Study on the Preparation, Characterization, and Stability of Freeze-Dried Curcumin-Loaded Cochleates
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
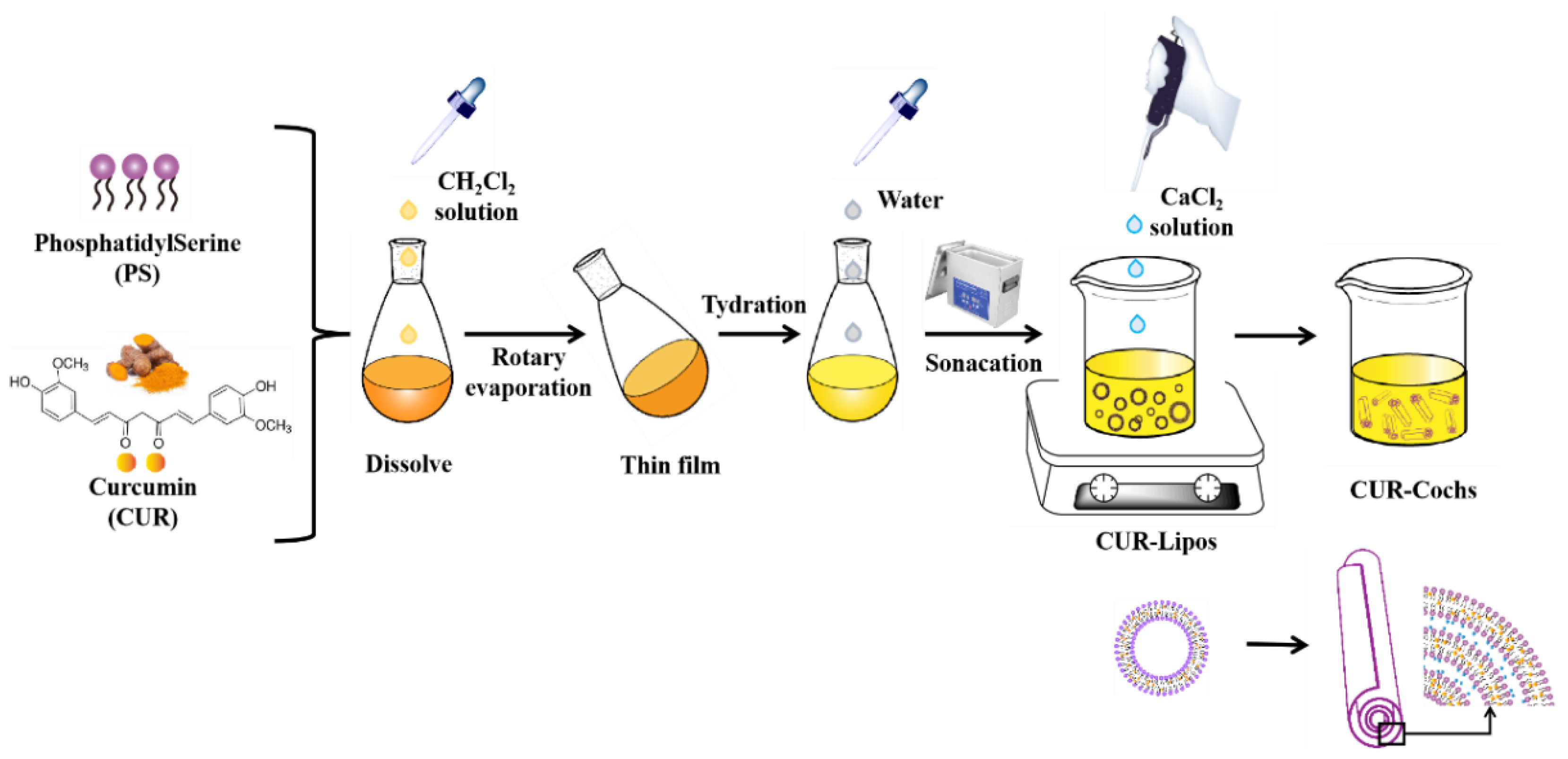
2.2. Preparation of CUR-Cochs by Trapping Method
2.3. Study on the Freeze-Drying Process of CUR-Cochs
2.4. Encapsulation Efficiency Analysis
2.5. Characterization of CUR-Cochs
2.5.1. Particle Size and Zeta Potential Analysis
2.5.2. TEM Observation
2.5.3. DSC Analysis
2.6. Study on the Stability of CUR-Cochs
2.6.1. Stability of CUR-Cochs against pH
2.6.2. Stability of CUR-Cochs in Different Temperature
2.7. In Vitro Bioaccessibility of CUR-Cochs
2.7.1. Simulated Gastrointestinal Digestion
2.7.2. CUR Stability and Bioaccessibility after Digestion
2.8. Cytotoxicity of Blank-Cochs by CCK8 Assay
2.9. Intracellular ROS Assay of CUR-Cochs
2.10. Statistical Analysis
3. Results and Discussion
3.1. Preparation Process of CUR-Cochs
3.2. Characterization of CUR-Cochs
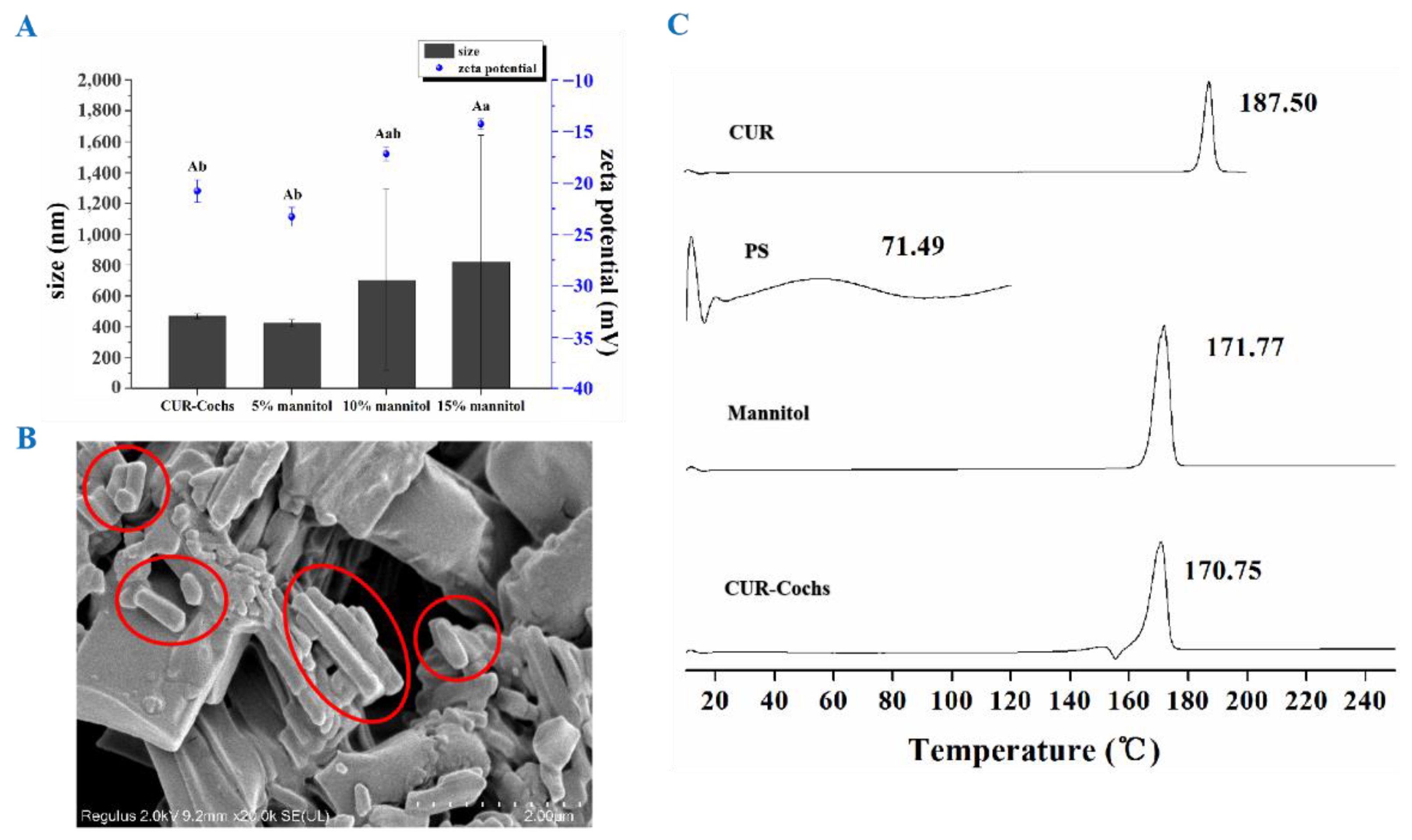
3.2.1. Particle Size and Zeta Potential Analysis
3.2.2. TEM Observation
3.3. Screening of CUR-to-Lipid Ratio
3.4. Selection of Freeze-Dried Protective Agent of CUR-Cochs
3.4.1. Appearance Observation
3.4.2. Particle Size and Zeta Potential
3.4.3. SEM Observation and DSC Analysis
3.5. Study on the Stability of CUR-Cochs
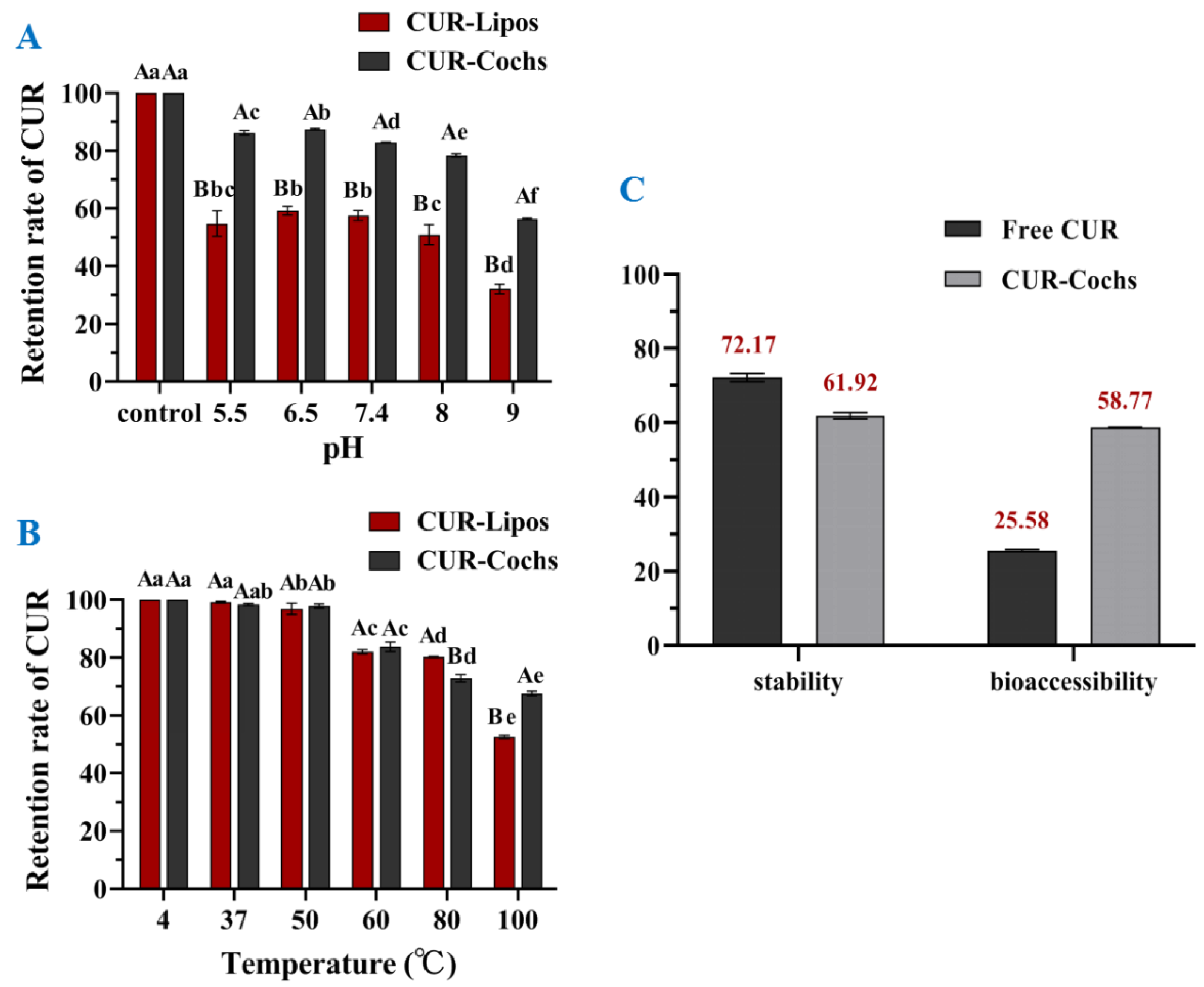
3.5.1. Stability against pH of CUR-Cochs
3.5.2. Thermal Stability of CUR-Cochs
3.6. In Vitro Bioaccessbility of CUR-Cochs
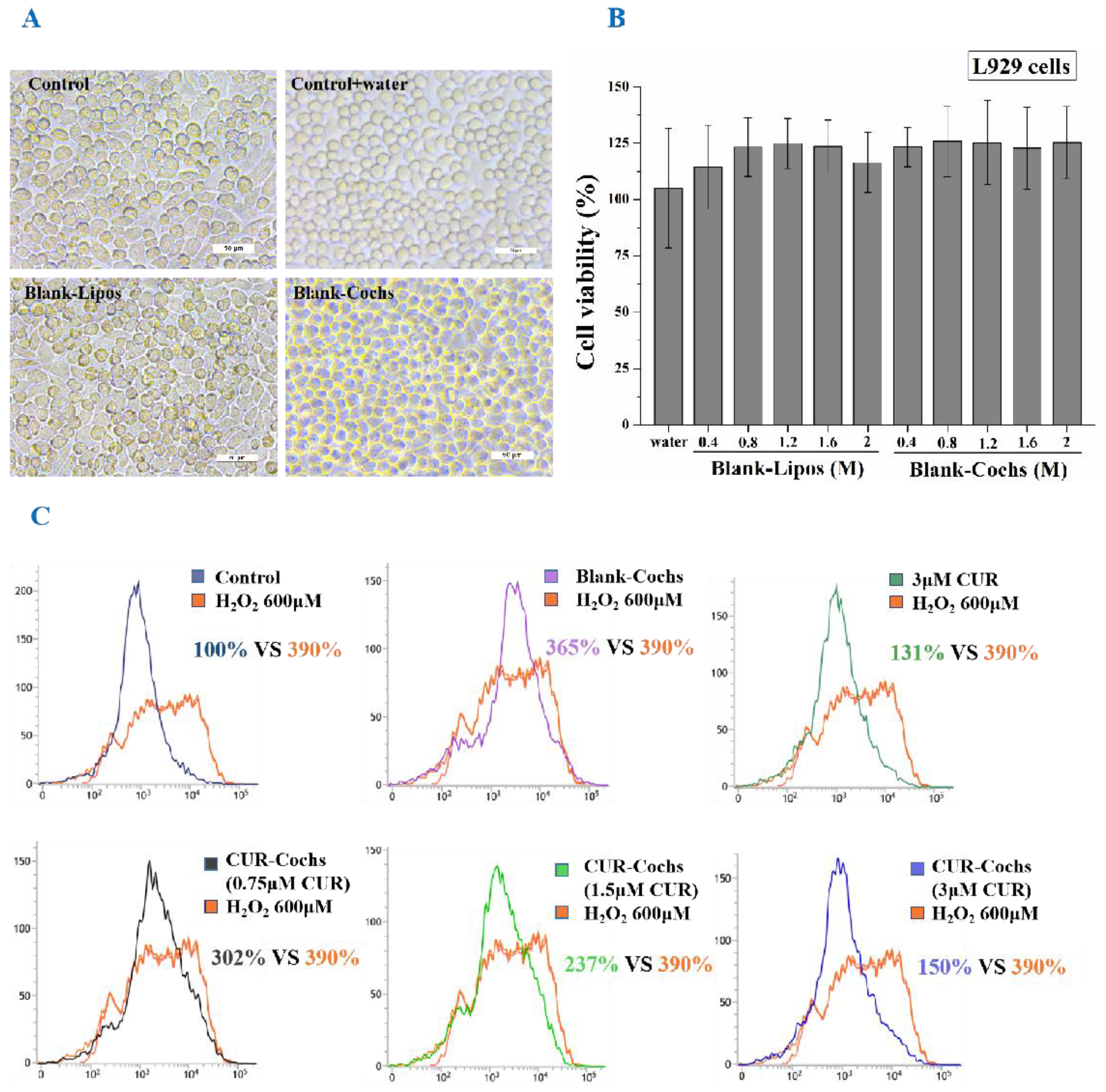
3.7. Measurement of Cytotoxicity of Blank-Cochs
3.8. Intracellular ROS Assay of CUR-Cochs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Typek, R.; Dawidowicz, A.L.; Bernacik, K.; Stankevic, M. Feruloyloacetone can be the main curcumin transformation product. Food Chem. 2019, 286, 136–140. [Google Scholar] [CrossRef] [PubMed]
- Mehanny, M.; Hathout, R.M.; Geneidi, A.S.; Mansour, S. Exploring the use of nanocarrier systems to deliver the magical molecule; curcumin and its derivatives. J. Control. Release 2016, 225, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Kotha, R.R.; Luthria, D.L. Curcumin: Biological, pharmaceutical, nutraceutical, and analytical aspects. Molecules 2019, 24, 2930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, T.; Wei, Y.; Lee, R.J.; Zhao, L. Liposomal curcumin and its application in cancer. Int. J. Nanomed. 2017, 12, 6027–6044. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shishodia, S. Molecular mechanisms of curcumin action: Gene expression. Biofactors 2013, 39, 37–55. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Singh, N. Molecular mechanism of curcumin induced cytotoxicity in human cervical carcinoma cells. Mol. Cell. Biochem. 2009, 325, 107–119. [Google Scholar] [CrossRef]
- Um, M.Y.; Hwang, K.H.; Choi, W.H.; Ahn, J.; Jung, C.H.; Ha, T.Y. Curcumin attenuates adhesion molecules and matrix metalloproteinase expression in hypercholesterolemic rabbits. Nutr. Res. 2014, 34, 886–893. [Google Scholar] [CrossRef]
- Goel, A.; Kunnumakkara, A.B.; Aggarwal, B.B. Curcumin as “curecumin”: From kitchen to clinic. Biochem. Pharmacol. 2008, 75, 787–809. [Google Scholar] [CrossRef] [Green Version]
- Joseph, A.I.; Edwards, R.L.; Luis, P.B.; Presley, S.H.; Porter, N.A.; Schneider, C. Stability and anti-inflammatory activity of the reduction-resistant curcumin analog, 2,6-dimethyl-curcumin. Org. Biomol. Chem. 2018, 16, 3273–3281. [Google Scholar] [CrossRef]
- Shaikh, J.; Ankola, D.D.; Beniwal, V.; Singh, D.; Kumar, M.N.V.R. Nanoparticle encapsulation improves oral bioavailability of curcumin by at least 9-fold when compared to curcumin administered with piperine as absorption enhancer. Eur. J. Pharm. Sci. 2009, 37, 223–230. [Google Scholar] [CrossRef]
- Selvam, C.; Jachak, S.M.; Thilagavathi, R.; Chakraborti, A.K. Design, synthesis, biological evaluation and molecular docking of curcumin analogues as antioxidant, cyclooxygenase inhibitory and anti-inflammatory agents. Bioorganic Med. Chem. Lett. 2005, 15, 1793–1797. [Google Scholar] [CrossRef]
- Hani, U.; Shivakumar, H.G. Solubility enhancement and delivery systems of curcumin a herbal medicine: A review. Curr. Drug Deliv. 2014, 11, 792–804. [Google Scholar] [CrossRef] [PubMed]
- Sanidad, K.Z.; Sukamtoh, E.; Xiao, H.; McClements, D.J.; Zhang, G. Curcumin: Recent advances in the development of strategies to improve oral bioavailability. Annu. Rev. Food Sci. Technol. 2019, 10, 597–617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, K.-Y.; Lin, L.-C.; Tseng, T.-Y.; Wang, S.-C.; Tsai, T.-H. Oral bioavailabilit of curcurnin in rat and the herbal analysis from curcuma longa by lc-ms/ms. J. Chromatogr. B-Anal. Technol. Biomed. Life Sci. 2007, 853, 183–189. [Google Scholar] [CrossRef]
- Jabczyk, M.; Nowak, J.; Hudzik, B.; Zubelewicz-Szkodzinska, B. Curcumin and its potential impact on microbiota. Nutrients 2021, 13, 2004. [Google Scholar] [CrossRef]
- Sivasami, P.; Hemalatha, T. Augmentation of therapeutic potential of curcumin using nanotechnology: Current perspectives. Artif. Cells Nanomed. Biotechnol. 2018, 46, S1004–S1015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rao, W.; Zhang, W.; Poventud-Fuentes, I.; Wang, Y.; Lei, Y.; Agarwal, P.; Weekes, B.; Li, C.; Lu, X.; Yu, J.; et al. Thermally responsive nanoparticle-encapsulated curcumin and its combination with mild hyperthermia for enhanced cancer cell destruction. Acta Biomater. 2014, 10, 831–842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nadimi, A.E.; Ebrahimipour, S.Y.; Afshar, E.G.; Falahati-Pour, S.K.; Ahmadi, Z.; Mohammadinejad, R.; Mohamadi, M. Nano-scale drug delivery systems for antiarrhythmic agents. Eur. J. Med. Chem. 2018, 157, 1153–1163. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.B.; Banerjee, A.; Onyuksel, H. Improvement of drug safety by the use of lipid-based nanocarriers. J. Control. Release 2012, 163, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Jaafar-Maalej, C.; Elaissari, A.; Fessi, H. Lipid-based carriers: Manufacturing and applications for pulmonary route. Expert Opin. Drug Deliv. 2012, 9, 1111–1127. [Google Scholar] [CrossRef]
- Chen, H.; Langer, R. Oral particulate delivery: Status and future trends. Adv. Drug Deliv. Rev. 1998, 34, 339–350. [Google Scholar] [CrossRef]
- Papahadjopoulos, D.; Vail, W.J.; Jacobson, K.; Poste, G. Cochleate lipid cylinders: Formation by fusion of unilamellar lipid vesicles. Biochim. Biophys. Acta 1975, 394, 483–491. [Google Scholar] [CrossRef]
- Pham, T.T.H.; Barratt, G.; Michel, J.P.; Loiseau, P.M.; Saint-Pierre-Chazalet, M. Interactions of antileishmanial drugs with monolayers of lipids used in the development of amphotericin b-miltefosine-loaded nanocochleates. Colloids Surf. B-Biointerfaces 2013, 106, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Zarif, L. Drug delivery by lipid cochleates. In Methods in Enzymology; Liposomes; Duzgunes, N., Ed.; Academic Press: Cambridge, MA, USA, 2005; Volume 391, pp. 314–329. [Google Scholar]
- McClements, D.J.; Li, Y. Review of in vitro digestion models for rapid screening of emulsion-based systems. Food Funct. 2010, 1, 32–59. [Google Scholar] [CrossRef]
- Hur, S.J.; Lim, B.O.; Decker, E.A.; McClements, D.J. In vitro human digestion models for food applications. Food Chem. 2011, 125, 1–12. [Google Scholar] [CrossRef]
- Ahmed, K.; Li, Y.; McClements, D.J.; Xiao, H. Nanoemulsion- and emulsion-based delivery systems for curcumin: Encapsulation and release properties. Food Chem. 2012, 132, 799–807. [Google Scholar] [CrossRef]
- Wan, J.-Z.; Wang, R.; Zhou, Z.-Y.; Deng, L.-L.; Zhang, C.-C.; Liu, C.-Q.; Zhao, H.-X.; Yuan, C.-F.; He, Y.-M.; Dun, Y.-Y.; et al. Saponins of panax japonicus confer neuroprotection against brain aging through mitochondrial related oxidative stress and autophagy in rats. Curr. Pharm. Biotechnol. 2020, 21, 667–680. [Google Scholar] [CrossRef]
- Wang, Y.J.; Pan, M.H.; Cheng, A.L.; Lin, L.I.; Ho, Y.S.; Hsieh, C.Y.; Lin, J.K. Stability of curcumin in buffer solutions and characterization of its degradation products. J. Pharm. Biomed. Anal. 1997, 15, 1867–1876. [Google Scholar] [CrossRef]
- Kolev, T.M.; Velcheva, E.A.; Stamboliyska, B.A.; Spiteller, M. DFT and experimental studies of the structure and vibrational spectra of curcumin. Int. J. Quantum Chem. 2005, 102, 1069–1079. [Google Scholar] [CrossRef]
- Dai, L.; Sun, C.X.; Li, R.R.; Mao, L.K.; Liu, F.G.; Gao, Y.X. Structural characterization, formation mechanism and stability of curcumin in zein-lecithin composite nanoparticles fabricated by antisolvent co-precipitation. Food Chem. 2017, 237, 1163–1171. [Google Scholar] [CrossRef]
- Witika, B.A.; Makoni, P.A.; Matafwali, S.K.; Mweetwa, L.L.; Shandele, G.C.; Walker, R.B. Enhancement of biological and pharmacological properties of an encapsulated polyphenol: Curcumin. Molecules 2021, 26, 4244. [Google Scholar] [CrossRef] [PubMed]
- Metzler, M.; Pfeiffer, E.; Schulz, S.I.; Dempe, J.S. Curcumin uptake and metabolism. Biofactors 2013, 39, 14–20. [Google Scholar] [CrossRef]
- Suresh, K.; Nangia, A. Curcumin: Pharmaceutical solids as a platform to improve solubility and bioavailability. CrystEngComm 2018, 20, 3277–3296. [Google Scholar] [CrossRef]
- Ahmad, M.Z.; Akhter, S.; Mohsin, N.; Abdel-Wahab, B.A.; Ahmad, J.; Warsi, M.H.; Rahman, M.; Mallick, N.; Ahmad, F.J. Transformation of curcumin from food additive to multifunctional medicine: Nanotechnology bridging the gap. Curr. Drug Discov. Technol. 2014, 11, 197–213. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, K.; Zia, K.M.; Zuber, M.; Nazli, Z.I.H.; Rehman, S.; Zia, F. Enhancement of bioactivity and bioavailability of curcumin with chitosan based materials. Korean J. Chem. Eng. 2016, 33, 3316–3329. [Google Scholar] [CrossRef]
- Zhang, Y.N.; Khan, A.R.; Fu, M.F.; Zhai, Y.J.; Ji, J.B.; Bobrovskaya, L.; Zhai, G.X. Advances in curcumin-loaded nanopreparations: Improving bioavailability and overcoming inherent drawbacks. J. Drug Target. 2019, 27, 917–931. [Google Scholar] [CrossRef]
- Rajasekaran, N.S.; Connell, P.; Christians, E.S.; Yan, L.-J.; Taylor, R.P.; Orosz, A.; Zhang, X.Q.; Stevenson, T.J.; Peshock, R.M.; Leopold, J.A.; et al. Human alpha b-crystallin mutation causes oxido-reductive stress and protein aggregation cardiomyopathy in mice. Cell 2007, 130, 427–439. [Google Scholar] [CrossRef] [Green Version]
- Trotter, E.W.; Grant, C.M. Thioredoxins are required for protection against a reductive stress in the yeast saccharomyces cerevisiae. Mol. Microbiol. 2002, 46, 869–878. [Google Scholar] [CrossRef]

| Protective Agents | Dosage (w/v) | Appearance |
|---|---|---|
| Lactose-1 | 5% | Slightly shrunken, yellow |
| Lactose-2 | 10% | Slightly shrunken, yellow |
| Lactose-3 | 15% | Slightly shrunken, yellow |
| Mannitol-1 | 5% | Smooth and full, light yellow |
| Mannitol-2 | 10% | Smooth and full, light yellow |
| Mannitol-3 | 15% | Smooth and full, light yellow |
| Trehalose-1 | 5% | Slightly collapsed, yellow |
| Trehalose-2 | 10% | Slightly collapsed, yellow |
| Trehalose-3 | 15% | Slightly collapsed, yellow |
| Glucose-1 | 5% | Severely shrunken and adherent, yellow |
| Glucose-2 | 10% | Severely shrunken and adherent, yellow |
| Glucose-3 | 15% | Severely shrunken and adherent, yellow |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.; Yue, B.; Liu, Z.; Luo, Y.; Ni, L.; Zhou, Z.; Ge, X. Study on the Preparation, Characterization, and Stability of Freeze-Dried Curcumin-Loaded Cochleates. Foods 2022, 11, 710. https://doi.org/10.3390/foods11050710
Chen L, Yue B, Liu Z, Luo Y, Ni L, Zhou Z, Ge X. Study on the Preparation, Characterization, and Stability of Freeze-Dried Curcumin-Loaded Cochleates. Foods. 2022; 11(5):710. https://doi.org/10.3390/foods11050710
Chicago/Turabian StyleChen, Lijuan, Bowen Yue, Zhiming Liu, Yali Luo, Lu Ni, Zhiyong Zhou, and Xuemei Ge. 2022. "Study on the Preparation, Characterization, and Stability of Freeze-Dried Curcumin-Loaded Cochleates" Foods 11, no. 5: 710. https://doi.org/10.3390/foods11050710
APA StyleChen, L., Yue, B., Liu, Z., Luo, Y., Ni, L., Zhou, Z., & Ge, X. (2022). Study on the Preparation, Characterization, and Stability of Freeze-Dried Curcumin-Loaded Cochleates. Foods, 11(5), 710. https://doi.org/10.3390/foods11050710






