Correlations between Electro-Diagnostic Findings, the Severity of Initial Infection, and the Rehabilitation Outcomes among COVID-19 Patients
Abstract
:Simple Summary
Abstract
1. Introduction
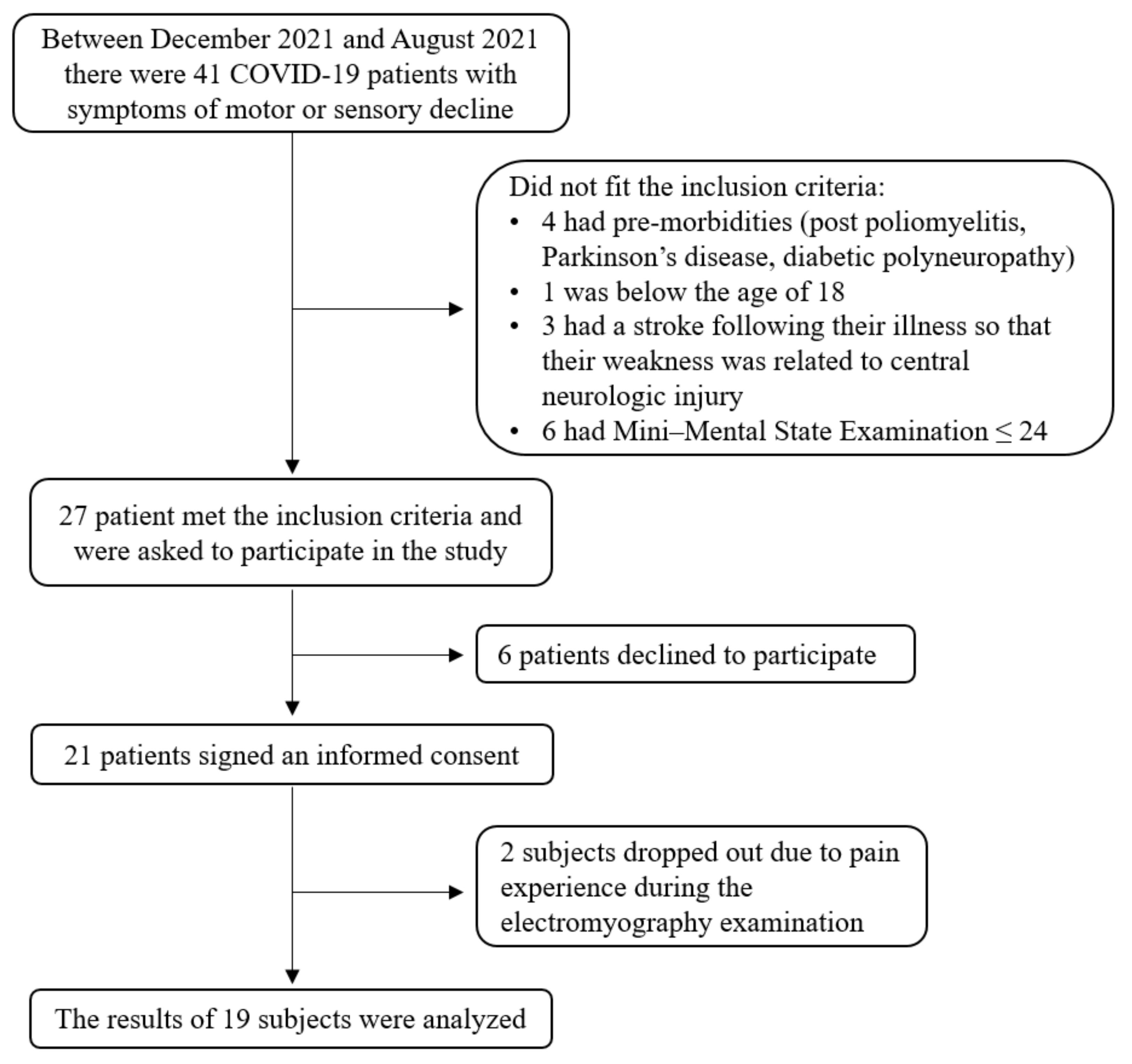
2. Methods and Materials
3. Results
3.1. Demographic Data (Descriptive Statistics)
3.2. Electrophysiological Findings
3.3. Rehabilitation Outcomes
3.4. Correlations between the WHO Clinical Progression Scale and Duration of Acute Hospitalization and Electrophysiological Factors
3.5. Correlations between the Rehabilitation Durations Functional and Electrophysiological Measurements
3.6. Regression Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mao, L.; Jin, H.; Wang, M.; Hu, Y.; Chen, S.; He, Q.; Chang, J.; Hong, C.; Zhou, Y.; Wang, D.; et al. Neurologic Manifestations of Hospitalized Patients With Coronavirus Disease 2019 in Wuhan, China. JAMA Neurol. 2020, 77, 683–690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Correia, A.O.; Feitosa, P.W.G.; de Moreira, J.L.S.; Nogueira, S.Á.R.; Fonseca, R.B.; Nobre, M.E.P. Neurological manifestations of COVID-19 and other coronaviruses: A systematic review. Neurol. Psychiatry Brain Res. 2020, 37, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Román, G.C.; Spencer, P.S.; Reis, J.; Buguet, A.; Faris, M.E.A.; Katrak, S.M.; Láinez, M.; Medina, M.T.; Meshram, C.; Mizusawa, H.; et al. The neurology of COVID-19 revisited: A proposal from the Environmental Neurology Specialty Group of the World Federation of Neurology to implement international neurological registries. J. Neurol. Sci. 2020, 414, 116884. [Google Scholar] [CrossRef] [PubMed]
- Maury, A.; Lyoubi, A.; Peiffer-Smadja, N.; de Broucker, T.; Meppiel, E. Neurological manifestations associated with SARS-CoV-2 and other coronaviruses: A narrative review for clinicians. Rev. Neurol. 2021, 177, 51–64. [Google Scholar] [CrossRef] [PubMed]
- Bagnato, S.; Ferraro, M.; Boccagni, C.; Battaglia, G.; D’Agostino, T.; Prestandrea, C.; Bellavia, M.A.; Rubino, F. COVID-19 Neuromuscular Involvement in Post-Acute Rehabilitation. Brain Sci. 2021, 11, 1611. [Google Scholar] [CrossRef] [PubMed]
- Miller, C.; O’Sullivan, J.; Jeffrey, J.; Power, D. Brachial Plexus Neuropathies During the COVID-19 Pandemic: A Retrospective Case Series of 15 Patients in Critical Care. Phys. Ther. 2021, 101, pzaa191. [Google Scholar] [CrossRef] [PubMed]
- Hameed, S.; Khan, A.F.; Khan, S. Electrodiagnostic findings in COVID-19 patients: A single center experience. Clin. Neurophysiol. 2021, 132, 3019–3024. [Google Scholar] [CrossRef]
- Bagnato, S.; Boccagni, C.; Marino, G.; Prestandrea, C.; D’Agostino, T.; Rubino, F. Critical illness myopathy after COVID-19. Int. J. Infect. Dis. 2020, 99, 276–278. [Google Scholar] [CrossRef] [PubMed]
- Finsterer, J.; Scorza, F.A.; Scorza, C.A.; Fiorini, C. Peripheral neuropathy in COVID-19 is due to immune-mechanisms, pre-existing risk factors, anti-viral drugs, or bedding in the Intensive Care Unit. Arq. Neuropsiquiatr. 2021, 79, 924–928. [Google Scholar] [CrossRef] [PubMed]
- Paliwal, V.K.; Garg, R.K.; Gupta, A.; Tejan, N. Neuromuscular presentations in patients with COVID-19. Neurol. Sci. 2020, 41, 3039–3056. [Google Scholar] [CrossRef] [PubMed]
- Tankisi, H.; de Carvalho, M.; ZʼGraggen, W.J. Critical Illness Neuropathy. J. Clin. Neurophysiol. 2020, 37, 205–207. [Google Scholar] [CrossRef] [PubMed]
- Cabañes-Martínez, L.; Villadóniga, M.; González-Rodríguez, L.; Araque, L.; Díaz-Cid, A.; Ruz-Caracuel, I.; Pian, H.; Sánchez-Alonso, S.; Fanjul, S.; del Álamo, M.; et al. Neuromuscular involvement in COVID-19 critically ill patients. Clin. Neurophysiol. 2020, 131, 2809–2816. [Google Scholar] [CrossRef] [PubMed]
- Santiago-Pérez, S.; Espinosa-García, S.; Martínez-Pérez, A.; Herráez-Sánchez, E.; Rizea, C.; Ruiz-Ávila, L.A. Neurophysiological Findings in Critical COVID-19 Patients With Peripheral Nervous System Manifestations. J. Clin. Neurophysiol. 2021. [Google Scholar] [CrossRef]
- Yu, C.; Helwig, E.J. Role of rehabilitation amidst the COVID-19 pandemic: A review. J. Transl. Med. 2021, 19, 376. [Google Scholar] [CrossRef] [PubMed]
- Albu, S.; Rivas Zozaya, N.; Murillo, N.; García-Molina, A.; Figueroa Chacón, C.A.; Kumru, H. Multidisciplinary outpatient rehabilitation of physical and neurological sequelae and persistent symptoms of covid-19: A prospective, observational cohort study. Disabil. Rehabil. 2021. [Google Scholar] [CrossRef] [PubMed]
- Piquet, V.; Luczak, C.; Seiler, F.; Monaury, J.; Martini, A.; Ward, A.B.; Gracies, J.M.; Motavasseli, D.; Lépine, E.; Chambard, L.; et al. Do Patients With COVID-19 Benefit from Rehabilitation? Functional Outcomes of the First 100 Patients in a COVID-19 Rehabilitation Unit. Arch. Phys. Med. Rehabil. 2021, 102, 1067–1074. [Google Scholar] [CrossRef] [PubMed]
- Abramoff, B.A.; Dillingham, T.R.; Caldera, F.E.; Ritchie, M.D.; Pezzin, L.E. Inpatient Rehabilitation Outcomes After Severe COVID-19 Infections: A Retrospective Cohort Study. Am. J. Phys. Med. Rehabil. 2021, 100, 1109–1114. [Google Scholar] [CrossRef]
- Imamura, M.; Mirisola, A.R.; Ribeiro, F.D.Q.; de Pretto, L.R.; Alfieri, F.M.; Delgado, V.R.; Battistella, L.R. Rehabilitation of patients after COVID-19 recovery: An experience at the Physical and Rehabilitation Medicine Institute and Lucy Montoro Rehabilitation Institute. Clinics 2021, 76, 1–9. [Google Scholar] [CrossRef]
- Ozyemisci Taskiran, O.; Turan, Z.; Tekin, S.; Senturk, E.; Topaloglu, M.; Yurdakul, F.; Ergonul, O.; Cakar, N. Physical rehabilitation in Intensive Care Unit in acute respiratory distress syndrome patients with COVID-19. Eur. J. Phys. Rehabil. Med. 2021, 57, 434–442. [Google Scholar] [CrossRef] [PubMed]
- Marshall, J.C.; Murthy, S.; Diaz, J.; Adhikari, N.; Angus, D.C.; Arabi, Y.M.; Baillie, K.; Bauer, M.; Berry, S.; Blackwood, B.; et al. A minimal common outcome measure set for COVID-19 clinical research. Lancet. Infect. Dis. 2020, 20, e192–e197. [Google Scholar] [CrossRef]
- Ojeda, A.; Calvo, A.; Cuñat, T.; Mellado Artigas, R.; Comino-Trinidad, O.; Aliaga, J.; Arias, M.; Ferrando, C.; Martínez, G.; Dürsteler, C. Characteristics and influence on quality of life of New-onset Pain in critical COVID-19 survivors. Eur. J. Pain 2021. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, B. Differential diagnosis: Nociceptive and neuropathic pain. Am. J. Manag. Care 2006, 12, S256–S262. [Google Scholar] [PubMed]
- Beninato, M.; Gill-Body, K.M.; Salles, S.; Stark, P.C.; Black-Schaffer, R.M.; Stein, J. Determination of the minimal clinically important difference in the FIM instrument in patients with stroke. Arch. Phys. Med. Rehabil. 2006, 87, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Perera, S.; Mody, S.H.; Woodman, R.C.; Studenski, S.A. Meaningful change and responsiveness in common physical performance measures in older adults. J. Am. Geriatr. Soc. 2006, 54, 743–749. [Google Scholar] [CrossRef]
- Werle, S.; Goldhahn, J.; Drerup, S.; Simmen, B.R.; Sprott, H.; Herren, D.B. Age- and gender-specific normative data of grip and pinch strength in a healthy adult Swiss population. J. Hand Surg. Eur. Vol. 2009, 34, 76–84. [Google Scholar] [CrossRef]
- Fritz, C.O.; Morris, P.E.; Richler, J.J. Effect size estimates: Current use, calculations, and interpretation. J. Exp. Psychol. Gen. 2012, 141, 2–18. [Google Scholar] [CrossRef] [Green Version]
- Agergaard, J.; Leth, S.; Pedersen, T.H.; Harbo, T.; Blicher, J.U.; Karlsson, P.; Østergaard, L.; Andersen, H.; Tankisi, H. Myopathic changes in patients with long-term fatigue after COVID-19. Clin. Neurophysiol. 2021, 132, 1974–1981. [Google Scholar] [CrossRef]
- Villa, D.; Ardolino, G.; Borellini, L.; Cogiamanian, F.; Vergari, M.; Savojardo, V.; Peyvandi, F.; Barbieri, S. Subclinical myopathic changes in COVID-19. Neurol. Sci. 2021, 42, 3973–3979. [Google Scholar] [CrossRef]
- Chang, L.G.; Zar, S.; Seidel, B.; Kurra, A.; Gitkind, A. COVID-19 Proned Ventilation and Its Possible Association With Foot Drop: A Case Series. Cureus 2021, 13, e14374. [Google Scholar] [CrossRef]
- Malik, G.R.; Wolfe, A.R.; Soriano, R.; Rydberg, L.; Wolfe, L.F.; Deshmukh, S.; Ko, J.H.; Nussbaum, R.P.; Dreyer, S.D.; Jayabalan, P.; et al. Injury-prone: Peripheral nerve injuries associated with prone positioning for COVID-19-related acute respiratory distress syndrome. Br. J. Anaesth. 2020, 125, e478–e480. [Google Scholar] [CrossRef]
- Daia, C.; Toader, C.; Scheau, C.; Onose, G. Motor demyelinating tibial neuropathy in COVID-19. J. Formos. Med. Assoc. 2021, 120, 2032–2036. [Google Scholar] [CrossRef] [PubMed]
- Simonelli, C.; Paneroni, M.; Vitacca, M.; Ambrosino, N. Measures of physical performance in COVID-19 patients: A mapping review. Pulmonology 2021, 27, 518–528. [Google Scholar] [CrossRef] [PubMed]
- Rooney, S.; Webster, A.; Paul, L. Systematic Review of Changes and Recovery in Physical Function and Fitness After Severe Acute Respiratory Syndrome-Related Coronavirus Infection: Implications for COVID-19 Rehabilitation. Phys. Ther. 2020, 100, 1717–1729. [Google Scholar] [CrossRef] [PubMed]
- Lopez, S.; Bini, F.; Del Percio, C.; Marinozzi, F.; Celletti, C.; Suppa, A.; Ferri, R.; Staltari, E.; Camerota, F.; Babiloni, C. Electroencephalographic sensorimotor rhythms are modulated in the acute phase following focal vibration in healthy subjects. Neuroscience 2017, 352, 236–248. [Google Scholar] [CrossRef]
- Mohamed, M.S.; Moulin, T.C.; Schiöth, H.B. Sex differences in COVID-19: The role of androgens in disease severity and progression. Endocrine 2021, 71, 3–8. [Google Scholar] [CrossRef]
| Measure | Wrist | Ankle | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Median Nerve | Ulnar Nerve | Sural Nerve | Peroneal Nerve | Tibial Nerve | ||||||
| Total (n = 19) | With Myopathy (n = 9) | Total (n = 19) | With Myopathy (n = 9) | Total (n = 19) | With Myopathy (n = 9) | Total (n = 19) | With Myopathy (n = 9) | Total (n = 19) | With Myopathy (n = 9) | |
| MNC latency (ms) | 5 (26.3%) | 2 (22.2%) | 4 (21.1%) | 1 (11.1%) | - | - | 5 (26.3%) | 2 (22.2%) | 3 (15.8%) | 1 (11.1%) |
| MNC velocity (m/s) | 3 (15.8%) | 1 (11.1%) | 4 (21.1%) | 2 (22.2%) | - | - | 7 (36.8%) | 6 (66.7%) | 10 (52.6%) | 6 (66.7%) |
| MNC amplitude (mV) | 5 (26.3%) | 2 (22.2%) | 9 (47.4%) | 4 (44.4%) | - | - | 14 (73.7%) | 7 (77.8%) | 13 (68.4%) | 8 (%88.9) |
| SNC velocity (ms) | 5 (26.3%) | 5 (55.6%) | 7 (36.8%) | 4 (44.4%) | 10 (52.6%) | 6 (66.7%) | - | - | - | - |
| SNC amplitude (µV) | 5 (26.3%) | 3 (33.3%) | 6 (31.6%) | 4 (44.4%) | 11 (57.9%) | 7 (77.8%) | - | - | - | - |
| Measure | Baseline | At Discharge | p | r |
|---|---|---|---|---|
| Cognitive FIM | 33 (30–35) | 34 (31–35) | 0.011 | −0.580 |
| Motor FIM | 75 (66–88) | 89 (84–90) | <0.001 | −0.815 |
| General FIM | 109 (100–119) | 121 (116–123) | <0.001 | −0.855 |
| Hand grip force (%) | 41.5 (16.6–49.5) | 51.5 (34.0–74.5) | <0.001 | −0.854 |
| 6MWT (m) | 180 (100–280) | 335 (285–429) | <0.001 | −0.877 |
| 10MWT (s) | 13 (9–16) | 8 (8–10) | <0.001 | −0.857 |
| Parameter Type | Nerve | WHO Clinical Progression Scale | Weeks of Acute Hospitalization |
|---|---|---|---|
| MNC latency (ms) | Median nerve | 0.595, 0.009 | 0.517, 0.049 |
| Ulnar nerve | 0.233, 0.353 | 0.071, 0.802 | |
| Peroneal nerve | −0.064, 0.800 | −0.281, 0.310 | |
| Tibial nerve | −0.042, 0.870 | 0.009, 0.975 | |
| MNC velocity (m/s) | Median nerve | −0.563, 0.012 | −0.008, 0.976 |
| Ulnar nerve | −0.113, 0.644 | −0.143, 0.598 | |
| Peroneal nerve | −0.131, 0.593 | −0.218, 0.416 | |
| Tibial nerve | −0.049, 0.841 | −0.092, 0.736 | |
| MNC amplitude (mV) | Median nerve | −0.474, 0.040 | −0.511, 0.043 |
| Ulnar nerve | −0.138, 0.572 | −0.220, 0.414 | |
| Peroneal nerve | −0.233, 0.336 | −0.429, 0.097 | |
| Tibial nerve | −0.489, 0.034 | −0.255, 0.340 | |
| SNC velocity (ms) | Median nerve | −0.193, 0.428 | −0.313, 0.238 |
| Ulnar nerve | −0.101, 0.692 | −0.179, 0.506 | |
| Sural nerve | −0.101, 0.691 | −0.179, 0.506 | |
| SNC amplitude (µV) | Median nerve | −0.428, 0.068 | −0.077, 0.778 |
| Ulnar nerve | −0.139, 0.570 | 0.095, 0.726 | |
| Sural nerve | −0.298, 0.216 | −0.283, 0.288 |
| Parameter Type | Nerve | Months of Rehabilitation | Grip Force | Motor FIM | 6MWT | 10MWT |
|---|---|---|---|---|---|---|
| MNC latency (ms) | Median nerve | 0.464, 0.053 | 0.210, 0.403 | −0.246, 0.326 | −0.160, 0.525 | 0.301, 0.225 |
| Ulnar nerve | 0.227, 0.365 | −0.015, 0.953 | −0.244, 0.329 | −0.195, 0.439 | 0.215, 0.391 | |
| Peroneal nerve | 0.014, 0.956 | 0.177, 0.483 | −0.237, 0.344 | −0.141, 0.578 | −0.171, 0.497 | |
| Tibial nerve | −0.111, 0.660 | 0.074, 0.771 | −0.493, 0.038 | −0.425, 0.079 | 0.290, 0.244 | |
| MNC velocity (m/s) | Median nerve | −0.376, 0.122 | 0.017, 0.946 | 0.540, 0.017 | 0.008, 0.974 | 0.028, 0.909 |
| Ulnar nerve | −0.063, 0.797 | 0.233, 0.337 | 0.299. 0.213 | −0.121, 0.623 | 0.371, 0.118 | |
| Peroneal nerve | −0.377, 0.111 | −0.093, 0.705 | 0.100, 0.685 | 0.039, 0.875 | −0.462, 0.047 | |
| Tibial nerve | −0.199, 0.414 | −0.014, 0.956 | 0.530, 0.020 | 0.278, 0.249 | −0.321, 0.181 | |
| MNC amplitude (mV) | Median nerve | −0.609, 0.006 | 0.183, 0.453 | 0.525, 0.021 | 0.203, 0.405 | 0.025, 0.918 |
| Ulnar nerve | −0.627, 0.004 | 0.000, 0.999 | 0.377, 0.112 | 0.379, 0.110 | −0.341, 0.153 | |
| Peroneal nerve | −0.534, 0.019 | 0.181, 0.459 | 0.590, 0.008 | 0.423, 0.071 | −0.056, 0.820 | |
| Tibial nerve | −0.479, 0.038 | 0.075, 0.760 | 0.535, 0.018 | 0.161, 0.511 | −0.370, 0.119 | |
| SNC velocity (ms) | Median nerve | −0.594, 0.007 | 0.161, 0.509 | 0.599, 0.007 | 0.384, 0.104 | −0.476, 0.040 |
| Ulnar nerve | −0.475, 0.046 | 0.057, 0.822 | 0.523, 0.026 | 0.371, 0.129 | −0.229, 0.360 | |
| Sural nerve | −0.402, 0.088 | 0.193, 0.429 | 0.397, 0.092 | 0.156, 0.524 | −0.301, 0.210 | |
| SNC amplitude (µV) | Median nerve | −0.562, 0.012 | −0.089, 0.718 | 0.285, 0.237 | 0.064, 0.796 | 0.082, 0.738 |
| Ulnar nerve | −0.017, 0.945 | 0.250, 0.302 | 0.089, 0.718 | −0.212, 0.383 | 0.189, 0.439 | |
| Sural nerve | −0.391, 0.098 | 0.141, 0.566 | 0.463, 0.046 | 0.244, 0.315 | −0.127, 0.605 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shabat, S.; Meiner, Z.; Tsenter, J.; Schwartz, I.; Portnoy, S. Correlations between Electro-Diagnostic Findings, the Severity of Initial Infection, and the Rehabilitation Outcomes among COVID-19 Patients. Biology 2022, 11, 277. https://doi.org/10.3390/biology11020277
Shabat S, Meiner Z, Tsenter J, Schwartz I, Portnoy S. Correlations between Electro-Diagnostic Findings, the Severity of Initial Infection, and the Rehabilitation Outcomes among COVID-19 Patients. Biology. 2022; 11(2):277. https://doi.org/10.3390/biology11020277
Chicago/Turabian StyleShabat, Sheer, Zeev Meiner, Jeanna Tsenter, Isabella Schwartz, and Sigal Portnoy. 2022. "Correlations between Electro-Diagnostic Findings, the Severity of Initial Infection, and the Rehabilitation Outcomes among COVID-19 Patients" Biology 11, no. 2: 277. https://doi.org/10.3390/biology11020277
APA StyleShabat, S., Meiner, Z., Tsenter, J., Schwartz, I., & Portnoy, S. (2022). Correlations between Electro-Diagnostic Findings, the Severity of Initial Infection, and the Rehabilitation Outcomes among COVID-19 Patients. Biology, 11(2), 277. https://doi.org/10.3390/biology11020277







