Sustained Functioning Impairments and Oxidative Stress with Neurobehavioral Dysfunction Associated with Oral Nicotine Exposure in the Brain of a Murine Model of Ehrlich Ascites Carcinoma: Modifying the Antioxidant Role of Chlorella vulgaris
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Tested Substances and Chemicals
2.2. Quantitative Assessment of Different Components of Chlorella vulgaris by HPLC
2.3. Animals
2.4. Experimental Design
2.5. Ehrlich Ascites Carcinoma Cells
2.6. Behavioral Response Evaluation
2.6.1. Open Field Test
2.6.2. Inclined Plane Test
2.6.3. Tail Suspension Test
2.6.4. The Postural Reflex Test
2.6.5. Swimming Performance Test
2.7. Sampling
2.8. Determination of the Levels of the Neurotransmitters
2.9. Assays of Antioxidant Enzymes and Oxidative Stress Indicators (MDA and PC) and Proinflammatory Cytokines
2.10. Comet Assay
2.11. Histopathological Evaluation
2.12. Bcl-2 and Caspase-3 Immunohistochemical Investigation
2.13. Statistical Analysis
3. Results
3.1. The HPLC Analysis of CV Main Components
3.2. Behavioral Observations
3.3. Mortalities
3.4. Effect of Nicotine and/or CV on the Antioxidant Enzymes and Oxidative Stress Biomarkers in EAC Swiss Female Mice
3.5. Effect of Nicotine Exposur, CV Exposure, or Both on Brain Neurotransmitter Levels
3.6. Effect of Nicotine Exposure, CV Exposure, or Both on Inflammatory Markers
3.7. Effect on DNA Damage (Comet Assay and 8-OHDG)
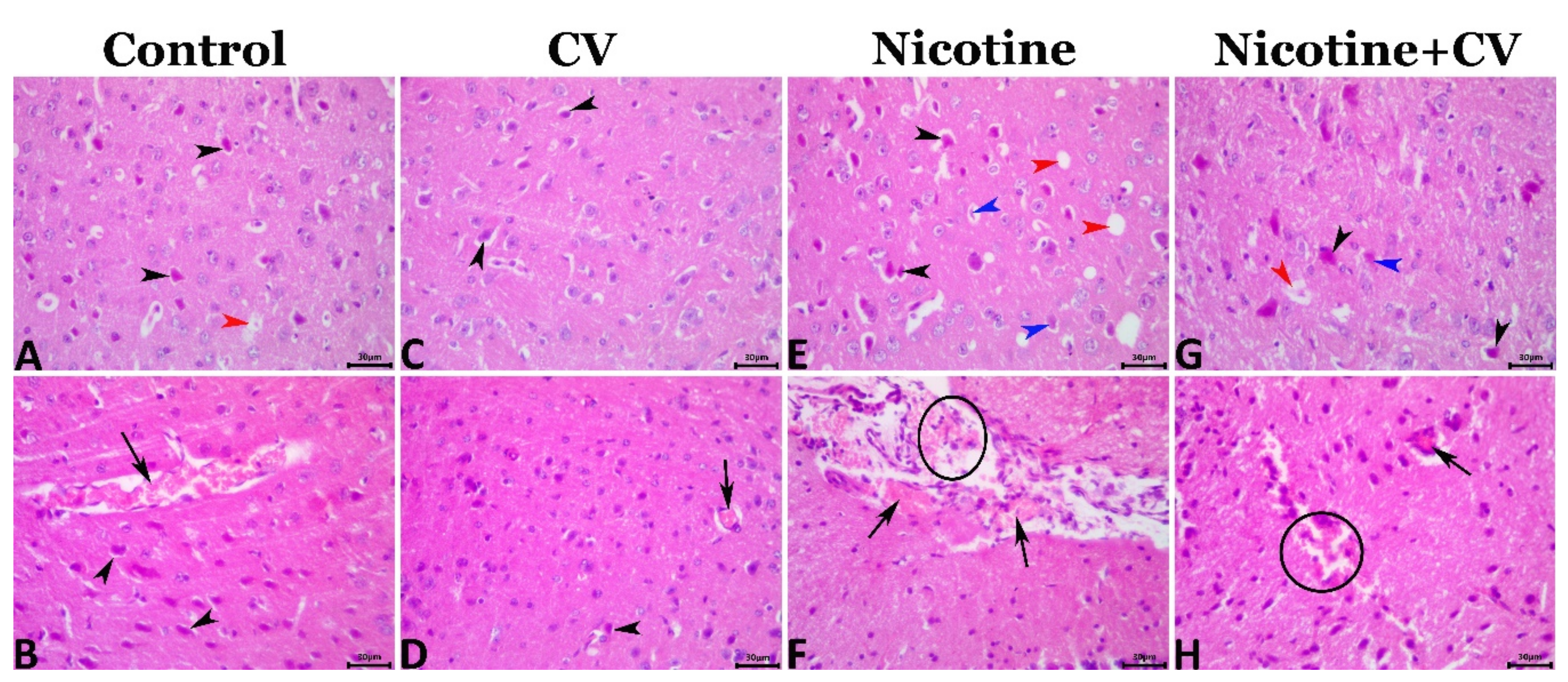
3.8. Pathological Findings
3.9. Immunohistochemical Findings
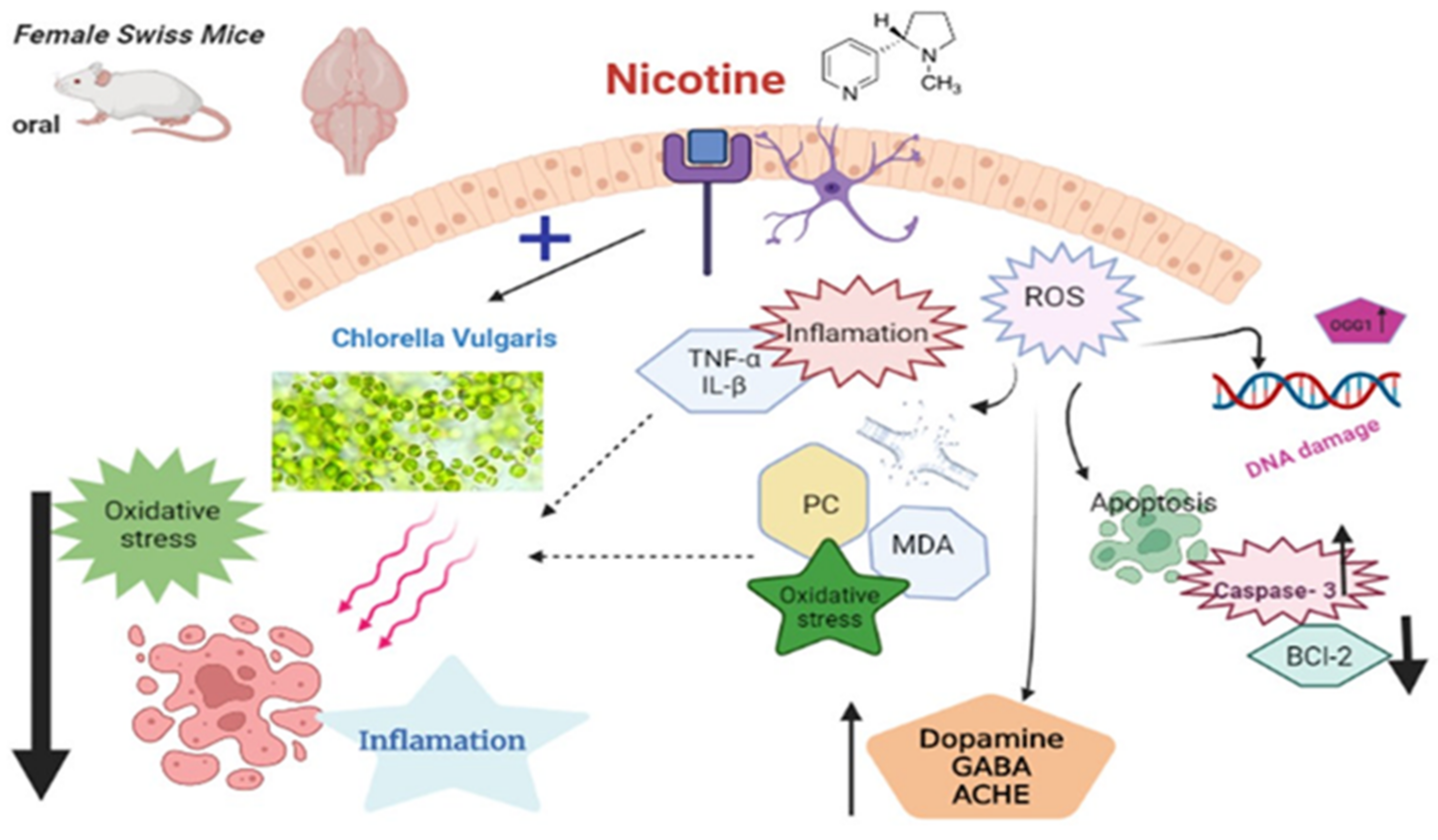
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ali, S.; Misganaw, A.; Worku, A.; Destaw, Z.; Negash, L.; Bekele, A.; Briant, P.S.; Johnson, C.O.; Alam, T.; Odell, C.; et al. The burden of cardiovascular diseases in Ethiopia from 1990 to 2017: Evidence from the Global Burden of Disease Study. Int. Health 2021, 13, 318–326. [Google Scholar] [CrossRef]
- Jensen, E.X.; Peheim, E.; Horber, F.F.; Fusch, C.; Jaeger, P. Impact of chronic cigarette smoking on body composition and fuel metabolism. J. Clin. Endocrinol. Metab. 1995, 80, 2181–2185. [Google Scholar] [CrossRef]
- Peto, R.; Boreham, J.; Lopez, A.D.; Thun, M.; Heath, C. Mortality from tobacco in developed countries: Indirect estimation from national vital statistics. Lancet 1992, 339, 1268–1278. [Google Scholar] [CrossRef]
- Jassem, J. Tobacco smoking after diagnosis of cancer: Clinical aspects. Transl. Lung Cancer Res. 2019, 8, S50–S58. [Google Scholar] [CrossRef]
- Courtney, R. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General, 2014 U.S. Department of Health and Human Services Atlanta, GA: Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health. 2014; p. 1081. 2015. Available online: http://www.surgeongeneral.gov/library/reports/50-years-of-progress (accessed on 9 December 2021).
- Liang, Z.; Wu, R.; Xie, W.; Xie, C.; Wu, J.; Geng, S.; Li, X.; Zhu, M.; Zhu, W.; Zhu, J.; et al. Effects of Curcumin on Tobacco Smoke-induced Hepatic MAPK Pathway Activation and Epithelial-Mesenchymal TransitionIn Vivo. Phytother. Res. 2017, 31, 1230–1239. [Google Scholar] [CrossRef]
- Zhao, H.; Albino, A.P.; Jorgensen, E.; Traganos, F.; Darzynkiewicz, Z. DNA damage response induced by tobacco smoke in normal human bronchial epithelial and A549 pulmonary adenocarcinoma cells assessed by laser scanning cytometry. Cytom. Part A 2009, 75, 840–847. [Google Scholar] [CrossRef] [Green Version]
- Ozaslan, M.; Karagoz, I.D.; Kilic, I.H.; Guldur, M.E. Ehrlich ascites carcinoma. Afr. J. Biotechnol. 2011, 10, 2375–2378. [Google Scholar]
- Da Silva, S.L.; Chaar, J.D.S.; Yano, T. Chemotherapeutic potential of two gallic acid derivative compounds from leaves of Casearia sylvestris Sw (Flacourtiaceae). Eur. J. Pharmacol. 2009, 608, 76–83. [Google Scholar] [CrossRef]
- Mohamed, A.A.-R.; Rahman, A.N.A.; Mohammed, H.H.; Ebraheim, L.L.; Abo-ElMaaty, A.M.; Ali, S.A.; Elhady, W.M. Neurobehavioral, apoptotic, and DNA damaging effects of sub-chronic profenofos exposure on the brain tissue of Cyprinus carpio L.: Antagonistic role of Geranium essential oil. Aquat. Toxicol. 2020, 224, 105493. [Google Scholar] [CrossRef]
- Rahman, A.N.A.; Mohamed, A.; Mohammed, H.H.; Elseddawy, N.M.; Salem, G.A.; El-Ghareeb, W.R. The ameliorative role of geranium (Pelargonium graveolens) essential oil against hepato-renal toxicity, immunosuppression, and oxidative stress of profenofos in common carp, Cyprinus carpio (L.). Aquaculture 2020, 517, 734777. [Google Scholar] [CrossRef]
- Elewa, Y.H.; Mohamed, A.A.-R.; Galal, A.A.; El-Naseery, N.I.; Ichii, O.; Kon, Y. Food Yellow4 reprotoxicity in relation to localization of DMC1 and apoptosis in rat testes: Roles of royal jelly and cod liver oil. Ecotoxicol. Environ. Saf. 2019, 169, 696–706. [Google Scholar] [CrossRef]
- Hashem, M.A.; Shoeeb, S.B.; Abd-Elhakim, Y.M.; Mohamed, W.A. The antitumor activity of Arthrospira platensis and/or cisplatin in a murine model of Ehrlich ascites carcinoma with hematinic and hepato-renal protective action. J. Funct. Foods 2020, 66, 103831. [Google Scholar] [CrossRef]
- Bito, T.; Okumura, E.; Fujishima, M.; Watanabe, F. Potential of Chlorella as a Dietary Supplement to Promote Human Health. Nutrients 2020, 12, 2524. [Google Scholar] [CrossRef]
- Galal, A.A.; Reda, R.M.; Mohamed, A. Influences of Chlorella vulgaris dietary supplementation on growth performance, hematology, immune response and disease resistance in Oreochromis niloticus exposed to sub-lethal concentrations of penoxsulam herbicide. Fish Shellfish Immunol. 2018, 77, 445–456. [Google Scholar] [CrossRef]
- Montoya, E.Y.O.; A Casazza, A.; Aliakbarian, B.; Perego, P.; Converti, A.; De Carvalho, J.C.M. Production of Chlorella vulgarisas a source of essential fatty acids in a tubular photobioreactor continuously fed with air enriched with CO2 at different concentrations. Biotechnol. Prog. 2014, 30, 916–922. [Google Scholar] [CrossRef]
- Rani, K.; Sandal, N.; Sahoo, P. A comprehensive review on chlorella-its composition, health benefits, market and regulatory scenario. Pharma Innov. J. 2018, 7, 584–589. [Google Scholar]
- Shibata, S.; Natori, Y.; Nishihara, T.; Tomisaka, K.; Matsumoto, K.; Sansawa, H.; Nguyen, V.C. Antioxidant and Anti-Cataract Effects of Chlorella on Rats with Streptozotocin-Induced Diabetes. J. Nutr. Sci. Vitaminol. 2003, 49, 334–339. [Google Scholar] [CrossRef]
- Konishi, F.; Tanaka, K.; Himeno, K.; Taniguchi, K.; Nomoto, K. Antitumor effect induced by a hot water extract of Chlorella vulgaris (CE): Resistance to meth-A tumor growth mediated by CE-induced polymorphonuclear leukocytes. Cancer Immunol. Immunother. 1985, 19, 73–78. [Google Scholar] [CrossRef]
- Tanaka, K.; Tomita, Y.; Tsuruta, M.; Konishi, F.; Okuda, M.; Himeno, K.; Nomoto, K. Oral Administration ofChlorella VulgarisAugments Concomitant Antitumor Immunity. Immunopharmacol. Immunotoxicol. 1990, 12, 277–291. [Google Scholar] [CrossRef]
- Guzman, S.; Gato, A.; Lamela, M.; Freire-Garabal, M.; Calleja, J. Anti-inflammatory and immunomodulatory activities of polysaccharide from Chlorella stigmatophora and Phaeodactylum tricornutum. Phytother. Res. 2003, 17, 665–670. [Google Scholar] [CrossRef]
- Miranda, M.S.; Sato, S.; Mancini-Filho, J. Antioxidant activity of the microalga Chlorella vulgaris cultered on special conditions. Boll. Chim. Farm. 2001, 140, 165–168. [Google Scholar]
- Beheshtipour, H.; Mortazavian, A.M.; Mohammadi, R.; Sohrabvandi, S.; Khosravi-Darani, K. Supplementation of Spirulina platensis and Chlorella vulgaris algae into probiotic fermented milks. Compr. Rev. Food Sci. Food Saf. 2013, 12, 144–154. [Google Scholar] [CrossRef]
- Grammes, F.; Reveco, F.E.; Romarheim, O.H.; Landsverk, T.; Mydland, L.T.; Øverland, M. Candida utilis and Chlorella vulgaris Counteract Intestinal Inflammation in Atlantic Salmon (Salmo salar L.). PLoS ONE 2013, 8, e83213. [Google Scholar] [CrossRef] [Green Version]
- Merchant, R.E.; Andre, C.A. A review of recent clinical trials of the nutritional supplement Chlorella pyrenoidosa in the treatment of fibromyalgia, hypertension, and ulcerative colitis. Altern. Ther. Health Med. 2001, 7, 79–92. [Google Scholar]
- Panahi, Y.; Darvishi, B.; Jowzi, N.; Beiraghdar, F.; Sahebkar, A. Chlorella vulgaris: A Multifunctional Dietary Supplement with Diverse Medicinal Properties. Curr. Pharm. Des. 2016, 22, 164–173. [Google Scholar] [CrossRef]
- Kim, S.-K. Marine Cosmeceuticals: Trends and Prospects; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- Lee, S.H.; Kang, H.J.; Lee, H.-J.; Kang, M.-H.; Park, Y.K. Six-week supplementation with Chlorella has favorable impact on antioxidant status in Korean male smokers. Nutrition 2010, 26, 175–183. [Google Scholar] [CrossRef]
- Elsawi, S.A.; Aly, H.F.; Elbatanony, M.M.; Maamoun, A.A.; Mowawad, D.M. Phytochemical evaluation of Lagerstroemia indica (L.) Pers leaves as anti-Alzheimer’s. J. Mater Environ. Sci. 2018, 9, 2575–2586. [Google Scholar]
- Sparks, J.A.; Pauly, J.R. Effects of continuous oral nicotine administration on brain nicotinic receptors and responsiveness to nicotine in C57Bl/6 mice. Psychopharmacology 1999, 141, 145–153. [Google Scholar] [CrossRef]
- Justo, G.Z.; Silva, M.R.; Queiroz, M.L. Effects of the green algae Chlorella vulgaris on the response of the host hematopoietic system to intraperitoneal Ehrlich ascites tumor transplantation in mice. Immunopharmacol. Immunotoxicol. 2001, 23, 119–132. [Google Scholar] [CrossRef]
- Mathews, H.L.; Stitzel, J.A. The effects of oral nicotine administration and abstinence on sleep in male C57BL/6J mice. Psychopharmacology 2019, 236, 1335–1347. [Google Scholar] [CrossRef]
- Scheid, M.; Boyse, E.A.; Carswell, E.A.; Old, L.J. Serologically Demonstrable Alloantigens of Mouse Epidermal Cells. J. Exp. Med. 1972, 135, 938–955. [Google Scholar] [CrossRef]
- Salem, F.S.; Badr, M.O.T.; Neamat-Allah, A.N.F. Biochemical and pathological studies on the effects of levamisole and chlorambucil on Ehrlich ascites carcinoma-bearing mice. Veter. Ital. 2011, 47, 89–95. [Google Scholar]
- Abd-Elhakim, Y.M.; Mohammed, H.H.; Mohamed, W.A.M. Imidacloprid Impacts on Neurobehavioral Performance, Oxidative Stress, and Apoptotic Events in the Brain of Adolescent and Adult Rats. J. Agric. Food Chem. 2018, 66, 13513–13524. [Google Scholar] [CrossRef]
- Contó, M.B.; de Carvalho, J.G.B.; Benedito, M.A.C. Behavioral differences between subgroups of rats with high and low threshold to clonic convulsions induced by DMCM, a benzodiazepine inverse agonist. Pharmacol. Biochem. Behav. 2005, 82, 417–426. [Google Scholar] [CrossRef]
- Abou-Donia, M.B.; Dechkovskaia, A.M.; Goldstein, L.B.; Abdel-Rahman, A.; Bullman, S.L.; Khan, W.A. Co-exposure to pyridostigmine bromide, DEET, and/or permethrin causes sensorimotor deficit and alterations in brain acetylcholinesterase activity. Pharmacol. Biochem. Behav. 2004, 77, 253–262. [Google Scholar] [CrossRef]
- Yonemori, F.; Yamaguchi, T.; Yamada, H.; Tamura, A. Evaluation of a Motor Deficit after Chronic Focal Cerebral Ischemia in Rats. J. Cereb. Blood Flow Metab. 1998, 18, 1099–1106. [Google Scholar] [CrossRef]
- Chermat, R.; Thierry, B.; Mico, J.A.; Steru, L.; Simon, P. Adaptation of the tail suspension test to the rat. J. Pharmacol. 1986, 17, 348–350. [Google Scholar]
- Bederson, J.B.; Pitts, L.H.; Germano, S.M.; Nishimura, M.C.; Davis, R.L.; Bartkowski, H.M. Evaluation of 2,3,5-triphenyltetrazolium chloride as a stain for detection and quantification of experimental cerebral infarction in rats. Stroke 1986, 17, 1304–1308. [Google Scholar] [CrossRef] [Green Version]
- Schapiro, S.; Salas, M.; Vukovich, K. Hormonal Effects on Ontogeny of Swimming Ability in the Rat: Assessment of Central Nervous System Development. Science 1970, 168, 147–151. [Google Scholar] [CrossRef]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Singh, N.P.; McCoy, M.T.; Tice, R.R.; Schneider, E.L. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp. Cell Res. 1988, 175, 184–191. [Google Scholar] [CrossRef] [Green Version]
- Ruehl-Fehlert, C.; Kittel, B.; Morawietz, G.; Deslex, P.; Keenan, C.; Mahrt, C.R.; Nolte, T.; Robinson, M.; Stuart, B.P.; Deschl, U. Revised guides for organ sampling and trimming in rats and mice–part 1: A joint publication of the RITA and NACAD groups. Exp. Toxicol. Pathol. 2003, 55, 91–106. [Google Scholar] [CrossRef] [Green Version]
- Bancroft, J.D.; Gamble, M. Theory and Practice of Histological Techniques; Elsevier Health Sciences: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Mohamed, A.A.-R.; Khater, S.I.; Arisha, A.H.; Metwally, M.M.; Mostafa-Hedeab, G.; El-Shetry, E.S. Chitosan-stabilized selenium nanoparticles alleviate cardio-hepatic damage in type 2 diabetes mellitus model via regulation of caspase, Bax/Bcl-2, and Fas/FasL-pathway. Gene 2021, 768, 145288. [Google Scholar] [CrossRef]
- Hsu, S.M.; Raine, L.; Fanger, H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: A comparison between ABC and unlabeled antibody (PAP) procedures. J. Histochem. Cytochem. 1981, 29, 577–580. [Google Scholar] [CrossRef] [Green Version]
- Breslau, N.; Fenn, N.; Peterson, E.L. Early smoking initiation and nicotine dependence in a cohort of young adults. Drug Alcohol Depend. 1993, 33, 129–137. [Google Scholar] [CrossRef] [Green Version]
- Hartz, S.M.; Horton, A.; Hancock, D.; Baker, T.B.; Caporaso, N.E.; Chen, L.-S.; Hokanson, J.E.; Lutz, S.M.; Marazita, M.L.; McNeil, D.W.; et al. Genetic correlation between smoking behaviors and schizophrenia. Schizophr. Res. 2018, 194, 86–90. [Google Scholar] [CrossRef]
- Martínez-Ortega, J.M.; Franco, S.; Rodríguez-Fernández, J.M.; Gutiérrez-Rojas, L.; Wang, S.; Gurpegui, M. Temporal sequencing of nicotine dependence and major depressive disorder: A U.S. national study. Psychiatry Res. 2017, 250, 264–269. [Google Scholar] [CrossRef]
- Moran, L.V.; Sampath, H.; Kochunov, P.; Hong, L.E. Brain Circuits That Link Schizophrenia to High Risk of Cigarette Smoking. Schizophr. Bull. 2013, 39, 1373–1381. [Google Scholar] [CrossRef] [Green Version]
- Sagud, M.; Peles, A.M.; Pivac, N. Smoking in schizophrenia: Recent findings about an old problem. Curr. Opin. Psychiatry 2019, 32, 402–408. [Google Scholar] [CrossRef]
- Trauth, J.; Seidler, F.; Slotkin, T. Persistent and delayed behavioral changes after nicotine treatment in adolescent rats. Brain Res. 2000, 880, 167–172. [Google Scholar] [CrossRef]
- Ajonijebu, D.; Adeniyi, P.; Adekeye, A.; Olatunji, B.P.; Ishola, A.; Ogundele, O.M. Nicotine-Cadmium Interaction Alters Exploratory Motor Function and Increased Anxiety in Adult Male Mice. J. Neurodegener. Dis. 2014, 2014, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Royal, W.; Bryant, J.; Davis, H.; Guo, M. Cigarette smoke and nicotine effects on behavior in HIV transgenic rats. Behav. Brain Res. 2021, 417, 113591. [Google Scholar] [CrossRef]
- Iwamoto, E.T. An assessment of the spontaneous activity of rats administered morphine, phencyclidine, or nicotine using automated and observational methods. Psychopharmacology 1984, 84, 374–382. [Google Scholar] [CrossRef]
- Acri, J.B.; Grunberg, N.E.; Morse, D.E. Effects of nicotine on the acoustic startle reflex amplitude in rats. Psychopharmacology 1991, 104, 244–248. [Google Scholar] [CrossRef]
- A Trauth, J.; Seidler, F.J.; A Slotkin, T. An animal model of adolescent nicotine exposure: Effects on gene expression and macromolecular constituents in rat brain regions. Brain Res. 2000, 867, 29–39. [Google Scholar] [CrossRef]
- Kelley, B.M.; Middaugh, L.D. Periadolescent Nicotine Exposure Reduces Cocaine Reward in Adult Mice. J. Addict. Dis. 1999, 18, 27–39. [Google Scholar] [CrossRef]
- McCarthy, D.M.; Morgan, T.J., Jr.; Lowe, S.E.; Williamson, M.J.; Spencer, T.J.; Biederman, J.; Bhide, P.G. Nicotine exposure of male mice produces behavioral impairment in multiple generations of descendants. PLoS Biol. 2018, 16, e2006497. [Google Scholar] [CrossRef] [Green Version]
- Mansvelder, H.; McGehee, D.S. Cellular and synaptic mechanisms of nicotine addiction. J. Neurobiol. 2002, 53, 606–617. [Google Scholar] [CrossRef]
- Slotkin, T.A. Nicotine and the adolescent brain Insights from an animal model. Neurotoxicology Teratol. 2002, 24, 369–384. [Google Scholar] [CrossRef]
- Kenny, P.J.; Markou, A. The ups and downs of addiction: Role of metabotropic glutamate receptors. Trends Pharmacol. Sci. 2004, 25, 265–272. [Google Scholar] [CrossRef]
- Janhunen, S.; Ahtee, L. Differential nicotinic regulation of the nigrostriatal and mesolimbic dopaminergic pathways: Implications for drug development. Neurosci. Biobehav. Rev. 2007, 31, 287–314. [Google Scholar] [CrossRef]
- Toth, E.; Sershen, H.; Hashim, A.; Vizi, E.S.; Lajtha, A. Effect of nicotine on extracellular levels of neurotransmitters assessed by microdialysis in various brain regions: Role of glutamic acid. Neurochem. Res. 1992, 17, 265–271. [Google Scholar] [CrossRef]
- Ribeiro, E.B.; Bettiker, R.L.; Bogdanov, M.; Wurtman, R.J. Effects of systemic nicotine on serotonin release in rat brain. Brain Res. 1993, 621, 311–318. [Google Scholar] [CrossRef]
- Takahashi, H.; Takada, Y.; Nagai, N.; Urano, T.; Takada, A. Nicotine increases stress-induced serotonin release by stimulating nicotinic acetylcholine receptor in rat striatum. Synapse 1998, 28, 212–219. [Google Scholar] [CrossRef]
- Alasmari, F.; Alexander, L.E.C.; Hammad, A.M.; Bojanowski, C.M.; Moshensky, A.; Sari, Y. Effects of Chronic Inhalation of Electronic Cigarette Vapor Containing Nicotine on Neurotransmitters in the Frontal Cortex and Striatum of C57BL/6 Mice. Front. Pharmacol. 2019, 10, 885. [Google Scholar] [CrossRef] [Green Version]
- Markou, A. Neurobiology of nicotine dependence. Philos. Trans. R. Soc. B Biol. Sci. 2008, 363, 3159–3168. [Google Scholar] [CrossRef] [Green Version]
- Sudheer, A.R.; Chandran, K.; Marimuthu, S.; Menon, V.P. Ferulic Acid Modulates Altered Lipid Profiles and Prooxidant/Antioxidant Status in Circulation During Nicotine-Induced Toxicity: A Dose-Dependent Study. Toxicol. Mech. Methods 2005, 15, 375–381. [Google Scholar] [CrossRef]
- Sudheer, A.R.; Muthukumaran, S.; Devipriya, N.; Menon, V.P. Ellagic acid, a natural polyphenol protects rat peripheral blood lymphocytes against nicotine-induced cellular and DNA damage in vitro: With the comparison of N-acetylcysteine. Toxicology 2007, 230, 11–21. [Google Scholar] [CrossRef]
- Chattopadhyay, K.; Chattopadhyay, B.D. Effect of nicotine on lipid profile, peroxidation & antioxidant enzymes in female rats with restricted dietary protein. Indian J. Med Res. 2008, 127, 127. [Google Scholar]
- Muthukumaran, S.; Sudheer, A.R.; Menon, V.P.; Nalini, N. Protective effect of quercetin on nicotine-induced prooxidant and antioxidant imbalance and DNA damage in Wistar rats. Toxicology 2008, 243, 207–215. [Google Scholar] [CrossRef]
- Zhang, J.; Jiang, S.; Watson, R.R. Antioxidant supplementation prevents oxidation and inflammatory responses induced by sidestream cigarette smoke in old mice. Environ. Health Perspect. 2001, 109, 1007–1009. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Tan, Y.L.; Zhou, D.F.; Haile, C.N.; Wu, G.Y.; Cao, L.Y.; A Kosten, T.; Kosten, T.R. Nicotine Dependence, Symptoms and Oxidative Stress in Male Patients with Schizophrenia. Neuropsychopharmacology 2007, 32, 2020–2024. [Google Scholar] [CrossRef]
- El-Shetry, E.S.; Mohamed, A.A.-R.; Khater, S.I.; Metwally, M.M.; Nassan, M.A.; Shalaby, S.; El-Mandrawy, S.A.; Bin Emran, T.; Abdel-Ghany, H.M. Synergistically enhanced apoptotic and oxidative DNA damaging pathways in the rat brain with lead and/or aluminum metals toxicity: Expression pattern of genes OGG1 and P53. J. Trace Elements Med. Biol. 2021, 68, 126860. [Google Scholar] [CrossRef]
- Mohamed, A.A.-R.; Rahman, A.A.; Salem, G.; Deib, M.; Nassan, M.; Rhouma, N.; Khater, S. The Antioxidant Role of a Taurine-Enriched Diet in Combating the Immunotoxic and Inflammatory Effects of Pyrethroids and/or Carbamates in Oreochromis niloticus. Animals 2021, 11, 1318. [Google Scholar] [CrossRef]
- Khanna, A.; Guo, M.; Mehra, M.; Royal, W. Inflammation and oxidative stress induced by cigarette smoke in Lewis rat brains. J. Neuroimmunol. 2013, 254, 69–75. [Google Scholar] [CrossRef] [Green Version]
- Cataldo, J.K.; Prochaska, J.J.; Glantz, S.A. Cigarette Smoking is a Risk Factor for Alzheimer’s Disease: An Analysis Controlling for Tobacco Industry Affiliation. J. Alzheimer’s Dis. 2010, 19, 465–480. [Google Scholar] [CrossRef] [Green Version]
- Barr, J.; Sharma, C.S.; Sarkar, S.; Wise, K.; Dong, L.; Periyakaruppan, A.; Ramesh, G.T. Nicotine induces oxidative stress and activates nuclear transcription factor kappa B in rat mesencephalic cells. Mol. Cell. Biochem. 2006, 297, 93–99. [Google Scholar] [CrossRef]
- Olasehinde, T.A.; Olaniran, A.O.; Okoh, A.I. Therapeutic Potentials of Microalgae in the Treatment of Alzheimer’s Disease. Molecules 2017, 22, 480. [Google Scholar] [CrossRef] [Green Version]
- Putteeraj, M.; Lim, W.L.; Teoh, S.L.; Yahaya, M.F. Flavonoids and its Neuroprotective Effects on Brain Ischemia and Neurodegenerative Diseases. Curr. Drug Targets 2018, 19, 1710–1720. [Google Scholar] [CrossRef]
- Ayaz, M.; Sadiq, A.; Junaid, M.; Ullah, F.; Ovais, M.; Ullah, I.; Ahmed, J.; Shahid, M. Flavonoids as Prospective Neuroprotectants and Their Therapeutic Propensity in Aging Associated Neurological Disorders. Front. Aging Neurosci. 2019, 11, 155. [Google Scholar] [CrossRef] [Green Version]
- Sun, L.; Zhang, J.; Lu, X.; Zhang, L.; Zhang, Y. Evaluation to the antioxidant activity of total flavonoids extract from persimmon (Diospyros kaki L.) leaves. Food Chem. Toxicol. 2011, 49, 2689–2696. [Google Scholar] [CrossRef]
- Wojdyło, A.; Oszmiański, J.; Czemerys, R. Antioxidant activity and phenolic compounds in 32 selected herbs. Food Chem. 2007, 105, 940–949. [Google Scholar] [CrossRef]
- Han, X.-Q.; Wu, X.-M.; Chai, X.-Y.; Chen, D.; Dai, H.; Dong, H.-L.; Ma, Z.-Z.; Gao, X.-M.; Tu, P.-F. Isolation, characterization and immunological activity of a polysaccharide from the fruit bodies of an edible mushroom, Sarcodon aspratus (Berk.) S. Ito. Food Res. Int. 2011, 44, 489–493. [Google Scholar] [CrossRef]
- Tanaka, K.; Yamada, A.; Noda, K.; Hasegawa, T.; Okuda, M.; Shoyama, Y.; Nomoto, K. A novel glycoprotein obtained from Chlorella vulgaris strain CK22 shows antimetastatic immunopotentiation. Cancer Immunol. Immunother. 1998, 45, 313–320. [Google Scholar] [CrossRef]
- El-Fayoumy, E.A.; Shanab, S.M.M.; Gaballa, H.S.; Tantawy, M.A.; Shalaby, E.A. Evaluation of antioxidant and anticancer activity of crude extract and different fractions of Chlorella vulgaris axenic culture grown under various concentrations of copper ions. BMC Complement. Med. Ther. 2021, 21, 1–16. [Google Scholar] [CrossRef]
- Nakashima, Y.; Ohsawa, I.; Konishi, F.; Hasegawa, T.; Kumamoto, S.; Suzuki, Y.; Ohta, S. Preventive effects of Chlorella on cognitive decline in age-dependent dementia model mice. Neurosci. Lett. 2009, 464, 193–198. [Google Scholar] [CrossRef]
- Wu, L.-C.; Ho, J.-A.A.; Shieh, M.-C.; Lu, I.-W. Antioxidant and Antiproliferative Activities of Spirulina and Chlorella Water Extracts. J. Agric. Food Chem. 2005, 53, 4207–4212. [Google Scholar] [CrossRef]
- Yun, H.; Kim, I.; Kwon, S.-H.; Kang, J.-S.; Om, A.-S. Protective Effect of Chlorella vulgaris against Lead-Induced Oxidative Stress in Rat Brains. J. Health Sci. 2011, 57, 245–254. [Google Scholar] [CrossRef] [Green Version]
- Spolaore, P.; Joannis-Cassan, C.; Duran, E.; Isambert, A. Commercial applications of microalgae. J. Biosci. Bioeng. 2006, 101, 87–96. [Google Scholar] [CrossRef] [Green Version]
- Safi, C.; Zebib, B.; Merah, O.; Pontalier, P.-Y.; Vaca-Garcia, C. Morphology, composition, production, processing and applications of Chlorella vulgaris: A review. Renew. Sustain. Energy Rev. 2014, 35, 265–278. [Google Scholar] [CrossRef] [Green Version]
- Vijayavel, K.; Anbuselvam, C.; Balasubramanian, M.P. Antioxidant effect of the marine algae Chlorella vulgaris against naphthalene-induced oxidative stress in the albino rats. Mol. Cell. Biochem. 2007, 303, 39–44. [Google Scholar] [CrossRef]
- Gurer, H.; Ercal, N. Can antioxidants be beneficial in the treatment of lead poisoning? Free. Radic. Biol. Med. 2000, 29, 927–945. [Google Scholar] [CrossRef]
- Abdel-Karim, O.H.; Gheda, S.F.; Ismail, G.A.; Abo-Shady, A.M. Phytochemical Screening and antioxidant activity of Chlorella vulgaris. Delta J. Sci. 2020, 41, 81–91. [Google Scholar] [CrossRef]
- Ru, I.T.K.; Sung, Y.Y.; Jusoh, M.; Wahid, M.E.A.; Nagappan, T. Chlorella vulgaris: A perspective on its potential for combining high biomass with high value bioproducts. Appl. Phycol. 2020, 1, 2–11. [Google Scholar] [CrossRef] [Green Version]


| Behavioral Tests | Experimental Groups | |||||
|---|---|---|---|---|---|---|
| Control | CV | Nicotine | Nicotine + CV | p-Value | ||
| Open field test | Ambulation frequency | 1.33 ± 0.88 c | 1.33 ± 0.33 c | 3.33 ± 0.33 a | 1.667 ± 0.33 b | 0.03 |
| Rearing frequency (anxiety-linked behaviour) | 0.662 ± 0.58 c | 0.667 ± 0.33 c | 3.663 ± 0.33 a | 1.333 ± 0.33 b | 0.034 | |
| Grooming frequency (anxiety-linked behaviour) | 1.000 ± 0.01 c | 1.001 ± 0.01 c | 2.333 ± 0.33 a | 1.333 ± 0.33 b | 0.044 | |
| Freezing time (Latency) (second) | 1.000 ± 0.02 c | 0.667 ± 0.01 c | 3.333 ± 0.34 a | 1.667 ± 0.14 b | 0.046 | |
| Inclined plain test | 46.667 ± 1.67 c | 48.333 ± 4.41 b | 45.33 ± 1.67 d | 50.667 ± 1.67 a | 0.034 | |
| Tail suspension test | 10.333 ± 0.88 b | 10.333 ± 0.33 b | 9.514 ± 0.58 c | 11.000 ± 0.58 a | 0.027 | |
| Posture reflex test | 0.000 ± 0.00 | 0.000 ± 0.00 | 1.000 ± 0.00 a | 0.333 ± 0.33 b | 0.041 | |
| Swimming performance test | 0.000 | 0.000 | 0.667 b | 1.667 a | 0.023 | |
| Oxidative Stress | SOD (U/mL) | CAT (U/L) | GSH (mmol/L) | MDA (nmol/mL) | PC (ng/mL) | Mortality |
|---|---|---|---|---|---|---|
| Control | 10.400 ± 1.16 a | 182.400 ± 5.26 a | 5.970 ± 0.68 a | 21.680 ± 1.22 c | 15.527 ± 0.35 c | 1/20 |
| CV | 11.340 ± 0.44 a | 193.333 ± 5.19 a | 6.890 ± 0.88 a | 19.157 ± 0.75 c | 12.967 ± 0.99 c | 0/20 |
| Nicotine | 3.017 ± 0.19 c | 84.900 ± 3.9 c | 1.573 ± 0.52 c | 57.607 ± 2.49 a | 130.600 ± 11.07 a | 9/20 |
| Nicotine + CV | 4.830 ± 0.57 b | 130.600 ± 11.07 b | 4.330 ± 0.52 b | 36.513 ± 0.62 b | 84.900 ± 3.9 b | 4/30 |
| Neurotransmitters in EAC | GABA (ng/mL) | DA(ng/mL) | SE (ng/mL) | AchE (mU/mL) |
| Control | 95.100 ± 7.13 c | 7.767 ± 0.76 c | 2.417 ± 0.49 c | 5.367 ± 0.56 c |
| CV | 92.467 ± 2.71 c | 7.017 ± 0.7 c | 2.587 ± 0.56 c | 4.215 ± 0.32 c |
| Nicotine | 106.667 ± 1.14 a | 23.267 ± 0.42 a | 12.977 ± 2.89 a | 8.067 ± 0.17 a |
| Nicotine + CV | 100.433 ± 2.21 bc | 13.333 ± 0.91 b | 5.790 ± 0.64 b | 5.741 ± 0.91 b |
| Inflammatory Response | TNFa (pg/mL) | IL-1B (pg/mL) | IL-10 (pg/mL) | 8-OHDG g/mol |
| Control | 324.567 ± 6.01 c | 451.367 ± 3.417 c | 100.367 ± 0.86 a | 4.957 ± 0.45 c |
| CV | 322.700 ± 5.1 c | 377.148 ± 3.12 d | 103.467 ± 1.1 a | 4.837 ± 0.36 c |
| Nicotine | 750.767 ± 3.29 a | 1309.533 ± 4.57 a | 29.200 ± 5.05 c | 14.933 ± 0.32 a |
| Nicotine + CV | 567.900 ± 11.2 b | 799.100 ± 5.09 b | 56.833 ± 0.95 b | 8.167 ± 0.65 b |
| Lesion | Control | CV | Nicotine | Nicotine + CV |
|---|---|---|---|---|
| Bcl-2 immunoexpression | 30.179 ± 0.23 b | 59.469 ± 0.85 a | 13.702 ± 0.21 c | 30.320 ± 0.37 b |
| Caspase 3 immunoexpression | 7.964 ± 0.18 c | 0.979 ± 0.09 d | 37.794 ± 0.53 a | 13.109 ± 0.24 b |
| Shrunken pyknotic neurons | 6.809 ± 0.22 c | 3.659 ± 0.27 d | 24.021 ± 0.44 a | 13.702 ± 0.2 b |
| Necrotic neurons | 2.969 ± 0.36 b | 1.325 ± 0.16 b | 10.936 ± 0.66 a | 7.505 ± 0.42 a |
| Perineural vacuolation | 6.039 ± 0.2 c | 2.980 ± 0.22 d | 21.824 ± 0.33 a | 11.949 ± 0.18 b |
| Neuropil microcavitation | 10.400 ± 0.2 b | 2.300 ± 0.22 c | 13.100 ± 0.37 a | 8.700 ± 0.24 b |
| Gliosis (astrocytosis and/or microgliosis) | 1.400 ± 0.16 b | 0.600 ± 0.1 b | 3.600 ± 0.23 a | 1.000 ± 0.11 b |
| Neuronophagia | 0.600 ± 0.1 ab | 0.200 ± 0.06 b | 1.400 ± 0.13 a | 0.400 ± 0.08 b |
| Cerebral congestion | 2.600 ± 0.16 b | 1.200 ± 0.1 b | 7.400 ± 0.67 a | 3.600 ± 0.16 b |
| Cerebral hemorrhage | 0.600 ± 0.1 b | 0.200 ± 0.06 b | 2.200 ± 0.15 a | 1.000 ± 0.11 b |
| Menengial congestion | 0.600 ± 0.1 ab | 0.400 ± 0.08 b | 1.400 ± 0.1 a | 0.400 ± 0.08 b |
| Meningeal hemorrhage | 0.400 ± 0.08 a | 0.200 ± 0.06 a | 1.000 ± 0.11 a | 0.400 ± 0.08 a |
| Bcl-2 immunoexpression | 30.179 ± 0.23 b | 59.469 ± 0.85 a | 13.702 ± 0.21 c | 30.320 ± 0.37 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohamed, A.A.-R.; Bohy, K.M.E.; Moustafa, G.G.; Mohammed, H.H.; Metwally, M.M.M.; Mohammed, H.E.D.; Nassan, M.A.; Saber, T.M. Sustained Functioning Impairments and Oxidative Stress with Neurobehavioral Dysfunction Associated with Oral Nicotine Exposure in the Brain of a Murine Model of Ehrlich Ascites Carcinoma: Modifying the Antioxidant Role of Chlorella vulgaris. Biology 2022, 11, 279. https://doi.org/10.3390/biology11020279
Mohamed AA-R, Bohy KME, Moustafa GG, Mohammed HH, Metwally MMM, Mohammed HED, Nassan MA, Saber TM. Sustained Functioning Impairments and Oxidative Stress with Neurobehavioral Dysfunction Associated with Oral Nicotine Exposure in the Brain of a Murine Model of Ehrlich Ascites Carcinoma: Modifying the Antioxidant Role of Chlorella vulgaris. Biology. 2022; 11(2):279. https://doi.org/10.3390/biology11020279
Chicago/Turabian StyleMohamed, Amany Abdel-Rahman, Khlood M. El Bohy, Gihan G. Moustafa, Hesham H. Mohammed, Mohamed M. M. Metwally, Heba El Desoukey Mohammed, Mohamed A. Nassan, and Taghred M. Saber. 2022. "Sustained Functioning Impairments and Oxidative Stress with Neurobehavioral Dysfunction Associated with Oral Nicotine Exposure in the Brain of a Murine Model of Ehrlich Ascites Carcinoma: Modifying the Antioxidant Role of Chlorella vulgaris" Biology 11, no. 2: 279. https://doi.org/10.3390/biology11020279
APA StyleMohamed, A. A.-R., Bohy, K. M. E., Moustafa, G. G., Mohammed, H. H., Metwally, M. M. M., Mohammed, H. E. D., Nassan, M. A., & Saber, T. M. (2022). Sustained Functioning Impairments and Oxidative Stress with Neurobehavioral Dysfunction Associated with Oral Nicotine Exposure in the Brain of a Murine Model of Ehrlich Ascites Carcinoma: Modifying the Antioxidant Role of Chlorella vulgaris. Biology, 11(2), 279. https://doi.org/10.3390/biology11020279






