1. Introduction
Cotton belongs to the genus
Gossypium, which comprises approximately 53 species [
1,
2]. The genus
Gossypium is unusually diverse; 46 species have been assigned to eight cytologically and geographically distinct groups with diploid genomes (A, B, C, D, E, F, G, and K) of 2n = 26, and 7 species have been assigned to a group with a tetraploid genome (AADD) of 2n = 52 [
1,
2,
3].
In diploid species, significant chromosome rearrangements have occurred during evolution [
4,
5]. Allopolyploid species likely arose 1–2 million years ago as a result of the spread of a taxon containing the A genome in the New World, followed by hybridization of this taxon with a local diploid containing the D genome and doubling of the number of chromosomes [
6].To date, the alleged donors of the genomes of tetraploid species are thought to come from African or Asian species with the A genome and from a species similar to the American diploid species with the D genome [
1,
7]. It has been shown that the subgenomes of allotetraploids—D
t and A
t (‘t’ indicates tetraploid)—are collinear with the genomes of
G. raimondii Ulbr. and
G. arboreum L., respectively [
8,
9].
The nascent A
tD
t allopolyploid spread throughout the American tropics and subtropics, diverging into seven species: AD1 =
G. hirsutum L., AD2 =
G. barbadense L., AD3 =
G. tomentosum Nutt. ex Seem., AD4 =
G. mustelinum Miers ex Watt., AD5 =
G. darwinii Watt., AD6 =
G. ekmanianum Wittm., and AD7 =
G. stephensii J. Gallagher, C. Grover, and Wendel. [
1,
10]. Two allopolyploid cotton species
G. hirsutum L. or Upland cotton and
G. barbadense L. (or Pima cotton) were independently domesticated [
11]. Genome doubling in tetraploids has led to a variety of molecular genetic interactions, including different rates of genome evolution, intergenomic gene transfer, and changes in gene expression [
6,
12,
13,
14].
To date, an increase in cotton yields has also been achieved through traditional breeding and intervarietal crossing. Cultivars have been created with better yield potential, higher fibre quality, and the ability to withstand changing climatic conditions, as well as early ripening times, a tolerance to high fertilizer contents, and the ability to efficiently use soil moisture reserves [
15]. However, most of these varieties were obtained by selection from a narrow gene pool and adapted to certain soil and climatic conditions. Thus, modern cultivated cotton shows a reduction in genetic diversity, which has resulted in a decrease in fibre quality and an increase in genetic vulnerability due to the close relationship of high-yielding varieties.
Enrichment of the
G. hirsutum genome with alleles from other economically valuable cotton species is very important [
15,
16,
17]. For example,
G. tomentosum is characterized as having heat resistance, while
G. mustelinum and
G. stocksii Mast. are characterized as having pest and disease resistance [
18]. The wild species
G. longicalyx J.B. Hutch and B.J.S. Lee (F1 genome) could be used as a donor of desirable traits for fibre fineness, length, and strength [
19].
It is known that G. barbadense is less productive and has a narrower range of agronomic adaptability, but it has significantly higher quality fibre (length, strength, and fibre fineness) than cultivated varieties of G. hirsutum, although the latter gives a higher yield. Given their complementary agronomic characteristics, numerous attempts have been made to hybridize the two species within the framework of traditional breeding. However, this work has been unsuccessful.
In cotton, interspecific hybrid progeny often have poor agronomic traits, distorted segregation, sterility, mote formation, and limited recombination due to genome incompatibility. A comparative analysis of the genomes of
G. barbadense and
G. hirsutum revealed large-scale inversions on various chromosomes of the A
t and D
tsubgenomes [
5]. The genetic effects of inversions are manifested in the suppression of the recombination of the inverted regions themselves and the regions adjacent to them, reducing population diversity and leading to population divergence. Thus, inversions between
G. hirsutum and
G. barbadense restrict recombination in F
1 hybrids, limiting genomic exchanges. Generally, F
1 hybrids of
G. hirsutum ×
G. barbadense are fertile, but the F
2 phenotypes and subsequent generations are biased towards one of the parents due to the manifestation of genetic breakdown.
Another method of transferring agronomically valuable traits from
G. barbadense to
G. hirsutum has been the creation of lines with introgressed segments of individual chromosomes from
G. barbadense and lines with chromosome substitutions of
G. barbadense L./
G. hirsutum L. [
20,
21,
22,
23].
D.M. Stelly et al. [
22] constructed 17 chromosome- or chromosomal-arm-substitution lines of
G.
barbadense in the genetic background of the
G.
hirsutum line Texas Marker-1 (TM-1). Previous analyses of CS lines have associated many novel traits with the substitution of chromosomes and chromosome segments.
Thus, the study of these lines made it possible to determine that the substitution of homologous chromosomes from
G. hirsutum L. into
G. barbadense L. (CS-B02, CS-B04,CS-B16, CS-B17, CS-B22Lo, CS-B22sh, and CS-B25) had an effect on fibre elongation, fibre yield, fibre strength, and micronair, when compared with the original lines TM-1 and Pima 3-79 [
24,
25,
26].
In creating
G. barbadense L./
G. hirsutum L.-substituted lines [
22,
23], monosomic individuals of TM-1
G. hirsutum L. were used or targeted transfer of certain chromosomes of
G. barbadense L. In this work, a similar scheme was used, and monosomic lines of
G. hirsutum from the Cytogenetic Collection of Uzbekistan were crossed with the
G. barbadense line Pima 3-79 to create
G. hirsutum/
G. barbadense substitution lines with improved agronomic traits. Identification of univalent chromosomes was carried out at the early stages of cotton-plant development using chromosome-specific molecular markers.
Previously, we obtained new monosomic cotton lines in a single genotypic background of the highly inbred
G. hirsutum L. line L-458 (A
tD
t genome) [
27,
28,
29,
30]; these lines resulted in long-term inbreeding and selection with the cotton cultivar 108-F. Univalent chromosomes of these lines were identified using a well-defined tester set of cotton translocation lines (USA) [
31,
32]. The identification of most monosomic lines was confirmed using SSR markers at the Center for Genomics and Bioinformatics of the Academy of Sciences of the Republic of Uzbekistan [
30,
31]. As a result, four monosomic lines from the Cytogenetic Collection of Uzbekistan (Mo11, Mo16, Mo19, and Mo93) were found to have chromosome
2 of the A
tsubgenome; 16 monosomic lines (Mo7, Mo31, Mo38, Mo58, Mo59, Mo60, Mo69, Mo70, Mo71, Mo72, Mo73, Mo75, Mo76, Mo79, Mo81, and Mo89) had chromosome
4 of the A
tsubgenome; five monosomic lines (Mo13, Mo34, Mo67, Mo92, and Mo95) had chromosome
6 of the A
tsubgenome; Mo27 had chromosome
7 of the A
tsubgenome; Mo48 line had chromosome
18 of the D
tsubgenome; and monotelodisomic line Mo21 lacks an arm of chromosome
11 of the A
tsubgenome [
31,
32,
33,
34].
Fivefold backcrossing was used to create
G. hirsutum/
G. barbadense near-isogenic substitution lines [
23]. Backcrossing was also used to prevent “genetic breakdown” in these interspecific crosses [
23]. Using this technique, a set of chromosome-segment-introgression lines (CSILs) were obtained [
20,
21,
23]. To our knowledge, data on the peculiarities of chromosome behaviour in interspecific aneuploid hybrids when obtaining these lines have not been published. Such information is necessary to understand the collinearity of
G. hirsutum chromosomes with
G. barbadense chromosomes, which reflects their compensatory ability, as well as to understand the contribution of individual chromosomes of these two species to the manifestation of “genetic breakdown” during interspecies crossing and subsequent backcrossing.
The aim of this work was to study interspecific aneuploid F1 hybrids obtained in a different genotypic background from crosses of monosomic cotton lines of the inbred G. hirsutum line L-458 with the G. barbadense L. line Pima 3-79, as well as their BC1F1 backcross offspring. In the course of this work, new monosomic lines of G. hirsutum L. were obtained; the identification of univalent chromosomes was carried out using molecular- genetics and translocation markers; and the crossing of monosomic lines with Pima 3-79 was performed. Additionally, the setting and germination of hybrid seeds were studied; the meiotic behaviour of chromosomes in monosomic F1 and BC1F1 plants has been investigated; and univalent chromosomes in F1 and BC1F1 hybrids have been identified using molecular genetic markers. Finally, a comparative morphobiological analysis of aneuploid F1 and BC1F1 hybrids was carried out and the prospect of using them to obtain lines with substitutions of specific chromosomes was assessed.
2. Results
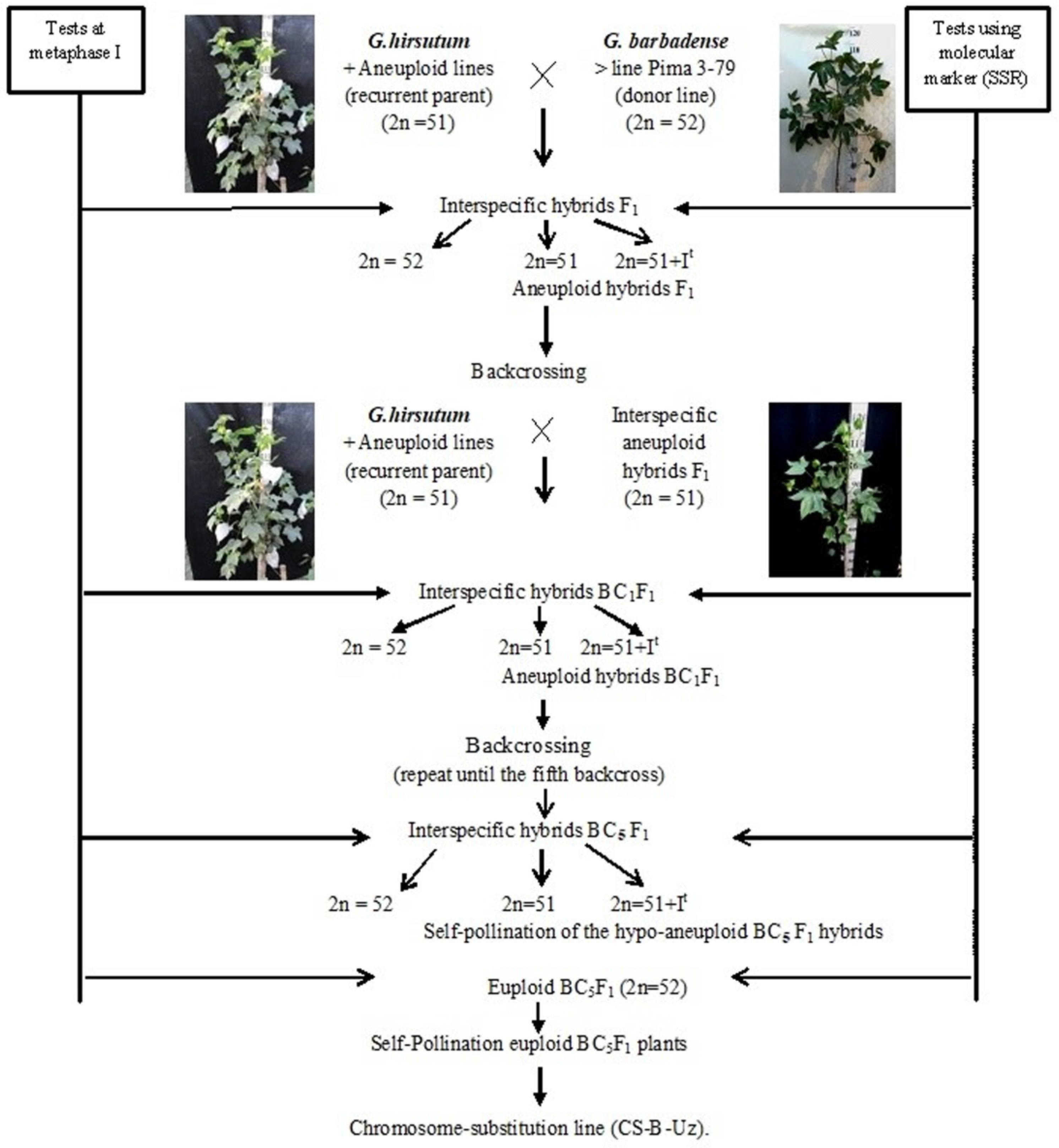
2.1. Development Scheme for Obtaining Cotton Chromosome-Substitution Lines
We developed a scheme for creating cotton lines with chromosome substitutions. According to the scheme (
Figure 1), the previously identified
G. hirsutum L. aneuploid lines were crossed with the
G. barbadense L. donor line Pima 3-79; the hybrid offspring from each crossing were studied at the metaphase I stage of meiosis; and SSR markers were used to confirm the chromosome identity. Furthermore, aneuploidy F
1 hybrid plants were crossed with the aneuploid
G. hirsutum L. line (recurrent parent), and the resulting BC
1F
1 backcross hybrids were studied using molecular marker analysis (SSR) at the plantlet stage to identify aneuploid BC
1F
1 hybrids, which were confirmed by cytogenetic analysis. The aneuploid BC
1F
1 hybrids were then recrossed with the recurrent parent. Furthermore, the obtained BC
2F
1 backcross hybrids were analysed using molecular markers (SSRs) at the plantlet stage. The identified BC
2F
1 aneuploid hybrids were re-examined at the metaphase I stage of meiosis to detect aneuploid BC
2F
1 hybrids. This work was repeated up to the fifth generation using backcrossing. Then, aneuploid hybrid BC
5F
1 plants were self-pollinated, and euploid plants from the self-pollinated BC
5F
1 generation were used as the founders of chromosome-substituted lines (
Figure 1).
Double screening of aneuploid hybrids with specific chromosome substitutions was carried out at all stages of cytogenetic (metaphase I of meiosis) and molecular marker analysis (chromosome-specific SSR markers) at the plantlet stage. Cytogenetic screening is necessary to exclude the phenomenon of a “univalent shift,” where as chromosome-specific molecular markers confirm the presence of a specific chromosome substitution of G. barbadense. This analysis was noticeably easier, since all aneuploid lines in our collection were created in a common genotypic background of the highly inbred G. hirsutum L. L-458 line, which was created through multiple generations of self-pollination (F20) of cultivar 108-F.
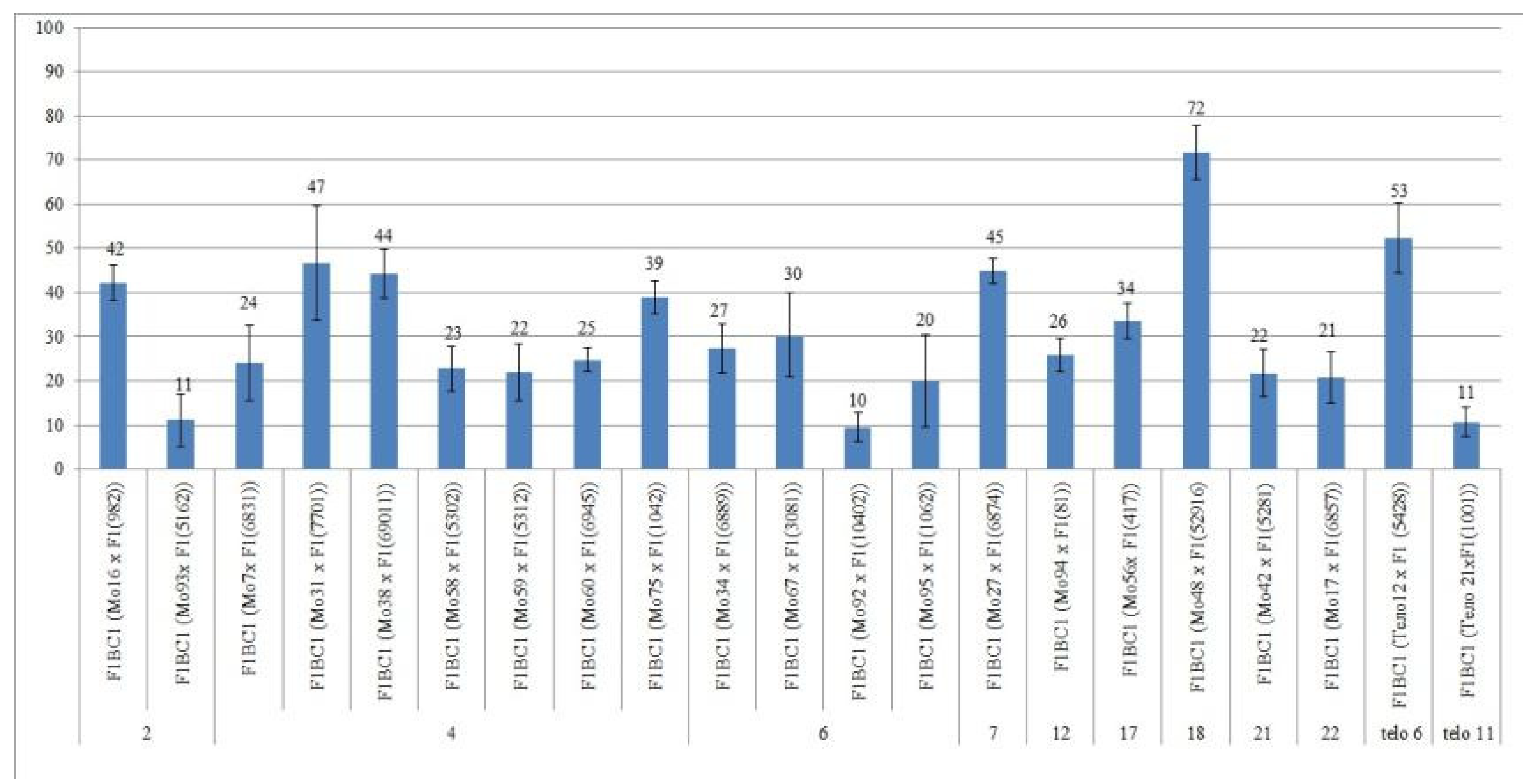
2.2. Features of Crossing, Setting, and Germination of Hybrid Seeds
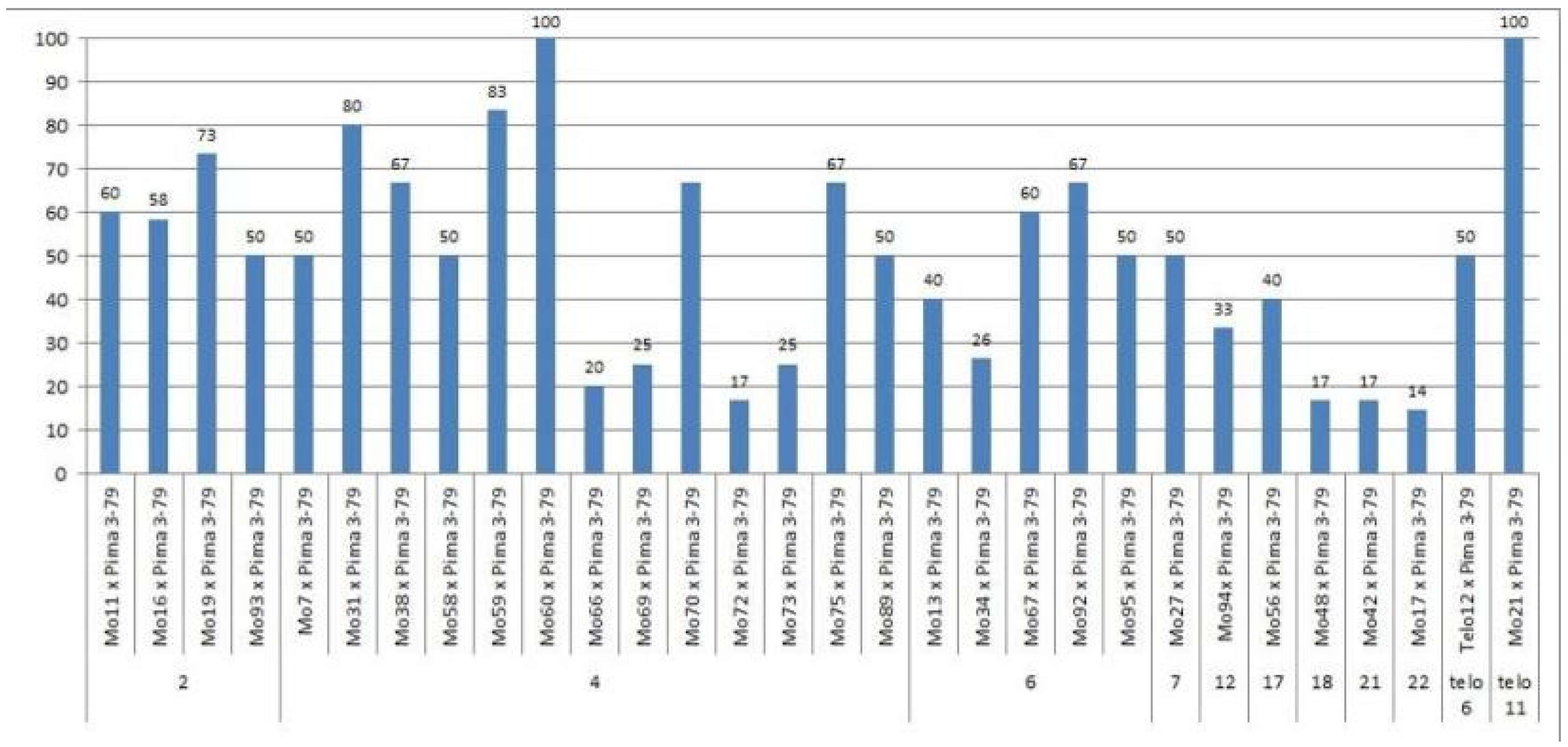
We crossed 30 monosomic and two monotelodisomic lines of
G. hirsutum L. from the Cytogenetic Collection of Uzbekistan with the
G. barbadense L. doubled haploid line Pima 3-79. The analysis of the crosses of the 30 aneuploid
G. hirsutum L. lines with Pima 3-79 revealed significant differences between the lines. Thus, four monosomic lines with deficiencies in chromosome
2 (Mo11, Mo16, Mo19, and Mo93) were characterized by a decrease in the crossing rate with small ranges of variation between the lines (from 50 to 73.33%) (
Figure 2). Four monosomic lines (Mo66, Mo69, Mo72, and Mo73) out of 13 lines with deficiencies in chromosome
4 were distinguished by a significant decrease in the crossing rate (from 16.66 to 25%); however, one line (Mo60) stood out as having the maximum crossability (100%) (
Figure 2).Five monosomic lines (Mo13, Mo34, Mo67, Mo92, and Mo95) with deficiencies in chromosome
6 also showed a significant decrease in the crossing rate (from 26.38 to 66.67%). Of the remaining six monosomic lines, three lines (Mo48, Mo42, and Mo17) with deficiencies in chromosomes
18,
21, and
22 from the D
tsubgenome were characterized as having the largest decrease in the crossing rate (16.66, 16.67, and 14.29%, respectively) (
Table S1,
Figure 2).
Two monotelodisomic lines (Telo12 and Mo21) with deficiencies in chromosome arms
6 and
11 differed significantly in their crossing rates (50 and 100%, respectively) with line Pima 3-79 (
Table S1,
Figure 2).
There were large differences in the setting of F0 hybrid seeds obtained from crosses of aneuploid cotton lines with the line Pima 3-79, with the exception of one crossing combination (Mo48 × Pima 3-79).
All four monosomic lines with deficiencies in chromosome
2 were characterized by a decrease in the setting of F
0 hybrid seeds (from 31.33 ± 5.09 to 60.71 ± 10.48%) compared with the control (73.83 ± 4.25) (
Table S2,
Figure 3).
The setting of F0 hybrid seeds obtained from crosses of 13 monosomic lines with deficiencies in chromosome 4 showed a wider range of decreases (from 26.03 ± 5.14 to 61.76 ± 8.33%). Five monosomic lines with deficiencies in chromosome 6 were also distinguished by a strong decrease in the setting of hybrid seeds, but with a narrower range (from 18.64 ± 5.07 to 35.48 ± 4.96).
Of the remaining six monosomic lines (Mo27, Mo94, Mo56, Mo48, Mo42, and Mo17), one line (Mo17) with a deficiency in chromosome
22 from the D
tsubgenome was characterized as having the strongest decrease in seed setting (up to 29.41%) compared to the control (73.83 ± 4.25%) (
Table S2,
Figure 3) and to other monosomic lines with deficiencies in chromosomes
7,
12,
17,
18, and
21.
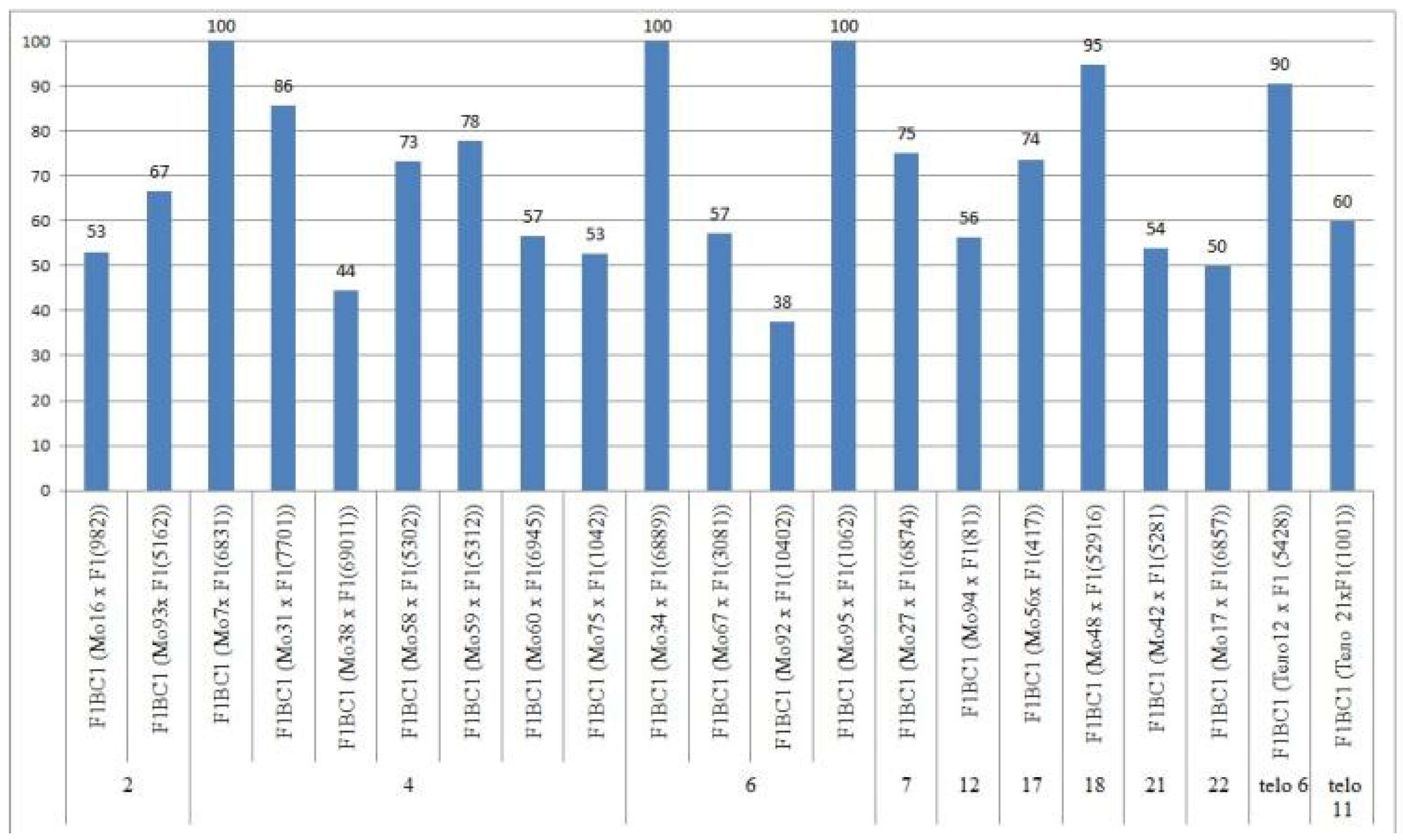
There was a large difference in the setting of F0 hybrid seeds obtained from crosses of two monotelodisomic lines with deficiencies in chromosome arms 6 and 11 (69.05 ± 7.13 and 16.00 ± 4.23%, respectively), which was explained by the arms belonging to different nonhomologous chromosomes.
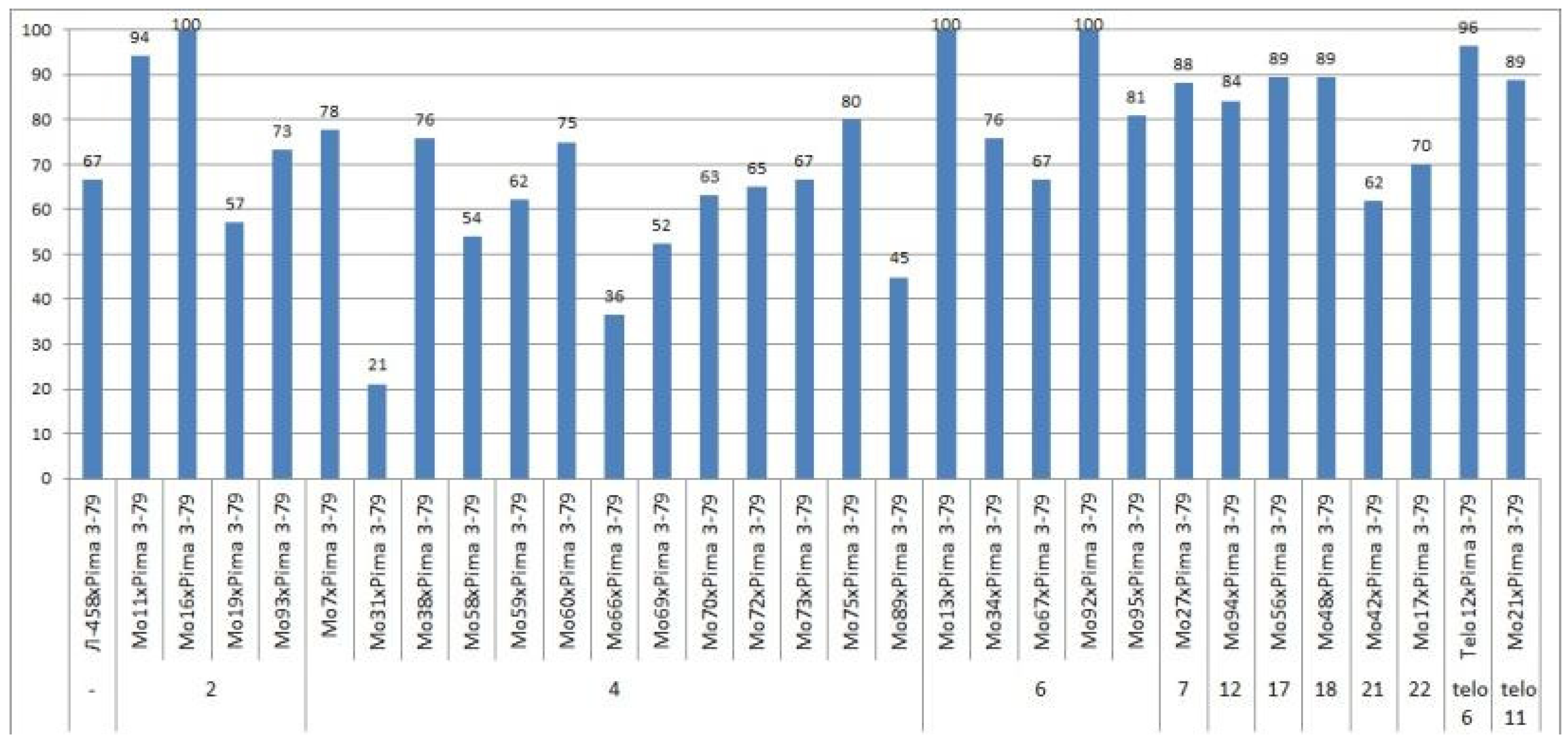
Only the F
0 hybrid seeds from the crosses of two monosomic lines with deficienciesin chromosome
2 (Mo19 and Mo93) showed differences in their reduced germination (57.14 and 73.33%) (
Table S3,
Figure 4).
The germination capacity of F
0 hybrid seeds obtained from crosses of one monosomic line (Mo31) with a deficiency in chromosome
4 showed the strongest decrease (21.05%), while in the other four variants of crosses (Mo58, Mo66, Mo69 and Mo89), a smaller decrease was observed (from 36.36 to 53.85%) (
Figure 4). The germination capacity of F
0 hybrid seeds obtained from crosses involving five monosomic lines with deficiencies in chromosome
6 was found to be very high (from 66.67 to 100%). The germination capacity of F
0 hybrid seeds obtained from crosses of monosomic lines with deficiencies in chromosomes
7,
12,
17, and
18 showed a slight decrease (up to 84.21%), while the germination of hybrid seeds from two lines with deficiencies in chromosomes
21 and
22 decreased to a higher degree (to 61.90 and 70.00%, respectively). The germination of hybrid seeds from crosses involving two monotelodisome lines with deficiencies in one arm of chromosomes
6 and
11 was high and did not differ significantly (
Table S3,
Figure 4).
In general, the germination capacity of F0 hybrid seeds obtained from crosses of aneuploid G. hirsutum L. lines with the G. barbadense L. line Pima 3-79 was high compared to the hybrid seeds of the control (66.67%).
2.3. Cytogenetic Characteristics of the Monosomic F1 Hybrids
Aneuploid F
1 hybrids were isolated in 27 interspecies F
1 hybrid families based on phenotypes and analysis of meiotic metaphase I, and in five variants, only several hybrid families were studied due to the low frequency of the target univalent transfer in monosomic offspring. In two of the monosomic F
1 families (Mo75 × Pima 3-79 and Mo95 × Pima 3-79), four monosomic hybrids were detected; in five monosomic F
1 hybrid families (Mo58, Mo59, Mo89, Mo34, and Mo94), three monosomic hybrids were isolated; in eight monosomic F
1 hybrid families (Mo16, Mo19, Mo93, Mo7, Mo31, Mo70, Mo56, and Mo17), two monosomic hybrids were detected; and in the remaining ten hybrid families (Mo11, Mo38, Mo60, Mo69, Mo13, Mo67, Mo92, Mo27, Mo48, and Mo42), one aneuploid F
1 hybrid was obtained. These results suggested differences in the ease of monosomic detection in various hybrid backgrounds and/or differences in the maternal transmission rates for various monosomes. Deficiencies in one chromosome arm occurred in the progenies of two monotelodisomic F
1 hybrids. In one of the aneuploid F
1 hybrid families, two hybrid monotelodisomic plants were detected (
Figure 5), but in another aneuploid F
1 hybrid family, one hybrid monotelodisomic plant was detected (
Table S4).
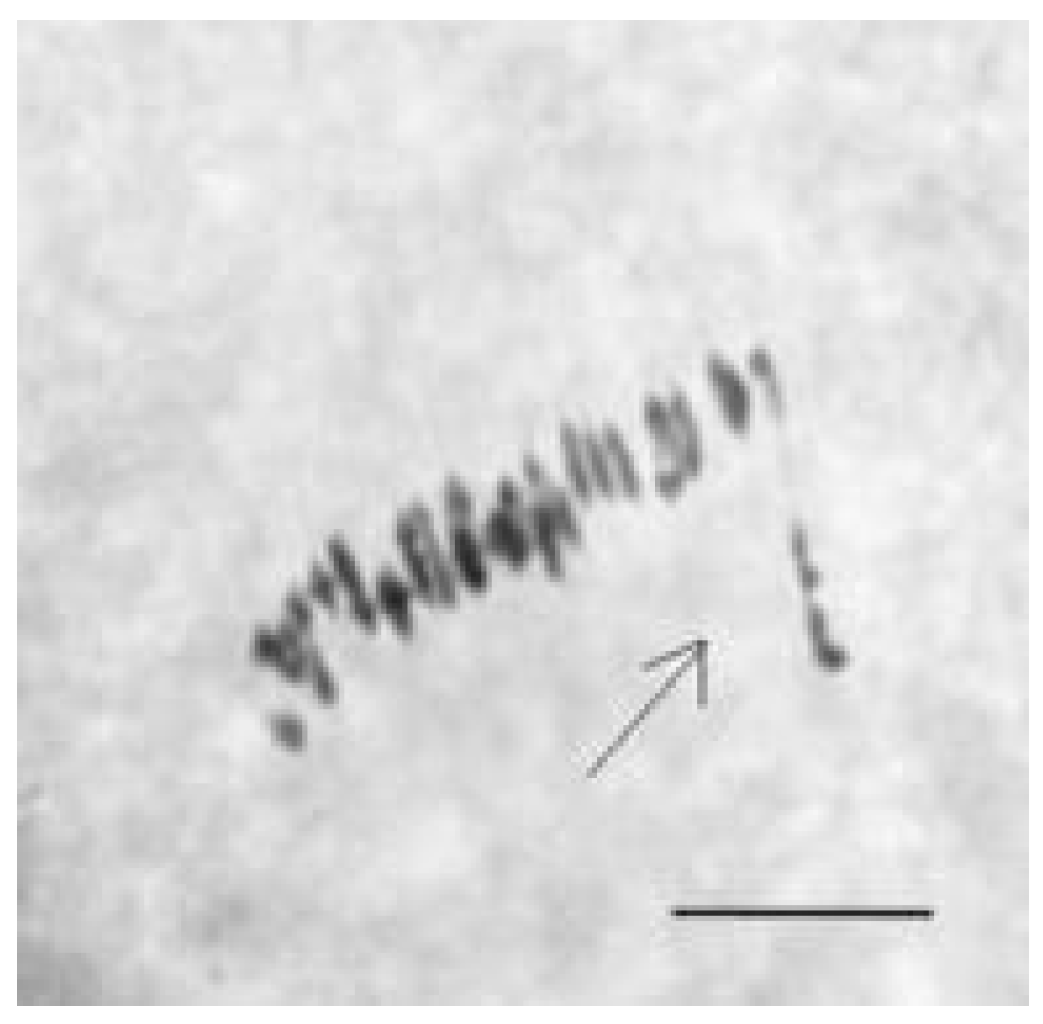
Metaphase I analysis in 49 monosomic F
1 hybrids revealed that 47 monosomic plants exhibited modal chromosome pairing of 25 bivalents and one univalent. A monosomic F
1 hybrid (2
8) from the Mo34 × Pima 3-79 family was characterized with the presence of additional univalents (1.09 ± 0.16 per cell). Another monosomic F
1 hybrid (106
5) from the Mo95 x Pima 3-79 family formed one quadrivalent in one PMC (0.07 ± 0.06 per cell) (
Table S5).
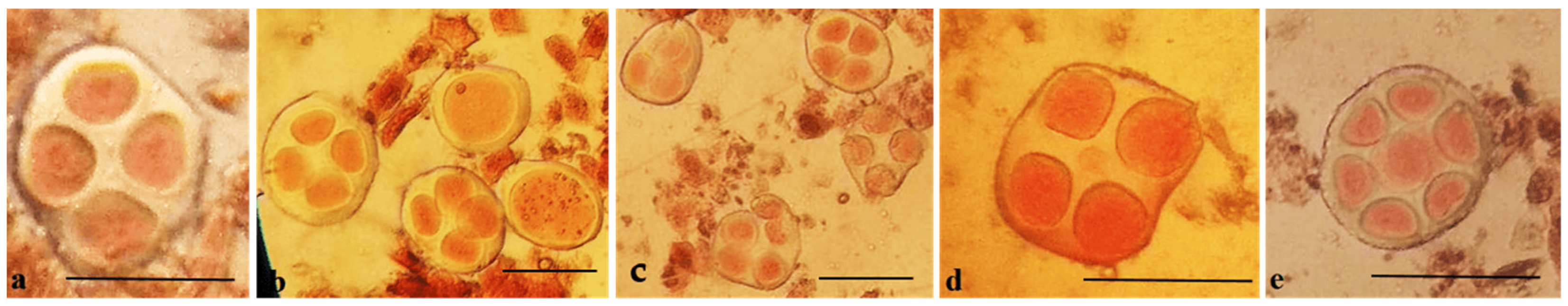
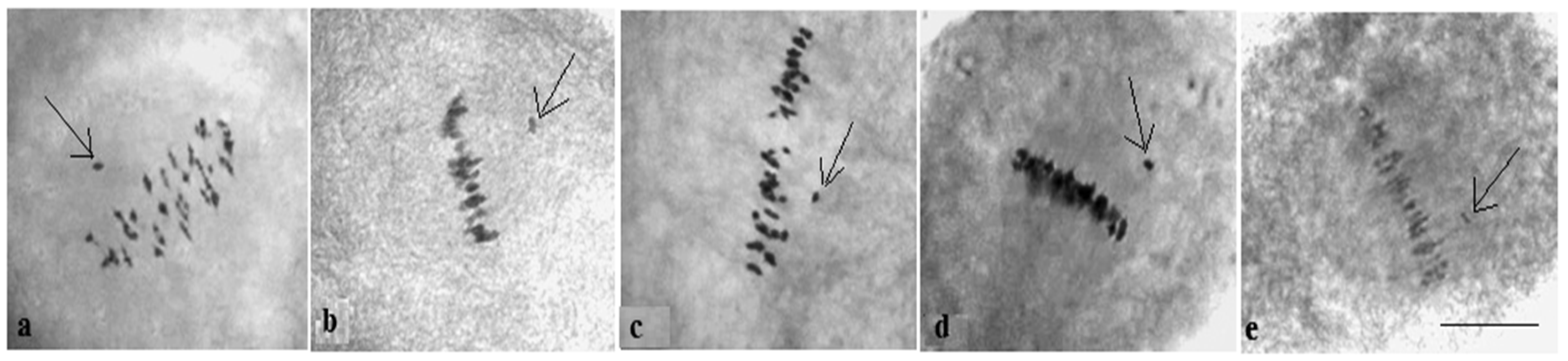
Analysis of the univalent size in monosomic interspecific F
1 hybrid revealed large-sized univalent in four families with monosomy for chromosome
2 (
Figure 6a) as well as in five families with monosomy for chromosome
6 (
Figure 6b,c) and in one family with monosomy for chromosome
12 (Mo94 × Pima 3-79) (
Figure 6d). F
1 hybrids from 10 families with monosomy for chromosome
4 (Mo7 × Pima 3-79, Mo31 × Pima 3-79, Mo38 × Pima 3-79, Mo58 × Pima 3-79, Mo59 × Pima 3-79, Mo60 × Pima 3-79, Mo69 × Pima 3-79, Mo70 × Pima 3-79, Mo75 × Pima 3-79, and Mo89 × Pima 3-79) had average-sized univalents (
Figure 7a,b), which confirmed that the univalents belong to the A
tsubgenome, and the “univalent shift” was absent. This phenomenon could lead to the production of substitution lines for target chromosomes.
The study of the size of univalents in three crosses with monosomy for chromosomes
17,
21, and
22 revealed a medium to small size of univalents (
Figure 7c); the Mo48 × Pima 3-79 family, which had a monosomy for chromosome
18, showed small-sized univalents (
Figure 7d), which confirmed that the monosomes belong to the D
tsubgenome.
At the telophase stage, the frequency of normal tetrad formation was analysed. The meiotic index, which was originally proposed by Love R.M. [
35] for the evaluation of meiosis in wheat, reports the normal tetrad percentage and is an indicator of meiotic stability. All F
1 hybrids with monosomy had a meiotic index (over 90.00%) higher than control plants, indicating that univalent chromosomes were included in micropores. However, six monosomic F
1 hybrids (Mo7 × Pima 3-79, Mo31 × Pima 3-79, Mo38 × Pima 3-79, Mo60 × Pima 3-79, Mo92 × Pima 3-79, and Mo94 × Pima 3-79) demonstrated an increase in tetrads with micronuclei from 1.15 ± 0.15% (Mo60 × Pima 3-79) to 2.03 ± 0.58% (Mo31 × Pima 3-79) in comparison with control plants (L-458 × Pima 3-79—0.36 ± 0.18%), which demonstrated disturbances in univalent disjunction and the formation of imbalanced gametes in them (
Table S6,
Figure 8 and
Figure 9).
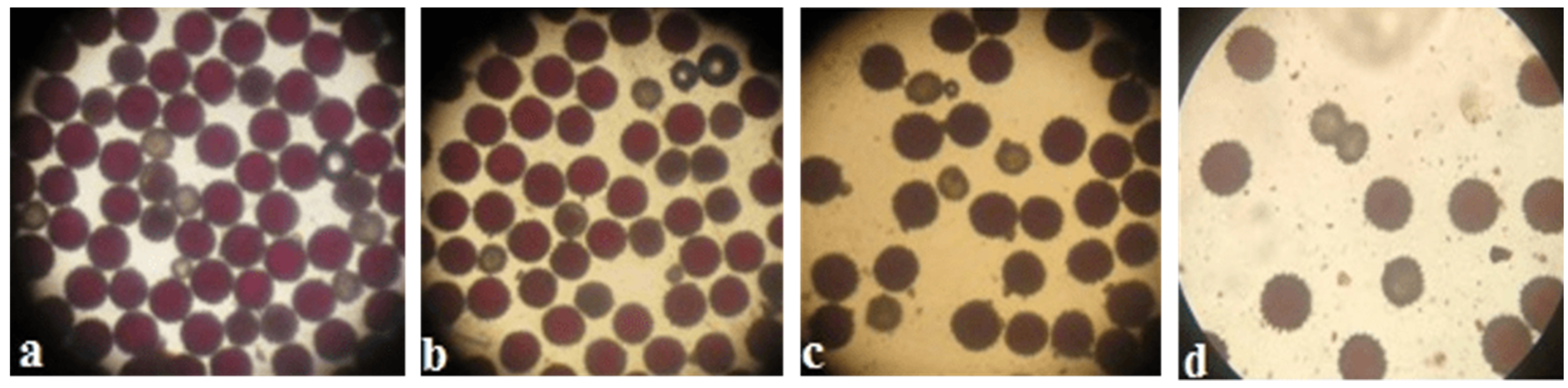
Pollen viability was high in some monosomic F
1 hybrids. However, most monosomic F
1 plants exhibited reduced pollen viability. Nine monosomic F
1 plants had great reductions in pollen viability (from 71.34 ± 1.28 to 79.89 ± 1.32%), but 30 monosomic F
1 plants had small reductions in pollen viability (from 80.79 ± 1.26 to 89.98 ± 1.21%)
. Early haplodeficient microspore abortion occurred prior to the pollen stage (
Table S7,
Figure 10).
2.4. Molecular Marker Analysis of Unknown Monosomics
To map SSR loci to chromosomes, we screened monosomic F1 hybrid plants for the L-458 allele using labelled and/or unlabelled primers. For SSR loci located at sites other than the chromatin-deficient segment, the L-458 marker was present, and F1 hybrids exhibited a heterozygous phenotype. In comparison, if an SSR locus was located on the segment missing from the F1 hybrid plant, the electropherogram would not show the L-458 allele and exhibit a hemizygous pattern for the donor allele from G. barbadense Pima 3-79.
Our results showed that one monosomic F
1 hybrid plant (Mo94 × Pima 3-79) deficient for a copy of an unknown chromosome showed the presence of only six
G. barbadense-specific SSR marker bands (BNL1227, BNL1707, BNL3261, BNL3594, BNL3835, and BNL3886) and the corresponding absence of the respective L-458 allele. The results helped determine the chromosomal identities of monosome Mo94 based on known chromosomal locations of the respective SSR markers. Because the abovementioned SSR markers were previously assigned to chromosome
12 of the A
tsubgenome [
36], the SSR-based results for Mo94 indicate that it is monosomic for chromosome
12 (
Table 1;
Figure 11).
SSR-based deficiency analysis of monosomic F
1 hybrid plants (Mo56 × Pima 3-79) deficient for an unknown chromosome showed the presence of only
G. barbadense-specific SSR marker bands for BNL1606, BNL2471, BNL2496, BNL3371, BNL3955, TMB0874, and TMB2018, with deficiencies in the respective L-458 alleles. As all of these SSR markers were previously assigned to chromosome
17 of the D
tsubgenome [
36,
37], the results indicated that Mo56 is monosomic for chromosome
17 (
Figure 12).
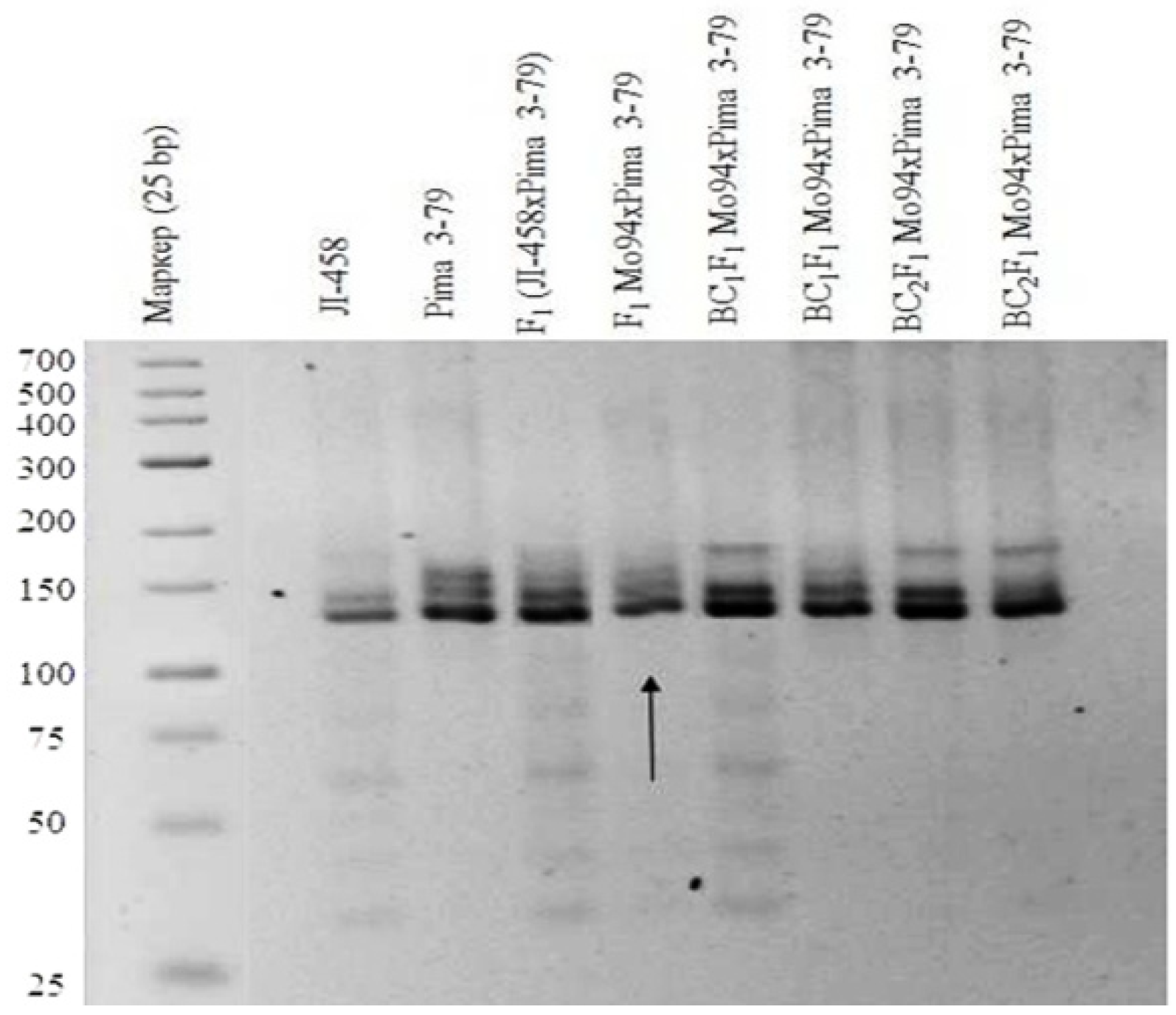
Our results also showed that a monosomic F
1 hybrid (Mo42 × Pima 3-79) deficient for an unknown chromosome only had one
G. barbadense-specific SSR marker BNL1705 and was deficient in the respective L-458 allele. Our results indicated that the univalent in Mo42 is chromosome
21 of the D
tsubgenome [
36], as this SSR marker has been assigned to this chromosome (
Table 1) (
Figure 13).
Line Mo17 was found to be monosomic for chromosome
22 of the D
tsubgenome based on the polymorphic BNL673 marker used here. Another previously identified marker, JESPR235, was also located on chromosome
20 or
22 of the D
tsubgenome [
34]. These results suggested that line Mo17 is monosomic for chromosome
22 of the D
tsubgenome [
36] (
Table 1).
Telo12 was found to be monotelodisomic based on the chromosome-specific SSR markers BNL2884 and TMB0154 that were polymorphic in the monosomic F
1 hybrids (Telo12 × Pima 3-79), and these markers were located on chromosome
6 of the A
tsubgenome [
36,
37] (
Table 1).
Thus, we identified chromosomes for four monosomic lines and one monotelodisomic line by means of F
1 aneuploid hybrids and chromosome-specific SSR markers that were previously assigned to specific cotton chromosomes based on deletion molecular analysis [
38].
2.5. Cytological Identification and Numeration of Unknown Monosomes
In our collection, monosomes were identified and numerated by analysing meiotic metaphase I configurations of monosomic translocation heterozygous F1 hybrids, which were obtained by numerous sexual crosses between monosomic lines and tester-translocation lines from a cytogenetic collection from the USA.
Cytological analysis of seven hybrid variants involving seven tester translocation set lines (TT1L-7L, TT2R-8Rb, TT3L-6L, TT3R-5R, TT4R-15L, TT9R-25, and TT10R-11R) suggested that Mo94 was not monosomic for chromosome 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 15, or 25 because the modal MI pairing configuration in the respective monosomic F1 hybrids included 23II + 1IV + 1I. In contrast, translocation line—TT11R-12L showed that Mo94 could be monosomic for chromosome 11 or 12 because in the monosomic F1 hybrid of Mo94 × TT11R-12L, the modal MI pairing configuration was 24II + 1III. Molecular-marker data indicated that Mo94 must be monosomic for chromosome 12 of the Atsubgenome.
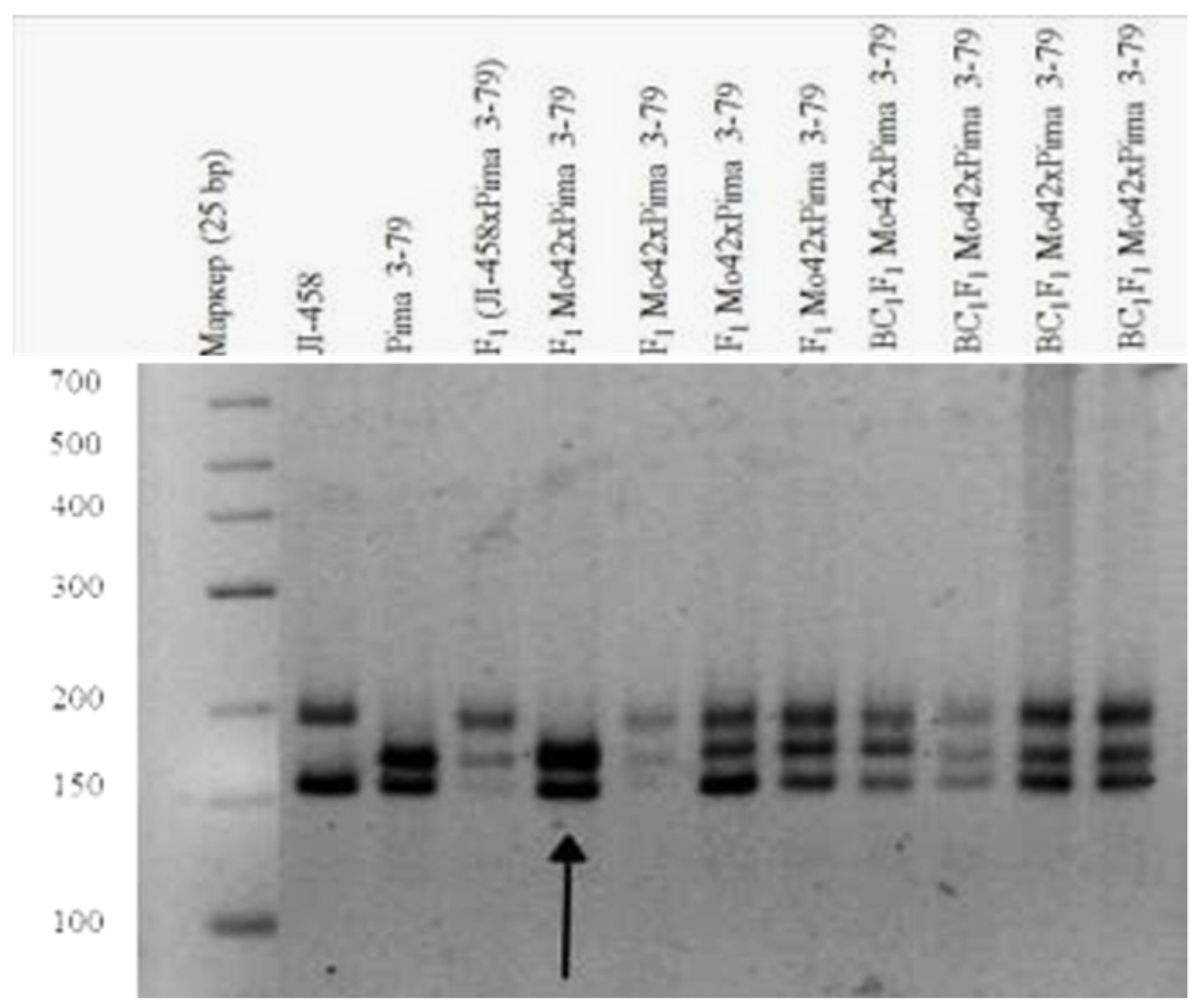
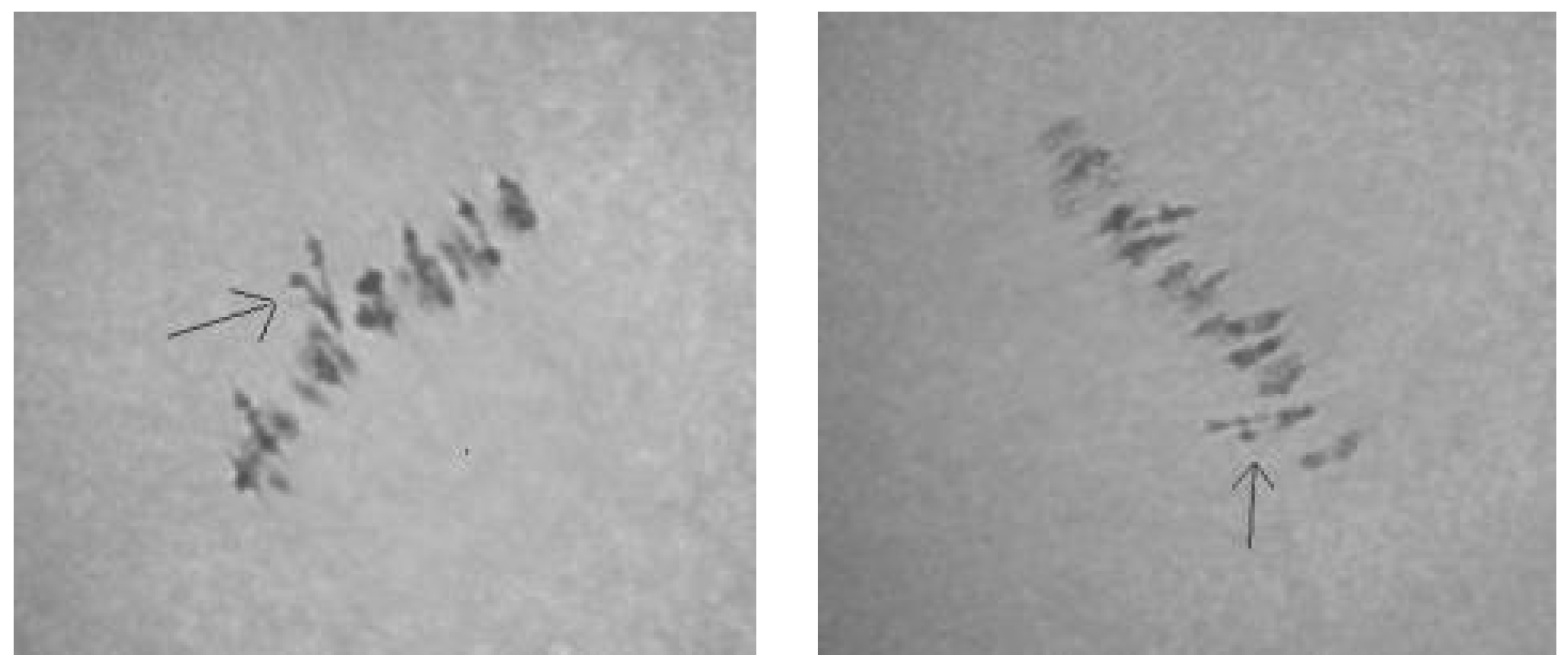
Tests involving two translocation lines (TT7R-21R and TT20R-21L) showed that Mo42 could be monosomic for chromosome 7, 20, or 21, because in the two monosomic hybrids of—Mo42 x TT7R-21R and Mo42 x TT20R-21L, the modal MI pairing configurations were 24
II + 1
III (
Figure 14). Since the monosomic line Mo42 shared a common chromosome
21 with the two translocation lines, the marker BNL1705 that was located on chromosome
21 of theD
tsubgenome could distinguish Mo42 from chromosome
21 of the D
tsubgenome.
Cytological tests of the four hybrid combinations of the four tester translocation lines (TT1L-7L, TT9R-25, TT11R-12L, and TT15R) suggested that Mo17 is not monosomic for chromosome 1, 7, 9, 11, 12, 15, 20, or 25 because the modal MI pairing configuration in the respective monosomic F1 hybrids included 23II +1IV +1I. In contrast, the test with translocation line TT20L-22R showed that Mo17 could be monosomic for chromosome 20 or 22, because in the monosomic F1 hybrid of Mo17 × TT20L-22R, the modal MI pairing configuration was 24II + 1III. Molecular-marker (BNL673) data indicated that Mo17 must be monosomic for chromosome 22 of the Dtsubgenome.
Unfortunately, it is not currently possible to obtain cytogenetic confirmation of the homology of the univalent chromosome in the monosomic line Mo56 due to the small number of buds and the difficulty of detecting meiosis in the hybrid monosomic plant.
2.6. Some Features of the Newly Identified Monosomic Lines of G. hirsutum L.
Different cotton chromosomal deficiencies had a specific influence on plant morphology and other characteristics, such as bushes, flowers, and bolls. The initial primary monosomic plant of Mo94 with a deficiency in chromosome 12 was obtained in the third generation by pollination with irradiated pollen at a dose of 20 Gy. The monosomic line Mo94 is characterized by a large univalent size, a high meiotic index (95.73 ± 0.42), a small number of tetrads with micronuclei (0.52 ± 0.05), and reduced pollen fertility (76.82 ± 1.72%), as well as a low frequency of transmission to the progeny (18.18%) and a reduced frequency of transmission of n-1 gametes. This Mo94 line was determined to have typical phenotypic characteristics (
Table 2;
Figure 15) and a low seed setting (26.67 ± 3.44) compared to the original L-458 inbred line (89.81 ± 1.55). This decrease in seed setting occurred due to the presence of a large number of unfertilized ovules in the form motes in the monosomic bolls, which, when combined with a decrease in the number of seeds per boll (8.80 ± 4.27), led to a decrease in the size of the bolls.
The initial plant of the Mo56 monosomic cotton line with a deficiency in chromosome
17 was obtained by irradiation of the seeds of the L-458 line with thermal neutrons at a dose of 35 Gy. This line was characterized by a medium-small-sized univalent, a high meiotic index (97.48 ± 0.43), a small number of tetrads with micronuclei (1.22 ± 0.30%), and reduced pollen fertility (87.71 ± 0.99%), as well as a very low frequency of transmission to the progeny (7.69%), which significantly reduced the frequency of transmission of haplo-deficient gametes. Monosomic line Mo56 was distinguished by a reduced seed setting (56.55 ± 3.36) compared to the original inbred line L-458 (89.81 ± 1.55%) (
Table 2,
Figure 16). This decrease in seed setting occurred due to the presence of a large number of unfertilized ovules in the form of motes in the monosomic bolls, which, when combined with a decrease in the number of seeds per boll (12.00 ± 0.52), led to a decrease in the size of the bolls. Monosomic lines with a deficiency in chromosome
17 are characterized by complex morphobiological characteristics.
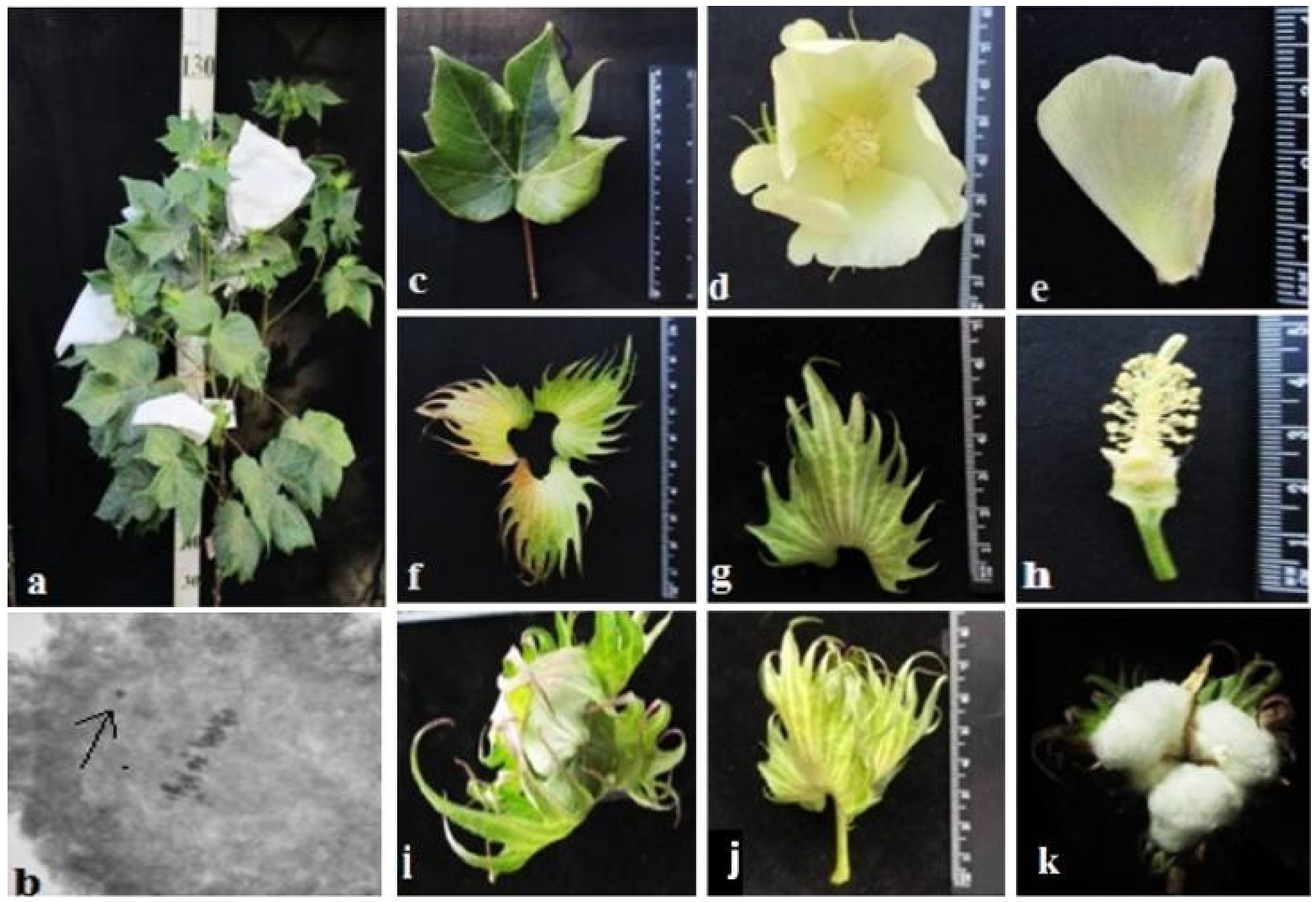
The initial plant of the Mo42 monosomic cotton line with a deficiency in chromosome
21 was obtained in the first generation by pollination with irradiated pollen from the L-458 line at a dose of 20 Gy. This line was characterized by a medium-small-sized univalent, a high meiotic index (95.87 ± 0.47), a small number of tetrads with micronuclei (0.45 ± 0.16%), and reduced pollen fertility (87.71 ± 1.53%), as well as a low frequency of transmission to the progeny (16.00%), which significantly reduced the frequency of transmission of haplo-deficient gametes. Monosomic line Mo42 was distinguished by a reduced seed setting (24.62 ± 3.72) compared to the original inbred line L-458 (89.81 ± 1.55%). This decrease in seed setting occurred due to the presence of a large number of unfertilized ovules in the form of motes in the monosomic bolls, which, combined with a decrease in the number of seeds per boll (6.60 ± 4.32), led to a decrease in the size of the bolls (
Table 2;
Figure 17).
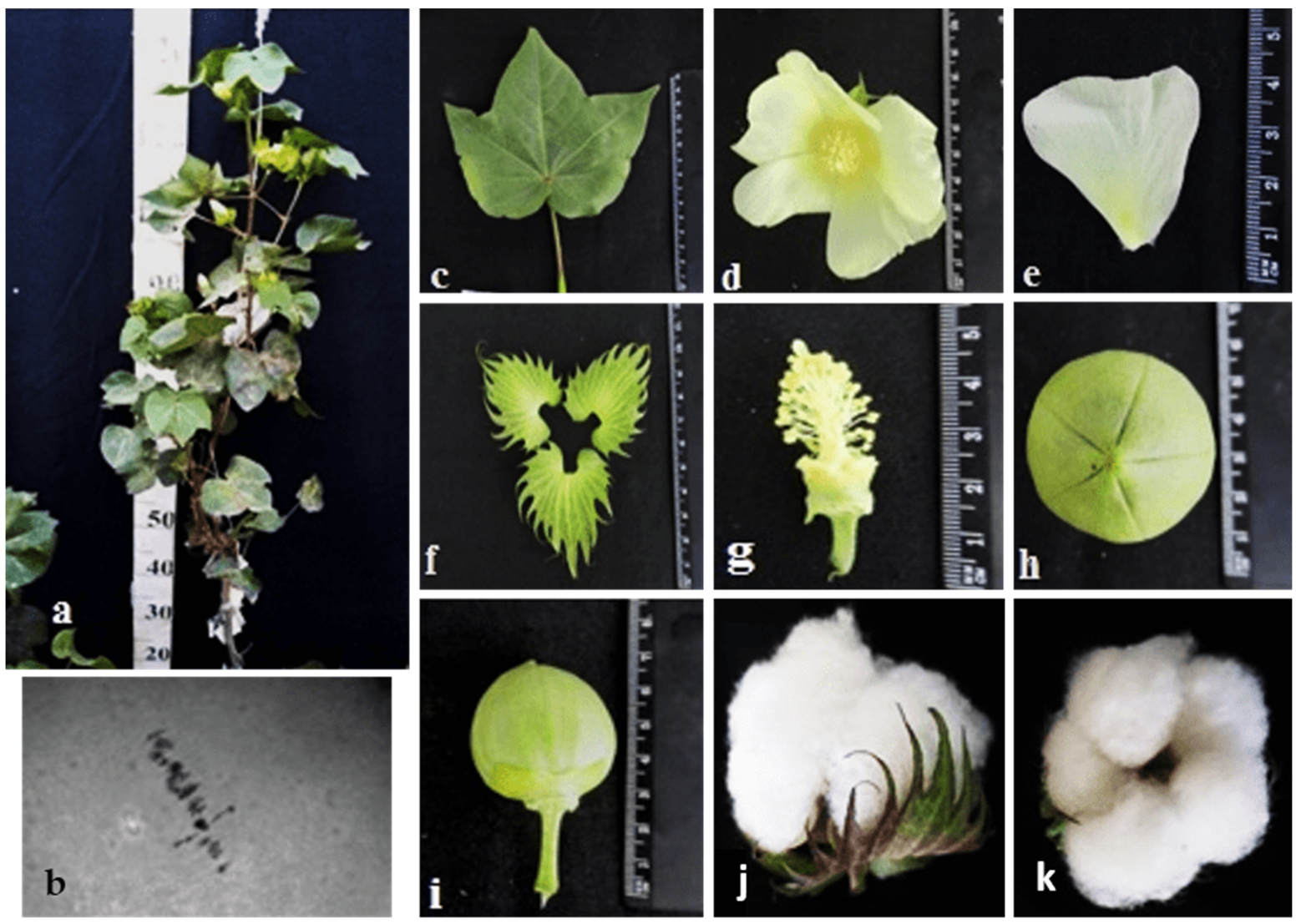
The initial plant of the Mo17 monosomic cotton line with a deficiency in chromosome
22 was induced in the first generation by pollination with irradiated pollen from the L-458 line at a dose of 25 Gy. This line was characterized by a medium-small-sized univalent, a high meiotic index (96.68 ± 0.43), a small number of tetrads with micronuclei (1.90 ± 0.22%), and high pollen fertility (95.16 ± 0.49%), as well as a low frequency of transmission to the progeny (19.35%), which significantly reduced the frequency of transmission of haplo-deficient gametes. Monosomic line Mo17 was distinguished by a reduced seed setting (58.64 ± 3.32) compared to the original inbred line L-458 (89.81 ± 1.55%). This decrease in seed setting occurred due to the presence of a large number of unfertilized ovules in the form of motes in the monosomic bolls, which, combinedwith the decreased number of seeds per boll (12.90 ± 0.77), led to a decrease in the size of the bolls (
Table 2;
Figure 18).
The initial plant of the monotelodisome line, Telo12, which is deficiency an arm of chromosome
6, was obtained in the second generation by pollination with irradiated pollen from the L-458 line at a dose of 20 Gy. This line was characterized by a high meiotic index (91.08 ± 1.38) and an increase in the percentage of tetrads with micronuclei (3.08 ± 0.89%), which disturbed monotelosome disjunction and resulted in imbalanced gametes. Telo12 had high pollen fertility (91.12 ± 1.80%) and a low frequency of transmission to the progeny (19.35%), which significantly reduced the frequency of transmission of haplo-deficient gametes. The monotelodisome line Telo12 was distinguished by a high seed setting rate (87.41 ± 2.69) compared to the original inbred line L-458 (89.81 ± 1.55%) and a decrease in the number of seeds per boll (26.00 ± 3.55) (
Table 2;
Figure 19).
2.7. Peculiarities of the Crossing, Setting, and Germination of BC1F1 Hybrid Seeds
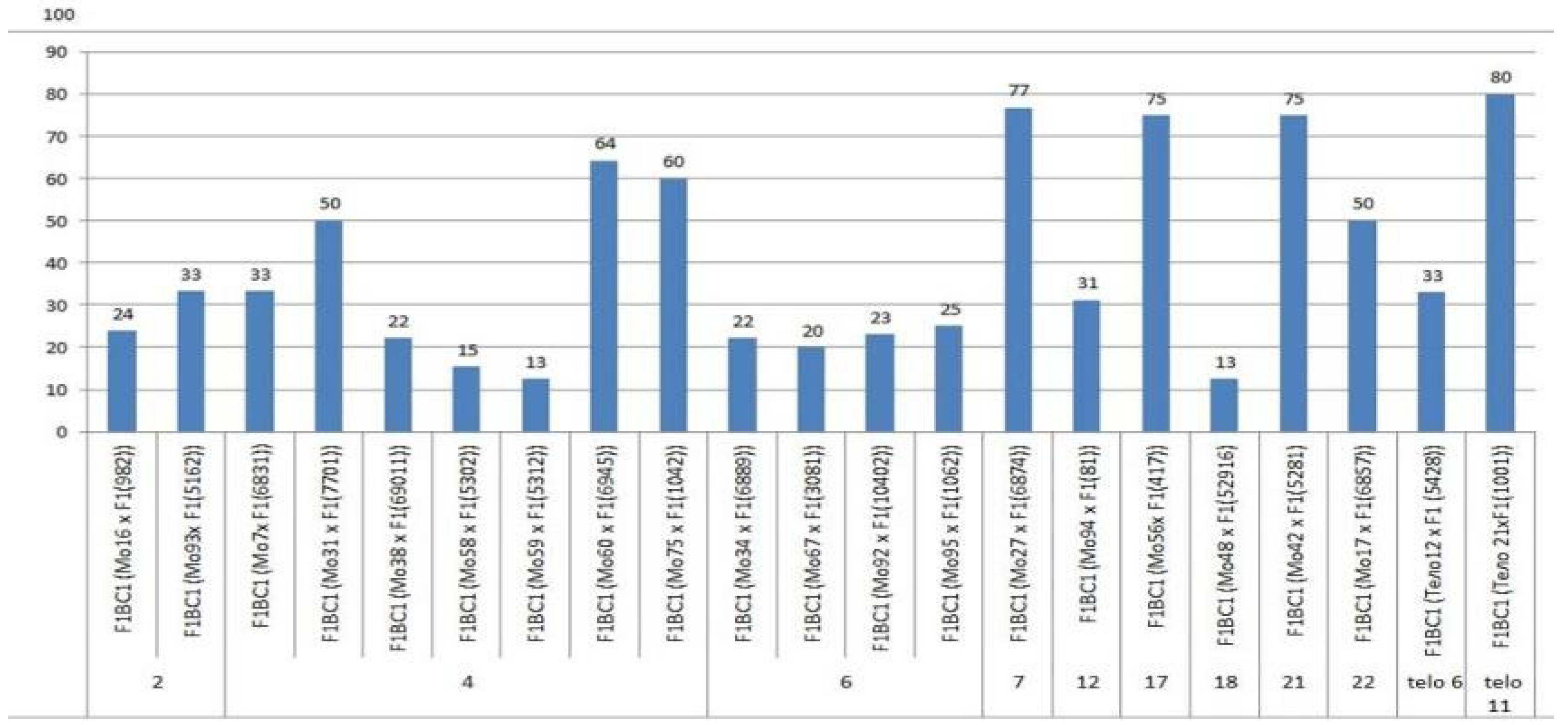
The aneuploid lines of our collection were crossed with interspecific aneuploid F
1 hybrids (Mo × Pima 3-79 or Telo× Pima 3-79). Most of the backcross hybrids were obtained from crosses of the original aneuploid lines with deficiencies in chromosomes
2,
4,
6,
7,
12,
17,
18,
21,
22, telo
6, and telo
11 with F
1 hybrids (Mo × Pima 3-79) characterized as having a strong decrease in the crossing rate (up to 12.50%). Only three monosomic lines (Mo56, Mo42, and Mo17) with deficiencies in chromosomes
17,
21, and
22, respectively, showed a strong increase in the crossing rate (up to 75, 75, and 50%, respectively) compared to the original F
1 hybrids (Mo × Pima 3-79) (
Figure 20). Five monosomic lines (Mo31, Mo60, Mo75, Mo27, and Mo21) showed a small decrease in the crossing rate (up to 50, 64.29, 60, 76.92, and 80%) compared to the F
1 hybrids (
Table S8). Unfortunately, in the monosomic F
1 hybrid (Mo13 × Pima 3-79) with a deficiency in chromosome
6, for many crosses (25 crosses), not a single boll was set with the monosomic line; therefore, it is not possible to study these BC
1F
1 hybrids at present.
The setting of BC
1F
1 hybrid seeds obtained from crosses of aneuploid lines with the F
1 hybrids (Mo × Pima 3-79 or Telo × Pima 3-79) was decreased in most lines. The exceptions included four monosomic lines (Mo16, Mo31, Mo67, and Mo27) with deficiencies in chromosomes
2,
4,
6, and
7, which, when backcrossed to the F
1 hybrids, showed a small increase in the setting of hybrid seeds (
Table S9,
Figure 21).
The germination capacity of the backcrossed hybrid seeds obtained from crosses of the original aneuploid lines with F
1 hybrids (Mo × Pima 3-79 or Telo × Pima 3-79) showed a strong decrease (up to 37.50%) in only one variant F
1BC
1 (Mo92 × F
1688
9) with the substitution of chromosome
6. In the remaining 13 variants, there was a slight decrease in the germination capacity of backcrossed seeds, and in seven variants, there was an increase in the germination capacity compared to the F
1 hybrids. In general, the backcrossed F
1BC
1 seeds were characterized as having a high germination capacity (
Table S10,
Figure 22).
2.8. Cytogenetic Characteristics of Aneuploid BC1F1 Hybrid Plants with Identified Monosomes
Among the resulting progeny, aneuploid BC
1F
1 hybrid plants were determined for 16 BC
1F
1 hybrid families based on hybrid phenotypes and meiotic metaphase I configuration analyses. Three monosomic plants were isolated from one aneuploid backcross BC
1F
1 family (Mo48); two hybrid monosomic plants were detected in each of six aneuploid backcross families (Mo60, Mo75, Mo34, Mo27, Mo94, and Mo17); and one hybrid monosomic plant was isolated from each of the remaining seven backcross families (Mo16, Mo38, Mo58, Mo59, Mo92, Mo56, and Mo42). Unfortunately, no monosomic plants were isolated from five aneuploid backcross families (Mo93, Mo7, Mo31, Mo67, and Mo95). These results suggested that there were differences in the transmission rate of monosomes in the various hybrid backgrounds and/or differences in the maternal transmission rates in different hybrid families. Deficiencies in one chromosome arm occurred in the progenies of two monotelodisomic BC
1F
1 hybrid plants. In one of the aneuploid BC
1F
1 families, three hybrid monotelodisomic plants were detected, but in another aneuploid backcross family, one hybrid monotelodisomic plant was isolated (
Table S11).
Meiotic metaphase I analysis of 14 monosomic BC
1F
1 plants revealed that 22 monosomes exhibited modal chromosome pairing with 25 bivalents and one univalent. One monosomic plant (110
3) from BC
1F
1 (Mo17 × F
1685
7) formed a quadrivalent (0.38 ± 0.17 per cell) (
Table S12).
Analysis of the size of the monosomes in monosomic backcross BC
1F
1 plants revealed large-sized univalents in one family with the substitution of chromosome
2 (BC
1F
1 (Mo16 × F
198
6)) and in two families with the substitution of chromosome
6 (BC
1F
1 (Mo34 × F
1688
9) and BC
1F
1 (Mo92 × F
1539
5)) (
Figure 23a), as well as in one family with the substitution of chromosome
12 (BC
1F
1 (Mo94 × F
18
1)) (
Figure 23b). Monosomic BC
1F
1 hybrids of five families with the substitution of chromosome
4 (BC
1F
1 (Mo38 × F
1690
11), BC
1F
1 (Mo58 × F
1530
3), BC
1F
1 (Mo59 × F
1531
8), BC
1F
1 (Mo60 × F
1694
5), and BC
1F
1 (Mo75 × F
1104
2)) (
Figure 23c–e), as well as with the substitution of chromosome
7 (BC
1F
1 (Mo27 × F
1687
4)) (
Figure 24a), had medium-sized univalent that confirmed that the monosomes were from the A
tsubgenome and the absence of “univalents shift.”
The study of the size of univalents in backcross plants of three variants (BC
1F
1(Mo56 × F
14
17), BC
1F
1(Mo42 × F
1528
1), and BC
1F
1(Mo17 × F
1685
7)) with the substitution of chromosomes
17,
21, and
22, respectively, revealed medium-small-sized univalents (
Figure 24c), and in backcross plants of one variant (BC
1F
1 (Mo48 × F
1529
16)) with substitution of chromosome
18, the univalent had a small size (
Figure 24b), which confirmed that the monosomes were from the D
tsubgenome.
All monosomic BC
1F
1 plants showed a higher meiotic index (more than 90%) than the control plants, which indicated that their univalent chromosomes underwent regular disjunction. However, five monosomic BC
1F
1 plants demonstrated an increase in the percentage of tetrads with micronuclei from 1.17 ± 0.26% (F
1BC
1 (Mo60 × F
1694
5) to 2.60 ± 0.81% (F
1BC
1 (Mo38 × F
1690
11)) in comparison with the control plants (L-458 × Pima 3-79—0.04 ± 0.04%), which demonstrated disturbances in monosome disjunction and the formation of imbalanced gametes in these BC
1F
1 hybrids. Moreover, two monotelodisomic BC
1F
1 plants from one family (F
1BC
1 (Mo21 × F
1100
1)) showed an increase in the percentage of tetrads with micronuclei from 2.46 ± 0.33% (292
1) to 2.55 ± 0.67% (291
1) in comparison with control plants (L-458 × Pima 3-79—0.04 ± 0.04%), which suggested disturbances in monotelodisome disjunction and the formation of imbalanced gametes in these BC
1F
1 hybrids (
Table S13,
Figure 25).
Pollen viability after 2% acetocarmine staining was detected in aneuploid BC
1F
1 families. Most monosomic hybrid BC
1F
1 plants exhibited reduced pollen viability. Specifically, three monosomic hybrid BC
1F
1 plants had greater reductions in pollen viability (from 67.27 ± 1.45 to 78.60 ± 1.53%), but nine monosomic hybrid F
1BC
1 plants showed small reductions in pollen viability (from 80.30 ± 1.71 to 89.79 ± 0.94%) (
Table S14,
Figure 26).
2.9. Identification of Univalent Chromosomes in Aneuploid F1BC1 Hybrids Obtained from Crosses between Aneuploid G. hirsutum L. Lines and Interspecific Aneuploid F1 Hybrids (Mo × Pima 3-79) Using Molecular Genetic Markers
Univalent chromosomes in aneuploid BC1F1 hybrids obtained from crosses of monosomic and monotelodisomic lines of G. hirsutum L. with interspecific aneuploid F1 hybrids (Mo × Pima 3-79) were identified using molecular genetic markers that were previously assigned to chromosomes.
To facilitate the detection of monosomic cytotypes and accelerate the production of backcross bolls in four BC
1F
1 hybrid families, molecular genetic analysis of hybrid plants at the plantlet stage was carried out before transplanting seedlings into greenhouse soil and carrying out cytogenetic analysis to identify monosomic forms (
Table 3).
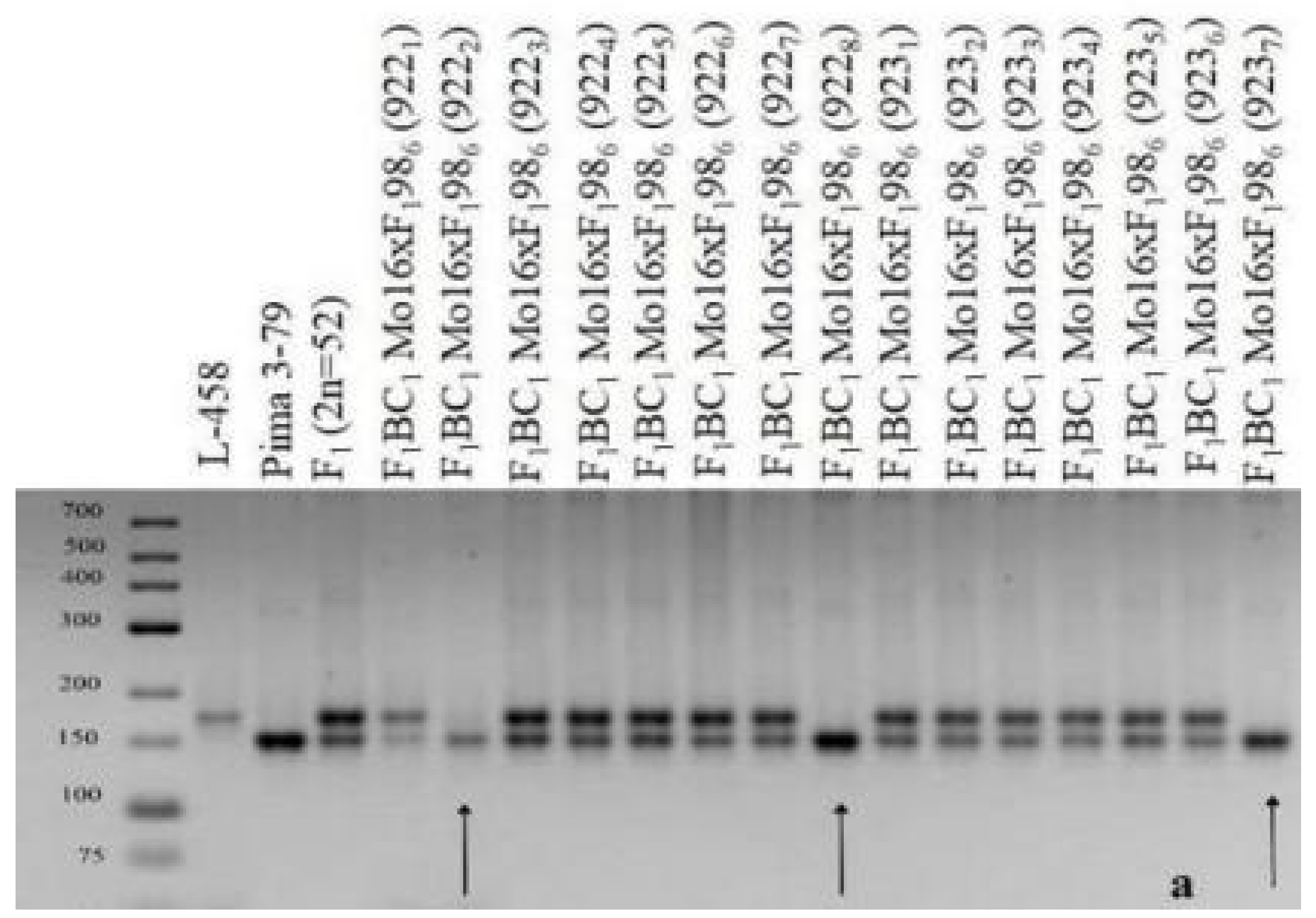
The results of this study showed that three backcross plantlets (922
2, 922
8, and 923
7) were found in two hybrid backcross families (922 and 923) with the substitution of chromosome
2 (BC
1F
1(Mo16 × F
198
6)); these plantlets were characterized by the presence of polymorphic alleles only from
G. barbadense, while alleles of the
G. hirsutum line L-458 were absent based on the localization of three chromosome-specific SSR markers: BNL834, TMB0471, and JESPR179 (
Table 3). Since the previously identified markers were located on chromosome
2 of the A
tsubgenome [
36,
39], it can be assumed that the above hybrid plants have substitutions of this chromosome (
Table 3;
Figure 27). Additional cytogenetic analysis revealed a monosomic state in another backcross hybrid (923
8), which has not been studied using molecular genetics due to poor development and few leaves present at the plantlet stage.
Four backcross plantlets were found (924
1, 925
4, 925
7, and 925
11) in two hybrid backcross families (924 and 925) with the substitution of chromosome
4 (BC
1F
1 (Mo31 × F
1770
1) and BC
1F
1 (Mo38 × F
1690
11)); these lines were characterized by the presence of polymorphic alleles only from
G. barbadense, while alleles of the
G. hirsutum line L-458 were absent based on the location of four chromosome-specific SSR markers: BNL2572, TMB0809, Gh117, and Gh107. Since the previously identified markers were located on chromosome
4 of the A
tsubgenome, we can assume that the above hybrid plantlets have substitutions of this chromosome (
Table 3). Further cytogenetic analysis confirmed the monosomic state of only two backcross hybrids (925
4 and 925
7). In this regard, it is of great interest to use previously identified chromosome-specific SSR markers from cotton to test backcrossed plantlets for the early detection of monosomic cytotypes and their early involvement in subsequent backcrossing.
Confirmation of the substitution of other chromosomes was carried out in eight cytogenetically studied monosomic BC
1F
1 hybrids by common methods. The analysis of one monosomic hybrid (117
5) from the variant BC
1F
1 (Mo60 × F
1694
5) with the substitution of chromosome
4 using molecular genetic markers showed the presence of polymorphic alleles only from
G. barbadense, while the alleles of the
G. hirsutum line L-458 were absent based on the chromosome-specific SSR markers Gh107, TMB0809, Gh117, CIR249, and JESPR234. Since the previously identified markers were located on chromosome
4 of the A
tsubgenome, it can be assumed that the substitution of this chromosome was confirmed in the studied monosomic hybrid (
Table 4 and
Table 5).
Additional molecular genetic analysis of the other two monosomic hybrids (298
2 and 298
3) in the BC
1F
1 family (Mo75 × F
1104
2) with the substitution of chromosome
4 revealed the presence of polymorphic alleles only from
G. barbadense, while the alleles from the
G. hirsutum line L-458 were absent based on the chromosome-specific SSR markers BNL2572, Gh107, TMB0809, Gh117, CIR249, and JESPR234. Since the previously listed markers were located on chromosome
4 of the A
tsubgenome [
36,
38], it can be assumed that the substitution of this chromosome 4 is confirmed in the above hybrid plants (
Table 4 and
Table 5).
A monosomic hybrid (293
3) from the BC
1F
1 family (Mo34 × F
1688
9) was studied using molecular genetic markers; the results showed only polymorphic alleles from
G. barbadense, while alleles from the
G. hirsutum line L-458 were not found based on the chromosome-specific SSR markers BNL1440, BNL3650, BNL2884, BNL1064, BNL3359, TMB1277, TMB0154, TMB0853, TMB1538, Gh039, and Gh082. Since the previously identified markers were located on chromosome
6 of the A
tsubgenome, it can be assumed that the substitution of this chromosome is confirmed in the above hybrid plants (
Table 4 and
Table 5,
Figure 28).
The molecular genetic analyses of another monosomic hybrid (1040
2) in the BC
1F
1 family (Mo92 × F
1539
5) with the substitution of chromosome
6 showed the presence of polymorphic alleles only from
G. barbadense, while the alleles from the
G. hirsutum line L-458 were absent based on the chromosome-specific SSR markers BNL1440, BNL3650, BNL2884, BNL3359, TMB1277, TMB0154, TMB0853, TMB1538, Gh039, and Gh082. Since the previously identified markers were located on chromosome
6 of the A
tsubgenome, it can be assumed that the substitution of this chromosome wasconfirmed in the studied monosomic hybrid (
Table 4 and
Table 5;
Figure 28).
The molecular genetic analysis of two monosomic hybrids (299
1 and 299
2) from the BC
1F
1 family (Mo94 × F
18
1) revealed only polymorphic alleles from
G. barbadense, while alleles from the
G. hirsutum line L-458 were not detected based on the chromosome-specific SSR markers BNL1227 and BNL3835 in hybrid 299
1 and BNL3594 in hybrid 299
2. Since the previously identified markers were located on chromosome
12 of the A
tsubgenome [
36], it can be assumed that the substitution of this chromosome was confirmed in the studied monosomic hybrids (
Table 4 and
Table 5).
The molecular genetic analysis of the monosomic hybrid (110
3) from the BC
1F
1 family (Mo17 × F
1685
7) also showed the presence of only polymorphic alleles from
G. barbadense, while the alleles from line L-458 were not detected based on the chromosome-specific SSR marker BNL673. Since this marker was previously localized to chromosome
22 of the D
tsubgenome [
36], it can be assumed that the substitution of this chromosome was confirmed in the studied monosomic hybrid (
Table 4 and
Table 5).
2.10. Analysis of the Unique Morphological Characteristics of F1 and BC1F1 Aneuploid Hybrids in Comparison with the Original Aneuploid Lines
Analysis of the unique morphological characteristics of aneuploid lines and F1 and BC1F1 hybrids obtained from crosses of aneuploid lines acting as recurrent parents with Pima 3-79 and with interspecific aneuploid F1(Mo × Pima 3-79) hybrids was carried out, and the results were compared with those for the original monosomic lines.
Monosomic lines with a deficiency in chromosome
2 were distinguished by small narrow leaves, shortened sympodial branches, and small round bolls, while F
1 hybrids showed an increase in leaves, flowers, and bolls. However, the monosomic BC
1F
1 hybrid showed a decrease in the plant growth and development rates, as well as a decrease in the size of the leaves, flowers, and bolls and the number of the bolls (
Figure 29).
Monosomic lines with a deficiency in chromosome
4 were characterized by dense and vibrant plants, long leaf lobes, elongated bracts and pedicels, and ribbed bolls; all these features were preserved in monosomic F
1 hybrids but with larger-sized peduncles and bolls, as well as an increase in the pubescence of the main stem. In monosomic BC
1F
1 hybrids with the substitution of chromosome
4, dense foliage, densely pubescent stems with short hairs and medium-pubescent leaves, as well as medium-sized leaves and long pedicels (
Figure 30), were found with the exception of the monosomic BC
1F
1 hybrid (Mo60 × F
1694
5), in which the peduncle length was short.
Monosomic lines with a deficiency in chromosome
6 were characterized by shortened sympodia, stiff stems, small leaves, small round bolls, and late flowering, while monosomic F
1 hybrids had larger dark green leaves, longer sympodia, and larger bolls. In monosomic BC
1F
1 hybrid plants, a decrease in the size of leaves, flowers, and bolls, as well as shortening of the internodes, was observed (
Figure 31).
Another monosomic line, Mo27, with a deficiency in chromosome
7 had characteristic features such as short sympodia, thick bracts and leaves, and small bolls. Monosomic F
1 hybrids were characterized as having large leaves, flowers, and bolls, while monosomic BC
1F
1 hybrids were distinguished by their pale green leaves and short sympodia (
Figure 32).
Monosomic line Mo94 with a deficiency in chromosome
12 showed a spreading form and a slightly leafy bush, shortened internodes, large leaves with slightly curved edges, and small bolls. Monosomic F
1 hybrids were characterized as having the same traits, along with weak foliage, large bolls, and a decrease in the number of bolls, while monosomic BC
1F
1 hybrid plants were more densely leafed, had elongated sympodial branches, and had large flowers and bolls (
Figure 33).
The monosomic line Mo56 with a deficiency in chromosome
17 had characteristic features such as a compact bush, dense foliage, small leaves, short stalks, small bolls, abundant budding, and a weak boll set. These traits were retained in monosomic F
1 hybrids, along with larger bolls and a decrease in the number of bolls. In addition, the monosomic BC
1F
1 hybrid had dense foliage and an increase in the number of bolls (
Figure 34).
The monosomic line Mo48 with a deficiency in chromosome
18 had small leaves, a long column and stigma, and short sympodia. These traits were retained in monosomic F
1 hybrids; in addition, medium-sized leaves and bolls were formed. Monosomic BC
1F
1 hybrids showed weak foliage, three-lobed leaves, and pubescent petioles and bracts (
Figure 35).
Monosomic line Mo42 with a deficiency in chromosome
21 had characteristic features such as a compact bush, weak foliage, small three-lobed leaves, shortened sympodial branches, and small ovoid bolls. These traits were preserved in the F
1 monosomic hybrid; in addition, large leaves and bolls were observed. In monosomic BC
1F
1 hybrids, the leaf shape was three-lobed, and the leaves and bolls were smaller than those of the monosomic F
1 hybrids (
Figure 36).
Another monosomic line, Mo17, with a deficiency in chromosome
22 was characterized as having vibrant plants, elongated leaf lobes with a slight curving of the edges upward, long bracts and pedicels, elongated ribbed bolls, and weak budding. These traits were retained in the F
1 monosomic hybrids, along with weak foliage. Monosomic BC
1F
1 hybrids differed in their dark green leaves and ovoid bolls of a medium size (
Figure 37).
The monotelodisome line Telo12 with a deficiency in an arm of chromosome
6 had characteristic features such as a compact bush, dense foliage, small leaves, and small, spherical bolls with elongated noses. These traits were preserved in the F
1 monotelodisome hybrids along with large leaves and long cuttings in leaves, while in BC
1F
1 hybrids, the bolls were larger and more ovoid (
Figure 38).
Another monotelodisome line, Mo21, with a deficiency in an arm of chromosome
11 was characterized by a spreading bush, dense foliage, dark green leaves of a medium size that were whole-edged and three-lobed, small bolls, and a very low seed setting. The monotelodisome F
1 hybrids had dark green, two- to three-lobed leaves, and spherical leaves with shallow-nosed bolls, while the BC
1F
1 monotelodisome hybrids presented leaves of a large size with long petioles and egg-shaped bolls (
Figure 39).
Since there are no detailed morphobiological descriptions of F1 and BC1F1 hybrids with substitutions on chromosomes 2, 4, 6, 7, and 12 and the arms of chromosomes 6 and 11 of the Atsubgenome and chromosomes 17, 18, 21, and 22 of the Dtsubgenome in G. barbadense, direct comparison of our data with that from the literature is not possible.
Thus, some aneuploid BC1F1 hybrids obtained from crosses of monosomic and monotelodisome lines with interspecific aneuploid F1 hybrids with substitutions of chromosomes 2, 4, 6, 7, and 12 and the arms of chromosomes 6 and 11 of the Atsubgenome and chromosomes 17, 18, 21, and 22 of the Dtsubgenome in G. barbadense showed some distinctive features. Such features included dense foliage, densely pubescent stems with short hairs, and medium-pubescent leaves in the monosomic BC1F1 hybrids (Mo58 × F15303 and Mo75 × F11042) (1151 and 2982, respectively), as well as medium-sized leaves and long pedicels (from 3 to 5 cm), with the exception of the monosomic BC1F1 hybrid (Mo60 × F16945), in which the peduncle length was short due the substitution of chromosome 4. Monosomic BC1F1 hybrids with the substitution of chromosome 6 were distinguished by a compact bush, very dense foliage, shortened sympodial branches, thicker and darker green leaves, short peduncles, and spherical bolls, while monotelodisome hybrids with the substitution of an arm of chromosome 6 showed larger leaves with an increase in the number of leaves with long petioles (up to 15 cm), as well as egg-shaped bolls. Monosomic BC1F1 hybrids with the substitution of chromosome 7 were distinguished by pale green-coloured leaves, a three-lobed and finger-lobed leaf shape, and a smaller number of teeth on the bracts (up to eight), while monosomic BC1F1 hybrids with the substitution of chromosome 18 were distinguished by a three-lobed leaf shape, shortening of the length of the petiole and dense pubescence, shortened stigma protrusion, and spherical bolls. Monotelodisome BC1F1 hybrids with the substitution of an arm of chromosome arm 11 were distinguished by thick, two- to three-lobed medium-sized leaves, a decrease in the number of teeth on the bracts, and spherical-shaped bolls. All aneuploid BC1F1 hybrids with substitutions of specific chromosomes or their arms were found to lack an anthocyanin spot on the petals. Therefore, it can be assumed that the persistence of changes in some traits in aneuploid BC1F1 hybrids in future backcross generations will indicate the connection of these changes with substituted chromosomes or specific chromosome arms.
3. Discussion
The creation of introgression lines can contribute to the expansion of genetic diversity, the decrease in genetic vulnerability, the increase in resistance to various pathogens, and the improvement in breeding indicators. In addition, obtaining stable lines containing the substitution of specific chromosomes allows the introgression of genetic material associated with valuable traits [
41]. The method used to develop chromosome-substitution lines in cotton has been discussed in numerous studies [
22]. The strategy is based on differences in the transmission rate between mega- and microgametophytes, that is, between the egg (seed or female parent) and the pollen (male parent) in cotton [
42]. The transmission of hypoaneuploidy in cotton through eggs is successful (up to 50%) for most chromosomes or chromosome arms, while transmission through pollen is rare or non-existent across all chromosomes and most large segmental deletions (for example, telosomes) [
43].
Later, S. Saha et al. [
23] described a method for obtaining chromosome-substitution lines in cotton that involved crossing the
G. barbadense line Pima 3-79, as the male parent, and a monosomic or monotelodisome line of
G. hirsutum as the female parent. Furthermore, interspecific F
1 progeny were screened phenotypically, cytogenetically, and in some cases using molecular marker analysis to identify plants deficient in a chromosome or chromosome arm. The identified interspecific aneuploid (BC
0F
1) plant was subsequently used as the male parent when backcrossed with a recurrent aneuploid plant. This procedure was repeated until the fifth backcross. Then, one euploid BC
5F
1 plant was self-pollinated to identify each substituted BC
5F
1S
1 line in which the (CS-B) chromosomes (or segments of the chromosome arms) of
G. hirsutum were substituted with the corresponding pair from
G. barbadense.
In this study, a scheme for the creation of chromosome-substituted cotton lines was developed, according to which all stages of hybridization and backcrossing were accompanied by double screening of specific chromosome substitutions using cytogenetic and molecular markers (SSRs) at early stages of development (plantlets). Here, the absence of amplification of an SSR locus that is associated with a specific chromosome in G. hirsutum, but the presence of markers from G. barbadense, was considered as a substitution of the homeologous chromosome of the first species with that of the second species. This scheme makes it possible to significantly accelerate the process of creating lines with substitutions of specific chromosomes and to control a possible “univalent shift.” The retarded development of monosomic plants and late budding and flowering in comparison with disomic siblings can delay backcrossing. After the early detection of monosomic cytotypes using the above markers at the plantlet stage, it becomes possible to quickly backcross hypoaneuploid hybrids at an earlier stage of development, even before their monosomic status is confirmed by cytogenetic analysis.
It is known that some previously obtained chromosome substitution lines from the USA collection did not receive further molecular-genetic confirmation (CS-B05sh, CS-B06, CS-B07, and CS-B15sh), and one of the DNA reserves that was previously considered to have arisen from a
G. barbadense plant hemizygous for Chr-17 was actually hemizygous for Chr-11 [
36,
40,
44]. It can be assumed that a “univalent shift” occurred during backcrossing, which led to the production of such lines.
In our work, a strong decrease in cross-breeding and the setting of hybrid seeds was revealed during the hybridization of aneuploid lines of
G. hirsutum L. with Pima 3-79 of
G. barbadense L., since many monosomic lines in our collection were initially characterized by a reduced seed set, as well as a small number of seeds per boll. It is known that a reduction in the size, number, or curvature of the boll due to a limitation in seed productivity and the presence of a large number of motes served as a marker for cytological aberrations in cotton [
45].
The study of the germination of hybrid seeds obtained from crosses between aneuploid lines of cotton and the donor G. barbadense L. line Pima 3-79 revealed a significant decrease in germination capacity in only one variant (Mo19) with a deficiency in chromosome 2 and four lines (Mo31, Mo66, Mo69, and Mo89) with a deficiency in chromosome 4. In the remaining variants, there was a slight decrease compared with the control. The germination of interspecific hybrid seeds is one of the most important indicators when working with aneuploid plants, since such plants have reduced growth and development rates, and the seeds of hypoaneuploid plants germinate much later than those of disomic sibs. Thus, nonsimultaneous germination of seeds from disomic cytotypes and cytotypes with haplo-deficiency was observed.
A comparative analysis of chromosome pairing in monosomes of F
1 hybrids obtained from crosses of 24 monosomic and two monotelodisome lines with the donor line Pima 3-79 revealed normal pairing of chromosomes with the formation of 25 bivalents and one univalent of different sizes in all studied PMCs. This result indicated the absence of the formation of additional univalents and served as a guarantee of the absence of a “univalent shift” in the tested monosomic plants resulting from a series of backcrosses. It is known that univalent chromosomes in three cotton monosomes (Mono12, Mono22, and Mono25) underwent misdivision much more often than other chromosomes [
42,
45,
46], which indicated their instability.
Our detection of a high meiotic index and the presence of a small number of tetrads with micronuclei in the analysis of the microspore stage in monosomic F1 hybrids indirectly indicated the regularity of meiotic division and confirmed the normal pairing of chromosomes in monosomic plants. The detection of some decrease in pollen fertility in monosomic hybrid F1 plants confirmed the existence of specific differences between them. In general, the regular pairing of chromosomes in interspecific aneuploid F1 hybrids, a high meiotic index, and a partial decrease in pollen fertility indicate latent structural variability that is not recorded at the metaphase I stage of meiosis.
The creation of a database of molecular markers (SSRs) and the assignment of such markers to chromosomes [
36,
37,
38,
39,
47,
48,
49] made it possible to successfully use these tools in experiments to identify specific chromosomes of cotton. The results reported here clearly demonstrated that the use of molecular deletion analysis [
38] to localize previously assigned chromosome-specific SSR markers made it possible to identify unknown univalent chromosomes in four new monosomic lines in our collection (Mo94, Mo56, Mo42, and Mo17). These lines have a deficiency in chromosome
12 from the A
tsubgenome and chromosomes
17,
21, and
22 from the D
tsubgenome, respectively; the monotelodisome line Telo12 has a deficiency in an arm of chromosome
6 from the A
tsubgenome.
Due to the small size, large number, and lack of differential banding, cotton chromosomes, similar to the chromosomes of maize [
50], barley [
51], peas [
52], rye [
53], and tomato [
54], are identified using translocation test lines created by Brown M.S. [
55]. The identification of cotton monosomes by the size of the univalents allows only conditionally attributing them to subgenomes, since it is known that the chromosomes of the A
hsubgenome are almost twice as long as those of the D
hsubgenome, but some of the smallest chromosomes of the A
hsubgenome are not much larger than the largest chromosomes of the D
hsubgenome; however, medium-sized chromosomes generally belong to the A
hsubgenome [
56].
The principle by which the identification of chromosomes using translocations was carried out has previously been described [
57]. In cotton, the detection of quadrivalent and univalent meiosis in the PMC in metaphase I in hybrid translocation monosomic plants indicates the nonhomology of chromosomes involved in translocation and in univalent chromosomes [
45]. When trivalents are found in PMCs of hybrid translocation monosomic plants, this result indicates the homology of the univalent and one of the chromosomes in the translocation. To determine which chromosome in the translocation is homologous to the univalent chromosome, crosses of this monosomic plant with other translocation lines were carried out, in which one of the chromosomes in translocations was the same as that of the original line. An analysis of chromosome associations in monosomic hybrids makes it possible to identify a univalent chromosome as a specific chromosome of a set [
42].
The results of the cytological tests of hybrids obtained from crosses of three monosomic lines with translocation test lines (F1Mo94 × TT11R-12L, F1Mo42 × TT7R-21R and F1Mo42 × TT20R-21L, and F1Mo17 × TT20L-22R) helped identify the trivalent chromosome associations of previously unknown chromosomes, such as chromosome 12 of the Atsubgenome and chromosomes 21 and 22 of the Dtsubgenome of cotton. In general, the use of chromosome-specific markers and translocation test lines greatly facilitated the identification of unknown monosomes in monosomic lines in our collection.
These monosomic cotton lines are characterized by complex phenotypic traits due to the deficiency in specific chromosomes. Since the monosomic lines in our collection were created in a single genotypic background, that of the L-458 line, obtained on the basis of the 108-F cultivar, and the monosomic lines of the collection from the USA have a different genetic background based on the Deltapine 14 variety, with subsequent backcrossing with the Texas Marker 1 line; the differences between the monosomic lines of different collections are acceptable. Backcrossing of monosomic cotton lines with the initial F
1 hybrids (Mo × Pima 3-79) led to a strong decrease in the crossing rate in many variants, with the exception of three variants involving the monosomic lines Mo56, Mo42, and Mo17 with deficiencies in chromosomes
17,
21, and
22. Studying the setting of F
1BC
1 hybrid seeds also revealed a general decrease in the setting rate in 17 variants of crosses compared to the F
1 hybrids (Mo × Pima 3-79), with the exception of variants involving four lines (Mo16, Mo31, Mo67, and Mo27). In general, the seed setting in F
1 crosses was significantly higher than that in backcrosses. It was previously noted that the difference in the number of motes in disomic and monosomic plants is sufficient to reveal the difference between monosomic individuals and normal sibs [
45].
The obtained results showed that it is important to consider the crossing and setting of hybrid seeds in the process of creating substitution lines, since for unknown reasons, F1BC1 hybrids have a strong decrease in these indicators, despite the regular pairing of chromosomes and the relatively high pollen fertility of F1 hybrids. This phenomenon was clearly demonstrated by monosomic line Mo13 with a deficiency in chromosome 6, which did not produce a single backcrossed boll when crossed with the monosomic interspecific F1 hybrid. Apparently, the decrease in the setting of hybrid BC1F1 backcross seeds was due to the specific features of those hybrids, along with the hybridity of the material and the low seed set in many of the original lines with haplo-deficiency.
Unfortunately, we were unable to compare our data with the results of American studies, since no data on the cytogenetic characteristics of hybrids were published when chromosome-substitution lines were created via backcrossing. Therefore, in future studies, how these indicators change with an increase in backcrossing generations will be examined.
A decrease in the germination of BC1F1 hybrid seeds compared to the initial F1 hybrids (Mo × Pima 3-79), with the exception of six BC1F1 families involving Mo7, Mo31, Mo58, Mo59, Mo34, and Mo95, could occur as a result of the effect of alien substitutions of various nonhomologous chromosomes and the reduced germination rates of backcross seeds with haplo-deficiencies.
It was found that the karyotypic transformations of plants during backcrossing of the original cotton lines with the pollen from F
1 hybrids were not accompanied by any visible disturbances in meiotic divisions, since a comparative analysis of chromosome pairing in 21 hybrid monosomic BC
1F
1 plants with substitutions of chromosomes
2,
4,
6,
7, and
12 from the A
tsubgenome and chromosomes
17,
18,
21, and
22 from the D
tsubgenome revealed normal chromosome pairing. In addition, the detection of a high meiotic index and a small number of tetrads with micronuclei served as an indirect confirmation of the regularity of meiosis and the absence of the appearance of additional univalents. However, the detection of a significant decrease in pollen fertility in monosomic hybrids with the substitution of specific chromosomes in different BC
1F
1 families indicated the existence of specific differences in pollen fertility in these plants. It is known that the microscopic assessment of pollen fertility after staining the progeny of cotton monosomes with acetocarmine is not entirely convincing as a method for separating monosomic and disomic plants due to the abortion of unbalanced microspores in early development [
45]. A strong decrease in pollen fertility combined with a high meiotic index occurred due to the formation of gametes with haplo-deficiencies, which looked like normal tetrads at the microspore stage.
In our study, hybrid plants with haplo-deficiencies were not found in all BC
1F
1 hybrid families, since it was not possible to detect monosomic plants in five of them (Mo7, Mo31, Mo67, Mo93, and Mo95) for various reasons. Success in the transmission of the monosomes was highly dependent on the rate of reproduction in the hybrid offspring. It is known that there are differences between specific chromosomes of the cotton genome in the viability of the haplo-deficient gametes produced [
45]. In addition, it was noted that the transfer of the monosomic state in cotton monosomes is limited in all by ovules, and the low frequency of transmission of the monosomes indicates a low viability of gametes with haplodeficiency [
42]. The data presented in this article suggest that the rate of transmission of haplo-deficiencies in cotton hybrids depends on the specificity of the monosome and the number of hybrid plants.
For molecular genetic confirmation of chromosome substitutions, accelerated detection of monosomic cytotypes, and rapid backcrossing of plants with haplo-deficiencies, chromosomal assignment of molecular markers was carried out at the plantlet stage (4–5 true leaves) for the first time in cotton plants with alien chromosome substitutions. The identification of seven monosomic cytotypes at the plantlet stage in four families with substitutions of chromosomes 2 and 4 using markers previously assigned to chromosomes is of great importance for identifying hybrids with alien chromosome substitutions. This method significantly speeds up the analysis of hybrid progeny and contributes to the detection of plants with haplo-deficiencies, which leads to an acceleration of backcrossing and the production of hybrid bolls. Confirmation of substitutions of alien chromosomes in other BC1F1 families, carried out in our study by the common molecular genetic analysis, based on six previously identified monosomic hybrids (Mo60, Mo75, Mo34, Mo92, Mo94, and Mo17), serves as the basis for further research on obtaining chromosome-substitution cotton lines. However, such studies require much more time than those using BC1F1 monosomes that were identified at the plantlet stage.
Comparative analysis of the morphobiological characteristics of monosomic F1 and BC1F1 hybrids in comparison with the original monosomic lines showed that monosomic hybrids of the first generation were more similar to the donor line, while BC1F1 hybrids with haplodeficiency shared more characteristics with the recurrent parent and presented previously unidentified characteristics. These new markers included strong pubescence of the main stem in the BC1F1 hybrid—with substitution of chromosome 4, pale green coloured leaves in the hybrid—with substitution of chromosome 7, dense foliage and large bolls in the hybrid—with substitution of chromosome 12, pubescence of the bracts and peduncles in the hybrid—with substitution of chromosome 18, dark green leaf colour in the hybrid—with the substitution of chromosome 22, lengthening of the cuttings and an increase in the size of the bolls in the hybrid—with the substitution of an arm of chromosome 6, and dark green leaves and two-three-lobed leaves in the hybrid—with the substitution of an arm of chromosome 11.
The results reported in recent years have clearly demonstrated the associations of substituted cotton chromosomes with various traits: CS-B04—higher fibre length; CS-B06—higher fibre yield; CS-B07—higher mass of one boll and micronaires; CS-B16—reduced seed yield, fibre yield, and fibre length but increased fibre yield and weight per boll; CS-B17—increased fibre yield, boll mass, and fibre elongation but reduced fibre yield, micronair, and fibre length; CS-B18—reduced seed and fibre yield and weight of one boll, but higher fibre and micronair yield; and CS-B25—significant association with all traits, except for seed yield [
24].
However, 103 SSR primers were not associated with specific chromosomes due to the absence of hypoaneuploid lines for some chromosomes in the genome. To date, for five chromosomes in the genome (
5,
8,
14,
15, and
22), no monosomic plants have been obtained, while for three chromosomes (
13,
19, and
24), no deficiencies have been found in either specific chromosomes or specific chromosome arms [
40].
An analysis of the studies including the association of markers assigned to chromosome
6 once again revealed inconsistencies between different studies, which raised the suspicion that the origin of some aneuploids may contribute most to the unexpected shortcomings of such studies [
36]. Later, the chromosome-substitution line CS-B06 was found not have a substitution for all of chromosome
6 from the A
tsubgenome, since extensive molecular genetic work with five SSR loci specific for chromosome
6 revealed the amplification of the Upland-type alleles, not the Pima 3-79 alleles, which collectively suggests that this lineage was missynthesized and/or not inbred [
44].
In the future, new chromosome substitution cotton lines will serve as the basis for molecular genetic studies and chromosome localization of new molecular markers, as well as improving the quality of cotton fibre by crossing these lines with cultivated varieties and enriching them with loci responsible for improving the quality of the fibre.