Modulatory Effects of Biosynthesized Gold Nanoparticles Conjugated with Curcumin and Paclitaxel on Tumorigenesis and Metastatic Pathways—In Vitro and In Vivo Studies
Abstract
:1. Introduction
2. Results
2.1. Characterization of Gold Nanoparticles (AuNPs) and Stability Study
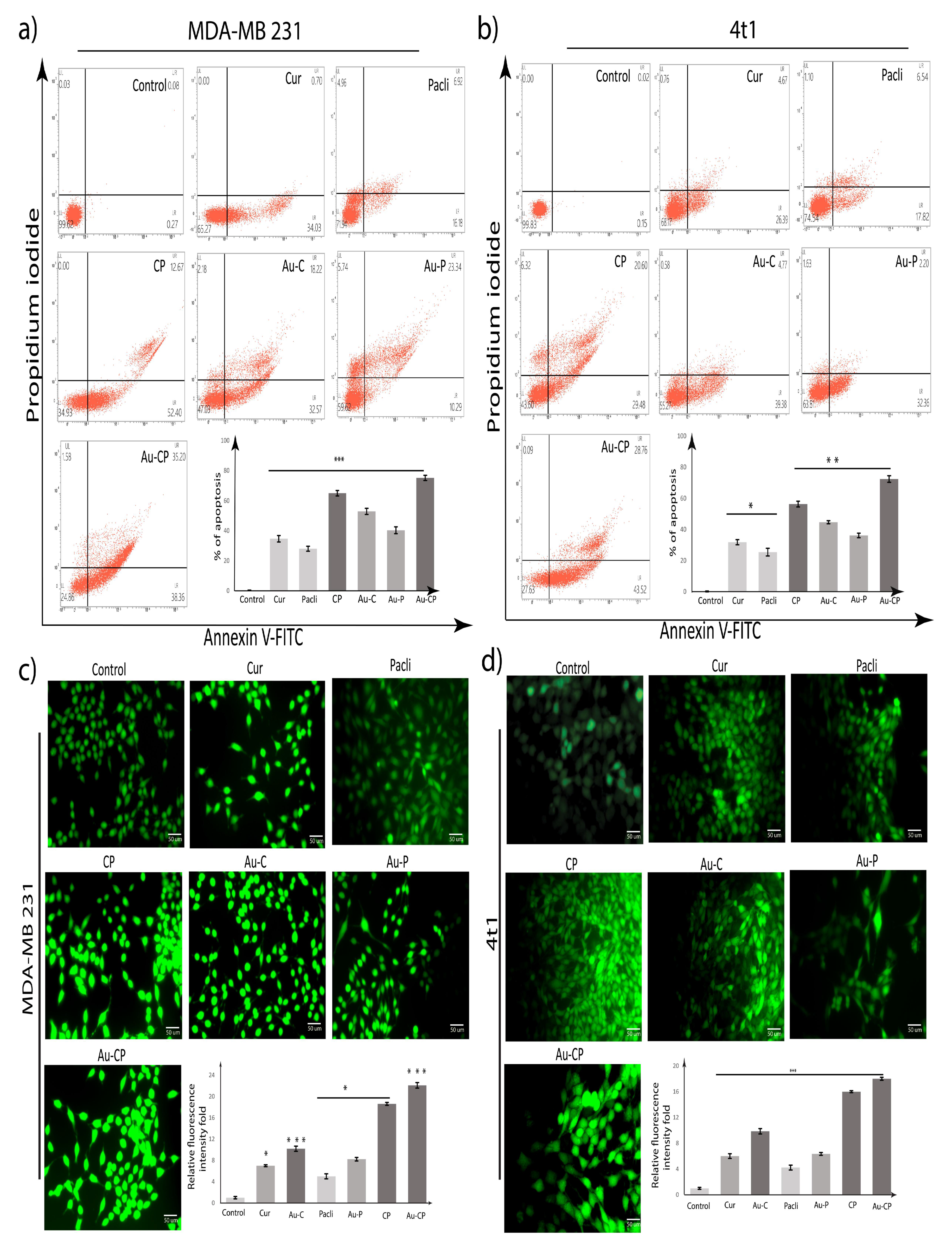
2.2. Apoptotic Assay
2.3. DCFDA Assay
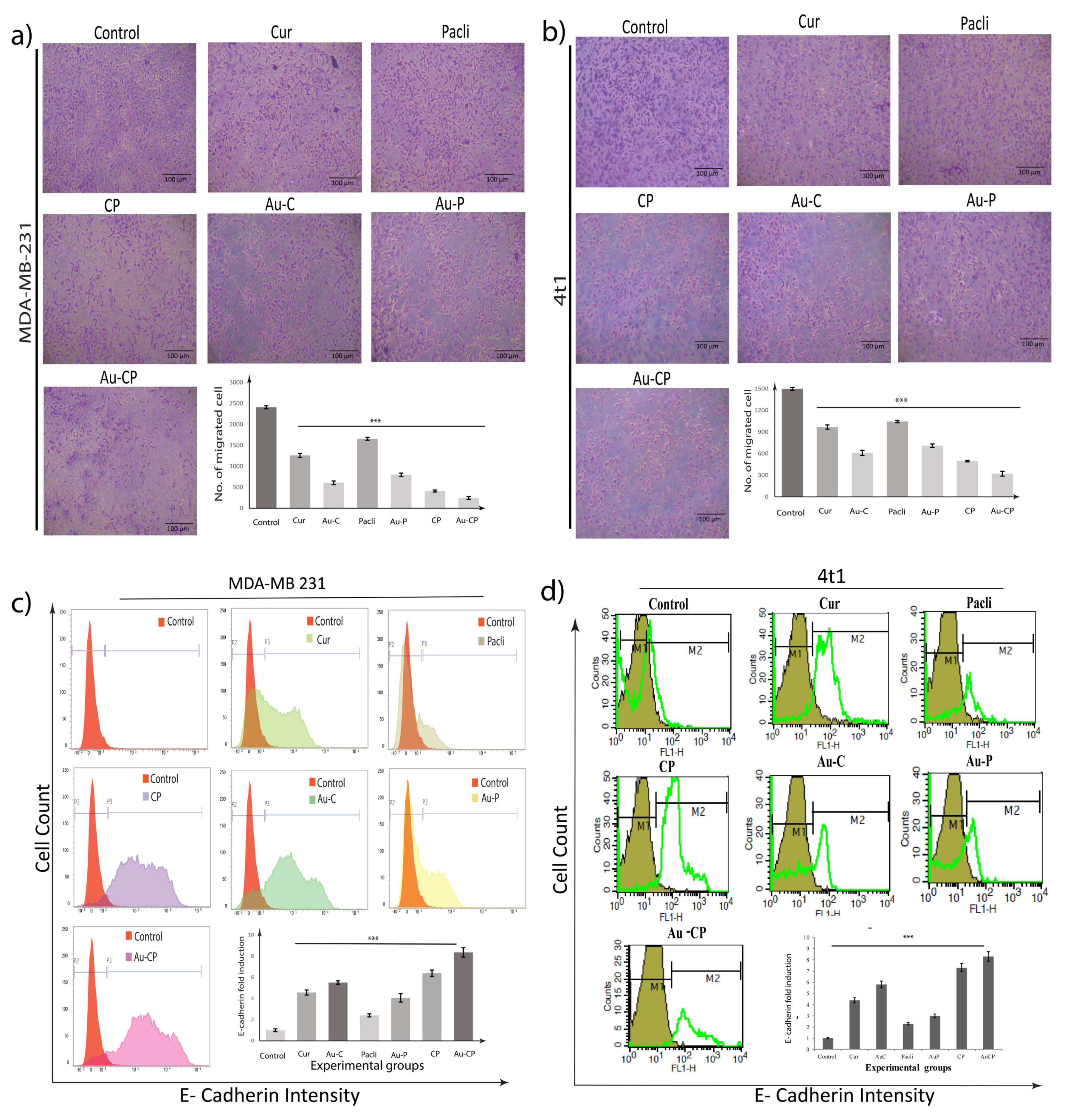
2.4. Retardation in MDA-MB 231 Cell Migration
2.5. Flow Cytometer Analysis—E-Cadherin Expression in 4T1 and MDA-MB 231 Cell Lines
2.6. Scratch Assay
2.7. Gene Marker Studies
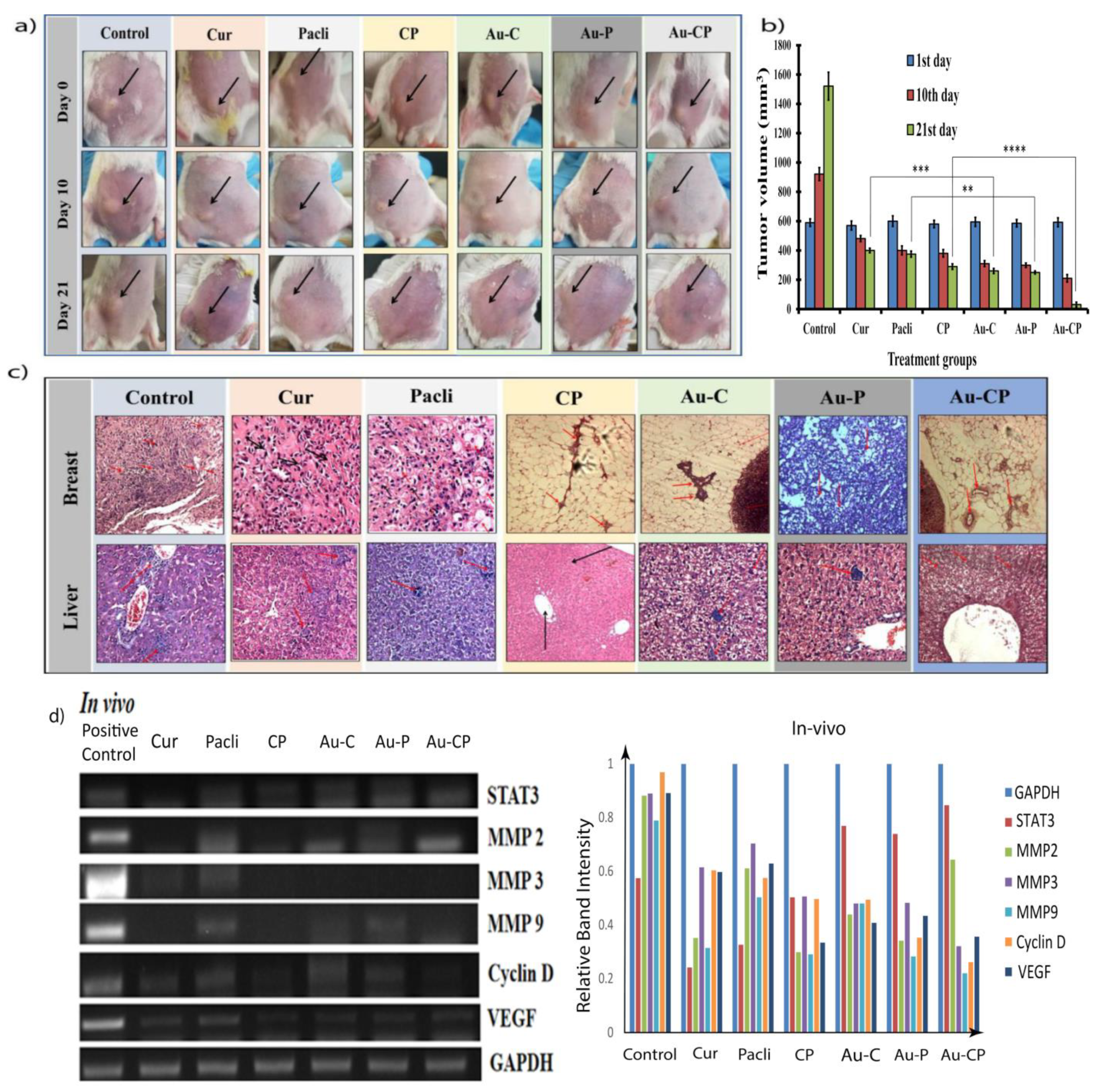
2.8. Tumor Induction and Treatment
2.9. Histopathological Studies
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Preparation of Stock Solutions
4.3. Synthesis of Gold Nanoparticles Using Phytochemicals (Curcumin and Paclitaxel)
Physicochemical Characterization of Curcumin and Paclitaxel Conjugated Gold Nanoparticles
4.4. Cell Culture
4.5. Cell Viability Assay/MTT Assay
4.6. Annexin V-FITC/PI Staining for Apoptosis Assay
4.7. Measurement of Cellular ROS Using DCFDA
4.8. Transwell Migration Assay
4.9. E-Cadherin Expression by Flow Cytometry
4.10. Scratch Assay
4.11. Quantitative RT-PCR
4.12. Tumor Induction in Mice
4.13. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- CYTECARE. Statistics of Breast Cancer in India. Available online: https://cytecare.com/blog/statistics-of-breast-cancer/ (accessed on 10 October 2020).
- Nam, J.; Son, S.; Park, K.S.; Zou, W.; Shea, L.D.; Moon, J.J. Cancer nanomedicine for combination cancer immunotherapy. Nat. Rev. Mater. 2019, 4, 398–414. [Google Scholar] [CrossRef]
- Tungsukruthai, S.; Petpiroon, N.; Chanvorachote, P. Molecular mechanisms of breast cancer metastasis and potential anti-metastatic compounds. Anticancer Res. 2018, 38, 2607–2618. [Google Scholar]
- Wang, C.; Li, J.; Ye, S.; Zhang, Y.; Li, P.; Wang, L.; Wang, T.H. Oestrogen inhibits VEGF expression and angiogenesis in triple-negative breast cancer by activating GPER-1. J. Cancer 2018, 9, 3802–3811. [Google Scholar] [CrossRef]
- Yang, L.; Lin, S.; Xu, L.; Lin, J.; Zhao, C.; Huang, X. Novel activators and small-molecule inhibitors of STAT3 in cancer. Cytokine Growth Factor Rev. 2019, 49, 10–22. [Google Scholar] [CrossRef]
- Qin, J.J.; Yan, L.; Zhang, J.; Zhang, W.D. STAT3 as a potential therapeutic target in triple negative breast cancer: A systematic review. J. Exp. Clin. Cancer Res. 2019, 38, 195. [Google Scholar] [CrossRef]
- Scripture, C.D.; Figg, W.D.; Sparreboom, A. Peripheral Neuropathy Induced by Paclitaxel: Recent Insights and Future Perspectives. Curr. Neuropharmacol. 2006, 4, 165–172. [Google Scholar] [CrossRef] [Green Version]
- Athigakunagorn, K.S.; Nantavithya, C.; Shotelesak, K. A Case Report of Cerebral Venous Thrombosis after Taking Tamoxifen in Breast Cancer Patient Case Report. J. Clin. Case Rep. 2018, 6, 1–34. [Google Scholar] [CrossRef]
- Avtanski, D.B.; Poretsky, L. Phyto-polyphenols as potential inhibitors of breast cancer metastasis. Mol. Med. 2018, 24, 24–29. [Google Scholar] [CrossRef]
- Zhou, Q.; Wang, X.; Liu, X.; Zhang, H.; Lu, Y.; Su, S. Curcumin enhanced antiproliferative effect of mitomycin C in human breast cancer MCF-7 cells in vitro and in vivo. Acta Pharmacol. Sin. 2011, 32, 1402–1410. [Google Scholar] [CrossRef] [Green Version]
- Landen, N.X.; Li, D.; Stahle, M. Transition from inflammation to proliferation: A critical step during wound healing. Cell. Mol. Life Sci. 2016, 73, 3861–3885. [Google Scholar] [CrossRef] [Green Version]
- Kanai, M.; Otsuka, Y.; Otsuka, K.; Sato, M.; Nishimura, T.; Mori, Y.; Kawaguchi, M.; Hatano, E.; Kodama, Y.; Matsumoto, S.; et al. A phase I study investigating the safety and pharmacokinetics of highly bioavailable curcumin (Theracurmin) in cancer patients. Cancer Chemother. Pharmacol. 2013, 71, 1521–1530. [Google Scholar] [CrossRef] [Green Version]
- Storka, A.; Vcelar, B.; Klickovic, U.; Gouya, G.; Weisshaar, S.; Aschauer, S.; Bolger, G.; Helson, L.; Wolzt, M. Safety, tolerability and pharmacokinetics of liposomal curcumin in healthy humans. Int. J. Clin. Pharmacol. Ther. 2015, 53, 54–65. [Google Scholar] [CrossRef] [PubMed]
- Dykman, L.A.; Khlebtsov, N.G. Immunologicalproperties of gold nanoparticles. Chem. Sci. 2017, 8, 1719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vemuri, S.K.; Banala, R.R.; Sudip, M.; Uppula, P.; Subbaiah, G.P.V.; Reddy, A.V.G.; Mallarvili, T. Novel biosynthesized gold nanoparticles as anti-cancer agents against breast cancer: Synthesis, biological evaluation, molecular modelling studies. Mater. Sci. Eng. C 2019, 99, 417–429. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Rong, Y.; Teng, Y.; Mu, J.; Zhuang, X.; Tseng, M.; Samykutty, A.; Zhang, L.; Yan, J.; Miller, D.; et al. Broccoli-Derived Nanoparticle Inhibits Mouse Colitis by Activating Dendritic Cell AMP-Activated Protein Kinase. Mol. Ther. 2017, 25, 1641–1654. [Google Scholar] [CrossRef] [Green Version]
- Nigjeh, S.E.; Yeap, S.K.; Nordin, N.; Rahman, H.; Rosli, R. In Vivo Anti-Tumor Effects of Citral on 4T1 Breast Cancer Cells via Induction of Apoptosis and Downregulation of Aldehyde Dehydrogenase Activity. Molecules 2019, 24, 3241. [Google Scholar] [CrossRef] [Green Version]
- Balakrishnan, S.; Mukherjee, S.; Das, S.; Bhat, F.; Rajasingh, A.P.; Patra, C.R. Gold nanoparticles–conjugated quercetin induces apoptosis via inhibition of EGFR/PI3K/Akt–mediated pathway in breast cancer cell lines (MCF-7 and MDA-MB 231). Cell Biochem. Funct. 2017, 35, 217–231. [Google Scholar] [CrossRef]
- Laha, D.; Pal, K.C.; Pravat, K.P.; Sumanta, K.S.; Kuladip, J.; Parimal, K. Fabrication of curcumin loaded folic acid tagged metal organic framework for triple negative breast cancer therapy in in vitro and in vivo system. New. J. Chem. 2019, 43, 217–229. [Google Scholar] [CrossRef]
- Vemuri, S.K.; Banala, R.R.; Subbaiah, G.P.V.; Srivastava, S.K.; Reddy, A.V.G.; Malarvili, T. The Anti-Cancer Activities of Natural Extract (NE) mix in Human Breast Cancer Cell Lines Are Mediated through Caspase-Dependent and p53-Independent Pathways. Egypt. J. Basic Appl. Sci. 2017, 4, 332–344. [Google Scholar] [CrossRef]
- Marina, A.D.; Anil, K.P.; Jiwen, Z.; Jeffrey, D.C.; Nader, A.; Parag, A.; Barry, W.N.B.S.; McNeil, S.E. Interaction of colloidal gold nanoparticles with human blood: Effects on particle size and analysis of plasma protein binding profiles. Nanomed. Nanotechnol. Biol. Med. 2009, 5, 106–107. [Google Scholar]
- Gunnarsson, S.B.; Bernfur, K.; Englund-Johansson, U.; Johansson, F.; Cedervall, T. Analysis of complexes formed by small gold nanoparticles in low concentration in cell culture media. PLoS ONE 2019, 14, e021821. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shakibaei, M.; Buhrmann, C.; Kraehe, P.; Shayan, P.; Lueders, C.; Goel, A. Curcumin chemosensitizes 5-fluorouracil resistant MMR-deficient human colon cancer cells in high density cultures. PLoS ONE 2014, 9, e85397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banerjee, S.; Singh, S.K.; Chowdhury, I.; Lillard, J.W., Jr.; Singh, R. Combinatorial effect of curcumin with docetaxel modulates apoptotic and cell survival molecules in prostate cancer. Front. Biosci. 2017, 9, 235–245. [Google Scholar]
- Lina, A.A.-A.; Farkaad, A.K.; Najihah, M.H.; Nurhidayatullaili, M.; Julkapli, A.S.; Jun, L.; Mohammed, A.A.; Wageeh, A.Y. The impact of curcumin-graphene based nanoformulation on cellular interaction and redox-activated apoptosis: An in vitro colon cancer study. Heliyon 2020, 6, e05360. [Google Scholar] [CrossRef]
- Karimi, F.; Shaabani, E.; Martínez-Rovira, I.; Yousef, I.; Ghahremani, M.H.; Kharrazi, S. Infrared microspectroscopy studies on the protective effect of curcumin coated gold nanoparticles against H2O2-induced oxidative stress in human neuroblastoma SK-N-SH cells. Analyst 2021, 146, 6902–6916. [Google Scholar] [CrossRef]
- Lee, W.H.; Loo, C.-Y.; Traini, D.; Young, P.M. Development and Evaluation of Paclitaxel and Curcumin Dry Powder for Inhalation Lung Cancer Treatment. Pharmaceutics 2021, 13, 9. [Google Scholar] [CrossRef] [PubMed]
- Chang, P.; Peng, S.; Lee, C.; Lu, C.; Tsai, S.; Shieh, T.; Wu, T.; Tu, M.; Chen, M.Y.; Yang, J.S. Curcumin-loaded nanoparticles induce apoptotic cell death through regulation of the function of MDR1 and reactive oxygen species in cisplatin-resistant CAR human oral cancer cells. Int. J. Oncol. 2013, 43, 1141–1150. [Google Scholar] [CrossRef] [Green Version]
- Chun, Y.S.; Bisht, S.; Chenna, V.; Pramanik, D.; Yoshida, T.; Hong, S.M.; de Wilde, R.F.; Zhang, Z.; Huso, D.L.; Zhao, M.; et al. Intraductal administration of a polymeric nanoparticle formulation of curcumin (NanoCurc) significantly attenuates incidence of mammary tumors in a rodent chemical carcinogenesis model: Implications for breast cancer chemoprevention in at-risk populations. Carcinogenesis 2012, 33, 2242–2249. [Google Scholar] [CrossRef] [Green Version]
- Zhai, Z.; Qu, X.; Li, H.; Ouyang, Z.; Yan, W.; Liu, G.; Liu, X.; Fan, Q.; Tang, T.; Dai, K.; et al. Inhibition of MDA-MB-231 breast cancer cell migration and invasion activity by andrographolide via suppression of nuclear factor-κB-dependent matrix metalloproteinase-9 expression. Mol. Med. Rep. 2015, 11, 1139–1145. [Google Scholar] [CrossRef]
- Chen, D.; Dai, F.; Chen, Z.; Wang, S.; Cheng, X.; Sheng, Q.; Lin, J.; Chen, W. Dimethoxy curcumin induces apoptosis by suppressing survivin and inhibits invasion by enhancing E-cadherin in colon cancer cells. Med. Sci. Monit. 2016, 22, 3215–3222. [Google Scholar] [CrossRef] [Green Version]
- Calaf, G.M.; Ponce-Cusi, R.; Carrión, F. Curcumin and paclitaxel induce cell death in breast cancer cell lines. Oncol. Rep. 2008, 40, 2381–2388. [Google Scholar] [CrossRef]
- Liang, C.C.; Park, A.Y.; Guan, J.L. In vitro scratch assay: A convenient and inexpensive method for analysis of cell migration in vitro. Nat. Protoc. 2007, 2, 329–333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kabała-Dzik, A.; Rzepecka-Stojko, A.; Robert, K.; Jastrzębska-Stojko, Z.; Rafał, S.; Robert, D.W.; Jerzy, S. Migration Rate Inhibition of Breast Cancer Cells Treated by Caffeic Acid and Caffeic Acid Phenethyl Ester: An In Vitro Comparison Study. Nutrients 2017, 9, 1144. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Chen, X.; Guan, S.; Yan, Y.; Lin, H.; Hua, Z.C. Curcumin inhibits angiogenesis and improves defective hematopoiesis induced by tumor-derived VEGF in tumor model through modulating VEGF-VEGFR2 signaling pathway. Oncotarget. 2015, 6, 19469–19482. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Gan, C.; Zhang, Y.; Yu, Y.; Fan, C.; Deng, Y.; Zhang, Q.; Yu, X.; Zhang, Y.; Wang, L.; et al. Inhibition of Stat3 Signaling Pathway by Natural Product Pectolinarigenin Attenuates Breast Cancer Metastasis. Front. Pharmacol. 2019, 10, 1195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, T.; Li, S.; Li, J.; Yin, F.; Hua, Y.; Wang, Z. Natural product pectolinarigenin inhibits osteosarcoma growth and metastasis via SHP-1-mediated STAT3 signaling inhibition. Cell Death Dis. 2016, 7, 305. [Google Scholar] [CrossRef] [Green Version]
- Quispe-Soto, E.T.; Calaf, G.M. Effect of curcumin and paclitaxel on breast carcinogenesis. Int. J. Oncol. 2016, 49, 2569–2577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, X.; Loc, W.S.; Dong, C.; Matters, G.L.; Butler, P.J.; Kester, M.; Meyers, C.; Jiang, Y.; Adair, J.H. The use of nanoparticulates to treat breast cancer. Nanomedicine 2017, 12, 2367–2388. [Google Scholar] [CrossRef]
- Mahesh, P.M.; Sagar, R.P.; Gaurav, A.S.; Mahesh, N.S.; Prashant, K.D.; Jitendra, B.N.; Bhijeet, D.K. Recent advances in phytochemical-based Nano-formulation for drug-resistant Cancer. Med. Drug Discov. 2021, 10, 10008. [Google Scholar]
- Balakrishnan, S.; Bhat, F.A.; Singh, P.R.; Mukherjee, S.; Elumalai, P.; Das, S.; Patra, C.R.; Arunakaran, J. Gold nanoparticle-conjugated quercetin inhibits epithelial-mesenchymal transition, angiogenesis and invasiveness via EGFR/VEGFR-2-mediated pathway in breast cancer. Cell Prolif. 2021, 49, 678–697. [Google Scholar] [CrossRef]
- Mantle, D.; Lennard, T.W.; Pickering, A.T. Therapeutic applications of medicinal plants in the treatment of breast cancer: A review of their pharmacology, efficacy and tolerability. Advers. Drug React. Toxicol. Rev. 2000, 19, 223–240. [Google Scholar]
- Kapinova, A.; Stefanicka, P.; Kubatka, P.; Zubor, P.; Uramova, S.; Kello, M. Are plant-based functional foods better choice against cancer than single phytochemicals? A critical review of current breast cancer research. Biomed. Pharmacother. 2017, 96, 1465–1477. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Chae, S.Y.; Park, J.O.; Lee, K.J.; Park, G. Gold-conjugated resveratrol nanoparticles attenuate the invasion and MMP-9 and COX-2 expression in breast cancer cells. Oncol. Rep. 2016, 35, 3248–3256. [Google Scholar] [CrossRef] [PubMed]
- Varlamova, E.G.; Goltyaev, M.V.; Mal’tseva, V.N.; Turovsky, E.A.; Sarimov, R.M.; Simakin, A.V.; Gudkov, S.V. Mechanisms of the Cytotoxic Effect of Selenium Nanoparticles in Different Human Cancer Cell Lines. Int. J. Mol. Sci. 2021, 22, 7798. [Google Scholar] [CrossRef] [PubMed]
- Turovsky, E.A.; Varlamova, E.G. Mechanism of Ca2+ Dependent Pro-Apoptotic Action of Selenium Nanoparticles, Mediated by Activation of Cx43 Hemichannels. Biology 2021, 10, 743. [Google Scholar] [CrossRef] [PubMed]
- Lui, D.; Chen, Z.; Medarde, F.; Santos, E. The effect of curcumin on breast cancer cells. J. Breast Cancer 2013, 16, 133–137. [Google Scholar]
- Liu, X.; Kim, C.N.; Yang, J.; Jemmerson, R.; Wang, X. Induction of apoptotic program in cell-free extracts: Requirement for dATP and cytochomec. Cell 1996, 86, 147–157. [Google Scholar] [CrossRef] [Green Version]
- Thakur, C.; Chen, B.; Li, L.; Zhang, Q.; Yang, Z.Q.; Chen, F. Loss of mdig expression enhances DNA and histone methylation and metastasis of aggressive breast cancer. Signal Transduct. Target. Ther. 2018, 3, 25. [Google Scholar] [CrossRef] [Green Version]
- Flores, F.F.; Suarez, J.A.Q.; Pardi, P.C.; Maria, D.A. DM-1, sodium 4-(5-(4-hydroxy-3-methoxyphenyl)-3-oxo-penta-1,4-dienyl)-2-methoxy-phenolate: A curcumin analog with a synergic effect in combination with paclitaxel in breast cancer treatment. Tumor Biol. 2012, 33, 775–785. [Google Scholar] [CrossRef]
- Banerjee, M.; Singh, P.; Panda, D. Curcumin suppresses the dynamic instability of microtubules, activates the mitotic checkpoint and induces apoptosis in MCF-7 cells. FEBS J. 2010, 277, 3437–3448. [Google Scholar] [CrossRef]
- Zhang, J.; Haines, C.; Watson, A.J.M.; Hart, A.R.; Platt, M.J.; Pardoll, D.M.; Cosgrove, S.E.; Gebo, K.A.; Sears, C.L. Oral antibiotic use and risk of colorectal cancer in the United Kingdom, 1989-2012: A matched case-control study. Gut 2019, 68, 1971–1978. [Google Scholar] [CrossRef] [PubMed]
- Karuppaiya, P.; Satheeshkumar, E.; Chao, W.T.; Kao, L.Y.; Chen, E.C.; Tsay, H.S. Anti-metastatic activity of biologically synthesized gold nanoparticles on human fibrosarcoma cell line HT-1080. Colloids Surf. B. Biointerfaces 2013, 110, 163–170. [Google Scholar] [CrossRef]
- Zhan, Y.; Chen, Y.; Liu, R.; Zhang, H.; Zhang, Y. Potentiation of paclitaxel activity by curcumin in human breast cancer cell by modulating apoptosis and inhibiting EGFR signaling. Arch. Pharm. Res. 2014, 37, 1086–1095. [Google Scholar] [CrossRef]
- Boztas, A.O.; Karakuzu, O.; Galante, G.; Ugur, Z.; Kocabas, F.; Altuntas, C.Z.; Yazaydin, A.O. Synergistic interaction of paclitaxel and curcumin with cyclodextrin polymer complexation in human cancer cells. Mol. Pharm. 2013, 10, 2676–2683. [Google Scholar] [CrossRef] [PubMed]
- Bayet-Robert, M.; Kwiatkowski, F.; Leheurteur, M.; Gachon, F.; Planchat, E.; Abrial, C.; Mouret-Reynier, M.A.; Durando, X.; Barthomeuf, C.; Chollet, P. Phase I dose escalation trial of docetaxel plus curcumin in patients with advanced and metastatic breast cancer. Cancer Biol. Ther. 2010, 9, 8–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, C.Q.; Xing, L.; Cui, P.F.; Qiao, J.B.; He, Y.J.; Chen, B.A.; Jin, L.; Jiang, H.L. Curcumin-coordinated nanoparticles with improved stability for reactive oxygen species-responsive drug delivery in lung cancer therapy. Int. J. Nanomed. 2017, 25, 855–869. [Google Scholar] [CrossRef] [Green Version]
- Epelbaum, R.; Schaffer, M.; Vizel, B.; Badmaev, V.; Bar-Sela, G. Curcumin and gemcitabine in patients with advanced pancreatic cancer. Nutr. Cancer 2010, 62, 1137–1141. [Google Scholar] [CrossRef]
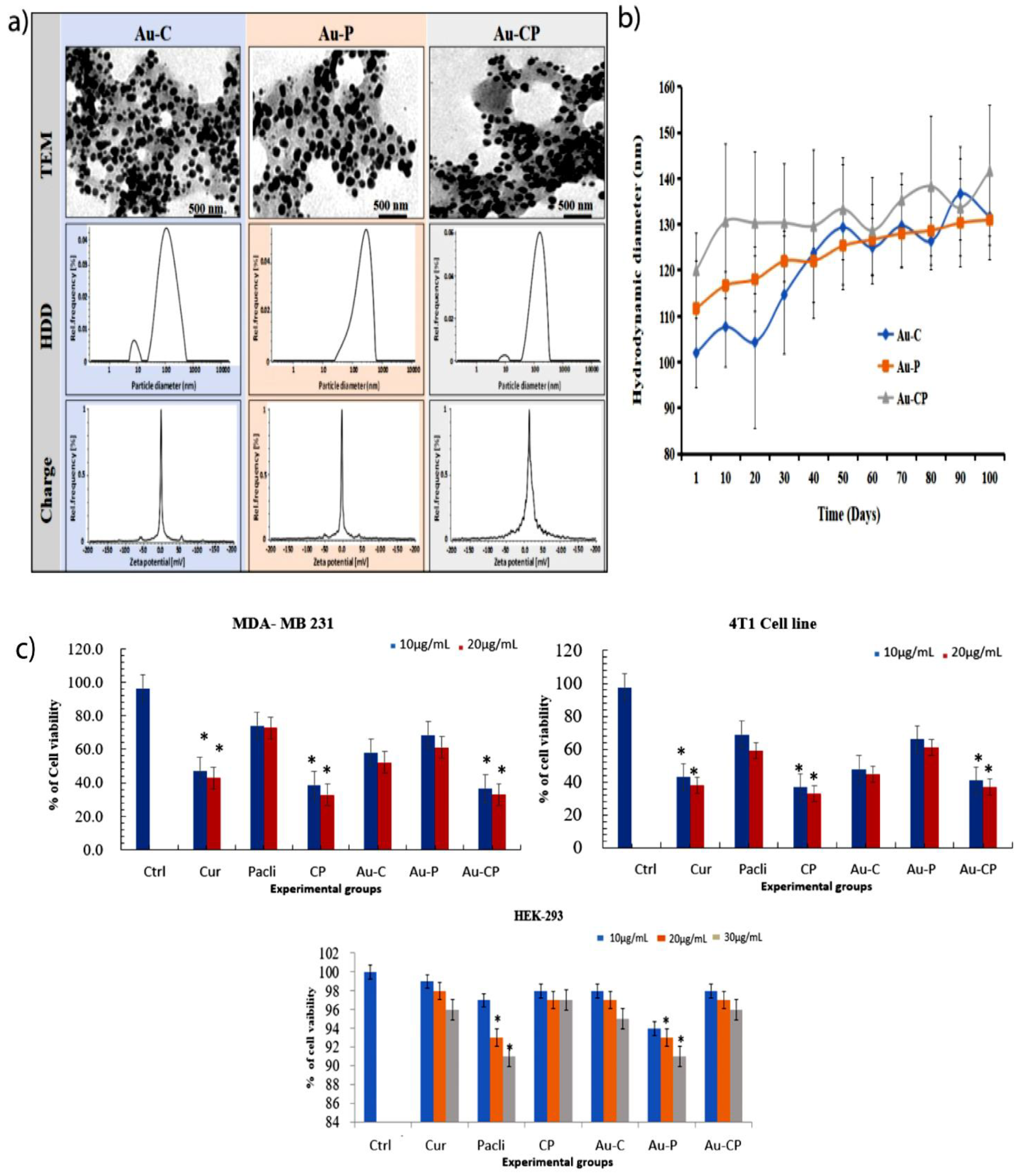

| Nanoparticles | In Water | In 10% Serum-Containing Medium | ||||
|---|---|---|---|---|---|---|
| HDD (mm) | Zeta Potentials (mV) | PDI% | HDD (mm) | Zeta Potentials (mV) | PDI% | |
| Au-C | 101.5 ± 15 | −0.2 ± 0.1 | 25.2 ± 2.5 | 152 ± 8 | −6.9 ± 1.5 | 23.1 ± 1.5 |
| Au-P | 115 ± 9 | −5.8 ± 2.1 | 23.9 ± 3.3 | 140 ± 15 | −8.2 ± 2.3 | 20.2 ± 4.3 |
| Au-CP | 128 ± 10 | −3.0 ± 1.1 | 25.5 ± 1.2 | 166 ± 6 | −3.9 ± 1.12 | 22.41 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vemuri, S.K.; Halder, S.; Banala, R.R.; Rachamalla, H.K.; Devraj, V.M.; Mallarpu, C.S.; Neerudu, U.K.; Bodlapati, R.; Mukherjee, S.; Venkata, S.G.P.; et al. Modulatory Effects of Biosynthesized Gold Nanoparticles Conjugated with Curcumin and Paclitaxel on Tumorigenesis and Metastatic Pathways—In Vitro and In Vivo Studies. Int. J. Mol. Sci. 2022, 23, 2150. https://doi.org/10.3390/ijms23042150
Vemuri SK, Halder S, Banala RR, Rachamalla HK, Devraj VM, Mallarpu CS, Neerudu UK, Bodlapati R, Mukherjee S, Venkata SGP, et al. Modulatory Effects of Biosynthesized Gold Nanoparticles Conjugated with Curcumin and Paclitaxel on Tumorigenesis and Metastatic Pathways—In Vitro and In Vivo Studies. International Journal of Molecular Sciences. 2022; 23(4):2150. https://doi.org/10.3390/ijms23042150
Chicago/Turabian StyleVemuri, Satish Kumar, Satyajit Halder, Rajkiran Reddy Banala, Hari Krishnreddy Rachamalla, Vijaya Madhuri Devraj, Chandra Shekar Mallarpu, Uttam Kumar Neerudu, Ravikiran Bodlapati, Sudip Mukherjee, Subbaiah Goli Peda Venkata, and et al. 2022. "Modulatory Effects of Biosynthesized Gold Nanoparticles Conjugated with Curcumin and Paclitaxel on Tumorigenesis and Metastatic Pathways—In Vitro and In Vivo Studies" International Journal of Molecular Sciences 23, no. 4: 2150. https://doi.org/10.3390/ijms23042150
APA StyleVemuri, S. K., Halder, S., Banala, R. R., Rachamalla, H. K., Devraj, V. M., Mallarpu, C. S., Neerudu, U. K., Bodlapati, R., Mukherjee, S., Venkata, S. G. P., Venkata, G. R. A., Thakkumalai, M., & Jana, K. (2022). Modulatory Effects of Biosynthesized Gold Nanoparticles Conjugated with Curcumin and Paclitaxel on Tumorigenesis and Metastatic Pathways—In Vitro and In Vivo Studies. International Journal of Molecular Sciences, 23(4), 2150. https://doi.org/10.3390/ijms23042150







