Photodynamic Effect of Riboflavin on Chitosan Coatings and the Application in Pork Preservation
Abstract
1. Introduction
2. Results and Discussions
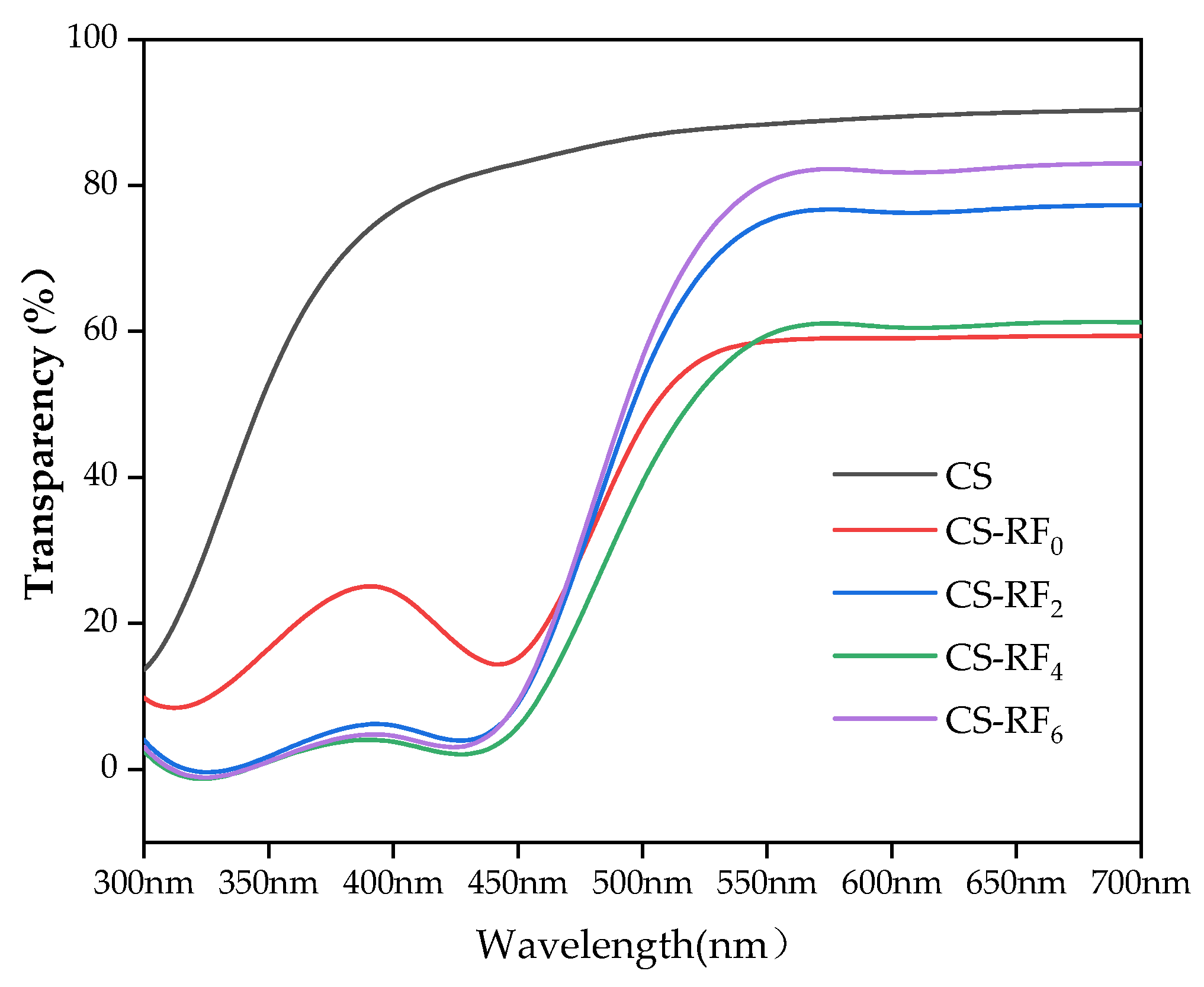
2.1. Optical Properties
2.2. Antibacterial Activity
2.3. ROS
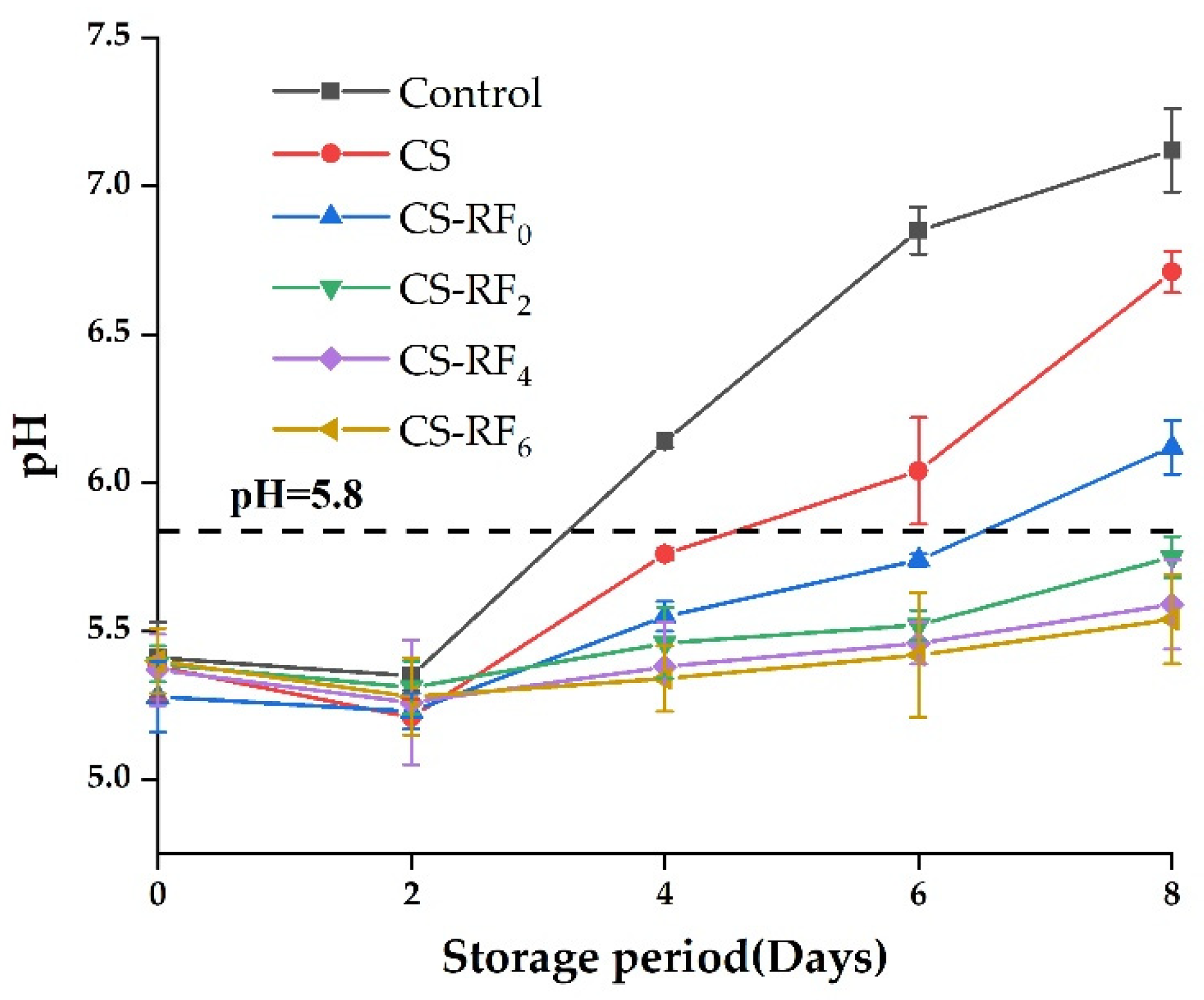
2.4. pH
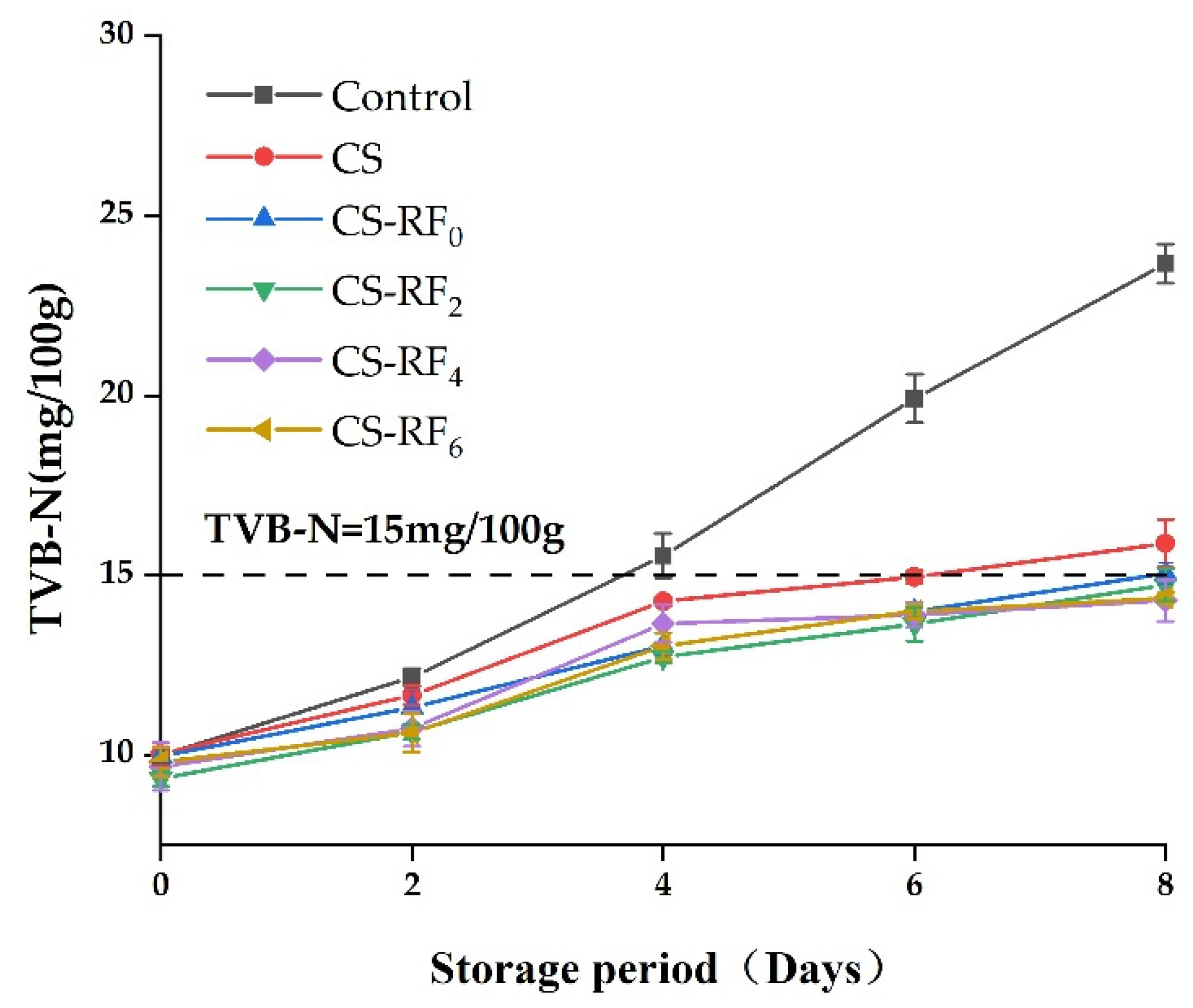
2.5. TVB-N
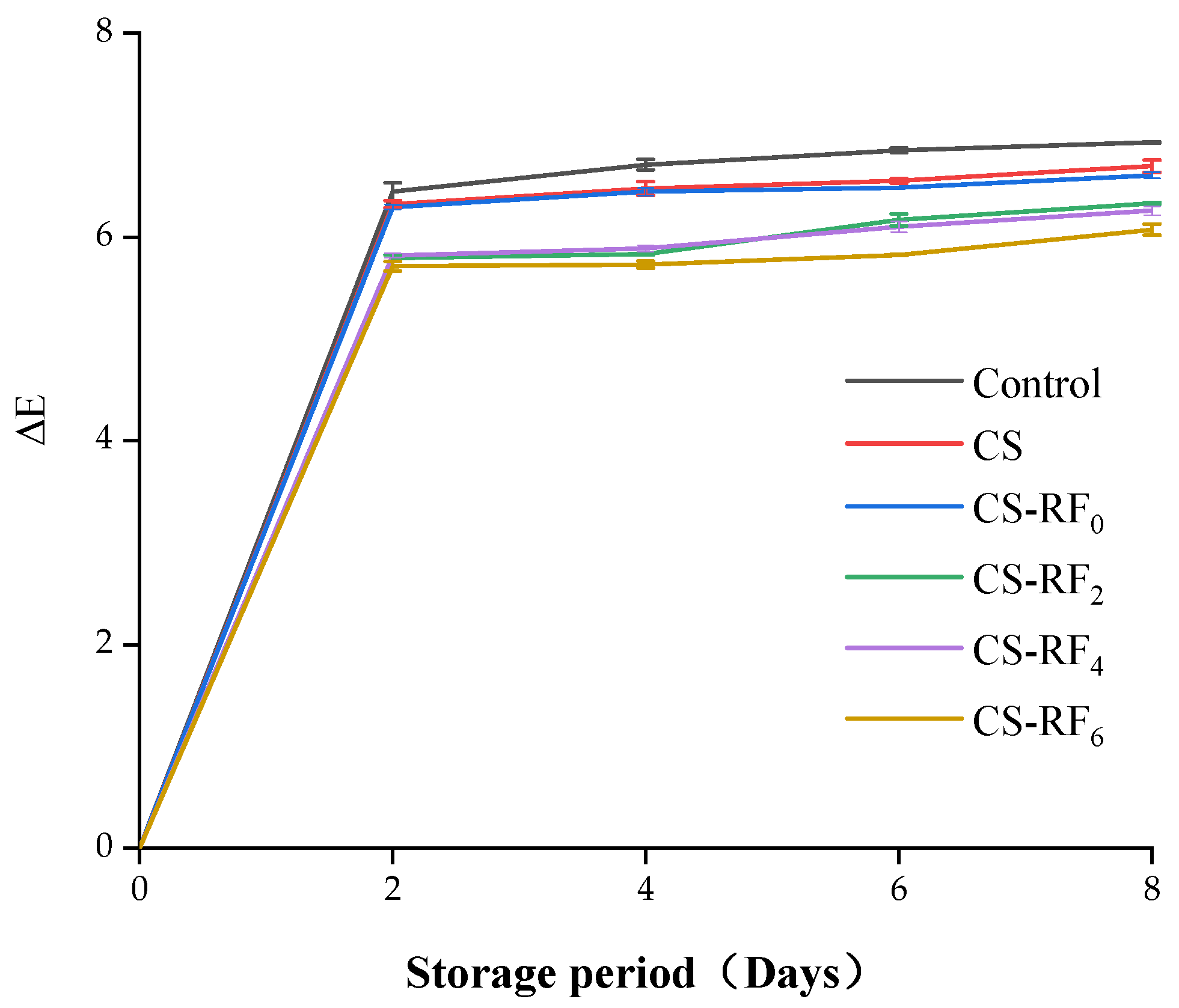
2.6. Color Characteristics
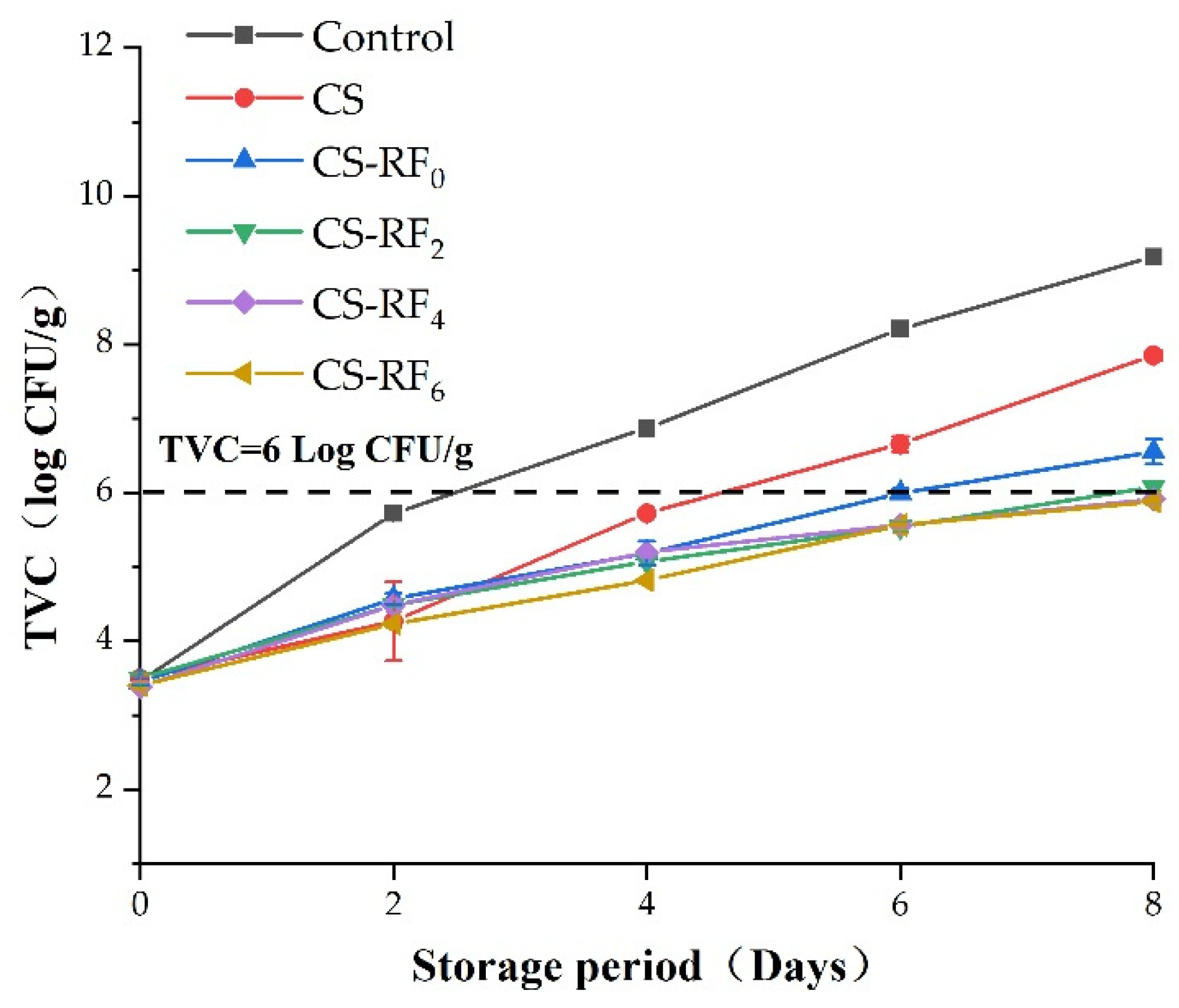
2.7. TVC
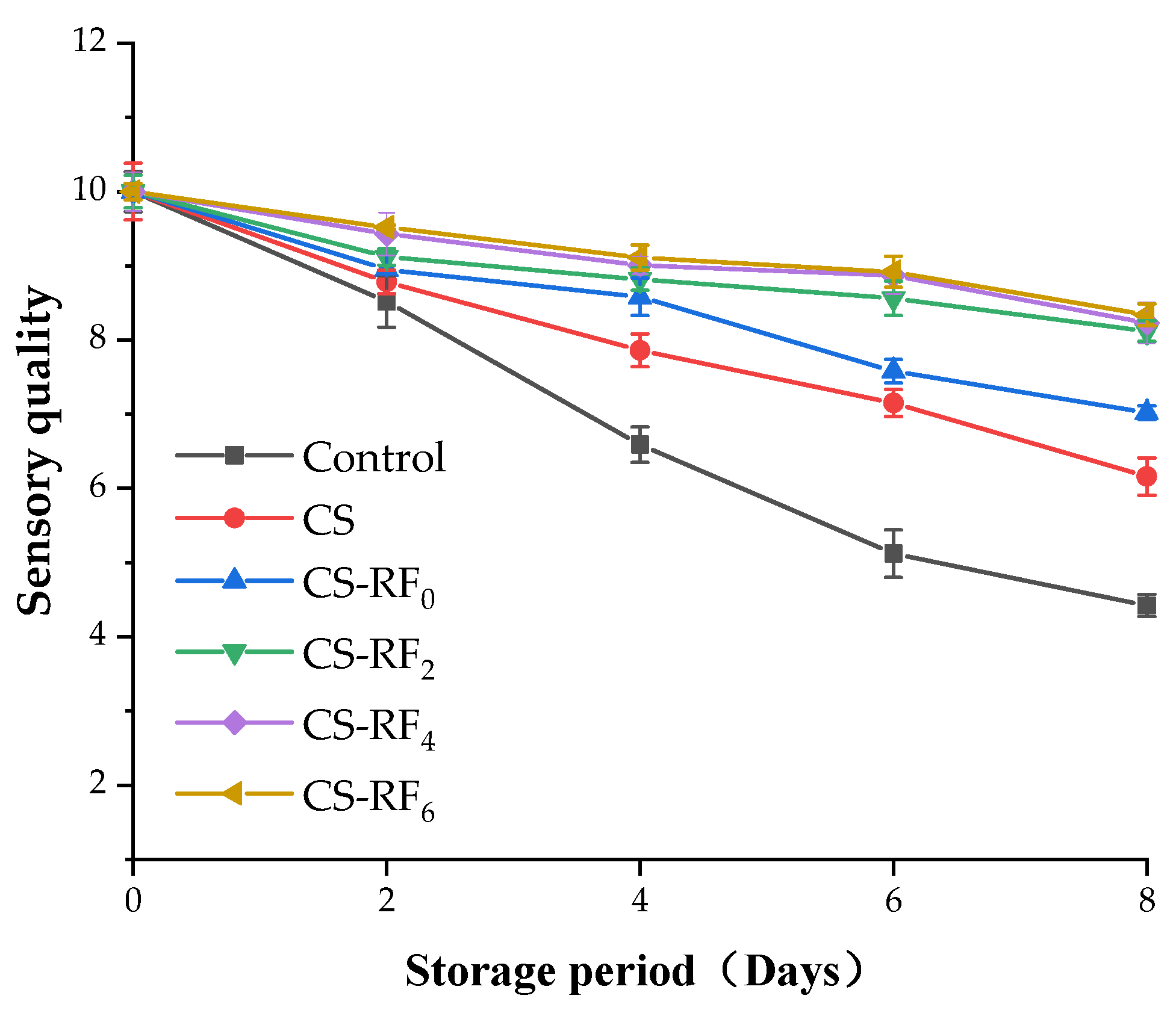
2.8. Sensory Qualities
3. Materials and Methods
3.1. Materials
3.2. Preparation of CS-RF Coatings
3.3. Determination of Light Transmittance of CS-RF Coatings
3.4. In Vitro Study on Antibacterial Activity
3.5. Measurement of Hydroxyl Radicals
3.6. Measurement of Singlet Oxygen
3.7. Measurement of Hydrogen Peroxide
3.8. Microbiological Analysis in Pork Packaging
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Javanmardi, F.; Rahmani, J.; Ghiasi, F.; Hashemi Gahruie, H.; Mousavi Khaneghah, A. The association between the preservative agents in foods and the risk of breast cancer. Nutr. Cancer 2020, 71, 1229–1240. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Naeem, H.; Zayed, N.; Mansour, H.A. Effect of chitosan and lauric arginate edible coating on bacteriological quality, deterioration criteria, and sensory attributes of frozen stored chicken meat. LWT 2021, 150, 111928. [Google Scholar] [CrossRef]
- Alirezalu, A.; Ahmadi, N.; Salehi, P.; Sonboli, A.; Alirezalu, K.; Mousavi Khaneghah, A.; Lorenzo, J.M. Physicochemical characterization, antioxidant activity, and phenolic compounds of hawthorn (Crataegus spp.) fruits species for potential use in food applications. Foods 2020, 9, 436. [Google Scholar] [CrossRef] [PubMed]
- Olmedilla-Alonso, B.; Jim’enez-Colmenero, F.; S’anchez-Muniz, F.J. Development and assessment of healthy properties of meat and meat products designed as functional foods. Meat Sci. 2013, 95, 919–930. [Google Scholar] [CrossRef]
- Kumar, S.; Mukherjee, A.; Dutta, J. Chitosan based nanocomposite films and coatings: Emerging antimicrobial food packaging alternatives. Trends Food Sci. Technol. 2020, 97, 196–209. [Google Scholar] [CrossRef]
- Han, J.H. Innovations in Food Packaging, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2005; pp. 239–262. [Google Scholar]
- Taghizadeh, M.; Mohammadifar, M.A.; Sadeghi, E.; Rouhi, M.; Mohammadi, R.; Askari, F.; Mortazavian, A.M.; Kariminejad, M. Photosensitizer-induced cross-linking: A novel approach for improvement of physicochemical and structural properties of gelatin edible films. Food Res. Int. 2018, 112, 90–97. [Google Scholar] [CrossRef]
- Yu, D.; Jiang, Q.; Xu, Y.; Xia, W. The shelf life extension of refrigerated grass carp (ctenopharyngodon idellus) fillets by chitosan coating combined with glycerol monolaurate. Int. J. Biol. Macromol. 2017, 101, 448–454. [Google Scholar] [CrossRef]
- Mincea, M.; Negrulescu, A.; Ostafe, V. Preparation, modification, and applications of chitin nanowhiskers: A review. Rev. Adv. Mater. Sci. 2012, 30, 225–242. [Google Scholar]
- Kumar, D.; Kumar, P.; Pandey, J. Binary grafted chitosan film: Synthesis, characterization, antibacterial activity and prospects for food packaging. Int. J. Biol. Macromol. 2018, 115, 341–348. [Google Scholar] [CrossRef]
- Haghighi, H.; Leugoue, S.K.; Pfeifer, F.; Siesler, H.W.; Licciardello, F.; Fava, P.; Pulvirenti, A. Development of antimicrobial films based on chitosan-polyvinyl alcohol blend enriched with ethyl lauroyl arginate (LAE) for food packaging applications. Food Hydrocoll. 2020, 100, 105419. [Google Scholar] [CrossRef]
- Wu, C.; Li, Y.; Wang, L.; Hu, Y.; Chen, J.; Liu, D.; Ye, X. Efficacy of chitosan-gallic acid coating on shelf life extension of refrigerated pacific mackerel fillets. Food Bioprocess. Technol. 2016, 9, 675–685. [Google Scholar]
- Souza, V.G.L.; Pires, J.R.A.; Vieira, E.T.; Coelhoso, I.M.; Duarte, M.P.; Fernando, A.L. Shelf life assessment of fresh poultry meat packaged in novel bionanocomposite of chitosan/montmorillonite incorporated with ginger essential oil. Coatings 2018, 8, 177. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, F.; Lai, S.; Wang, H.; Yang, H. Impact of soybean protein isolate-chitosan edible coating on the softening of apricot fruit during storage. LWT 2018, 96, 604–611. [Google Scholar] [CrossRef]
- Jamroz, E.; Kulawik, P.; Kopel, P. The effect of nanofillers on the functional properties of biopolymer-based films: A review. Polymers 2019, 11, 675. [Google Scholar] [CrossRef] [PubMed]
- Nohr, D.; Biesalski, H.K. Vitamins in milk and dairy products: B-Group vitamins. In Advanced Dairy Chemistry Volume 3: Lactose, Water, Salts and Vitamins; Fox, P.F., Ed.; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2009; pp. 591–630. [Google Scholar]
- Amin, F.; Khan, W.; Bano, B. Oxidation of cystatin imparted by riboflavin generated free radicals: Spectral analysis. Int. J. Biol. Macromol. 2019, 124, 1281–1291. [Google Scholar] [CrossRef]
- Dainty, J.R.; Bullock, N.R.; Hart, D.J.; Hewson, A.T.; Turner, R.; Finglas, P.M.; Powers, H.J. Quantification of the bioavailability of riboflavin from foods by use of stable-isotope labels and kinetic modeling. Am. J. Clin. Nutr. 2007, 85, 1557–1564. [Google Scholar] [CrossRef][Green Version]
- Powers, H.J. Riboflavin (vitamin B-2) and health. Am. J. Clin. Nutr. 2003, 77, 1352–1360. [Google Scholar] [CrossRef]
- Su, L.; Huang, J.; Li, H.; Pan, Y.; Zhu, B.; Zhao, Y.; Liu, H. Chitosan-riboflavin composite film based on photodynamic inactivation technology for antibacterial food packaging. Int. J. Biol. Macromol. 2021, 172, 231–240. [Google Scholar] [CrossRef]
- Northrop-Clewes, C.A.; Thurnham, D.I. The discovery and characterization of riboflavin. Ann. Nutr. Metab. 2012, 61, 224–230. [Google Scholar] [CrossRef]
- Rostas, A.; Einholz, C.; Illarionov, B.; Heidinger, L.; Said, T.A.; Bauss, A.; Fischer, M.; Bacher, A.; Weber, S.; Schleicher, E. Long-lived hydrated FMN radicals: EPR characterization and implications for catalytic variability in flavoproteins. J. Am. Chem. Soc. 2018, 140, 16521–16527. [Google Scholar] [CrossRef]
- Yamashita, M.; Rosatto, S.S.; Kubota, L.T. Electrochemical comparative study of riboflavin, FMN and FAD immobilized on the silica gel modified with zirconium oxide. J. Braz. Chem. Soc. 2002, 13, 635–641. [Google Scholar] [CrossRef]
- Tirella, A.; Liberto, T.; Ahluwalia, A. Riboflavin and collagen: New crosslinking methods to tailor the stiffness of hydrogels. Mater. Lett. 2012, 74, 58–61. [Google Scholar] [CrossRef]
- Orsuwan, A.; Kwon, S.; Bumbudsanpharoke, N.; Ko, S. Novel LDPE-riboflavin composite film with dual function of broad-spectrum light barrier and antimicrobial activity. Food Control 2019, 100, 176–182. [Google Scholar] [CrossRef]
- Ahmad, M.; Benjakul, S.; Prodpran, T.; Agustini, T.W. Physico-mechanical and antimicrobial properties of gelatin film from the skin of unicorn leatherjacket incorporated with essential oils. Food Hydrocoll. 2012, 28, 189–199. [Google Scholar] [CrossRef]
- Hosseinnejad, M.; Jafari, S.M. Evaluation of different factors affecting antimicrobial properties of chitosan. Int. J. Biol. Macromol. 2016, 85, 467–475. [Google Scholar] [CrossRef]
- Raafat, D.; Sahl, H.G. Chitosan and its antimicrobial potential—A critical literature survey. Microb. Biotechnol. 2009, 2, 186–201. [Google Scholar] [CrossRef]
- Kim, M.J.; Lianto, D.K.; Koo, G.H.; Yuk, H.G. Antibacterial mechanism of riboflavin-mediated 460 nm light emitting diode illumination against Listeria monocytogenes in phosphate-buffered saline and on smoked salmon. Food Control 2021, 124, 107930. [Google Scholar] [CrossRef]
- Li, H.; Tan, L.; Chen, B.; Huang, J.; Zeng, Q.; Liu, H.; Wang, J.J. Antibacterial potency of riboflavin-mediated photodynamic inactivation against Salmonella and its influences on tuna quality. LWT 2021, 146, 111462. [Google Scholar] [CrossRef]
- Bertolotti, S.G.; Previtali, C.M.; Rufs, A.M.; Encinas, M.V. Riboflavin/triethanolamine as photoinitiator system of vinyl polymerization. A mechanistic study by laser flash photolysis. Macromolecules 1999, 32, 2920–2924. [Google Scholar] [CrossRef]
- Nguyen, A.K.; Gittard, S.D.; Koroleva, A.; Schlie, S.; Gaidukeviciute, A.; Chichkov, B.N.; Narayan, R.J. Two-photon polymerization of polyethylene glycol diacrylate scaffolds with riboflavin and triethanolamine used as a water-soluble photoinitiator. Regen. Med. 2013, 8, 725–738. [Google Scholar] [CrossRef]
- Righetti, P.G.; Gelfi, C.; Bosisio, A.B. Polymerization kinetics of polyacrylamide gels. III. Effect of catalysts. Electrophoresis 1981, 2, 291–295. [Google Scholar] [CrossRef]
- Wang, T.; Bruin, G.J.; Kraak, J.C.; Poppe, H. Preparation of polyacrylamide gel-filled fused-silica capillaries by photopolymerization with riboflavin as the initiator. Anal. Chem. 1991, 63, 2207–2208. [Google Scholar] [CrossRef]
- Tomal, W.; Ortyl, J. Water-soluble photoinitiators in biomedical applications. Polymers 2020, 12, 1073. [Google Scholar] [CrossRef] [PubMed]
- Allushi, A.; Kutahya, C.; Aydogan, C.; Kreutzer, J.; Yilmaz, G.; Yagci, Y. Conventional Type II photoinitiators as activators for photoinduced metal-free atom transfer radical polymerization. Polym. Chem. 2017, 8, 1972–1977. [Google Scholar] [CrossRef]
- Sathe, P.; Richter, J.; Myint, M.T.Z.; Dobretsov, S.; Dutta, J. Self-decontaminating photocatalytic zinc oxide nanorod coatings for prevention of marine microfouling: A mesocosm study. Biofouling 2016, 32, 383–395. [Google Scholar] [CrossRef]
- Beyer, W.F.; Fridovich, I. Assaying for superoxide dismutase activity: Some large consequences of minor changes in conditions. Anal. Biochem. 1987, 161, 559–566. [Google Scholar] [CrossRef]
- Zhang, Z.; Si, Y.; Sun, G. Photo-activities of vitamin k Derivatives and potential applications as daylight activated antimicrobial agents. ACS Sustain. Chem. Eng. 2019, 7, 18493–18504. [Google Scholar] [CrossRef]
- Zhang, Z.; El-Moghazy, A.Y.; Wisuthiphaet, N.; Nitin, N.; Castillo, D.; Murphy, B.G.; Sun, G. Daylight-induced antibacterial and antiviral nanofibrous membranes containing vitamin K derivatives for personal protective equipment. ACS Appl. Mater. Interfaces 2020, 12, 49416–49430. [Google Scholar] [CrossRef]
- Cullere, M.; Dalle Zotte, A.; Tasoniero, G.; Giaccone, V.; Szendrő, Z.; Szín, M.; Matics, Z. Effect of diet and packaging system on the microbial status, pH, color and sensory traits of rabbit meat evaluated during chilled storage. Meat Sci. 2018, 141, 36–43. [Google Scholar] [CrossRef]
- Miao, J.; Peng, W.; Liu, G.; Chen, Y.; Chen, F.; Cao, Y. Biopreservative effect of the natural antimicrobial substance from Lactobacillus paracasei subsp. tolerans FX-6 on fresh pork during chilled storage. Food Control 2015, 56, 53–56. [Google Scholar] [CrossRef]
- Huang, B.; Zhang, Z.; Ding, N.; Zhuang, Y.; Zhang, G.; Fei, P. Preparation of acylated chitosan with caffeic acid in non-enzymatic and enzymatic systems: Characterization and application in pork preservation. Int. J. Biol. Macromol. 2022, 194, 246–253. [Google Scholar] [CrossRef]
- Chaparrohernandez, S.; Ruízcruz, S.; Marquezríos, E.; Oca Nohiguera, V.M.; Valenzuelalopez, C.C.; Ornelaspaz, J.D.J.; Del Toro, L. Effect of chitosan carvacrol edible coatings on the quality and shelf life of tilapia (Oreochromis niloticus) fillets stored in ice. Food Sci. Technol. 2015, 35, 734–741. [Google Scholar] [CrossRef]
- Feng, X.; Bansal, N.; Yang, H. Fish gelatin combined with chitosan coating inhibits myofibril degradation of golden pomfret (Trachinotus blochii) fillet during cold storage. Food Chem. 2016, 200, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Chi, H.; Yao, H.; Lu, Z.; Bie, X.; Zhang, C.; Chen, M. The antibacterial activity of plantaricin GZ1-27 against MRSA and its bio-preservative effect on chilled pork in combination with chitosan. Int. J. Food Microbiol. 2022, 365, 109539. [Google Scholar] [CrossRef] [PubMed]
- Jonaidi Jafari, N.; Kargozari, M.; Ranjbar, R.; Rostami, H.; Hamedi, H. The effect of chitosan coating incorporated with ethanolic extract of propolis on the quality of refrigerated chicken fillet. J. Food Process. Pres. 2018, 42, 13336. [Google Scholar] [CrossRef]
- Insausti, K.; Beriain, M.J.; Purroy, A.; Alberti, P.; Gorraiz, C.; Alzueta, M.J. Shelf life of beef from local Spanish cattle breeds stored under modified atmosphere. Meat Sci. 2001, 57, 273–281. [Google Scholar] [CrossRef]
- Zhou, Z.; Liu, Y.L.; Liu, Z.J.; Fan, L.; Dong, T.; Jin, Y.; Sun, W. Sustained-release antibacterial pads based on nonwovens polyethylene terephthalate modified by β-cyclodextrin embedded with cinnamaldehyde for cold fresh pork preservation. Food Packag. Shelf Life 2020, 26, 100554. [Google Scholar] [CrossRef]
- Yun, D.; Qin, Y.; Zhang, J.; Zhang, M.; Qian, C.; Liu, J. Development of chitosan films incorporated with rambutan (Nephelium lappaceum L.) peel extract and their application in pork preservation. Int. J. Biol. Macromol. 2021, 189, 900–909. [Google Scholar] [CrossRef]
- Kerry, J.P.; O’Grady, M.N.; Hogan, S.A. Past, current and potential utilization of active and intelligent packaging systems for meat and musclebased products: A review. Meat Sci. 2006, 74, 113–130. [Google Scholar] [CrossRef]
- Morelli, E.; Noel, V.; Rosset, P.; Poumeyrol, G. Performance and conditions of use of refrigerated display cabinets among producer/vendors of foodstuffs. Food Control 2012, 26, 363–368. [Google Scholar] [CrossRef]
- Song, X.; Wang, L.; Liu, T.; Liu, Y.; Wu, X.; Liu, L. Mandarin (Citrus reticulata L.) essential oil incorporated into chitosan nanoparticles: Characterization, anti-biofilm properties and application in pork preservation. Int. J. Biol. Macromol. 2021, 185, 620–628. [Google Scholar] [CrossRef] [PubMed]
- Siripatrawan, U.; Noipha, S. Active film from chitosan incorporating green tea extract for shelf life extension of pork sausages. Food Hydrocoll. 2012, 27, 102–108. [Google Scholar] [CrossRef]
- Alirezalu, K.; Movlan, H.S.; Yaghoubi, M.; Pateiro, M.; Lorenzo, J.M. ε-polylysine coating with stinging nettle extract for fresh beef preservation. Meat Sci. 2021, 176, 108474. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Ma, H.; Sun, M.; Lin, Y.; Bai, F.; Li, J.; Zhang, B. A novel bacteriocin DY4-2 produced by Lactobacillus plantarum from cutlassfish and its application as bio-preservative for the control of Pseudomonas fluorescens in fresh turbot (Scophthalmus maximus) fillets. Food Control 2018, 89, 22–31. [Google Scholar] [CrossRef]
- Ashrafi, A.; Jokar, M.; Mohammadi Nafchi, A. Preparation and characterization of biocomposite film based on chitosan and kombucha tea as active food packaging. Int. J. Biol. Macromol. 2018, 108, 444–454. [Google Scholar] [CrossRef]
- Halim, A.L.A.; Kamari, A.; Phillip, E. Chitosan, gelatin and methylcellulose films incorporated with tannic acid for food packaging. Int. J. Biol. Macromol. 2018, 120, 1119–1126. [Google Scholar] [CrossRef]
- Kang, J.; Jin, W.; Wang, J.; Sun, Y.; Wu, X.; Liu, L. Antibacterial and anti-biofilm activities of peppermint essential oil against Staphylococcus aureus. LWT 2019, 101, 639–645. [Google Scholar] [CrossRef]
- Diao, W.R.; Hu, Q.P.; Zhang, H.; Xu, J.G. Chemical composition, antibacterial activity and mechanism of action of essential oil from seeds of fennel (Foeniculum vulgare Mill.). Food Control 2014, 35, 109–116. [Google Scholar] [CrossRef]
- Liu, N.; Sun, G. Production of reactive oxygen species by photoactive anthraquinone compounds and their applications in wastewater treatment. Ind. Eng. Chem. Res. 2011, 50, 5326–5333. [Google Scholar] [CrossRef]
- Bors, W.; Michel, C.; Saran, M. On the nature of biochemically generated hydroxyl radicals. Studies using the bleaching of p-Nitrosodimethylaniline as a direct assay method. Eur. J. Biochem. 1979, 95, 621–627. [Google Scholar] [CrossRef]
- Kraljic, I.; Mohsni, S.E. A new method for the detection of singlet oxygen in aqueous solutions. Photochem. Photobiol. 1978, 28, 577–581. [Google Scholar] [CrossRef]
- Si, Y.; Zhang, Z.; Wu, W.; Fu, Q.; Huang, K.; Nitin, N.; Ding, B.; Sun, G. Daylight-driven rechargeable antibacterial and antiviral nanofibrous membranes for bioprotective applications. Sci. Adv. 2018, 4, 5931. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liu, T.; Liu, L.; Liu, Y.; Wu, X. Impacts of chitosan nanoemulsions with thymol or thyme essential oil on volatile compounds and microbial diversity of refrigerated pork meat. Meat Sci. 2022, 185, 108706. [Google Scholar] [CrossRef] [PubMed]
- Saricaoglu, F.T.; Turhan, S. Performance of mechanically deboned chicken meat protein coatings containing thyme or clove essential oil for storage quality improvement of beef sucuks. Meat Sci. 2019, 158, 107912. [Google Scholar] [CrossRef]
- Chen, X.; Zhao, J.; Zhu, L.; Luo, X.; Mao, Y.; Hopkins, D.L.; Dong, P. Effect of modified atmosphere packaging on shelf life and bacterial community of roast duck meat. Food Res. Int. 2020, 137, 109645. [Google Scholar] [CrossRef]
- He, Q.; Xiao, K. Quality of broccoli (Brassica oleracea L. var. italica) in modified atmosphere packaging made by gas barrier-gas promoter blending materials. Postharvest Biol. Technol. 2018, 144, 63–69. [Google Scholar] [CrossRef]
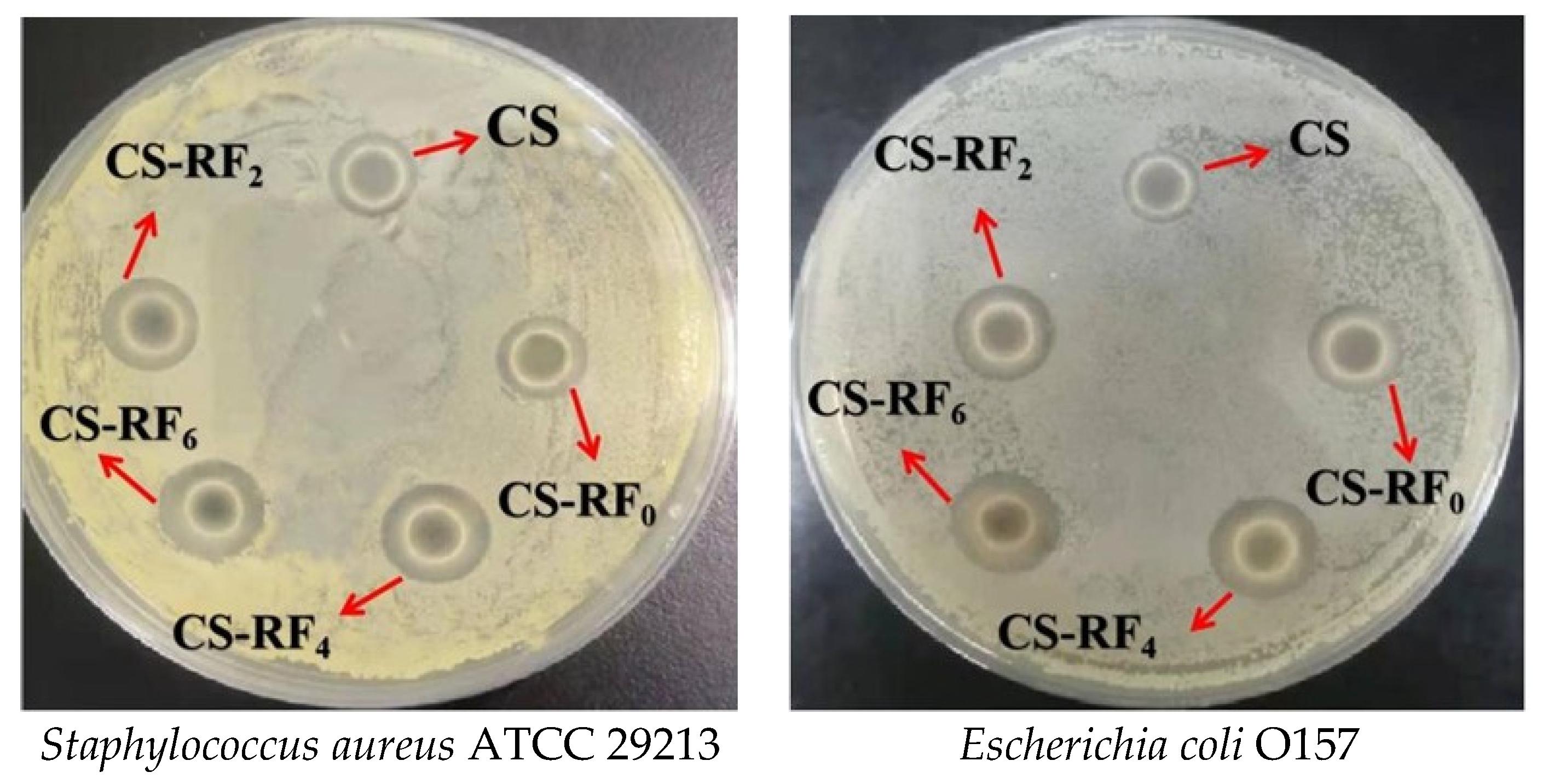
| Samples | S. aureus (mm) | E. coli (mm) |
|---|---|---|
| CS | 2.27 ± 0.40 a | 1.10 ± 0.36 a |
| CS-RF0 | 3.03 ± 0.20 b | 1.97 ± 0.21 b |
| CS-RF2 | 5.53 ± 0.49 c | 4.33 ± 0.31 c |
| CS-RF4 | 6.80 ± 0.26 d | 5.73 ± 0.25 d |
| CS-RF6 | 7.61 ± 0.32 e | 6.37 ± 0.15 e |
| Samples | ∙OH (μg/g) | H2O2 (μg/g) | 1O2 |
|---|---|---|---|
| CS | - | - | - |
| CS-RF0 | - | - | - |
| CS-RF2 | 465.48 ± 14.60 a | 58.57 ± 3.72 a | 0.17 ± 0.03 a |
| CS-RF4 | 549.33 ± 22.65 b | 69.88 ± 2.51 b | 0.44 ± 0.08 b |
| CS-RF6 | 1549.08 ± 129.41 c | 95.48 ± 1.69 c | 0.27 ± 0.06 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pei, J.; Zhu, S.; Liu, Y.; Song, Y.; Xue, F.; Xiong, X.; Li, C. Photodynamic Effect of Riboflavin on Chitosan Coatings and the Application in Pork Preservation. Molecules 2022, 27, 1355. https://doi.org/10.3390/molecules27041355
Pei J, Zhu S, Liu Y, Song Y, Xue F, Xiong X, Li C. Photodynamic Effect of Riboflavin on Chitosan Coatings and the Application in Pork Preservation. Molecules. 2022; 27(4):1355. https://doi.org/10.3390/molecules27041355
Chicago/Turabian StylePei, Jiliu, Shengyu Zhu, Yu Liu, Yukang Song, Feng Xue, Xiaohui Xiong, and Chen Li. 2022. "Photodynamic Effect of Riboflavin on Chitosan Coatings and the Application in Pork Preservation" Molecules 27, no. 4: 1355. https://doi.org/10.3390/molecules27041355
APA StylePei, J., Zhu, S., Liu, Y., Song, Y., Xue, F., Xiong, X., & Li, C. (2022). Photodynamic Effect of Riboflavin on Chitosan Coatings and the Application in Pork Preservation. Molecules, 27(4), 1355. https://doi.org/10.3390/molecules27041355






