mech. 2010, 1799, 854–865. [Google Scholar] [CrossRef] [PubMed]
- Hung, H.Y.; Qian, K.; Morris-Natschke, S.L.; Hsu, C.S.; Lee, K.H. Recent discovery of plant-derived anti-diabetic natural products. Nat. Prod. Rep. 2012, 29, 580–606. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, M.; Yadav, P.; Vashishth, D.; Sharma, K.; Kumar, A.; Chahal, J.; Dalal, S.; Kataria, S.K. A review on obesity management through natural compounds and a green nanomedicine-based approach. Molecules 2021, 26, 3278. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, G.A.; Ibrahim, S.R.M.; Elkhayat, E.S.; El Dine, R.S. Natural anti-obesity agents. Bull. Fac. Pharm. Cairo Univ. 2014, 52, 269–284. [Google Scholar] [CrossRef] [Green Version]
- Oh, S.; Kim, K.; Chung, Y.; Shong, M.; Park, S. Anti-obesity Agents: A Focused Review on the Structural Classification of Therapeutic Entities. Curr. Top. Med. Chem. 2009, 9, 466–481. [Google Scholar] [CrossRef] [Green Version]
- Hatware, K.V.; Sharma, S.; Patil, K.; Shete, M.; Karri, S.; Gupta, G. Evidence for gastroprotective, anti-inflammatory and antioxidant potential of methanolic extract of Cordia dichotoma leaves on indomethacin and stress induced gastric lesions in Wistar rats. Biomed. Pharmacother. 2018, 103, 317–325. [Google Scholar] [CrossRef]
- Das, R.; Mitra, S.; Tareq, A.M.; Emran, T.B.; Hossain, M.J.; Alqahtani, A.M.; Alghazwani, Y.; Dhama, K.; Simal-Gandara, J. Medicinal plants used against hepatic disorders in Bangladesh: A comprehensive review. J. Ethnopharmacol. 2022, 282, 114588. [Google Scholar] [CrossRef]
- Sun, N.N.; Wu, T.Y.; Chau, C.F. Natural dietary and herbal products in anti-obesity treatment. Molecules 2016, 21, 1351. [Google Scholar] [CrossRef]
- Karri, S.; Sharma, S.; Hatware, K.; Patil, K. Natural anti-obesity agents and their therapeutic role in management of obesity: A future trend perspective. Biomed. Pharmacother. 2019, 110, 224–238. [Google Scholar] [CrossRef]
- Lahlou, M. The Success of Natural Products in Drug Discovery. Pharmacol. Pharm. 2013, 4, 17–31. [Google Scholar] [CrossRef] [Green Version]
- Lam, K.S. New aspects of natural products in drug discovery. Trends Microbiol. 2007, 15, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Kingston, D.G.I. Modern natural products drug discovery and its relevance to biodiversity conservation. J. Nat. Prod. 2011, 74, 496–511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mishra, B.B.; Tiwari, V.K. Natural products: An evolving role in future drug discovery. Eur. J. Med. Chem. 2011, 46, 4769–4807. [Google Scholar] [CrossRef] [PubMed]
- Kostrzewa, T.; Przychodzen, P.; Gorska-Ponikowska, M.; Kuban-Jankowska, A. Curcumin and cinnamaldehyde as PTP1B inhibitors with antidiabetic and anticancer potential. Anticancer Res. 2019, 39, 745–749. [Google Scholar] [CrossRef]
- Dias, D.A.; Urban, S.; Roessner, U. A Historical overview of natural products in drug discovery. Metabolites 2012, 2, 303–336. [Google Scholar] [CrossRef] [Green Version]
- Zaid, H.; Saad, B. State of the Art of Diabetes Treatment in Greco-Arab and Islamic Medicine. Bioact. Food Diet. Interv. Diabetes 2013, 327–337. [Google Scholar] [CrossRef]
- Zaid, H.; Saad, B.; Mahdi, A.A.; Tamrakar, A.K.; Haddad, P.S.; Afifi, F.U. Medicinal Plants and Natural Active Compounds for Diabetes and/or Obesity Treatment. Evid.-Based Complement. Altern. Med. 2015, 2015, 202874. [Google Scholar] [CrossRef]
- Pankaj Modi Diabetes Beyond Insulin: Review of New Drugs for Treatment of Diabetes Mellitus. Curr. Drug Discov. Technol. 2007, 4, 39–47. [CrossRef]
- Rahman, M.M.; Islam, M.R.; Islam, M.T.; Harun-Or-rashid, M.; Islam, M.; Abdullah, S.; Uddin, M.B.; Das, S.; Rahaman, M.S.; Ahmed, M.; et al. Stem Cell Transplantation Therapy and Neurological Disorders: Current Status and Future Perspectives. Biology 2022, 11, 147. [Google Scholar] [CrossRef]
- Neustadt, J.; Pieczenik, S.R. Medication-induced mitochondrial damage and disease. Mol. Nutr. Food Res. 2008, 52, 780–788. [Google Scholar] [CrossRef]
- Cao, Y.; Liu, X.M. Should we still be concerned about the potential side effects of glucagon-like peptide-1 receptor agonists on thyroid C cells? Endocrine 2015, 48, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.K.; Prasad, S.K.; Kumar, R.; Hemalatha, S. An overview on antidiabetic medicinal plants having insulin mimetic property. Asian Pac. J. Trop. Biomed. 2012, 2, 320–330. [Google Scholar] [CrossRef] [Green Version]
- Arulselvan, P.; Ghofar, H.A.A.; Karthivashan, G.; Halim, M.F.A.; Ghafar, M.S.A.; Fakurazi, S. Antidiabetic therapeutics from natural source: A systematic review. Biomed. Prev. Nutr. 2014, 4, 607–617. [Google Scholar] [CrossRef]
- Gothai, S.; Ganesan, P.; Park, S.Y.; Fakurazi, S.; Choi, D.K.; Arulselvan, P. Natural phyto-bioactive compounds for the treatment of type 2 diabetes: Inflammation as a target. Nutrients 2016, 8, 461. [Google Scholar] [CrossRef] [PubMed]
- Redinger, R.N. The pathophysiology of obesity and its clinical manifestations. Gastroenterol. Hepatol. 2007, 3, 856–863. [Google Scholar]
- Nagaraju, G.P.; Aliya, S.; Alese, O.B. Role of adiponectin in obesity related gastrointestinal carcinogenesis. Cytokine Growth Factor Rev. 2015, 26, 83–93. [Google Scholar] [CrossRef]
- Chung, S.J.; Nagaraju, G.P.; Nagalingam, A.; Muniraj, N.; Kuppusamy, P.; Walker, A.; Woo, J.; Győrffy, B.; Gabrielson, E.; Saxena, N.K.; et al. ADIPOQ/adiponectin induces cytotoxic autophagy in breast cancer cells through STK11/LKB1-mediated activation of the AMPK-ULK1 axis. Autophagy 2017, 13, 1386–1403. [Google Scholar] [CrossRef]
- Muppala, S.; Konduru, S.K.P.; Merchant, N.; Ramsoondar, J.; Rampersad, C.K.; Rajitha, B.; Mukund, V.; Kancherla, J.; Hammond, A.; Barik, T.K.; et al. Adiponectin: Its role in obesity-associated colon and prostate cancers. Crit. Rev. Oncol. Hematol. 2017, 116, 125–133. [Google Scholar] [CrossRef]
- Deng, Z.B.; Liu, Y.; Liu, C.; Xiang, X.; Wang, J.; Cheng, Z.; Shah, S.V.; Zhang, S.; Zhang, L.; Zhuang, X.; et al. Immature myeloid cells induced by a high-fat diet contribute to liver inflammation. Hepatology 2009, 50, 1412–1420. [Google Scholar] [CrossRef] [Green Version]
- Hossain, P.; Kawar, B.; El Nahas, M. Obesity and Diabetes in the Developing World—A Growing Challenge. N. Engl. J. Med. 2007, 356, 213–215. [Google Scholar] [CrossRef] [Green Version]
- Jung, H.S.; Lim, Y.; Kim, E.K. Therapeutic phytogenic compounds for obesity and diabetes. Int. J. Mol. Sci. 2014, 15, 21505–21537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jebb, S. Obesity: Causes and consequences. Women’s Health Med. 2004, 1, 38–41. [Google Scholar] [CrossRef]
- Kopelman, P.G. Obesity as a medical problem. Nature 2000, 404, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Jahan, I.; Tona, M.R.; Sharmin, S.; Sayeed, M.A.; Tania, F.Z.; Paul, A.; Chy, M.; Uddin, N.; Rakib, A.; Emran, T.B.; et al. GC-MS phytochemical profiling, pharmacological properties, and in silico studies of Chukrasia velutina leaves: A novel source for bioactive agents. Molecules 2020, 25, 3536. [Google Scholar] [CrossRef] [PubMed]
- Friedman, J.M. Obesity: Causes and control of excess body fat. Nature 2009, 459, 340–342. [Google Scholar] [CrossRef]
- Näslund, E.; Hellström, P.M. Appetite signaling: From gut peptides and enteric nerves to brain. Physiol. Behav. 2007, 92, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Dutta, T.; Paul, A.; Majumder, M.; Sultan, R.A.; Emran, T.B. Pharmacological evidence for the use of Cissus assamica as a medicinal plant in the management of pain and pyrexia. Biochem. Biophys. Rep. 2020, 21, 100715. [Google Scholar] [CrossRef] [PubMed]
- Padwal, R.S.; Majumdar, S.R. Drug treatments for obesity: Orlistat, sibutramine, and rimonabant. Lancet 2007, 369, 71–77. [Google Scholar] [CrossRef]
- Association, A.D. Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 2004, 27, s5–s10. [Google Scholar] [CrossRef] [Green Version]
- Chakraborty, A.J.; Uddin, T.M.; Zidan, M.; Redwan, B.M.; Mitra, S.; Das, R.; Nainu, F.; Dhama, K.; Roy, A.; Hossain, M.; et al. Allium cepa: A Treasure of Bioactive Phytochemicals with Prospective Health Benefits. Evid. Based Complement. Altern. Med. 2022, 2022, 4586318. [Google Scholar] [CrossRef]
- Ripsin, C.M.; Kang, H.; Urban, R.J. Management of blood glucose in type 2 diabetes mellitus. Am. Fam. Physician 2009, 79, 29–36. [Google Scholar]
- Men, P.; Qu, S.; Song, Z.; Liu, Y.; Li, C.; Zhai, S. Lixisenatide for Type 2 Diabetes Mellitus Patients Inadequately Controlled on Oral Antidiabetic Drugs: A Mixed-Treatment Comparison Meta-analysis and Cost–Utility Analysis. Diabetes Ther. 2020, 11, 1745–1755. [Google Scholar] [CrossRef]
- Tirla, A.; Vesa, C.M.; Cavalu, S. Severe Cardiac and Metabolic Pathology Induced by Steroid Abuse in a Young Individual. Diagnostics 2021, 11, 1313. [Google Scholar] [CrossRef]
- Xu, L.; Li, Y.; Dai, Y.; Peng, J. Natural products for the treatment of type 2 diabetes mellitus: Pharmacology and mechanisms. Pharmacol. Res. 2018, 130, 451–465. [Google Scholar] [CrossRef]
- Oguma, Y.; Sesso, H.D.; Paffenbarger, R.S.; Lee, I.M. Weight change and risk of developing type 2 diabetes. Obes. Res. 2005, 13, 945–951. [Google Scholar] [CrossRef]
- Wannamethee, S.G.; Shaper, A.G.; Walker, M. Overweight and obesity and weight change in middle aged men: Impact on cardiovascular disease and diabetes. J. Epidemiol. Community Health 2005, 59, 134–139. [Google Scholar] [CrossRef] [Green Version]
- Nagaya, T.; Yoshida, H.; Takahashi, H.; Kawai, M. Increases in body mass index, even within non-obese levels, raise the risk for Type 2 diabetes mellitus: A follow-up study in a Japanese population. Diabet. Med. 2005, 22, 1107–1111. [Google Scholar] [CrossRef]
- Meigs, J.B.; Wilson, P.W.F.; Fox, C.S.; Vasan, R.S.; Nathan, D.M.; Sullivan, L.M.; D’Agostino, R.B. Body mass index, metabolic syndrome, and risk of type 2 diabetes or cardiovascular disease. J. Clin. Endocrinol. Metab. 2006, 91, 2906–2912. [Google Scholar] [CrossRef]
- Antonescu, A.-I.; Miere, F.; Fritea, L.; Ganea, M.; Zdrinca, M.; Dobjanschi, L.; Antonescu, A.; Vicas, S.I.; Bodog, F.; Sindhu, R.K.; et al. Perspectives on the Combined Effects of Ocimum basilicum and Trifolium pratense Extracts in Terms of Phytochemical Profile and Pharmacological Effects. Plants 2021, 10, 1390. [Google Scholar] [CrossRef]
- Weyer, C.; Funahashi, T.; Tanaka, S.; Hotta, K.; Matsuzawa, Y.; Pratley, R.E.; Tataranni, P.A. Hypoadiponectinemia in obesity and type 2 diabetes: Close association with insulin resistance and hyperinsulinemia. J. Clin. Endocrinol. Metab. 2001, 86, 1930–1935. [Google Scholar] [CrossRef] [PubMed]
- De Ferranti, S.; Mozaffarian, D. The perfect storm: Obesity, adipocyte dysfunction, and metabolic consequences. Clin. Chem. 2008, 54, 945–955. [Google Scholar] [CrossRef] [Green Version]
- Deng, Y.; Scherer, P.E. Adipokines as novel biomarkers and regulators of the metabolic syndrome. Ann. N. Y. Acad. Sci. 2010, 1212, E1–E19. [Google Scholar] [CrossRef] [PubMed]
- Pandya, H.; Lakhani, J.D.; Patel, N. Obesity is becoming synonym for diabetes in rural areas of India also—An alarming situation. Int. J. Biol. Med. Res. 2011, 2, 556–560. [Google Scholar]
- Snehalatha, C.; Viswanathan, V.; Ramachandran, A. Cutoff values for normal anthropometric variables in Asian Indian adults. Diabetes Care 2003, 26, 1380–1384. [Google Scholar] [CrossRef] [Green Version]
- The, N.S.; Richardson, A.S.; Gordon-Larsen, P. Timing and duration of obesity in relation to diabetes: Findings from an ethnically diverse, nationally representative sample. Diabetes Care 2013, 36, 865–872. [Google Scholar] [CrossRef] [Green Version]
- Wormser, D.; Kaptoge, S.; Di Angelantonio, E.; Wood, A.M.; Pennells, L.; Thompson, A.; Sarwar, N.; Kizer, J.R.; Lawlor, D.A.; Nordestgaard, B.G.; et al. Separate and combined associations of body-mass index and abdominal adiposity with cardiovascular disease: Collaborative analysis of 58 prospective studies. Lancet 2011, 377, 1085–1095. [Google Scholar] [CrossRef] [Green Version]
- Lindgren, C.M.; Heid, I.M.; Randall, J.C.; Lamina, C.; Steinthorsdottir, V.; Qi, L.; Speliotes, E.K.; Thorleifsson, G.; Willer, C.J.; Herrera, B.M.; et al. Genome-wide association scan meta-analysis identifies three loci influencing adiposity and fat distribution. PLoS Genet. 2009, 5, e1000508. [Google Scholar] [CrossRef]
- Scherag, A.; Dina, C.; Hinney, A.; Vatin, V.; Scherag, S.; Vogel, C.I.G.; Müller, T.D.; Grallert, H.; Wichmann, H.E.; Balkau, B.; et al. Two new loci for body-weight regulation identified in a joint analysis of genome-wide association studies for early-onset extreme obesity in French and German study groups. PLoS Genet. 2010, 6, e1000916. [Google Scholar] [CrossRef] [Green Version]
- Hayes, M.G.; Pluzhnikov, A.; Miyake, K.; Sun, Y.; Ng, M.C.Y.; Roe, C.A.; Below, J.E.; Nicolae, R.I.; Konkashbaev, A.; Bell, G.I.; et al. Identification of type 2 diabetes genes in Mexican Americans through genome-wide association studies. Diabetes 2007, 56, 3033–3044. [Google Scholar] [CrossRef]
- Rampersaud, E.; Damcott, C.M.; Fu, M.; Shen, H.; McArdle, P.; Shi, X.; Shelton, J.; Yin, J.; Chang, Y.P.C.; Ott, S.H.; et al. Identification of novel candidate genes for type 2 diabetes from a genome-wide association scan in the old order amish: Evidence for replication from diabetes-related quantitative traits and from independent populations. Diabetes 2007, 56, 3053–3062. [Google Scholar] [CrossRef] [Green Version]
- Lander, E.S. Initial impact of the sequencing of the human genome. Nature 2011, 470, 187–197. [Google Scholar] [CrossRef]
- Bogardus, C. Missing heritability and GWAS utility. Obesity 2009, 17, 209–210. [Google Scholar] [CrossRef] [Green Version]
- Loos, R.J.F.; Bouchard, C. Obesity—Is it a genetic disorder? J. Intern. Med. 2003, 254, 401–425. [Google Scholar] [CrossRef]
- Elbers, C.C.; Onland-Moret, N.C.; Franke, L.; Niehoff, A.G.; van der Schouw, Y.T.; Wijmenga, C. A strategy to search for common obesity and type 2 diabetes genes. Trends Endocrinol. Metab. 2007, 18, 19–26. [Google Scholar] [CrossRef]
- Hu, H.; Li, X. Networking pathways unveils association between obesity and non-insulin dependent diabetes mellitus. Pacific Symp. Biocomput. 2008, 2008, 255–266. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.H.; Joung, H.Y.; Kang, S.A.; Pyun, K.H.; Shim, I. Ginsenoside Rb1 as a suppressor in central modulation of feeding in the rat. Appetite 2007, 49, 303. [Google Scholar] [CrossRef]
- Xie, J.T.; Zhou, Y.P.; Dey, L.; Attele, A.S.; Wu, J.A.; Gu, M.; Polonsky, K.S.; Yuan, C.S. Ginseng berry reduces blood glucose and body weight in db/db mice. Phytomedicine 2002, 9, 254–258. [Google Scholar] [CrossRef]
- Attele, A.S.; Zhou, Y.P.; Xie, J.T.; Wu, J.A.; Zhang, L.; Dey, L.; Pugh, W.; Rue, P.A.; Polonsky, K.S.; Yuan, C.S. Antidiabetic effects of Panax ginseng berry extract and the identification of an effective component. Diabetes 2002, 51, 1851–1858. [Google Scholar] [CrossRef] [Green Version]
- MacLean, D.B.; Luo, L.G. Increased ATP content/production in the hypothalamus may be a signal for energy-sensing of satiety: Studies of the anorectic mechanism of a plant steroidal glycoside. Brain Res. 2004, 1020, 154–162. [Google Scholar] [CrossRef]
- van Heerden, F.R.; Marthinus Horak, R.; Maharaj, V.J.; Vleggaar, R.; Senabe, J.V.; Gunning, P.J. An appetite suppressant from Hoodia species. Phytochemistry 2007, 68, 2545–2553. [Google Scholar] [CrossRef]
- Jain, S.; Singh, S.N. Metabolic effect of short term administration of Hoodia gordonii, an herbal appetite suppressant. S. Afr. J. Bot. 2013, 86, 51–55. [Google Scholar] [CrossRef] [Green Version]
- Rahman, M.; Uddin, M.; Reza, A.S.M.; Tareq, A.M.; Emran, T.B.; Simal-Gandara, J. Ethnomedicinal value of antidiabetic plants in Bangladesh: A comprehensive review. Plants 2021, 10, 729. [Google Scholar] [CrossRef]
- Vuong, T.; Benhaddou-Andaloussi, A.; Brault, A.; Harbilas, D.; Martineau, L.C.; Vallerand, D.; Ramassamy, C.; Matar, C.; Haddad, P.S. Antiobesity and antidiabetic effects of biotransformed blueberry juice in KKA y mice. Int. J. Obes. 2009, 33, 1166–1173. [Google Scholar] [CrossRef] [Green Version]
- Grace, M.H.; Ribnicky, D.M.; Kuhn, P.; Poulev, A.; Logendra, S.; Yousef, G.G.; Raskin, I.; Lila, M.A. Hypoglycemic activity of a novel anthocyanin-rich formulation from lowbush blueberry, Vaccinium angustifolium Aiton. Phytomedicine 2009, 16, 406–415. [Google Scholar] [CrossRef] [Green Version]
- Basu, A.; Lyons, T.J. Strawberries, blueberries, and cranberries in the metabolic syndrome: Clinical perspectives. J. Agric. Food Chem. 2012, 60, 5687–5692. [Google Scholar] [CrossRef]
- Molan, A.L.; Lila, M.A.; Mawson, J. Satiety in rats following blueberry extract consumption induced by appetite-suppressing mechanisms unrelated to in vitro or in vivo antioxidant capacity. Food Chem. 2008, 107, 1039–1044. [Google Scholar] [CrossRef]
- Prior, R.L.; Wilkes, S.E.; Rogers, T.R.; Khanal, R.C.; Wu, X.; Howard, L.R. Purified blueberry anthocyanins and blueberry juice alter development of obesity in mice fed an obesogenic high-fat diet. J. Agric. Food Chem. 2010, 58, 3970–3976. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X.; Gu, L.; Hager, T.J.; Hager, A.; Howard, L.R. Whole berries versus berry anthocyanins: Interactions with dietary fat levels in the C57BL/6J mouse model of obesity. J. Agric. Food Chem. 2008, 56, 647–653. [Google Scholar] [CrossRef]
- Yun, J.W. Possible anti-obesity therapeutics from nature—A review. Phytochemistry 2010, 71, 1625–1641. [Google Scholar] [CrossRef]
- Azzu, V.; Jastroch, M.; Divakaruni, A.S.; Brand, M.D. The regulation and turnover of mitochondrial uncoupling proteins. Biochim. Biophys. Acta-Bioenerg. 2010, 1797, 785–791. [Google Scholar] [CrossRef] [Green Version]
- Sridhar, K.R.; Bhat, R. Lotus—A potential nutraceutical source. J. Agric. Technol. Bhat J. Agric. Technol. 2007, 3, 143–155. [Google Scholar]
- Ono, Y.; Hattori, E.; Fukaya, Y.; Imai, S.; Ohizumi, Y. Anti-obesity effect of Nelumbo nucifera leaves extract in mice and rats. J. Ethnopharmacol. 2006, 106, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.H.; Kim, E.S.; Lee, C.; Kim, S.; Cho, S.H.; Hwang, B.Y.; Lee, M.K. Chemical constituents from Nelumbo nucifera leaves and their anti-obesity effects. Bioorg. Med. Chem. Lett. 2013, 23, 3604–3608. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Rahaman, M.S.; Islam, M.R.; Hossain, M.E.; Mithi, F.M.; Ahmed, M.; Saldías, M.; Akkol, E.K.; Sobarzo-Sánchez, E. Multifunctional Therapeutic Potential of Phytocomplexes and Natural Extracts for Antimicrobial Properties. Antibiotics 2021, 10, 1076. [Google Scholar] [CrossRef]
- Mitra, S.; Rauf, A.; Tareq, A.M.; Jahan, S.; Emran, T.B.; Shahriar, T.G.; Dhama, K.; Alhumaydhi, F.A.; Aljohani, A.S.M.; Rebezov, M.; et al. Potential health benefits of carotenoid lutein: An updated review. Food Chem. Toxicol. 2021, 154, 112328. [Google Scholar] [CrossRef]
- Liu, S.; Li, D.; Huang, B.; Chen, Y.; Lu, X.; Wang, Y. Inhibition of pancreatic lipase, α-glucosidase, α-amylase, and hypolipidemic effects of the total flavonoids from Nelumbo nucifera leaves. J. Ethnopharmacol. 2013, 149, 263–269. [Google Scholar] [CrossRef]
- Islam, M.S.; Choi, H. Dietary red chilli (Capsicum frutescens L.) is insulinotropic rather than hypoglycemic in type 2 diabetes model of rats. Phyther. Res. 2008, 22, 1025–1029. [Google Scholar] [CrossRef]
- Rousset, S.; Alves-Guerra, M.C.; Mozo, J.; Miroux, B.; Cassard-Doulcier, A.M.; Bouillaud, F.; Ricquier, D. The Biology of Mitochondrial Uncoupling Proteins. Diabetes 2004, 53, S130–S135. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.S.; Kim, C.T.; Kim, I.H.; Kim, Y. Effects of capsaicin on lipid catabolism in 3T3-L1 adipocytes. Phyther. Res. 2011, 25, 935–939. [Google Scholar] [CrossRef]
- Ohnuki, K.; Niwa, S.; Maeda, S.; Inoue, N.; Yazawa, S.; Fushiki, T. CH-19 Sweet, a Non-Pungent Cultivar of Red Pepper, Increased Body Temperature and Oxygen Consumption in Humans. Biosci. Biotechnol. Biochem. 2001, 65, 2033–2036. [Google Scholar] [CrossRef]
- Yoneshiro, T.; Aita, S.; Kawai, Y.; Iwanaga, T.; Saito, M. Nonpungent capsaicin analogs (capsinoids) increase energy expenditure through the activation of brown adipose tissue in humans. Am. J. Clin. Nutr. 2012, 95, 845–850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janssens, P.L.H.R.; Hursel, R.; Martens, E.A.P.; Westerterp-Plantenga, M.S. Acute Effects of Capsaicin on Energy Expenditure and Fat Oxidation in Negative Energy Balance. PLoS ONE 2013, 8, e67786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balentine, D.A.; Wiseman, S.A.; Bouwens, L.C.M. The chemistry of tea flavonoids. Crit. Rev. Food Sci. Nutr. 1997, 37, 693–704. [Google Scholar] [CrossRef] [PubMed]
- Bose, M.; Lambert, J.D.; Ju, J.; Reuhl, K.R.; Shapses, S.A.; Yang, C.S. The green tea polyphenol, (−)-epigallocatechin-3-gallate, inhibits obesity and metabolic syndrome in high-fat fed mice. FASEB J. 2008, 22, 702–709. [Google Scholar] [CrossRef]
- Raederstorff, D.G.; Schlachter, M.F.; Elste, V.; Weber, P. Effect of EGCG on lipid absorption and plasma lipid levels in rats. J. Nutr. Biochem. 2003, 14, 326–332. [Google Scholar] [CrossRef]
- Hursel, R.; Viechtbauer, W.; Dulloo, A.G.; Tremblay, A.; Tappy, L.; Rumpler, W.; Westerterp-Plantenga, M.S. The effects of catechin rich teas and caffeine on energy expenditure and fat oxidation: A meta-analysis. Obes. Rev. 2011, 12, e573–e581. [Google Scholar] [CrossRef] [Green Version]
- Vendrame, S.; Daugherty, A.; Kristo, A.S.; Klimis-Zacas, D. Wild blueberry (Vaccinium angustifolium)-enriched diet improves dyslipidaemia and modulates the expression of genes related to lipid metabolism in obese Zucker rats. Br. J. Nutr. 2014, 111, 194–200. [Google Scholar] [CrossRef] [Green Version]
- Sheng, X.; Zhang, Y.; Gong, Z.; Huang, C.; Zang, Y.Q. Improved insulin resistance and lipid metabolism by cinnamon extract through activation of peroxisome proliferator-activated receptors. PPAR Res. 2008, 2008, 581348. [Google Scholar] [CrossRef] [Green Version]
- Sartorius, T.; Peter, A.; Schulz, N.; Drescher, A.; Bergheim, I.; MacHann, J.; Schick, F.; Siegel-Axel, D.; Schürmann, A.; Weigert, C.; et al. Cinnamon extract improves insulin sensitivity in the brain and lowers liver fat in mouse models of obesity. PLoS ONE 2014, 9, e92358. [Google Scholar] [CrossRef] [Green Version]
- Ziegenfuss, T.N.; Hofheins, J.E.; Mendel, R.W.; Landis, J.; Anderson, R.A. Effects of a Water-Soluble Cinnamon Extract on Body Composition and Features of the Metabolic Syndrome in Pre-Diabetic Men and Women. J. Int. Soc. Sports Nutr. 2006, 3, 45–53. [Google Scholar] [CrossRef] [Green Version]
- Foster, G.D.; Wyatt, H.R.; Hill, J.O.; McGuckin, B.G.; Brill, C.; Mohammed, B.S.; Szapary, P.O.; Rader, D.J.; Edman, J.S.; Klein, S. A Randomized Trial of a Low-Carbohydrate Diet for Obesity. N. Engl. J. Med. 2003, 348, 2082–2090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhong, L.; Furne, J.K.; Levitt, M.D. An extract of black, green, and mulberry teas causes malabsorption of carbohydrate but not of triacylglycerol in healthy volunteers. Am. J. Clin. Nutr. 2006, 84, 551–555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanhineva, K.; Törrönen, R.; Bondia-Pons, I.; Pekkinen, J.; Kolehmainen, M.; Mykkänen, H.; Poutanen, K. Impact of dietary polyphenols on carbohydrate metabolism. Int. J. Mol. Sci. 2010, 11, 11. [Google Scholar] [CrossRef] [PubMed]
- Asano, N.; Yamashita, T.; Yasuda, K.; Ikeda, K.; Kizu, H.; Kameda, Y.; Kato, A.; Nash, R.J.; Lee, H.S.; Ryu, K.S. Polyhydroxylated alkaloids isolated from mulberry trees (Morus alba L.) and silkworms (Bombyx mori L.). J. Agric. Food Chem. 2001, 49, 4208–4213. [Google Scholar] [CrossRef]
- Juhel, C.; Armand, M.; Pafumi, Y.; Rosier, C.; Vandermander, J.; Lairon, D. Green tea extract (AR25®) inhibits lipolysis of triglycerides in gastric and duodenal medium in vitro. J. Nutr. Biochem. 2000, 11, 45–51. [Google Scholar] [CrossRef]
- Birari, R.B.; Bhutani, K.K. Pancreatic lipase inhibitors from natural sources: Unexplored potential. Drug Discov. Today 2007, 12, 879–889. [Google Scholar] [CrossRef]
- Bhathena, S.J.; Velasquez, M.T. Beneficial role of dietary phytoestrogens in obesity and diabetes. Am. J. Clin. Nutr. 2002, 76, 1191–1201. [Google Scholar] [CrossRef] [Green Version]
- Ali, A.A.; Velasquez, M.T.; Hansen, C.T.; Mohamed, A.I.; Bhathena, S.J. Modulation of carbohydrate metabolism and peptide hormones by soybean isoflavones and probiotics in obesity and diabetes. J. Nutr. Biochem. 2005, 16, 693–699. [Google Scholar] [CrossRef]
- Purkins, L.; Love, E.R.; Eve, M.D.; Wooldridge, C.L.; Cowan, C.; Smart, T.S.; Johnson, P.J.; Rapeport, W.G. The influence of diet upon liver function tests and serum lipids in healthy male volunteers resident in a Phase I unit. Br. J. Clin. Pharmacol. 2004, 57, 199–208. [Google Scholar] [CrossRef] [Green Version]
- DeFuria, J.; Bennett, G.; Strissel, K.J.; Perfield, J.W.; Milbury, P.E.; Greenberg, A.S.; Obin, M.S. Dietary blueberry attenuates whole-body insulin resistance in high fat-fed mice by reducing adipocyte death and its inflammatory sequelae. J. Nutr. 2009, 139, 1510–1516. [Google Scholar] [CrossRef]
- Surmi, B.K.; Hasty, A.H. Macrophage infiltration into adipose tissue: Initiation, propagation and remodeling. Future Lipidol. 2008, 3, 545–556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mills, C.D. M1 and M2 macrophages: Oracles of health and disease. Crit. Rev. Immunol. 2012, 32, 463–488. [Google Scholar] [CrossRef] [Green Version]
- Seymour, E.M.; Tanone, I.I.; Urcuyo-Llanes, D.E.; Lewis, S.K.; Kirakosyan, A.; Kondoleon, M.G.; Kaufman, P.B.; Bolling, S.F. Blueberry intake alters skeletal muscle and adipose tissue peroxisome proliferator-activated receptor activity and reduces insulin resistance in obese rats. J. Med. Food 2011, 14, 1511–1518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stull, A.J.; Cash, K.C.; Johnson, W.D.; Champagne, C.M.; Cefalu, W.T. Bioactives in blueberries improve insulin sensitivity in obese, insulin-resistant men and women. J. Nutr. 2010, 140, 1764–1768. [Google Scholar] [CrossRef] [PubMed]
- Weidner, C.; De Groot, J.C.; Prasad, A.; Freiwald, A.; Quedenau, C.; Kliem, M.; Witzke, A.; Kodelja, V.; Han, C.T.; Giegold, S.; et al. Amorfrutins are potent antidiabetic dietary natural products. Proc. Natl. Acad. Sci. USA 2012, 109, 7257–7262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Emran, T.B.; Dutta, M.; Uddin, M.M.N.; Nath, A.K.; Uddin, M.Z. Antidiabetic potential of the leaf extract of Centella asiatica in alloxan induced diabetic rats. Jahangirnagar Univ. J. Biol. Sci. 2015, 4, 51–59. [Google Scholar] [CrossRef] [Green Version]
- Moller, D.E. New drug targets for type 2 diabetes and the metabolic syndrome. Nature 2001, 414, 821–827. [Google Scholar] [CrossRef]
- Kaplan, F.; al-Majali, K.; Betteridge, D.J. PPARs, insulin resistance and type 2 diabetes. Eur. J. Cardiovasc. Prev. Rehabil. 2001, 8, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Basch, E.; Ulbricht, C.; Kuo, G.; Szapary, P.; Smith, M. Therapeutic applications of fenugreek. Altern. Med. Rev. 2003, 8, 20–27. [Google Scholar]
- Zia, T.; Hasnain, S.N.; Hasan, S.K. Evaluation of the oral hypoglycaemic effect of Trigonella foenum-graecum L. (methi) in normal mice. J. Ethnopharmacol. 2001, 75, 191–195. [Google Scholar] [CrossRef]
- Tiran, D. The use of fenugreek for breast feeding women. Complement. Ther. Nurs. Midwifery 2003, 9, 155–156. [Google Scholar] [CrossRef]
- Ota, A.; Ulrih, N.P. An overview of herbal products and secondary metabolites used for management of type two diabetes. Front. Pharmacol. 2017, 8, 436. [Google Scholar] [CrossRef] [PubMed]
- Uemura, T.; Hirai, S.; Mizoguchi, N.; Goto, T.; Lee, J.Y.; Taketani, K.; Nakano, Y.; Shono, J.; Hoshino, S.; Tsuge, N.; et al. Diosgenin present in fenugreek improves glucose metabolism by promoting adipocyte differentiation and inhibiting inflammation in adipose tissues. Mol. Nutr. Food Res. 2010, 54, 1596–1608. [Google Scholar] [CrossRef]
- Gupta, A.; Gupta, R.; Lal, B. Effect of Trigonella foenum-graecum (Fenugreek) Seeds on Glycaemic Control and Insulin Resistance in Type 2 Diabetes Mellitus: A Double Blind Placebo Controlled Study. J. Assoc. Physicians India 2001, 49, 1057–1061. [Google Scholar] [PubMed]
- Rahman, J.; Tareq, A.M.; Hossain, M.; Sakib, S.A.; Islam, M.N.; Ali, M.; Uddin, A.B.M.; Hoque, M.; Nasrin, M.; Emran, T.B.; et al. Biological evaluation, DFT calculations and molecular docking studies on the antidepressant and cytotoxicity activities of Cycas pectinata Buch.-Ham. Compounds. Pharmaceuticals 2020, 13, 232. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.D.; Sarkar, A.; Hazra, D.K.; Mishra, B.; Singh, J.B.; Sharma, S.K.; Maheshwari, B.B.; Maheshwari, P.K. Use of Fenugreek seed powder in the management of non-insulin dependent diabetes mellitus. Nutr. Res. 1996, 16, 1331–1339. [Google Scholar] [CrossRef]
- Kabir, M.S.H.; Hossain, M.M.; Kabir, M.I.; Rahman, M.M.; Hasanat, A.; Emran, T.B.; Rahman, M.A. Phytochemical screening, Antioxidant, Thrombolytic, alpha-amylase inhibition and cytotoxic activities of ethanol extract of Steudnera colocasiifolia K. Koch leaves. J. Young Pharm. 2016, 8, 391. [Google Scholar] [CrossRef] [Green Version]
- Rafehi, H.; Ververis, K.; Karagiannis, T.C. Controversies surrounding the clinical potential of cinnamon for the management of diabetes. Diabetes Obes. Metab. 2012, 14, 493–499. [Google Scholar] [CrossRef]
- Qin, B.; Panickar, K.S.; Anderson, R.A. Cinnamon: Potential role in the prevention of insulin resistance, metabolic syndrome, and type 2 diabetes. J. Diabetes Sci. Technol. 2010, 4, 685–693. [Google Scholar] [CrossRef] [Green Version]
- Imparl-Radosevich, J.; Deas, S.; Polansky, M.M.; Baedke, D.A.; Ingebritsen, T.S.; Anderson, R.A.; Graves, D.J. Regulation of PTP-1 and insulin receptor kinase by fractions from cinnamon: Implications for cinnamon regulation of insulin signalling. Horm. Res. 1998, 50, 177–182. [Google Scholar] [CrossRef]
- Cao, H.; Polansky, M.M.; Anderson, R.A. Cinnamon extract and polyphenols affect the expression of tristetraprolin, insulin receptor, and glucose transporter 4 in mouse 3T3-L1 adipocytes. Arch. Biochem. Biophys. 2007, 459, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Mitra, S.; Tareq, A.M.; Das, R.; Emran, T.B.; Nainu, F.; Chakraborty, A.J.; Ahmad, I.; Tallei, T.E.; Idris, A.M.; Simal-Gandara, J. Polyphenols: A first evidence in the synergism and bioactivities. Food Rev. Int. 2022, 2022, 1–23. [Google Scholar] [CrossRef]
- Shanmugasundaram, E.R.B.; Rajeswari, G.; Baskaran, K.; Kumar, B.R.R.; Shanmugasundaram, K.R.; Ahmath, B.K. Use of Gymnema sylvestre leaf extract in the control of blood glucose in insulin-dependent diabetes mellitus. J. Ethnopharmacol. 1990, 30, 281–294. [Google Scholar] [CrossRef]
- Siddiqui, A.A.; Bahar, A.; Anshul, D. Development in the chemistry and pharmacology of Gymnema sylvestre. J. Med. Aromat. Plant Sci. 2000, 22, 223–231. [Google Scholar]
- Tiwari, A.K.; Rao, J.M. Diabetes mellitus and multiple therapeutic approaches of phytochemicals: Present status and future prospects. Curr. Sci. 2002, 83, 30–38. [Google Scholar]
- Baskaran, K.; Ahamath, B.K.; Shanmugasundaram, K.R.; Shanmugasundaram, E.R.B. Antidiabetic effect of a leaf extract from Gymnema sylvestre in non-insulin-dependent diabetes mellitus patients. J. Ethnopharmacol. 1990, 30, 295–305. [Google Scholar] [CrossRef]
- Balasubramaniam, K.; Seevaratnam, S.; Ageswaran, A.; Arasaratnam, V.; Thirumagal, K. Hypoglycemic effect of Gymnema sylvestre on diabetic patients. Jaffna Med. J. 1988, 23, 49–53. [Google Scholar]
- Al Mahmud, Z.; Emran, T.B.; Qais, N.; Bachar, S.C.; Sarker, M.; Uddin, M.M.N. Evaluation of analgesic, anti-inflammatory, thrombolytic and hepatoprotective activities of roots of Premna esculenta (Roxb). J. Basic Clin. Physiol. Pharmacol. 2016, 2, 63–70. [Google Scholar] [CrossRef]
- Tiwari, P.; Mishra, B.N.; Sangwan, N.S. Phytochemical and pharmacological properties of Gymnema sylvestre: An important medicinal plant. Biomed. Res. Int. 2014, 2014, 830285. [Google Scholar] [CrossRef] [Green Version]
- Fujii, M.; Takei, I.; Umezawa, K. Antidiabetic effect of orally administered conophylline-containing plant extract on streptozotocin-treated and Goto-Kakizaki rats. Biomed. Pharmacother. 2009, 63, 710–716. [Google Scholar] [CrossRef]
- Kawakami, M.; Hirayama, A.; Tsuchiya, K.; Ohgawara, H.; Nakamura, M.; Umezawa, K. Promotion of β-cell differentiation by the alkaloid conophylline in porcine pancreatic endocrine cells. Biomed. Pharmacother. 2010, 64, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Saito, R.; Yamada, S.; Yamamoto, Y.; Kodera, T.; Hara, A.; Tanaka, Y.; Kimura, F.; Takei, I.; Umezawa, K.; Kojima, I. Conophylline suppresses pancreatic stellate cells and improves islet fibrosis in Goto-Kakizaki rats. Endocrinology 2012, 153, 621–630. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cai, J.; Ruan, H.; Pi, H.; Wu, J. Antihyperglycemic activity of kinsenoside, a high yielding constituent from Anoectochilus roxburghii in streptozotocin diabetic rats. J. Ethnopharmacol. 2007, 114, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Subash-Babu, P.; Ignacimuthu, S.; Agastian, P.; Varghese, B. Partial regeneration of β-cells in the islets of Langerhans by Nymphayol a sterol isolated from Nymphaea stellata (Willd.) flowers. Bioorg. Med. Chem. 2009, 17, 2864–2870. [Google Scholar] [CrossRef] [PubMed]
- Diabetes, C.O.F.; Management, D. Herbal Support for Diabetes Management. Clin. Nutr. Insights 1998, 6, 1–4. [Google Scholar]
- Saxena, A.; Vikram, N.K. Role of Selected Indian Plants in Management of Type 2 Diabetes: A Review. J. Altern. Complement. Med. 2004, 10, 369–378. [Google Scholar] [CrossRef] [Green Version]
- Grover, J.K.; Yadav, S.; Vats, V. Medicinal plants of India with anti-diabetic potential. J. Ethnopharmacol. 2002, 81, 81–100. [Google Scholar] [CrossRef]
- Noor-E-Tabassum; Das, R.; Lami, M.S.; Chakraborty, A.J.; Mitra, S.; Tallei, T.E.; Idroes, R.; Mohamed, A.A.-R.; Hossain, M.J.; Dhama, K.; et al. Ginkgo biloba: A Treasure of Functional Phytochemicals with Multimedicinal Applications. Evid. Based Complement. Altern. Med. 2022, 2022, 8288818. [Google Scholar] [CrossRef]
- Bhatt, J.K.; Thomas, S.; Nanjan, M.J. Resveratrol supplementation improves glycemic control in type 2 diabetes mellitus. Nutr. Res. 2012, 32, 537–541. [Google Scholar] [CrossRef]
- Szkudelska, K.; Szkudelski, T. Resveratrol, obesity and diabetes. Eur. J. Pharmacol. 2010, 635, 1–8. [Google Scholar] [CrossRef]
- Mitra, S.; Paul, S.; Roy, S.; Sutradhar, H.; Emran, T.B.; Nainu, F.; Khandaker, M.U.; Almalki, M.; Wilairatana, P.; Mubarak, M.S. Exploring the Immune-Boosting Functions of Vitamins and Minerals as Nutritional Food Bioactive Compounds: A Comprehensive Review. Molecules 2022, 27, 555. [Google Scholar] [CrossRef] [PubMed]
- Aribal-Kocatürk, P.; Özelçi Kavas, G.; İren Büyükkağnici, D. Pretreatment effect of resveratrol on streptozotocin-induced diabetes in rats. Biol. Trace Elem. Res. 2007, 118, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Ramadori, G.; Gautron, L.; Fujikawa, T.; Vianna, C.R.; Elmquist, J.K.; Coppari, R. Central administration of resveratrol improves diet-induced diabetes. Endocrinology 2009, 150, 5326–5333. [Google Scholar] [CrossRef] [PubMed]
- Ungvari, Z.; Csiszar, A. Resveratrol Confers Endothelial Protection in Insulin-Dependent Diabetes Mellitus. Cardiovasc. Drugs Ther. 2011, 25, 111–113. [Google Scholar] [CrossRef]
- Zhang, H.; Morgan, B.; Potter, B.J.; Ma, L.; Dellsperger, K.C.; Ungvari, Z.; Zhang, C. Resveratrol improves left ventricular diastolic relaxation in type 2 diabetes by inhibiting oxidative/nitrative stress: In vivo demonstration with magnetic resonance imaging. Am. J. Physiol.-Heart Circ. Physiol. 2010, 299, H985–H994. [Google Scholar] [CrossRef] [Green Version]
- Resmi, H. The combination of bortezomib and resveratrol may prevent muscle wasting in diabetes. Med. Hypotheses 2011, 76, 291–292. [Google Scholar] [CrossRef]
- Hong, Y.J.; Kim, N.; Lee, K.; Hee Sonn, C.; Eun Lee, J.; Tae Kim, S.; Ho Baeg, I.; Lee, K.M. Korean red ginseng (Panax ginseng) ameliorates type 1 diabetes and restores immune cell compartments. J. Ethnopharmacol. 2012, 144, 225–233. [Google Scholar] [CrossRef]
- Huang, J.P.; Huang, S.S.; Deng, J.Y.; Chang, C.C.; Day, Y.J.; Hung, L.M. Insulin and resveratrol act synergistically, preventing cardiac dysfunction in diabetes, but the advantage of resveratrol in diabetics with acute heart attack is antagonized by insulin. Free Radic. Biol. Med. 2010, 49, 1710–1721. [Google Scholar] [CrossRef]
- Venturini, C.D.; Merlo, S.; Souto, A.A.; Fernandes, M.D.C.; Gomez, R.; Rhoden, C.R. Resveratrol and red wine function as antioxidants in the central nervous system without cellular proliferative effects during experimental diabetes. Oxid. Med. Cell. Longev. 2010, 3, 434–441. [Google Scholar] [CrossRef]
- Do, G.M.; Jung, U.J.; Park, H.J.; Kwon, E.Y.; Jeon, S.M.; Mcgregor, R.A.; Choi, M.S. Resveratrol ameliorates diabetes-related metabolic changes via activation of AMP-activated protein kinase and its downstream targets in db/db mice. Mol. Nutr. Food Res. 2012, 56, 1282–1291. [Google Scholar] [CrossRef]
- Ding, D.F.; You, N.; Wu, X.M.; Xu, J.R.; Hu, A.P.; Ye, X.L.; Zhu, Q.; Jiang, X.Q.; Miao, H.; Liu, C.; et al. Resveratrol attenuates renal hypertrophy in early-stage diabetes by activating AMPK. Am. J. Nephrol. 2010, 31, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Mishra, R.; Sellin, D.; Radovan, D.; Gohlke, A.; Winter, R. Inhibiting islet amyloid polypeptide fibril formation by the red wine compound resveratrol. ChemBioChem 2009, 10, 445–449. [Google Scholar] [CrossRef] [PubMed]
- Ku, C.R.; Lee, H.J.; Kim, S.K.; Lee, E.Y.; Lee, M.K.; Lee, E.J. Resveratrol prevents streptozotocin-induced diabetes by inhibiting the apoptosis of pancreatic β-cell and the cleavage of poly(ADP-ribose) polymerase. Endocr. J. 2012, 59, 103–109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vetterli, L.; Brun, T.; Giovannoni, L.; Bosco, D.; Maechler, P. Resveratrol potentiates glucose-stimulated insulin secretion in INS-1E β-cells and human islets through a SIRT1-dependent mechanism. J. Biol. Chem. 2011, 286, 6049–6060. [Google Scholar] [CrossRef] [Green Version]
- Haigis, M.C.; Sinclair, D.A. Mammalian sirtuins: Biological insights and disease relevance. Annu. Rev. Pathol. Mech. Dis. 2010, 5, 253–295. [Google Scholar] [CrossRef] [Green Version]
- Pacholec, M.; Bleasdale, J.E.; Chrunyk, B.; Cunningham, D.; Flynn, D.; Garofalo, R.S.; Griffith, D.; Griffor, M.; Loulakis, P.; Pabst, B.; et al. SRT1720, SRT2183, SRT1460, and resveratrol are not direct activators of SIRT1. J. Biol. Chem. 2010, 285, 8340–8351. [Google Scholar] [CrossRef] [Green Version]
- Kantsadi, A.L.; Apostolou, A.; Theofanous, S.; Stravodimos, G.A.; Kyriakis, E.; Gorgogietas, V.A.; Chatzileontiadou, D.S.M.; Pegiou, K.; Skamnaki, V.T.; Stagos, D.; et al. Biochemical and biological assessment of the inhibitory potency of extracts from vinification byproducts of Vitis vinifera extracts against glycogen phosphorylase. Food Chem. Toxicol. 2014, 67, 35–43. [Google Scholar] [CrossRef]
- Aoki, F.; Honda, S.; Kishida, H.; Kitano, M.; Arai, N.; Tanaka, H.; Yokota, S.; Nakagawa, K.; Asakura, T.; Nakai, Y.; et al. Suppression by licorice flavonoids of abdominal fat accumulation and body weight gain in high-fat diet-induced obese C57BL/6J mice. Biosci. Biotechnol. Biochem. 2007, 71, 206–214. [Google Scholar] [CrossRef]
- Kim, H.K.; Nelson-Dooley, C.; Della-Fera, M.A.; Yang, J.Y.; Zhang, W.; Duan, J.; Hartzell, D.L.; Hamrick, M.W.; Baile, C.A. Genistein decreases food intake, body weight, and fat pad weight and causes adipose tissue apoptosis in ovariectomized female mice. J. Nutr. 2006, 136, 409–414. [Google Scholar] [CrossRef] [Green Version]
- Naaz, A.; Yellayi, S.; Zakroczymski, M.A.; Bunick, D.; Doerge, D.R.; Lubahn, D.B.; Helferich, W.G.; Cooke, P.S. The soy isoflavone genistein decreases adipose deposition in mice. Endocrinology 2003, 144, 3315–3320. [Google Scholar] [CrossRef]
- Brennan, A.M.; Mantzoros, C.S. Drug Insight: The role of leptin in human physiology and pathophysiology—Emerging clinical applications. Nat. Clin. Pract. Endocrinol. Metab. 2006, 2, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Ae Park, S.; Choi, M.S.; Cho, S.Y.; Seo, J.S.; Jung, U.J.; Kim, M.J.; Sung, M.K.; Park, Y.B.; Lee, M.K. Genistein and daidzein modulate hepatic glucose and lipid regulating enzyme activities in C57BL/KsJ-db/db mice. Life Sci. 2006, 79, 1207–1213. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.S.; Jung, U.J.; Yeo, J.; Kim, M.J.; Lee, M.K. Genistein and daidzein prevent diabetes onset by elevating insulin level and altering hepatic gluconeogenic and lipogenic enzyme activities in non-obese diabetic (NOD) mice. Diabetes. Metab. Res. Rev. 2008, 24, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Zhen, W.; Yang, Z.; Carter, J.D.; Si, H.; Reynolds, K.A. Genistein acutely stimulates insulin secretion in pancreatic β-cells through a cAMP-dependent protein kinase pathway. Diabetes 2006, 55, 1043–1050. [Google Scholar] [CrossRef] [Green Version]
- Fu, Z.; Liu, D. Long-term exposure to genistein improves insulin secretory function of pancreatic β-cells. Eur. J. Pharmacol. 2009, 616, 321–327. [Google Scholar] [CrossRef] [Green Version]
- Oetjen, E. Amorfrutins are potent antidiabetic dietary natural products. Yearb. Endocrinol. 2013, 2013, 25. [Google Scholar] [CrossRef]
- Kamisoyama, H.; Honda, K.; Tominaga, Y.; Yokota, S.; Hasegawa, S. Investigation of the anti-obesity action of licorice flavonoid oil in diet-induced obese rats. Biosci. Biotechnol. Biochem. 2008, 72, 3225–3231. [Google Scholar] [CrossRef] [Green Version]
- Tominaga, Y.; Nakagawa, K.; Mae, T.; Kitano, M.; Yokota, S.; Arai, T.; Ikematsu, H.; Inoue, S. Licorice flavonoid oil reduces total body fat and visceral fat in overweight subjects: A randomized, double-blind, placebo-controlled study. Obes. Res. Clin. Pract. 2009, 3, 169–178. [Google Scholar] [CrossRef]
- Mominur Rahman, M.; Islam, F.; Saidur Rahaman, M.; Sultana, N.A.; Fahim, N.F.; Ahmed, M. Studies on the prevalence of HIV/AIDS in Bangladesh including other developing countries. Adv. Tradit. Med. 2021, 21, 1–12. [Google Scholar] [CrossRef]
- Saito, M.; Yoneshiro, T. Capsinoids and related food ingredients activating brown fat thermogenesis and reducing body fat in humans. Curr. Opin. Lipidol. 2013, 24, 71–77. [Google Scholar] [CrossRef] [Green Version]
- Gram, D.X.; Ahrén, B.; Nagy, I.; Olsen, U.B.; Brand, C.L.; Sundler, F.; Tabanera, R.; Svendsen, O.; Carr, R.D.; Santha, P.; et al. Capsaicin-sensitive sensory fibers in the islets of Langerhans contribute to defective insulin secretion in Zucker diabetic rat, an animal model for some aspects of human type 2 diabetes. Eur. J. Neurosci. 2007, 25, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Grover, J.K.; Vats, V.; Rathi, S.S.; Dawar, R. Traditional Indian anti-diabetic plants attenuate progression of renal damage in streptozotocin induced diabetic mice. J. Ethnopharmacol. 2001, 76, 233–238. [Google Scholar] [CrossRef]
- Huang, H.L.; Hong, Y.W.; Wong, Y.H.; Chen, Y.N.; Chyuan, J.H.; Huang, C.J.; Chao, P. min Bitter melon (Momordica charantia L.) inhibits adipocyte hypertrophy and down regulates lipogenic gene expression in adipose tissue of diet-induced obese rats. Br. J. Nutr. 2008, 99, 230–239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kar, S.; Roy, K. QSAR of phytochemicals for the design of better drugs. Expert Opin. Drug Discov. 2012, 7, 877–902. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.W.; Zhou, J.C.; Hu, Z.W. Autophagy as a target for development of anti-diabetes drugs derived from natural compounds. J. Asian Nat. Prod. Res. 2017, 19, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Wais, M.; Nazish, I.; Samad, A.; Beg, S.; Abusufyan, S.; Ajaz Ajaj, S.; Aqil, M. Herbal Drugs for Diabetic Treatment: An Updated Review of Patents. Recent Pat. Antiinfect. Drug Discov. 2012, 7, 53–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, S.R.; Chang, C.H.; Tsai, M.J.; Cheng, H.; Chen, J.C.; Leong, M.K.; Weng, C.F. The perceptions of natural compounds against dipeptidyl peptidase 4 in diabetes: From in silico to in vivo. Ther. Adv. Chronic Dis. 2019, 10, 2040622319875305. [Google Scholar] [CrossRef] [Green Version]
- Omar, B.; Ahrén, B. Pleiotropic mechanisms for the glucose-lowering action of DPP-4 inhibitors. Diabetes 2014, 63, 2196–2202. [Google Scholar] [CrossRef] [Green Version]
- Da Silva Júnior, W.S.; De Godoy-Matos, A.F.; Kraemer-Aguiar, L.G. Dipeptidyl peptidase 4: A new link between diabetes mellitus and atherosclerosis? Biomed. Res. Int. 2015, 2015, 816164. [Google Scholar] [CrossRef]
- Hussain, H.; Green, I.R.; Abbas, G.; Adekenov, S.M.; Hussain, W.; Ali, I. Protein tyrosine phosphatase 1B (PTP1B) inhibitors as potential anti-diabetes agents: Patent review (2015–2018). Expert Opin. Ther. Pat. 2019, 29, 689–702. [Google Scholar] [CrossRef]
- Banu, S.; Bhowmick, A. Therapeutic Targets of Type 2 Diabetes: An Overview. MOJ Drug Des. Dev. Ther. 2017, 1, 2–7. [Google Scholar] [CrossRef] [Green Version]
- Kumar, G.S.; Page, R.; Peti, W. The mode of action of the Protein tyrosine phosphatase 1B inhibitor Ertiprotafib. PLoS ONE 2020, 15, e0240044. [Google Scholar] [CrossRef] [PubMed]
- Tomasik, P.; Horton, D. Enzymatic conversions of starch. Adv. Carbohydr. Chem. Biochem. 2012, 68, 59–436. [Google Scholar] [CrossRef] [PubMed]
- Türkan, F.; Taslimi, P.; Saltan, F.Z. Tannic acid as a natural antioxidant compound: Discovery of a potent metabolic enzyme inhibitor for a new therapeutic approach in diabetes and Alzheimer’s disease. J. Biochem. Mol. Toxicol. 2019, 33, e22340. [Google Scholar] [CrossRef]
- Hossain, U.; Das, A.K.; Ghosh, S.; Sil, P.C. An overview on the role of bioactive α-glucosidase inhibitors in ameliorating diabetic complications. Food Chem. Toxicol. 2020, 145, 111738. [Google Scholar] [CrossRef] [PubMed]
- Khursheed, R.; Singh, S.K.; Wadhwa, S.; Gulati, M.; Awasthi, A. Therapeutic potential of mushrooms in diabetes mellitus: Role of polysaccharides. Int. J. Biol. Macromol. 2020, 164, 1194–1205. [Google Scholar] [CrossRef]
- Reis, A.A.d.S.; Santos, R.d.S.; Cruz, A.H.d.S.; Silva, E.G.d.; Cruz, A.D.d.; Pedrino, G.R. The Effect of Nrf2 on Diabetic Complications. In A Master Regul. Oxidative Stress—The Transcription Factor Nrf2; IntechOpen: London, UK, 2016. [Google Scholar] [CrossRef] [Green Version]
- Oh, Y.S. Plant-derived compounds targeting pancreatic beta cells for the treatment of diabetes. Evid.-Based Complement. Altern. Med. 2015, 2015, 629863. [Google Scholar] [CrossRef] [Green Version]
- Jiménez-Osorio, A.S.; González-Reyes, S.; Pedraza-Chaverri, J. Natural Nrf2 activators in diabetes. Clin. Chim. Acta. 2015, 448, 182–192. [Google Scholar] [CrossRef]
- Veeresham, C.; Rama Rao, A.; Asres, K. Aldose reductase inhibitors of plant origin. Phyther. Res. 2014, 28, 317–333. [Google Scholar] [CrossRef]
- Chen, S.; Khoury, C.; Ziyadeh, F.N. Pathophysiology and Pathogenesis of Diabetic Nephropathy, 5th ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2013; Volume 2, ISBN 9780123814623. [Google Scholar]
- Maitra, S.; Dutta, D. Downregulation of Hexose Sugar Metabolism in Diabetes Decreases the Rate of Wound Healing; Elsevier Inc.: Amsterdam, The Netherlands, 2020; ISBN 9780128164136. [Google Scholar]
- Patel, D.K.; Kumar, R.; Sairam, K.; Hemalatha, S. Pharmacologically tested aldose reductase inhibitors isolated from plant sources—A concise report. Chin. J. Nat. Med. 2012, 10, 388–400. [Google Scholar] [CrossRef]
- Gao, Y.; Zhu, J.; Li, Z.; Zhu, W.; Shi, J.; Jia, Q.; Li, Y. Recent progress in natural products as DPP-4 inhibitors. Future Med. Chem. 2015, 7, 1079–1089. [Google Scholar] [CrossRef] [PubMed]
- Le, H.L.; To, D.C.; Tran, M.H.; Do, T.T.; Nguyen, P.H. Natural PTP1B Inhibitors From Polygonum cuspidatum and Their 2-NBDG Uptake Stimulation. Nat. Prod. Commun. 2020, 15, 1934578X20961201. [Google Scholar] [CrossRef]
- Zhao, B.T.; Nguyen, D.H.; Le, D.D.; Choi, J.S.; Min, B.S.; Woo, M.H. Protein tyrosine phosphatase 1B inhibitors from natural sources. Arch. Pharm. Res. 2018, 41, 130–161. [Google Scholar] [CrossRef] [PubMed]
- Tagde, P.; Tagde, P.; Islam, F.; Tagde, S.; Shah, M.; Hussain, Z.D.; Rahman, M.H.; Najda, A.; Alanazi, I.S.; Germoush, M.O.; et al. The multifaceted role of curcumin in advanced nanocurcumin form in the treatment and management of chronic disorders. Molecules 2021, 26, 7109. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Narwal, S.; Kumar, V.; Prakash, O. α-glucosidase inhibitors from plants: A natural approach to treat diabetes. Pharmacogn. Rev. 2011, 5, 19–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verma, S.K.; Thareja, S. An Overview on Chemistry of Natural Aldose Reductase Inhibitors for the Management of Diabetic Complications, 1st ed.; Elsevier B.V.: Amsterdam, The Netherlands, 2020; Volume 65, ISBN 9780128179055. [Google Scholar]
- Chooi, Y.C.; Ding, C.; Magkos, F. The epidemiology of obesity. Metabolism 2019, 92, 6–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Darbre, P.D. Endocrine Disruptors and Obesity. Curr. Obes. Rep. 2017, 6, 18–27. [Google Scholar] [CrossRef] [Green Version]
- Jaeger, C.; Tischkau, S.A. Role of Aryl Hydrocarbon Receptor in Circadian Clock Disruption and Metabolic Dysfunction. Environ. Health Insights 2016, 10, 133–141. [Google Scholar] [CrossRef] [Green Version]
- McMillan, B.J.; Bradfield, C.A. The aryl hydrocarbon receptor is activated by modified low-density lipoprotein. Proc. Natl. Acad. Sci. USA 2007, 104, 1412–1417. [Google Scholar] [CrossRef] [Green Version]
- Moyer, B.J.; Rojas, I.Y.; Kerley-Hamilton, J.S.; Hazlett, H.F.; Nemani, K.V.; Trask, H.W.; West, R.J.; Lupien, L.E.; Collins, A.J.; Ringelberg, C.S.; et al. Inhibition of the aryl hydrocarbon receptor prevents Western diet-induced obesity. Model for AHR activation by kynurenine via oxidized-LDL, TLR2/4, TGFβ, and IDO1. Toxicol. Appl. Pharmacol. 2016, 300, 13–24. [Google Scholar] [CrossRef] [Green Version]
- Kerley-Hamilton, J.S.; Trask, H.W.; Ridley, C.J.A.; Dufour, E.; Ringelberg, C.S.; Nurinova, N.; Wong, D.; Moodie, K.L.; Shipman, S.L.; Moore, J.H.; et al. Obesity is mediated by differential aryl hydrocarbon receptor signaling in mice fed a western diet. Environ. Health Perspect. 2012, 120, 1252–1259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fukuda, I.; Sakane, I.; Yabushita, Y.; Kodoi, R.; Nishiumi, S.; Kakuda, T.; Sawamura, S.I.; Kanazawa, K.; Ashida, H. Pigments in Green Tea Leaves (Camellia sinensis) Suppress Transformation of the Aryl Hydrocarbon Receptor Induced by Dioxin. J. Agric. Food Chem. 2004, 52, 2499–2506. [Google Scholar] [CrossRef] [PubMed]
- Moyer, B.J.; Rojas, I.Y.; Kerley-Hamilton, J.S.; Nemani, K.V.; Trask, H.W.; Ringelberg, C.S.; Gimi, B.; Demidenko, E.; Tomlinson, C.R. Obesity and fatty liver are prevented by inhibition of the aryl hydrocarbon receptor in both female and male mice. Nutr. Res. 2017, 44, 38–50. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.T.; Hochfeld, W.E.; Myburgh, R.; Pepper, M.S. Adipocyte and adipogenesis. Eur. J. Cell Biol. 2013, 92, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Kowalska, K. Natural compounds involved in adipose tissue mass control in in vitro studies. Postepy Hig. Med. Dosw. (Online) 2011, 65, 515–523. [Google Scholar] [CrossRef] [PubMed]
- Colitti, M.; Stefanon, B. Different anti-adipogenic effects of bio-compounds on primary visceral pre-adipocytes and adipocytes. EXCLI J. 2016, 15, 362. [Google Scholar] [CrossRef]
- Lubrano-Berthelier, C.; Cavazos, M.; Le Stunff, C.; Haas, K.; Shapiro, A.; Zhang, S.; Bougnerës, P.; Vaisse, C. The Human MC4R Promoter: Characterization and Role in Obesity. Diabetes 2003, 52, 2996–3000. [Google Scholar] [CrossRef] [Green Version]
- Huszar, D.; Lynch, C.A.; Fairchild-Huntress, V.; Dunmore, J.H.; Fang, Q.; Berkemeier, L.R.; Gu, W.; Kesterson, R.A.; Boston, B.A.; Cone, R.D.; et al. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell 1997, 88, 131–141. [Google Scholar] [CrossRef] [Green Version]
- Yeo, G.S.H.; Lank, E.J.; Farooqi, I.S.; Keogh, J.; Challis, B.G.; O’Rahilly, S. Mutations in the human melanocortin-4 receptor gene associated with severe familial obesity disrupts receptor function through multiple molecular mechanisms. Hum. Mol. Genet. 2003, 12, 561–574. [Google Scholar] [CrossRef]
- Lotta, L.A.; Mokrosiński, J.; Mendes de Oliveira, E.; Li, C.; Sharp, S.J.; Luan, J.; Brouwers, B.; Ayinampudi, V.; Bowker, N.; Kerrison, N.; et al. Human Gain-of-Function MC4R Variants Show Signaling Bias and Protect against Obesity. Cell 2019, 177, 597–607. [Google Scholar] [CrossRef] [Green Version]
- Brumm, H.; Mühlhaus, J.; Bolze, F.; Scherag, S.; Hinney, A.; Hebebrand, J.; Wiegand, S.; Klingenspor, M.; Grüters, A.; Krude, H.; et al. Rescue of melanocortin 4 receptor (MC4R) nonsense mutations by aminoglycoside-mediated read-through. Obesity 2012, 20, 1074–1081. [Google Scholar] [CrossRef] [PubMed]
- René, P.; Le Gouill, C.; Pogozheva, I.D.; Lee, G.; Mosberg, H.I.; Farooqi, I.S.; Valenzano, K.J.; Bouvier, M. Pharmacological chaperones restore function to MC4R mutants responsible for severe early-onset obesity. J. Pharmacol. Exp. Ther. 2010, 335, 520–532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, D.; Saxena, N.K. Mouse Models to Study the Effect of Natural Products on Obesity-Associated NAFLD/NASH. In Murine Models, Energy Balance, and Cancer; Springer: Cham, Switzerland, 2015; pp. 247–270. [Google Scholar] [CrossRef]
- Fang, X.; Sweeney, G. Mechanisms regulating energy metabolism by adiponectin in obesity and diabetes. Biochem. Soc. Trans. 2006, 34, 798–801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawano, J.; Arora, R. The Role of Adiponectin in Obesity, Diabetes, and Cardiovascular Disease. J. Cardiometab. Syndr. 2009, 4, 44–49. [Google Scholar] [CrossRef]
- Xu, A.; Wang, H.; Hoo, R.L.C.; Sweeney, G.; Vanhoutte, P.M.; Wang, Y.; Wu, D.; Chu, W.; Qin, G.; Lam, K.S.L. Selective Elevation of Adiponectin Production by the Natural Compounds Derived from a Medicinal Herb Alleviates Insulin Resistance and Glucose Intolerance in Obese Mice. Endocrinology 2009, 150, 625–633. [Google Scholar] [CrossRef]
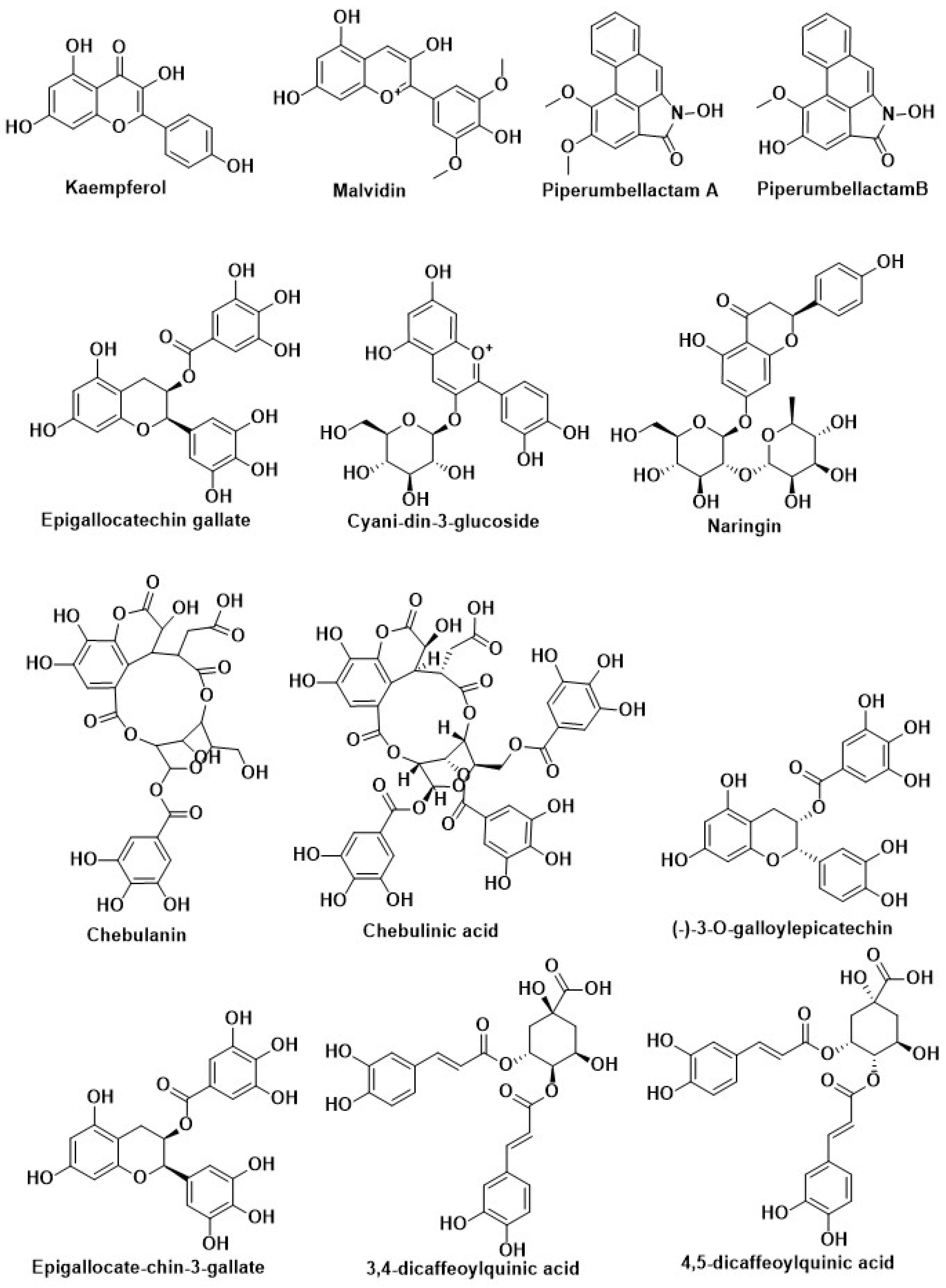
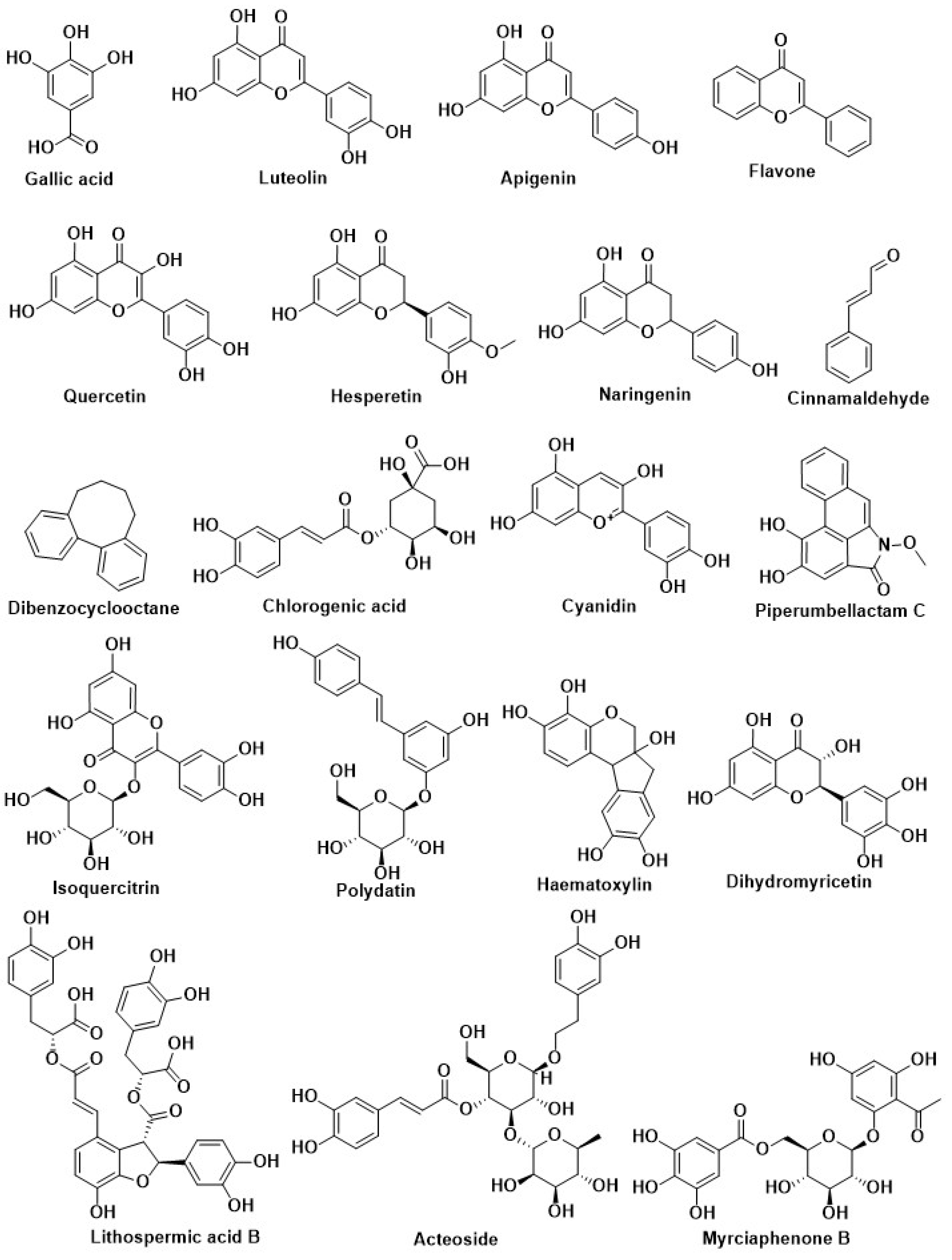
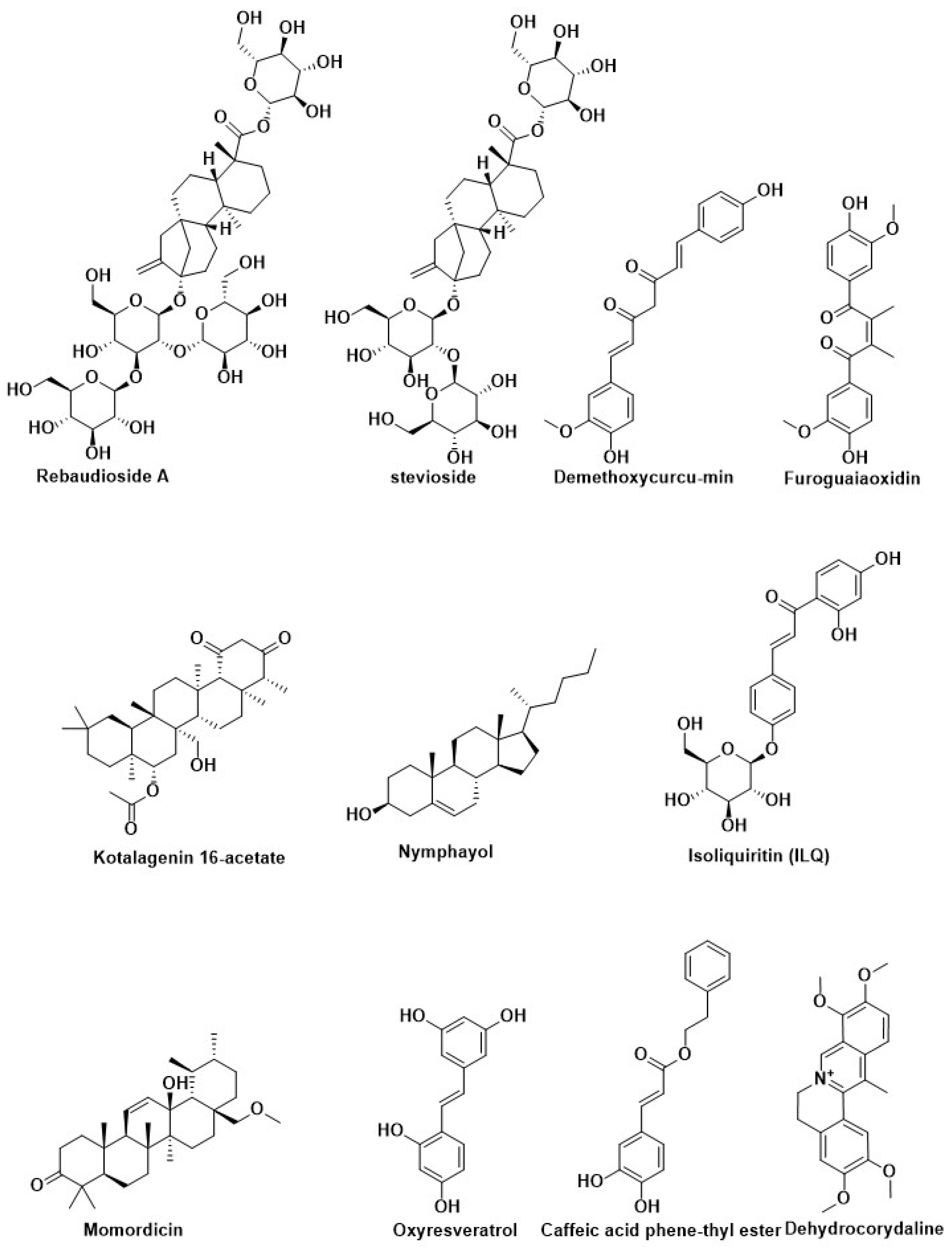
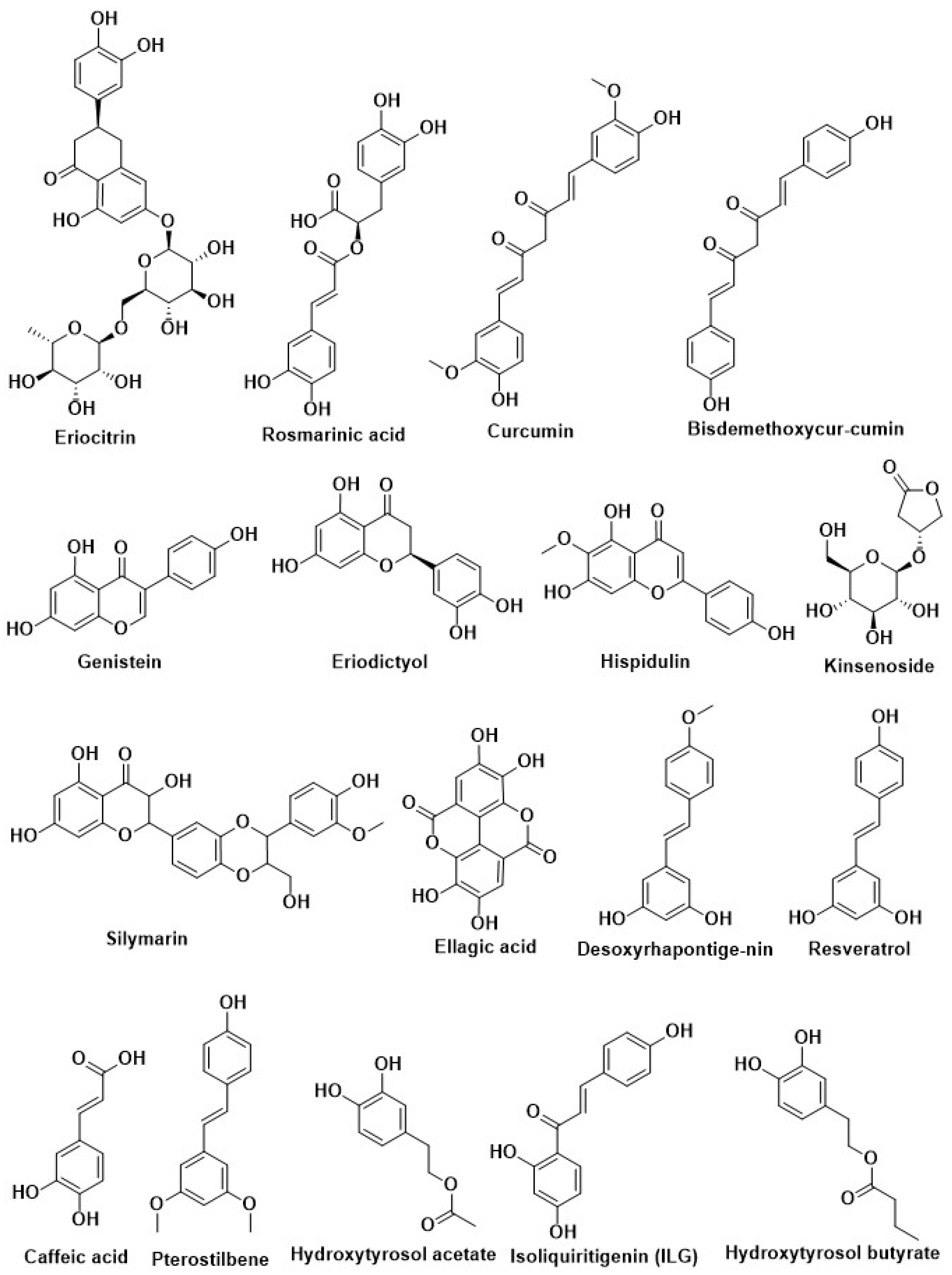

| Compound Name | Herbal Sources | Mode of Action | References |
|---|---|---|---|
| Kaempferol | Citrus, berry, grape, and soybean | Inhibition of DPP-4 | [204] |
| Malvidin | |||
| Epigallocatechin gallate | |||
| Cyanidin-3-glucoside | |||
| Gallic acid | |||
| Luteolin | |||
| Apigenin | |||
| Quercetin | |||
| Flavone | |||
| Hesperetin | |||
| Naringenin | |||
| Eriocitrin | |||
| Resveratrol | |||
| Caffeic acid | |||
| Cyanidin | |||
| Genistein | |||
| Isoquercitrin | Flowers of Gossypium herbaceum L. (Malvaceae) and leaves of Apocynumcannabinum L. (Apocynaceae) | ||
| Naringenin | Rosmarinus officinalis L. (Labiatae) and greenhouse-grown Mexican Lippia graveolens Kunth (Labiatae) | ||
| Eriodictyol | |||
| Hispidulin | |||
| Cirsimaritin | |||
| Rosmarinic acid | |||
| Carnosol | |||
| Naringin | Citrus aurantium L. (Rutaceae) and Peels of Citrus maxima Merr. | ||
| Berberine | Chinese herb Coptis chinensis French. (Ranunculaceae) | ||
| Rebaudioside A | Stevia rebaudiana (Bertoni) Hemsl (Asteraceae) | ||
| stevioside | |||
| Curcumin | Curcuma longa | Inhibition of PTP1B | [14,205,206,207] |
| Cinnamaldehyde | Cinnamon trees | ||
| ethyl acetate (EtOAc) | Methanolic extract of the root of P. cuspidatum | ||
| Eicosenoic acid | Bark of Phellodendronamurense Rupr | ||
| vaccenic acid | |||
| oleic acid | |||
| linoleic acid | |||
| petroselinic acid | |||
| palmitoleic acid | |||
| palmitic acid | Agrimonia pilosa | ||
| Vasicine | Methanolic extract of Adhatoda vasica | Inhibition of α-Glycosidase | [208] |
| Vasicinol | |||
| Piperumbellactam A | Branches of Piper umbellatum | ||
| PiperumbellactamB | |||
| Piperumbellactam C | |||
| 3,4-dicaffeoylquinic acid | Methanolic extract from flower buds of Tussilago farfara | ||
| 4,5-dicaffeoylquinic acid | |||
| Chebulanin | 70% methanolic extract from dried Terminalia chebula (Combretaceae) fruits | ||
| Chebulagic acid | |||
| Chebulinic acid | |||
| (-)-3-O-galloylepicatechin | 50% methanolic extract from Bergenia cilata | ||
| Curcumin | Curcuma longa (turmeric) | ||
| Demethoxycurcumin | |||
| Bisdemethoxycurcumin | |||
| Resveratrol | Grapes and red wine | Activation of Nrf2 | [208] |
| Pterostilbene | Blueberry | ||
| Caffeic acid | Coffee | ||
| Desoxyrhapontigenin | Rheum undulatum L. | ||
| Oxyresveratrol | Mulberry | ||
| Polydatin | Polygonum cuspidatum | ||
| Caffeic acid phenethyl ester | Honeybee propolis | ||
| Hydroxytyrosol acetate | Olive | ||
| Hydroxytyrosol butyrate | |||
| Epigallocatechin gallate (EGCG) | Green tea | ||
| Hesperetin | Aurantium | ||
| Isoliquiritin (ILQ) | Glycyrrhiza | ||
| Isoliquiritigenin (ILG) | |||
| Kinsenoside | Anoectochilus roxburghii | Modification of pancreatic beta-cell | [198] |
| Silymarin | Silybum marianum | ||
| Berberine | Rhizomacoptidis | ||
| Nymphayol | Nymphaea stellate | ||
| Momordicin | Momordica charantia | ||
| Genistein | Glycine max | ||
| Conophylline | Ervatamia microphylla | ||
| Curcumin | Curcuma longa | ||
| Capsaicin | Capsicum annuum | ||
| Epigallocatechin-3-gallate | Camellia sinensis | ||
| Curcumin | Curcuma longa (Turmeric) | Inhibition of Aldose reductase enzyme | [209] |
| Ellagic acid | Phyllanthus niruni L. (Euphorbiaceae) | ||
| Berberine | Mahonia aquifolium (Oregon grape), Tinosporacordifolia, Coptis chinensis (Chinese goldthread), Berberis vulgaris (European barberry), Philodendron bipinnatifidum (Phellodendron), Coptistrifolia (Goldthread), Berberis aristata (tree turmeric), Cortex phellodendri, Cosciniumfenestratum(Yellow vine), and Hydrastis canadensis (Goldenseal), Coptis japonica (Japanese goldthread) | ||
| Quercetin | Tomato, red grapes, leafy green vegetables, broccoli, citrus fruit | ||
| Maesanin | Fruits of Maesa lanceolata (Myrsinaceae) | ||
| Brevifolin carboxylic acid | Phyllanthus nirun | ||
| Dehydrocorydaline | Tuber of Corydalis turstchaninovii | ||
| Flaviolin | Fruits of Maesalanceolata (Myrsinaceae) | ||
| Salvianolic acid A | Salvia miltiorhiza | ||
| Lithospermic acid B | Root of Salvia deserta | ||
| Kotalagenin 16-acetate | Root of Salacia oblonga Wall (Celastraceae) | ||
| Acteoside | Monochasmasavatierii, Plantagoasiatica | ||
| Myrciaphenone B | Myrcia multiflora (Myrtaceae) | ||
| Chlorogenic acid | Chrysanthemunindicum L. (Compositae) | ||
| Gossypol | Gossypium Sp. (Malvaceae) | ||
| Dibenzocyclooctane | Schisandra chinensis | ||
| Brazilin | Caesalphiniasappan (Leguminosae) | ||
| Haematoxylin | Haematoxylum campechianum | ||
| Furoguaiaoxidin | Resin of Guaiacum officinale L. | ||
| Resveratrol | Grapes, red wine, and peanuts | Regulation of autophagy | [185] |
| Berberine | Coptischinensis | ||
| Quercetin | Vegetables, fruits, and teas | ||
| Dihydromyricetin | Ampelopsis grossedentata | ||
| Epigallocatechin gallate (EGCG) | Green tea |
| Compound Name | Herbal Sources | Mode of Action | References |
|---|---|---|---|
| Lutein | Green tea leaves | Inhibition of Aryl hydrocarbon receptors | [85,216] |
| Chlorophyll a | |||
| Chlorophyll b | |||
| (-)-Epigallocatechin gallate | |||
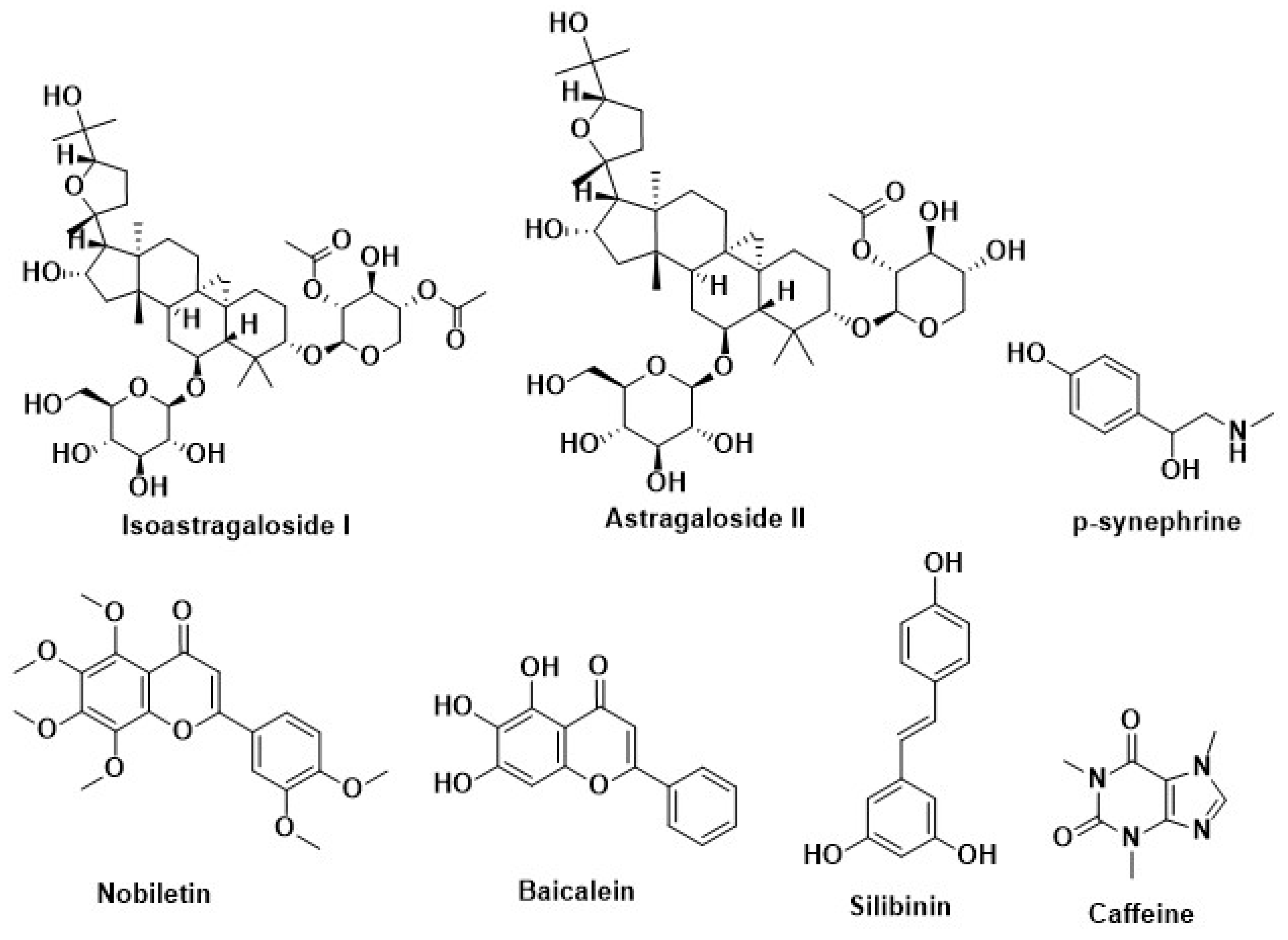
| Silymarin | Milk thistle (Silybummarianum SL) | Inhibition of adipogenesis by methylxanthine | [220] |
| Caffeine | Coffeacanephora, various tea brush, and yerba maté | ||
| Curcumin | Curcuma longa | ||
| p-synephrine | Citrus aurantium | ||
| Resveratrol | Berries of the wine grape | ||
| Silibinin | Milk thistle (Silybummarianum) | Recover the disruption of melanocortin 4 receptor (MC4R) protein | [227] |
| Lycopene | Tomato, watermelon, papaya, orange, grapefruit | ||
| Nobiletin | Citrus fruit | ||
| Baicalein | Scutellariabaicalensis Georgi | ||
| Quercetin | Broccoli, onion | ||
| Astragaloside II | Radix astragali | Increase the secretion of adiponectin | [230] |
| Isoastragaloside I |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahman, M.M.; Islam, M.R.; Shohag, S.; Hossain, M.E.; Rahaman, M.S.; Islam, F.; Ahmed, M.; Mitra, S.; Khandaker, M.U.; Idris, A.M.; et al. The Multifunctional Role of Herbal Products in the Management of Diabetes and Obesity: A Comprehensive Review. Molecules 2022, 27, 1713. https://doi.org/10.3390/molecules27051713
Rahman MM, Islam MR, Shohag S, Hossain ME, Rahaman MS, Islam F, Ahmed M, Mitra S, Khandaker MU, Idris AM, et al. The Multifunctional Role of Herbal Products in the Management of Diabetes and Obesity: A Comprehensive Review. Molecules. 2022; 27(5):1713. https://doi.org/10.3390/molecules27051713
Chicago/Turabian StyleRahman, Md. Mominur, Md. Rezaul Islam, Sheikh Shohag, Md. Emon Hossain, Md. Saidur Rahaman, Fahadul Islam, Muniruddin Ahmed, Saikat Mitra, Mayeen Uddin Khandaker, Abubakr M. Idris, and et al. 2022. "The Multifunctional Role of Herbal Products in the Management of Diabetes and Obesity: A Comprehensive Review" Molecules 27, no. 5: 1713. https://doi.org/10.3390/molecules27051713
APA StyleRahman, M. M., Islam, M. R., Shohag, S., Hossain, M. E., Rahaman, M. S., Islam, F., Ahmed, M., Mitra, S., Khandaker, M. U., Idris, A. M., Chidambaram, K., Emran, T. B., & Cavalu, S. (2022). The Multifunctional Role of Herbal Products in the Management of Diabetes and Obesity: A Comprehensive Review. Molecules, 27(5), 1713. https://doi.org/10.3390/molecules27051713











