Cryopreservation in a Standard Freezer: −28 °C as Alternative Storage Temperature for Amniotic Membrane Transplantation
Abstract
1. Introduction
2. Materials and Methods
3. Results
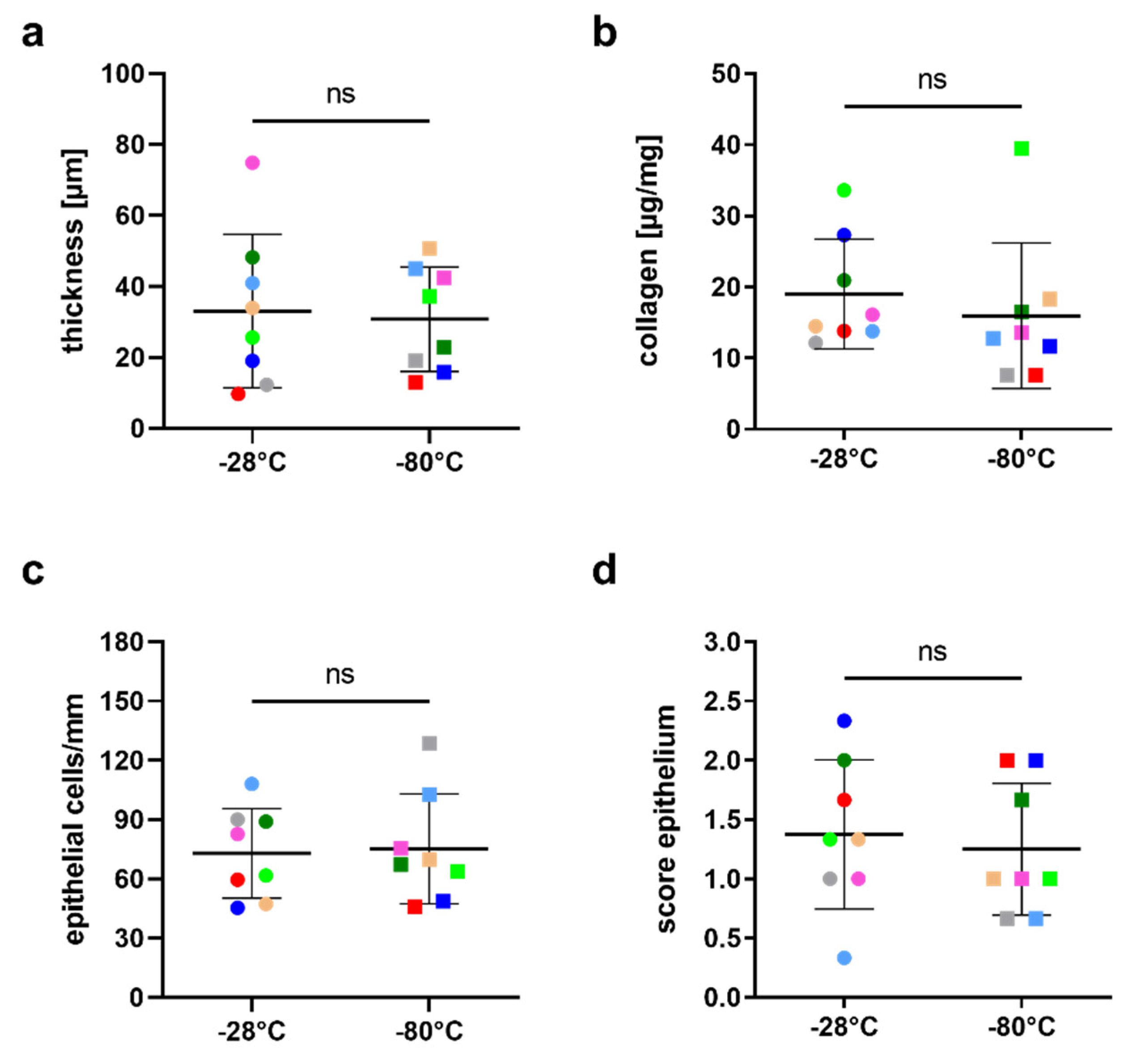
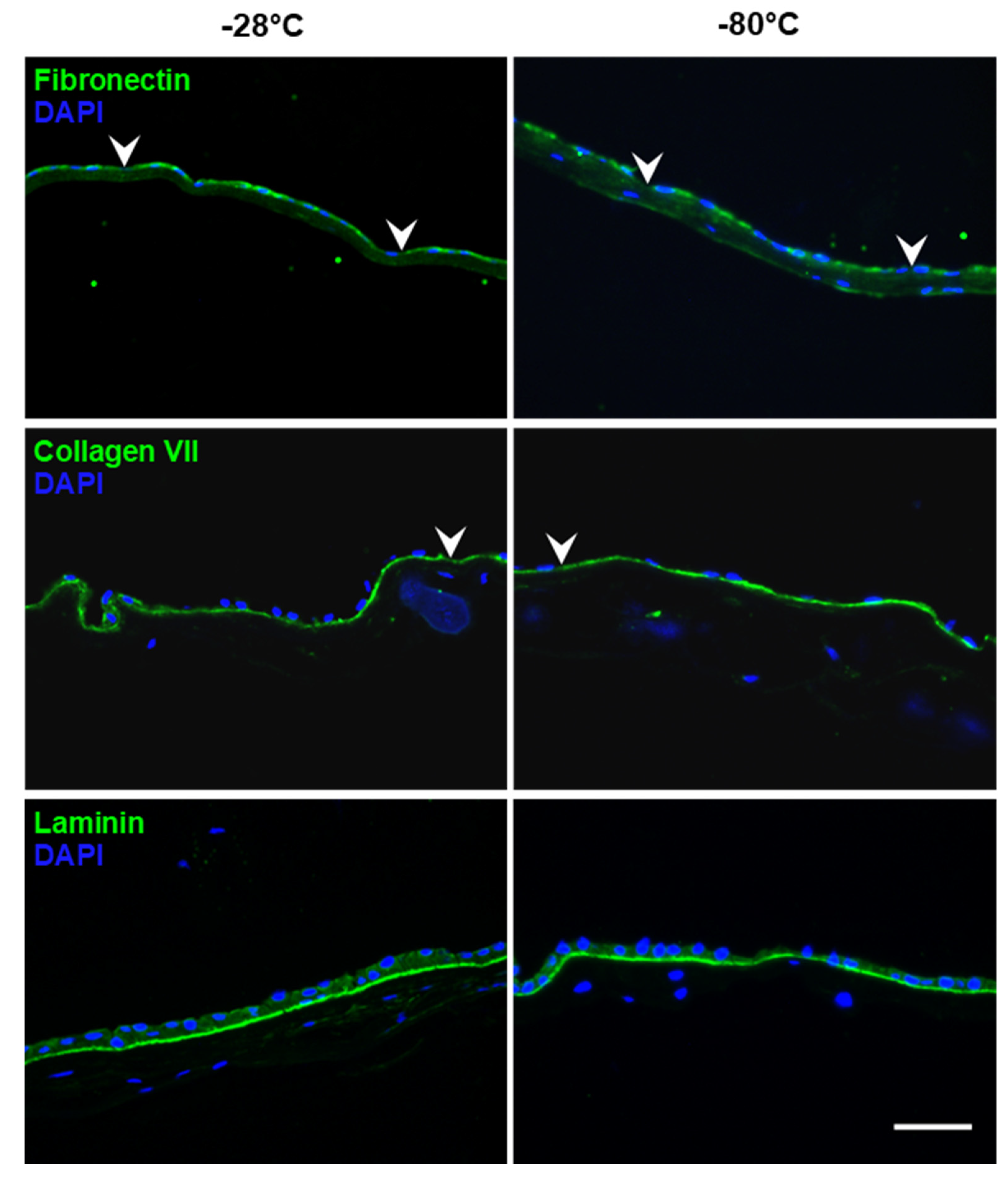
3.1. Integrity of hAM Stroma, Epithelium and Epithelial Basement Membrane
3.2. Influence of the Storage Temperature on hAM Biomechanics
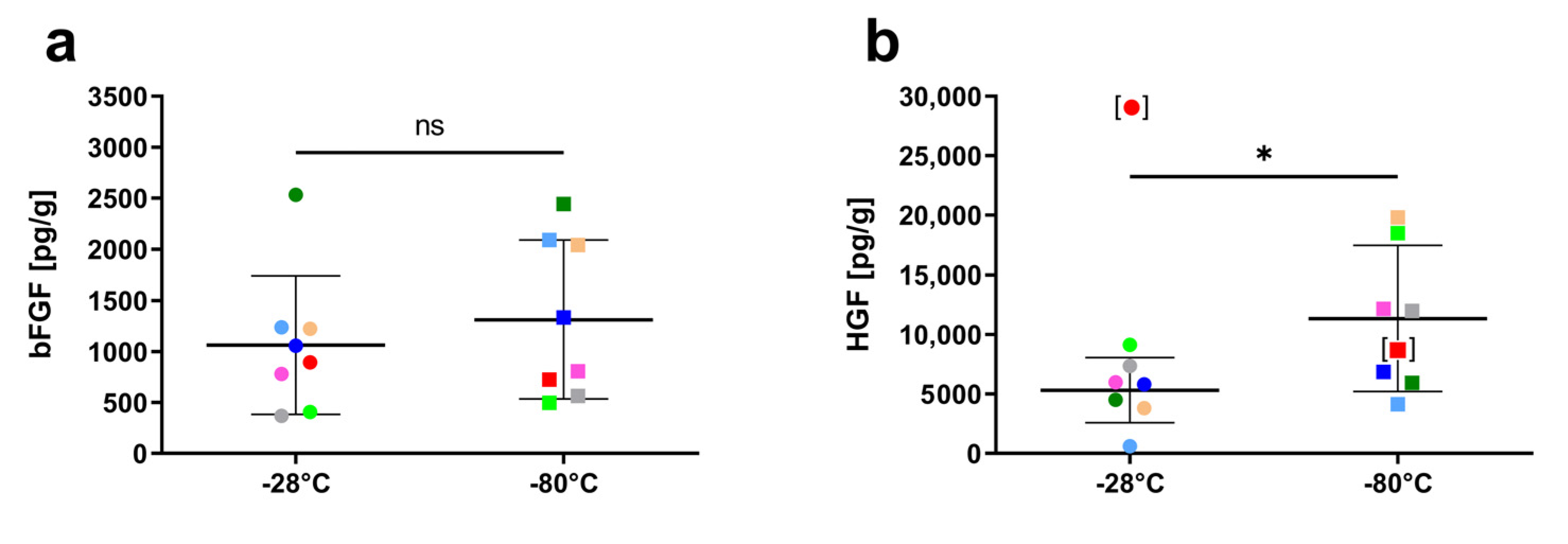
3.3. Influence of Storage Temperature on Growth Factor Content
3.4. Sterility
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bourne, G.L. The anatomy of the human amnion and chorion. Proc. R. Soc. Med. 1966, 59, 1127–1128. [Google Scholar] [PubMed]
- De Rotth, A. Plastic repair of conjunctival defects with fetal membranes. Arch. Ophthalmol. 1940, 23, 522–525. [Google Scholar] [CrossRef]
- Kim, J.C.; Tseng, S.C. Transplantation of preserved human amniotic membrane for surface reconstruction in severely damaged rabbit corneas. Cornea 1995, 14, 473–484. [Google Scholar] [CrossRef]
- Rahman, I.; Said, D.G.; Maharajan, V.S.; Dua, H.S. Amniotic membrane in ophthalmology: Indications and limitations. Eye 2009, 23, 1954. [Google Scholar] [CrossRef] [PubMed]
- Walkden, A. Amniotic membrane transplantation in ophthalmology: An updated perspective. Clin. Ophthalmol. 2020, 14, 2057–2072. [Google Scholar] [CrossRef]
- Malhotra, C.; Jain, A.K. Human amniotic membrane transplantation: Different modalities of its use in ophthalmology. World J. Transplant. 2014, 4, 111. [Google Scholar] [CrossRef]
- Borrelli, M.; Geerling, G.; Spaniol, K.; Witt, J. Eye socket regeneration and reconstruction. Curr. Eye Res. 2020, 45, 253–264. [Google Scholar] [CrossRef]
- Lee, S.-H.; Tseng, S.C. Amniotic membrane transplantation for persistent epithelial defects with ulceration. Am. J. Ophthalmol. 1997, 123, 303–312. [Google Scholar] [CrossRef]
- Kruse, F.E.; Joussen, A.M.; Rohrschneider, K.; You, L.; Sinn, B.; Baumann, J.; Völcker, H.E. Cryopreserved human amniotic membrane for ocular surface reconstruction. Graefes Arch. Clin. Exp. Ophtalmol. 2000, 238, 68–75. [Google Scholar] [CrossRef]
- Chen, H.-J.; Pires, R.T.F.; Tseng, S.C.G. Amniotic membrane transplantation for severe neurotrophic corneal ulcers. Br. J. Ophthalmol. 2000, 84, 826–833. [Google Scholar] [CrossRef]
- Shimmura, S.; Shimazaki, J.; Ohashi, Y.; Tsubota, K. Antiinflammatory effects of amniotic membrane transplantation in ocular surface disorders. Cornea 2001, 20, 408–413. [Google Scholar] [CrossRef]
- Hao, Y.; Ma, D.H.-K.; Hwang, D.G.; Kim, W.-S.; Zhang, F. Identification of antiangiogenic and antiinflammatory proteins in human amniotic membrane. Cornea 2000, 19, 348–352. [Google Scholar] [CrossRef]
- Galask, R.P.; Snyder, I.S. Antimicrobial factors in amniotic fluid. Am. J. Obstet. Gynecol. 1970, 106, 59–65. [Google Scholar] [CrossRef]
- Weidinger, A.; Poženel, L.; Wolbank, S.; Banerjee, A. Sub-regional differences of the human amniotic membrane and their potential impact on tissue regeneration application. Front. Bioeng. Biotechnol. 2021, 13, 613804. [Google Scholar] [CrossRef] [PubMed]
- Magatti, M.; Vertua, E.; Cargnoni, A.; Silini, A.; Parolini, O. The immunomodulatory properties of amniotic cells: The two sides of the coin. Cell Transplant. 2018, 27, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Thomasen, H.; Pauklin, M.; Steuhl, K.-P.; Meller, D. Comparison of cryopreserved and air-dried human amniotic membrane for ophthalmologic applications. Graefes Arch. Clin. Exp. Ophtalmol. 2009, 247, 1691–1700. [Google Scholar] [CrossRef]
- Gündüz, K.; Uçakhan, Ö.; Kanpolat, A.; Günalp, I. Nonpreserved human amniotic membrane transplantation for conjunctival reconstruction after excision of extensive ocular surface neoplasia. Eye 2006, 20, 351–357. [Google Scholar] [CrossRef][Green Version]
- Adds, P. Amniotic membrane grafts, “fresh” or frozen? A clinical and in vitro comparison. Br. J. Ophthalmol. 2001, 85, 905–907. [Google Scholar] [CrossRef] [PubMed]
- Jirsova, K.; Jones, G.L.A. Amniotic membrane in ophthalmology: Properties, preparation, storage and indications for grafting—A review. Cell Tissue Bank. 2017, 18, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Hahn, A.; Thanos, M.; Reinhard, T.; Seitz, B.; Steuhl, K.-P.; Meller, D. Procedural guidelines. Good practice procedures for acquisition and preparation of cryopreserved human amniotic membranes from donor placentas. Ophthalmologe 2010, 107, 1020–1031. [Google Scholar] [CrossRef]
- Sibinga, C.T.S.; Abdella, Y.E.; Seghatchian, J. Poor economics—Transforming challenges in transfusion medicine and science into opportunities. Transfus. Apher. Sci. 2020, 59, 102752. [Google Scholar] [CrossRef] [PubMed]
- Bialasiewicz, A.A.; Shenoy, R.; Al-Muniri, A.; Thakral, A. Diseases of the adnexa in the tropics: Amnion membrane transplantation for noninfectious trachoma-associated corneal ulcers. Ophthalmologe 2006, 103, 940–944. [Google Scholar] [CrossRef]
- Ramakrishnan, K.; Jayaraman, V. Management of partial-thickness burn wounds by amniotic membrane: A cost-effective treatment in developing countries. Burns 1997, 23, S33–S36. [Google Scholar] [CrossRef]
- Jain, R.; Hans, R.; Totadri, S.; Trehan, A.; Sharma, R.R.; Menon, P.; Kapoor, R.; Saxena, A.K.; Mittal, B.R.; Bhatia, P.; et al. Autologous stem cell transplant for high-risk neuroblastoma: Achieving cure with low-cost adaptations. Pediatr. Blood Cancer 2020, 67, e28273. [Google Scholar] [CrossRef]
- Wagner, M.; Walter, P.; Salla, S.; Johnen, S.; Plange, N.; Rütten, S.; Goecke, T.W.; Fuest, M. Cryopreservation of amniotic membrane with and without glycerol additive. Graefes Arch. Clin. Exp. Ophtalmol. 2018, 256, 1117–1126. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Barton, K.; Budenz, D.L.; Khaw, P.T.; Tseng, S.C. Glaucoma filtration surgery using amniotic membrane transplantation. Investig. Ophthalmol. Vis. Sci. 2001, 42, 1762–1768. [Google Scholar]
- Kinoshita, S.; Koizumi, N.; Nakamura, T. Transplantable cultivated mucosal epithelial sheet for ocular surface reconstruction. Exp. Eye Res. 2004, 78, 483–491. [Google Scholar] [CrossRef]
- Menzel-Severing, J.; Kruse, F.E.; Schlötzer-Schrehardt, U. Stem cell-based therapy for corneal epithelial reconstruction: Present and future. Can. J. Ophthalmol. 2013, 48, 13–21. [Google Scholar] [CrossRef]
- Dua, H.S.; Gomes, J.A.P.; King, A.J.; Maharajan, V. The amniotic membrane in ophthalmology. Surv. Ophthalmol. 2004, 49, 51–77. [Google Scholar] [CrossRef]
- Koizumi, N.; Inatomi, T.; Sotozono, C.; Fullwood, N.J.; Quantock, A.J.; Kinoshita, S. Growth factor mRNA and protein in preserved human amniotic membrane. Curr. Eye Res. 2000, 20, 173–177. [Google Scholar] [CrossRef]
- Thomasen, H.; Schroeter, J.; Reinhard, T.; Seitz, B.; Steuhl, K.-P.; Meller, D. Good practice procedures for acquisition and preparation of cryopreserved human amniotic membranes from donor placentas. Ophthalmologe 2018, 115, 855–867. [Google Scholar] [CrossRef] [PubMed]
- Dua, H.S.; Maharajan, V.S.; Hopkinson, A. Controversies and limitations of amniotic membrane in ophthalmic surgery. In Cornea and External Eye Disease; Reinhard, T., Larkin, D., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 21–33. [Google Scholar]
- Dua, H.S.; Rahman, I.; Miri, A.; Said, D.G. Variations in amniotic membrane: Relevance for clinical applications. Br. J. Ophthalmol. 2010, 94, 963–964. [Google Scholar] [CrossRef][Green Version]
- Connon, C.J.; Doutch, J.; Chen, B.; Hopkinson, A.; Mehta, J.S.; Nakamura, T.; Kinoshita, S.; Meek, K.M. The variation in transparency of amniotic membrane used in ocular surface regeneration. Br. J. Ophthalmol. 2010, 94, 1057–1061. [Google Scholar] [CrossRef]
- Niknejad, H.; Deihim, T.; Solati-Hashjin, M.; Peirovi, H. The effects of preservation procedures on amniotic membrane’s ability to serve as a substrate for cultivation of endothelial cells. Cryobiology 2011, 63, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Von Versen-Höynck, F.; Syring, C.; Bachmann, S.; Möller, D.E. The influence of different preservation and sterilisation steps on the histological properties of amnion allografts—Light and scanning electron microscopic studies. Cell Tissue Bank. 2004, 5, 45–56. [Google Scholar] [CrossRef]
- Witt, J.; Borrelli, M.; Mertsch, S.; Geerling, G.; Spaniol, K.; Schrader, S. Evaluation of plastic-compressed collagen for conjunctival repair in a rabbit model. Tissue Eng. Part A 2019, 25, 1084–1095. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Ares, M.T.; López-Valladares, M.J.; Touriño, R.; Vieites, B.; Gude, F.; Silva, M.T.; Couceiro, J. Effects of lyophilization on human amniotic membrane. Acta Ophthalmol. 2009, 87, 396–403. [Google Scholar] [CrossRef]
- Nakamura, T.; Yoshitani, M.; Rigby, H.; Fullwood, N.J.; Ito, W.; Inatomi, T.; Sotozono, C.; Shimizu, Y.; Kinoshita, S. Sterilized, freeze-dried amniotic membrane: A useful substrate for ocular surface reconstruction. Investig. Opthalmol. Vis. Sci. 2004, 45, 93–99. [Google Scholar] [CrossRef]
- Fukuda, K.; Chikama, T.-I.; Nakamura, M.; Nishida, T. Differential distribution of subchains of the basement membrane components type IV collagen and laminin among the amniotic membrane, cornea, and conjunctiva. Cornea 1999, 18, 73–79. [Google Scholar] [CrossRef]
- Modesti, A.; Scarpa, S.; D’Orazi, G.; Simonelli, L.; Caramia, F.G. Localization of type IV and V collagens in the stroma of human amnion. Prog. Clin. Biol. Res. 1989, 296, 459–463. [Google Scholar] [PubMed]
- Tseng, S.C.; Espana, E.M.; Kawakita, T.; Di Pascuale, M.A.; Li, W.; He, H.; Liu, T.-S.; Cho, T.-H.; Gao, Y.-Y.; Yeh, L.-K.; et al. How does amniotic membrane work? Ocul. Surf. 2004, 2, 177–187. [Google Scholar] [CrossRef]
- Elder, E.; Chen, Z.; Ensley, A.; Nerem, R.; Brockbank, K.; Song, Y. Enhanced tissue strength in cryopreserved, collagen-based blood vessel constructs. Transplant. Proc. 2005, 37, 4625–4629. [Google Scholar] [CrossRef]
- George, A.K.; Dalvi, Y.B.; Balram, B.; Kj, N.; Anil, S. Amnion and chorion membranes for root coverage procedures: An in vitro evaluation of its physical characteristics. Periodontics Prosthodont. 2018, 4, 7. [Google Scholar] [CrossRef]
- Witt, J.; Mertsch, S.; Borrelli, M.; Dietrich, J.; Geerling, G.; Schrader, S.; Spaniol, K. Decellularised conjunctiva for ocular surface reconstruction. Acta Biomater. 2018, 67, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Koizumi, N.; Fullwood, N.J.; Bairaktaris, G.; Inatomi, T.; Kinoshita, S.; Quantock, A.J. Cultivation of corneal epithelial cells on intact and denuded human amniotic membrane. Investig. Ophthalmol. Vis. Sci. 2000, 41, 2506–2513. [Google Scholar]
- Koizumi, N.; Inatomi, T.; Quantock, A.J.; Fullwood, N.J.; Dota, A.; Kinoshita, S. Amniotic membrane as a substrate for cul-tivating limbal corneal epithelial cells for autologous transplantation in rabbits. Cornea 2000, 19, 65–71. [Google Scholar] [CrossRef]
- Werner, S.; Grose, R. Regulation of wound healing by growth factors and cytokines. Physiol. Rev. 2003, 83, 835–870. [Google Scholar] [CrossRef]
- Hopkinson, A.; McIntosh, R.S.; Tighe, P.; James, D.K.; Dua, H.S. Amniotic membrane for ocular surface reconstruction: Donor variations and the effect of handling on TGF-β content. Investig. Opthalmol. Vis. Sci. 2006, 47, 4316–4322. [Google Scholar] [CrossRef]
- López-Valladares, M.J.; Rodríguez-Ares, M.T.; Touriño, R.; Gude, F.; Silva, M.T.; Couceiro, J. Donor age and gestational age influence on growth factor levels in human amniotic membrane. Acta Ophthalmol. 2010, 88, e211–e216. [Google Scholar] [CrossRef]

| Donor | Age in Years | Storage in Months |
|---|---|---|
| hAM1 | 34 | 7 |
| hAM2 | 31 | 12 |
| hAM3 | 39 | 11 |
| hAM4 | 34 | 8 |
| hAM5 | 35 | 7 |
| hAM6 | 40 | 7 |
| hAM7 | 30 | 7 |
| hAM8 | 30 | 7 |
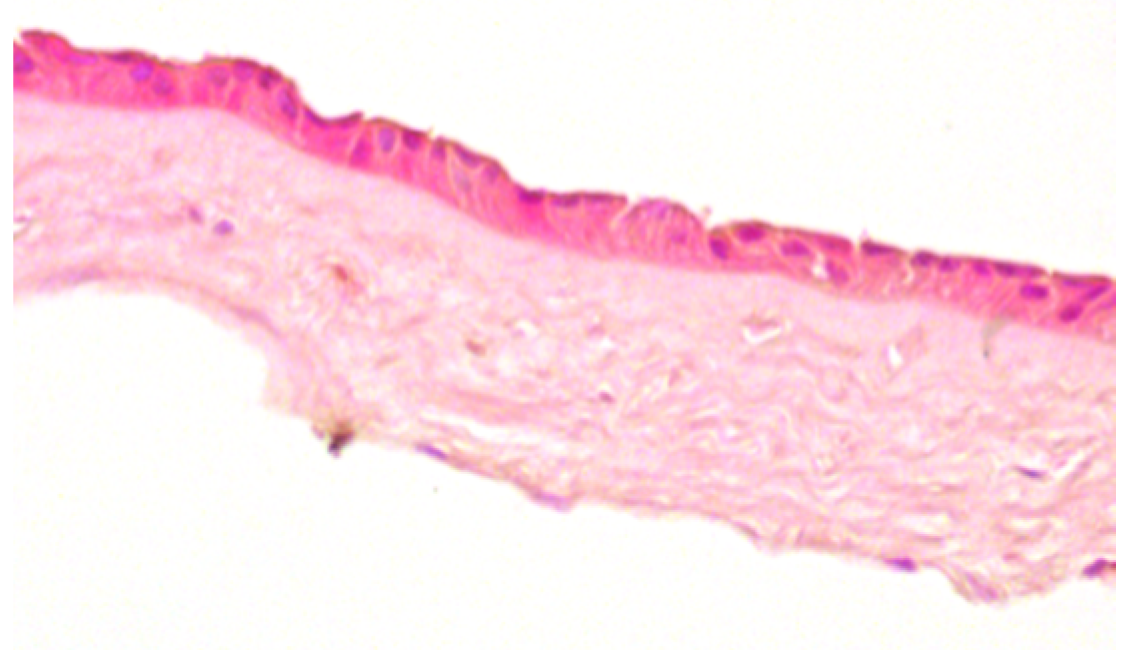
| Score | Criteria | Representative Image |
|---|---|---|
| 0 |
|  |
| 1 |
|  |
| 2 |
|  |
| 3 |
|  |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Witt, J.; Grumm, L.; Salla, S.; Geerling, G.; Menzel-Severing, J. Cryopreservation in a Standard Freezer: −28 °C as Alternative Storage Temperature for Amniotic Membrane Transplantation. J. Clin. Med. 2022, 11, 1109. https://doi.org/10.3390/jcm11041109
Witt J, Grumm L, Salla S, Geerling G, Menzel-Severing J. Cryopreservation in a Standard Freezer: −28 °C as Alternative Storage Temperature for Amniotic Membrane Transplantation. Journal of Clinical Medicine. 2022; 11(4):1109. https://doi.org/10.3390/jcm11041109
Chicago/Turabian StyleWitt, Joana, Luis Grumm, Sabine Salla, Gerd Geerling, and Johannes Menzel-Severing. 2022. "Cryopreservation in a Standard Freezer: −28 °C as Alternative Storage Temperature for Amniotic Membrane Transplantation" Journal of Clinical Medicine 11, no. 4: 1109. https://doi.org/10.3390/jcm11041109
APA StyleWitt, J., Grumm, L., Salla, S., Geerling, G., & Menzel-Severing, J. (2022). Cryopreservation in a Standard Freezer: −28 °C as Alternative Storage Temperature for Amniotic Membrane Transplantation. Journal of Clinical Medicine, 11(4), 1109. https://doi.org/10.3390/jcm11041109






